Rare Drivers at Low Prevalence with High Cancer Effects in T-Cell and B-Cell Pediatric Acute Lymphoblastic Leukemia
Abstract
1. Introduction
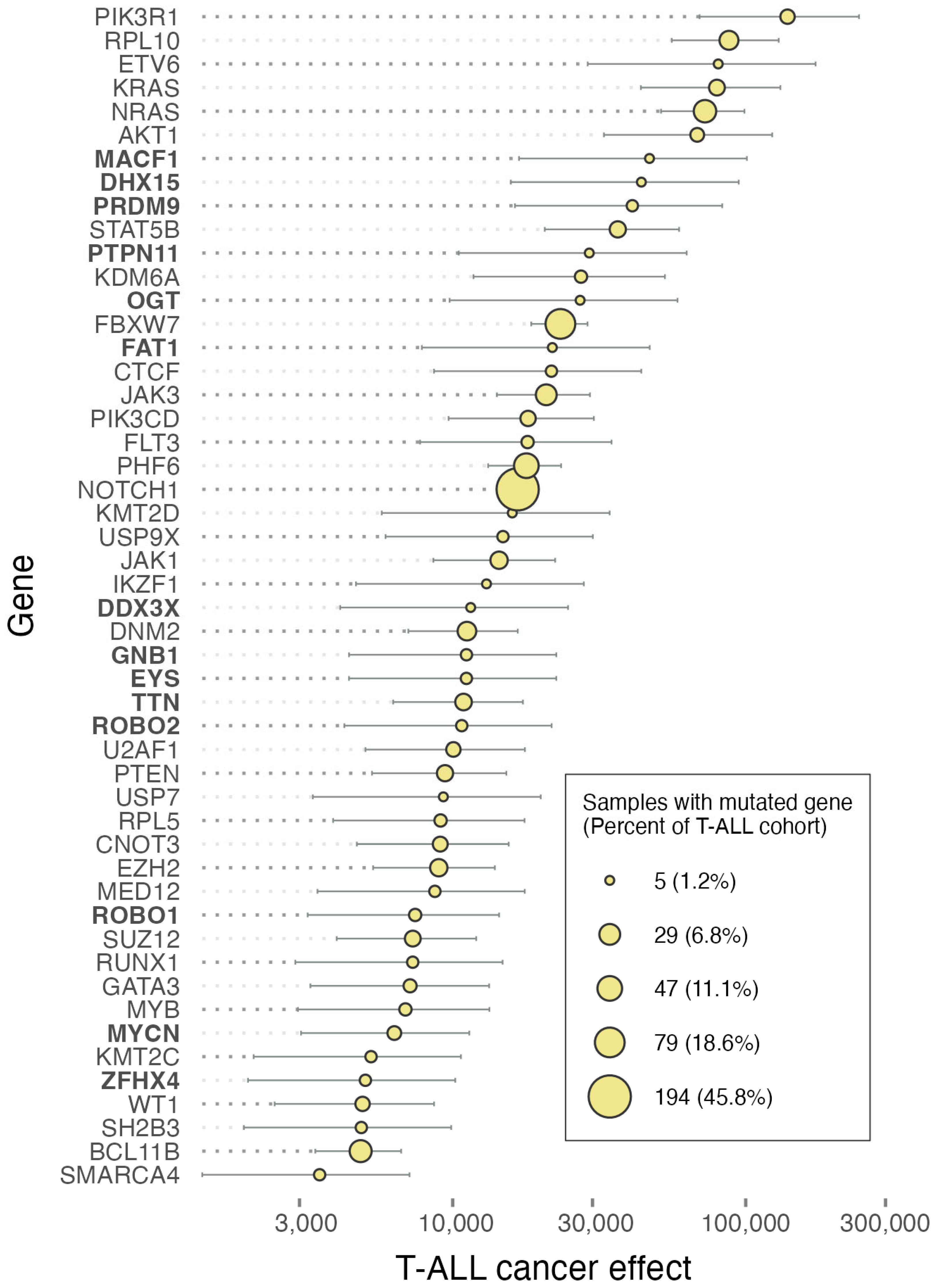
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brady, S.W.; Roberts, K.G.; Gu, Z.; Shi, L.; Pounds, S.; Pei, D.; Cheng, C.; Dai, Y.; Devidas, M.; Qu, C.; et al. The Genomic Landscape of Pediatric Acute Lymphoblastic Leukemia. Nat. Genet. 2022, 54, 1376–1389. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Easton, J.; Shao, Y.; Maciaszek, J.; Wang, Z.; Wilkinson, M.R.; McCastlain, K.; Edmonson, M.; Pounds, S.B.; Shi, L.; et al. The Genomic Landscape of Pediatric and Young Adult T-Lineage Acute Lymphoblastic Leukemia. Nat. Genet. 2017, 49, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Wang, B.-Y.; Zhang, W.-N.; Huang, J.-Y.; Li, B.-S.; Zhang, M.; Jiang, L.; Li, J.-F.; Wang, M.-J.; Dai, Y.-J.; et al. Genomic Profiling of Adult and Pediatric B-Cell Acute Lymphoblastic Leukemia. eBioMedicine 2016, 8, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Cannataro, V.L.; Gaffney, S.G.; Townsend, J.P. Effect Sizes of Somatic Mutations in Cancer. J. Natl. Cancer Inst. 2018, 110, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Churchman, M.L.; Roberts, K.G.; Moore, I.; Zhou, X.; Nakitandwe, J.; Hagiwara, K.; Pelletier, S.; Gingras, S.; Berns, H.; et al. PAX5-Driven Subtypes of B-Progenitor Acute Lymphoblastic Leukemia. Nat. Genet. 2019, 51, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Cannataro, V.L.; Mandell, J.D.; Townsend, J.P. Attribution of Cancer Origins to Endogenous, Exogenous, and Preventable Mutational Processes. Mol. Biol. Evol. 2022, 39, msac084. [Google Scholar] [CrossRef] [PubMed]
- Mandell, J.D.; Cannataro, V.L.; Townsend, J.P. Estimation of Neutral Mutation Rates and Quantification of Somatic Variant Selection Using cancereffectsizeR. Cancer Res. 2023, 83, 500–505. [Google Scholar] [CrossRef]
- Silva, A.; Yunes, J.A.; Cardoso, B.A.; Martins, L.R.; Jotta, P.Y.; Abecasis, M.; Nowill, A.E.; Leslie, N.R.; Cardoso, A.A.; Barata, J.T. PTEN Posttranslational Inactivation and Hyperactivation of the PI3K/Akt Pathway Sustain Primary T Cell Leukemia Viability. J. Clin. Investig. 2008, 118, 3762–3774. [Google Scholar] [CrossRef] [PubMed]
- Girardi, T.; Vereecke, S.; Sulima, S.O.; Khan, Y.; Fancello, L.; Briggs, J.W.; Schwab, C.; de Beeck, J.O.; Verbeeck, J.; Royaert, J.; et al. The T-Cell Leukemia-Associated Ribosomal RPL10 R98S Mutation Enhances JAK-STAT Signaling. Leukemia 2018, 32, 809–819. [Google Scholar] [CrossRef]
- Wen, Z.; Yun, G.; Hebert, A.; Kong, G.; Ranheim, E.A.; Finn, R.; Rajagoplan, A.; Li, S.; Zhou, Y.; Yu, M.; et al. Nras Q61R/+ and Kras−/− Cooperate to Downregulate Rasgrp1 and Promote Lympho-Myeloid Leukemia in Early T-Cell Precursors. Blood 2021, 137, 3259–3271. [Google Scholar] [CrossRef]
- Mazzucchelli, R.I.; Riva, A.; Durum, S.K. The Human IL-7 Receptor Gene: Deletions, Polymorphisms and Mutations. Semin. Immunol. 2012, 24, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-M.; Kang, T.-I.; So, J.-S. Roles of XBP1s in Transcriptional Regulation of Target Genes. Biomedicines 2021, 9, 791. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Lee, S.H.; Yoon, S.-R.; Kim, M.S.; Piao, Z.-H.; Myung, P.-K.; Kim, T.-D.; Jung, H.; Choi, I. TOX Regulates the Differentiation of Human Natural Killer Cells from Hematopoietic Stem Cells in Vitro. Immunol. Lett. 2011, 136, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Maestre, L.; García-García, J.F.; Jiménez, S.; Reyes-García, A.I.; García-González, Á.; Montes-Moreno, S.; Arribas, A.J.; González-García, P.; Caleiras, E.; Banham, A.H.; et al. High-Mobility Group Box (TOX) Antibody a Useful Tool for the Identification of B and T Cell Subpopulations. PLoS ONE 2020, 15, e0229743. [Google Scholar] [CrossRef] [PubMed]
- Jenks, S.A.; Cashman, K.S.; Zumaquero, E.; Marigorta, U.M.; Patel, A.V.; Wang, X.; Tomar, D.; Woodruff, M.C.; Simon, Z.; Bugrovsky, R.; et al. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity 2018, 49, 725–739.e6. [Google Scholar] [CrossRef]
- Carr, R.M.; Vorobyev, D.; Lasho, T.; Marks, D.L.; Tolosa, E.J.; Vedder, A.; Almada, L.L.; Yurcheko, A.; Padioleau, I.; Alver, B.; et al. RAS Mutations Drive Proliferative Chronic Myelomonocytic Leukemia via a KMT2A-PLK1 Axis. Nat. Commun. 2021, 12, 2901. [Google Scholar] [CrossRef]
- Molina-Arcas, M.; Moore, C.; Rana, S.; van Maldegem, F.; Mugarza, E.; Romero-Clavijo, P.; Herbert, E.; Horswell, S.; Li, L.-S.; Janes, M.R.; et al. Development of Combination Therapies to Maximize the Impact of KRAS-G12C Inhibitors in Lung Cancer. Sci. Transl. Med. 2019, 11, eaaw7999. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, D.; Li, Z.; Li, X.; Jin, M.; Jia, N.; Cui, X.; Hu, G.; Tang, T.; Yu, Q. Pan-Cancer Analysis on the Role of PIK3R1 and PIK3R2 in Human Tumors. Sci. Rep. 2022, 12, 5924. [Google Scholar] [CrossRef]
- Tharin, Z.; Richard, C.; Derangère, V.; Ilie, A.; Arnould, L.; Ghiringhelli, F.; Boidot, R.; Ladoire, S. PIK3CA and PIK3R1 Tumor Mutational Landscape in a Pan-Cancer Patient Cohort and Its Association with Pathway Activation and Treatment Efficacy. Sci. Rep. 2023, 13, 4467. [Google Scholar] [CrossRef]
- Wang, X.; Ding, J.; Meng, L.-H. PI3K Isoform-Selective Inhibitors: Next-Generation Targeted Cancer Therapies. Acta Pharmacol. Sin. 2015, 36, 1170–1176. [Google Scholar] [CrossRef]
- Belli, C.; Repetto, M.; Anand, S.; Porta, C.; Subbiah, V.; Curigliano, G. The Emerging Role of PI3K Inhibitors for Solid Tumour Treatment and beyond. Br. J. Cancer 2023, 128, 2150–2162. [Google Scholar] [CrossRef] [PubMed]
- Barata, J.T. Anatomy of a Crime: How IL7R and NRAS Join Forces to Drive T-Cell Acute Lymphoblastic Leukemia. Haematologica 2024, 109, 1637–1639. [Google Scholar] [CrossRef] [PubMed]
- Winer, H.; Li, W.; Rodrigues, G.; Gower, T.; Meyer, T.J.; Hixon, J.; Durum, S.K. Mechanism of Co-Operation of Mutant α and Mutant in Acute Lymphoblastic Leukemia: Role of MYC. Haematologica 2023, 109, 1726–1740. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.O.L.; Cramer, S.D.; Winer, H.Y.; Hixon, J.A.; Li, W.; Yunes, J.A.; Durum, S.K. Mutations That Collaborate with IL-7Ra Signaling Pathways to Drive ALL. Adv. Biol. Regul. 2021, 80, 100788. [Google Scholar] [CrossRef] [PubMed]
- Dunna, N.R.; Vuree, S.; Anuradha, C.; Sailaja, K.; Surekha, D.; Digumarti, R.R.; Rao, V.R.; Yadav, S.K.; Reddy, R.; Vishnupriya, S. NRAS Mutations in de Novo Acute Leukemia: Prevalence and Clinical Significance. Indian J. Biochem. Biophys. 2014, 51, 207–210. [Google Scholar] [PubMed]
- Qian, L.; Chen, K.; Wang, C.; Chen, Z.; Meng, Z.; Wang, P. Targeting NRAS-Mutant Cancers with the Selective STK19 Kinase Inhibitor Chelidonine. Clin. Cancer Res. 2020, 26, 3408–3419. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Uribe, P.; Adrianzen-Ruesta, M.P.; Deng, Z.; Echevarria-Vargas, I.; Mender, I.; Saheb, S.; Liu, Q.; Altieri, D.C.; Murphy, M.E.; Shay, J.W.; et al. Exploiting TERT Dependency as a Therapeutic Strategy for NRAS-Mutant Melanoma. Oncogene 2018, 37, 4058–4072. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jiao, B.; Wang, P.; Zhang, B.; Gao, J.; Li, D.; Xie, X.; Yao, Y.; Yan, L.; Qin, Z.; et al. GOLGA7 Is Essential for NRAS Trafficking from the Golgi to the Plasma Membrane but Not for Its Palmitoylation. Cell Commun. Signal. 2024, 22, 98. [Google Scholar] [CrossRef] [PubMed]
- Vujic, I.; Posch, C.; Sanlorenzo, M.; Yen, A.J.; Tsumura, A.; Kwong, A.; Feichtenschlager, V.; Lai, K.; Arneson, D.V.; Rappersberger, K.; et al. Mutant NRASQ61 Shares Signaling Similarities across Various Cancer Types—Potential Implications for Future Therapies. Oncotarget 2014, 5, 7936–7944. [Google Scholar] [CrossRef]
- Sulima, S.O.; Kampen, K.R.; Vereecke, S.; Pepe, D.; Fancello, L.; Verbeeck, J.; Dinman, J.D.; De Keersmaecker, K. Ribosomal Lesions Promote Oncogenic Mutagenesis. Cancer Res. 2019, 79, 320–327. [Google Scholar] [CrossRef]
- Pollutri, D.; Penzo, M. Ribosomal Protein L10: From Function to Dysfunction. Cells 2020, 9, 2503. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, K.M.; Lindström, M.S. Role of Ribosomal Protein Mutations in Tumor Development (Review). Int. J. Oncol. 2016, 48, 1313–1324. [Google Scholar] [CrossRef]
- Bacci, L.; Indio, V.; Rambaldelli, G.; Bugarin, C.; Magliocchetti, F.; Del Rio, A.; Pollutri, D.; Melchionda, F.; Pession, A.; Lanciotti, M.; et al. Mutational Analysis of Ribosomal Proteins in a Cohort of Pediatric Patients with T-Cell Acute Lymphoblastic Leukemia Reveals Q123R, a Novel Mutation in RPL10. Front. Genet. 2022, 13, 1058468. [Google Scholar] [CrossRef]
- Kampen, K.R.; Sulima, S.O.; Verbelen, B.; Girardi, T.; Vereecke, S.; Rinaldi, G.; Verbeeck, J.; Op de Beeck, J.; Uyttebroeck, A.; Meijerink, J.P.P.; et al. The Ribosomal RPL10 R98S Mutation Drives IRES-Dependent BCL-2 Translation in T-ALL. Leukemia 2019, 33, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Bardelli, V.; Arniani, S.; Pierini, V.; Di Giacomo, D.; Pierini, T.; Gorello, P.; Mecucci, C.; La Starza, R. T-Cell Acute Lymphoblastic Leukemia: Biomarkers and Their Clinical Usefulness. Genes 2021, 12, 1118. [Google Scholar] [CrossRef]
- Alghandour, R.; Sakr, D.H.; Shaaban, Y. Philadelphia-like Acute Lymphoblastic Leukemia: The Journey from Molecular Background to the Role of Bone Marrow Transplant-Review Article. Ann. Hematol. 2023, 102, 1287–1300. [Google Scholar] [CrossRef]
- Campos, L.W.; Pissinato, L.G.; Yunes, J.A. Deleterious and Oncogenic Mutations in the IL7RA. Cancers 2019, 11, 1952. [Google Scholar] [CrossRef]
- Thomas, K.R.; Allenspach, E.J.; Camp, N.D.; Wray-Dutra, M.N.; Khim, S.; Zielinska-Kwiatkowska, A.; Timms, A.E.; Loftus, J.P.; Liggitt, H.D.; Georgopoulos, K.; et al. Activated Interleukin-7 Receptor Signaling Drives B-Cell Acute Lymphoblastic Leukemia in Mice. Leukemia 2022, 36, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.L.; Veloso, A.; Garcia, E.G.; Iyer, S.; Pereira, C.; Barreto, V.M.; Langenau, D.M.; Barata, J.T. Mutant IL7R Collaborates with MYC to Induce T-Cell Acute Lymphoblastic Leukemia. Leukemia 2022, 36, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.R.M.; Neto, J.L.; Cachucho, A.; Euzébio, M.; Meng, X.; Kim, R.; Fernandes, M.B.; Raposo, B.; Oliveira, M.L.; Ribeiro, D.; et al. Interleukin-7 Receptor α Mutational Activation Can Initiate Precursor B-Cell Acute Lymphoblastic Leukemia. Nat. Commun. 2021, 12, 7268. [Google Scholar] [CrossRef] [PubMed]
- Ojha, R.; Amaravadi, R.K. Targeting the Unfolded Protein Response in Cancer. Pharmacol. Res. 2017, 120, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Maly, D.J.; Papa, F.R. Druggable Sensors of the Unfolded Protein Response. Nat. Chem. Biol. 2014, 10, 892–901. [Google Scholar] [CrossRef]
- Salimi, A.; Schemionek-Reinders, M.; Huber, M.; Vieri, M.; Patterson, J.B.; Alten, J.; Brümmendorf, T.H.; Kharabi Masouleh, B.; Appelmann, I. XBP1 Promotes NRAS Pre-B Acute Lymphoblastic Leukaemia through IL-7 Receptor Signalling and Provides a Therapeutic Vulnerability for Oncogenic RAS. J. Cell. Mol. Med. 2023, 27, 3363–3377. [Google Scholar] [CrossRef] [PubMed]
- Lobbardi, R.; Pinder, J.; Martinez-Pastor, B.; Theodorou, M.; Blackburn, J.S.; Abraham, B.J.; Namiki, Y.; Mansour, M.; Abdelfattah, N.S.; Molodtsov, A.; et al. TOX Regulates Growth, DNA Repair, and Genomic Instability in T-Cell Acute Lymphoblastic Leukemia. Cancer Discov. 2017, 7, 1336–1353. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.I.; Cannataro, V.L.; Townsend, J.P.; Newman, S.; Stern, D.F.; Zhao, H. Identifying Modules of Cooperating Cancer Drivers. Mol. Syst. Biol. 2021, 17, e9810. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Murillo, J.A.; Townsend, J.P. Pairwise and Higher-Order Epistatic Effects among Somatic Cancer Mutations across Oncogenesis. Math. Biosci. 2023, 366, 109091. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Taghi, K.A.; Duault, C.; Aramburo, S.; Sanchez, O.A.; Lee, S.J.; Chan, A.; McDonald, T.; Huang, M.; Lacayo, N.J.; et al. Intrinsic Suppression of Type I Interferon Production Underlies the Therapeutic Efficacy of IL-15-Producing Natural Killer Cells in B-Cell Acute Lymphoblastic Leukemia. J. Immunother. Cancer 2023, 11, e006649. [Google Scholar] [CrossRef]
- Spinella, J.-F.; Cassart, P.; Richer, C.; Saillour, V.; Ouimet, M.; Langlois, S.; St-Onge, P.; Sontag, T.; Healy, J.; Minden, M.D.; et al. Genomic Characterization of Pediatric T-Cell Acute Lymphoblastic Leukemia Reveals Novel Recurrent Driver Mutations. Oncotarget 2016, 7, 65485–65503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, H.; Wang, H.; Qian, X.; Gao, S.; Xia, J.; Liu, J.; Cheng, Y.; Man, J.; Zhai, X. Genetic Mutational Analysis of Pediatric Acute Lymphoblastic Leukemia from a Single Center in China Using Exon Sequencing. BMC Cancer 2020, 20, 211. [Google Scholar] [CrossRef]
- Ueno, H.; Yoshida, K.; Shiozawa, Y.; Nannya, Y.; Iijima-Yamashita, Y.; Kiyokawa, N.; Shiraishi, Y.; Chiba, K.; Tanaka, H.; Isobe, T.; et al. Landscape of Driver Mutations and Their Clinical Impacts in Pediatric B-Cell Precursor Acute Lymphoblastic Leukemia. Blood Adv. 2020, 4, 5165–5173. [Google Scholar] [CrossRef]
- Martincorena, I.; Raine, K.M.; Gerstung, M.; Dawson, K.J.; Haase, K.; Van Loo, P.; Davies, H.; Stratton, M.R.; Campbell, P.J. Universal Patterns of Selection in Cancer and Somatic Tissues. Cell 2017, 171, 1029–1041.e21. [Google Scholar] [CrossRef] [PubMed]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehár, J.; Kryukov, G.V.; Sonkin, D.; et al. The Cancer Cell Line Encyclopedia Enables Predictive Modelling of Anticancer Drug Sensitivity. Nature 2012, 483, 603–607. [Google Scholar] [CrossRef]
- Roadmap Epigenomics Consortium; Kundaje, A.; Meuleman, W.; Ernst, J.; Bilenky, M.; Yen, A.; Heravi-Moussavi, A.; Kheradpour, P.; Zhang, Z.; Wang, J.; et al. Integrative Analysis of 111 Reference Human Epigenomes. Nature 2015, 518, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Manders, F.; Brandsma, A.M.; de Kanter, J.; Verheul, M.; Oka, R.; van Roosmalen, M.J.; van der Roest, B.; van Hoeck, A.; Cuppen, E.; van Boxtel, R. MutationalPatterns: The One Stop Shop for the Analysis of Mutational Processes. BMC Genom. 2022, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Kim, J.; Haradhvala, N.J.; Huang, M.N.; Tian Ng, A.W.; Wu, Y.; Boot, A.; Covington, K.R.; Gordenin, D.A.; Bergstrom, E.N.; et al. The Repertoire of Mutational Signatures in Human Cancer. Nature 2020, 578, 94–101. [Google Scholar] [CrossRef]
- Cannataro, V.L.; Gaffney, S.G.; Stender, C.; Zhao, Z.-M.; Philips, M.; Greenstein, A.E.; Townsend, J.P. Heterogeneity and Mutation in KRAS and Associated Oncogenes: Evaluating the Potential for the Evolution of Resistance to Targeting of KRAS G12C. Oncogene 2018, 37, 2444–2455. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandell, J.D.; Diviti, S.; Xu, M.; Townsend, J.P. Rare Drivers at Low Prevalence with High Cancer Effects in T-Cell and B-Cell Pediatric Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2024, 25, 6589. https://doi.org/10.3390/ijms25126589
Mandell JD, Diviti S, Xu M, Townsend JP. Rare Drivers at Low Prevalence with High Cancer Effects in T-Cell and B-Cell Pediatric Acute Lymphoblastic Leukemia. International Journal of Molecular Sciences. 2024; 25(12):6589. https://doi.org/10.3390/ijms25126589
Chicago/Turabian StyleMandell, Jeffrey D., Saathvika Diviti, Mina Xu, and Jeffrey P. Townsend. 2024. "Rare Drivers at Low Prevalence with High Cancer Effects in T-Cell and B-Cell Pediatric Acute Lymphoblastic Leukemia" International Journal of Molecular Sciences 25, no. 12: 6589. https://doi.org/10.3390/ijms25126589
APA StyleMandell, J. D., Diviti, S., Xu, M., & Townsend, J. P. (2024). Rare Drivers at Low Prevalence with High Cancer Effects in T-Cell and B-Cell Pediatric Acute Lymphoblastic Leukemia. International Journal of Molecular Sciences, 25(12), 6589. https://doi.org/10.3390/ijms25126589






