Antibacterial Effect of Spanish Honeys of Different Botanical Origins against Staphylococcus epidermidis
Abstract
1. Introduction
2. Results and Discussion
2.1. Composition and Characterisation of Honey Samples
2.2. Colour of Honey Samples
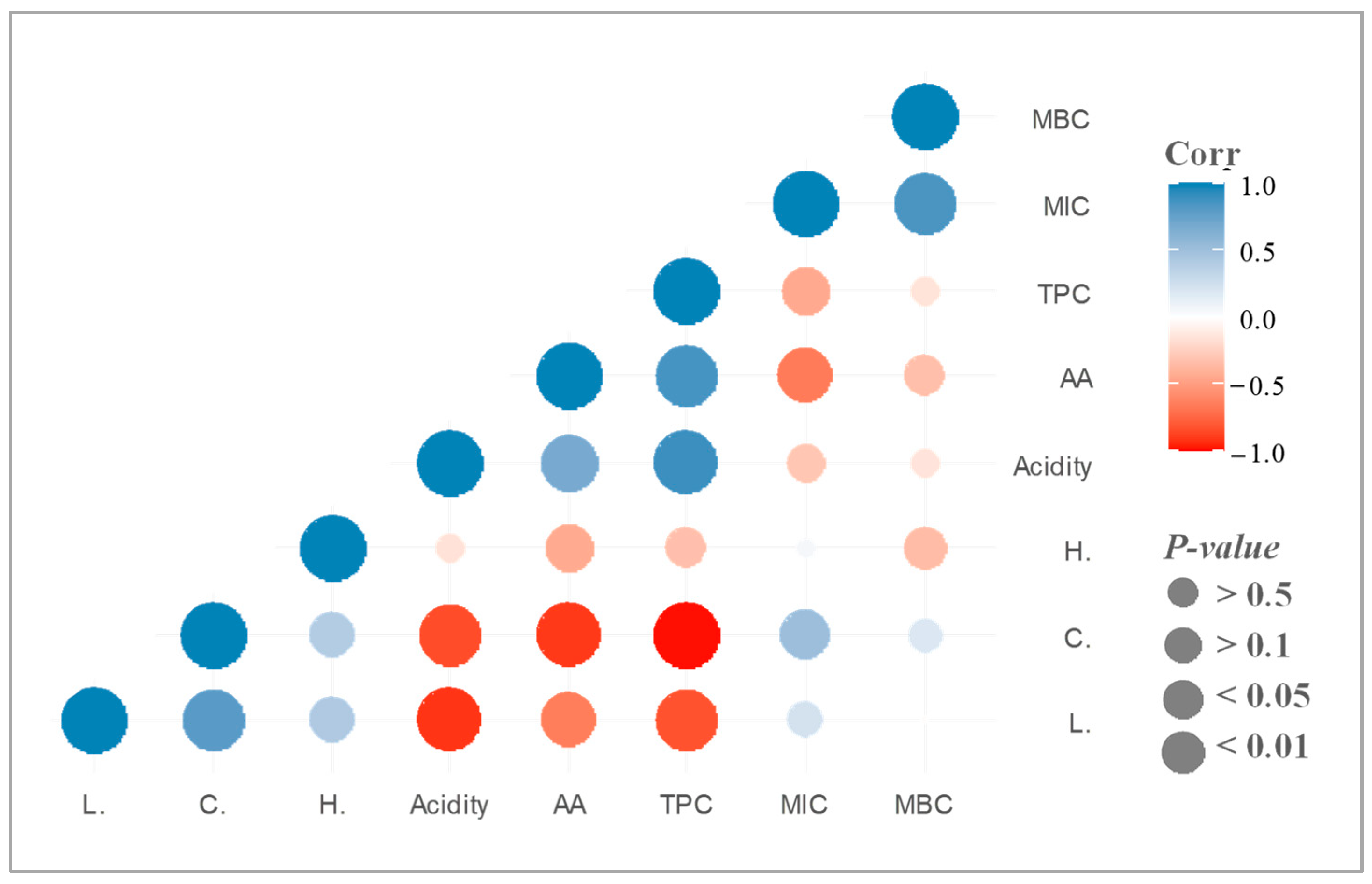
2.3. Total (Poly)phenol Content and Antioxidant Activity
2.4. Antibacterial Activity
3. Materials and Methods
3.1. Samples
3.2. Determination of pH and Acidity
3.3. Moisture Content and °Brix
3.4. Colour Determination by Pfund Scale and CIE Lab Colour Space
3.5. Total Phenolic Content and Antioxidant Capacity Analysis
3.6. Evaluation of the Antibacterial Activity
3.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eteraf-Oskouei, T.; Najafi, M. Traditional and Modern Uses of Natural Honey in Human Diseases: A Review. Iran. J. Basic Med. Sci. 2013, 16, 731. [Google Scholar] [PubMed]
- Escuredo, O.; Shantal Rodríguez-Flores, M.; Rojo-Martínez, S.; Seijo, M.C. Contribution to the Chromatic Characterization of Unifloral Honeys from Galicia (NW Spain). Foods 2019, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Terzo, S.; Mulè, F.; Amato, A. Honey and obesity-related dysfunctions: A summary on health benefits. J. Nutr. Biochem. 2020, 82, 108401. [Google Scholar] [CrossRef] [PubMed]
- Nyarko, K.; Boozer, K.; Greenlief, C.M. Profiling of the Polyphenol Content of Honey from Different Geographical Origins in the United States. Molecules 2023, 28, 5011. [Google Scholar] [CrossRef] [PubMed]
- Koulis, G.A.; Tsagkaris, A.S.; Aalizadeh, R.; Dasenaki, M.E.; Panagopoulou, E.I.; Drivelos, S.; Halagarda, M.; Georgiou, C.A.; Proestos, C.; Thomaidis, N.S. Honey Phenolic Compound Profiling and Authenticity Assessment Using HRMS Targeted and Untargeted Metabolomics. Molecules 2021, 26, 2769. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Datta, N.; Tomás-Barberán, F.A.; Ferreres, F.; Martos, I.; Singanusong, R. Flavonoids, phenolic acids and abscisic acid in Australian and New Zealand Leptospermum honeys. Food Chem. 2003, 81, 159–168. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Estevinho, L.M.; Dias, L.G.; Castro, J.J.; Tomás, F.A.; Barberán, T.-B.; Tornadijo, M.E.; Fresno-Baro, J.J. Bioactive Components and Antioxidant and Antibacterial Activities of Different Varieties of Honey: A Screening Prior to Clinical Application. J. Agric. Food Chem. 2018, 67, 688–698. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Junie, L.M.; Vică, M.L.; Glevitzky, M.; Matei, H.V. Physico-chemical characterisation and antibacterial activity of different types of honey tested on strains isolated from hospitalised patients. J. Apic. Sci. 2016, 60, 5–17. [Google Scholar] [CrossRef]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154. [Google Scholar] [CrossRef]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188. [Google Scholar] [CrossRef] [PubMed]
- Proaño, A.; Coello, D.; Villacrés-Granda, I.; Ballesteros, I.; Debut, A.; Vizuete, K.; Brenciani, A.; Álvarez-Suarez, J.M. The osmotic action of sugar combined with hydrogen peroxide and bee-derived antibacterial peptide Defensin-1 is crucial for the antibiofilm activity of eucalyptus honey. LWT 2021, 136, 110379. [Google Scholar] [CrossRef]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 2464507. [Google Scholar] [CrossRef] [PubMed]
- Masad, R.J.; Haneefa, S.M.; Mohamed, Y.A.; Al-Sbiei, A.; Bashir, G.; Fernandez-Cabezudo, M.J.; Al-Ramadi, B.K. The immunomodulatory effects of honey and associated flavonoids in cancer. Nutrients 2021, 13, 1269. [Google Scholar] [CrossRef]
- Otto, M. Molecular basis of Staphylococcus epidermidis infections. Semin. Immunopathol. 2012, 34, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Yaghoobi, R.; Kazerouni, A.; kazerouni, O. Evidence for Clinical Use of Honey in Wound Healing as an Anti-bacterial, Anti-inflammatory Anti-oxidant and Anti-viral Agent: A Review. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Tashkandi, H. Honey in wound healing: An updated review. Open Life Sci. 2021, 16, 1091. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; De Meyer, L.; Nelis, H.J.; Coenye, T. Biofilm inhibitory and eradicating activity of wound care products against staphylococcus aureus and staphylococcus epidermidis biofilms in an in vitro chronic wound model. J. Appl. Microbiol. 2013, 114, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Morroni, G.; Alvarez-Suarez, J.M.; Brenciani, A.; Simoni, S.; Fioriti, S.; Pugnaloni, A.; Giampieri, F.; Mazzoni, L.; Gasparrini, M.; Marini, E.; et al. Comparison of the antimicrobial activities of four honeys from three countries (New Zealand, Cuba, and Kenya). Front. Microbiol. 2018, 9, 382109. [Google Scholar] [CrossRef]
- Vîjan, L.E.; Mazilu, I.C.; Enache, C.; Enache, S.; Topală, C.M. Botanical Origin Influence on Some Honey Physicochemical Characteristics and Antioxidant Properties. Foods 2023, 12, 2134. [Google Scholar] [CrossRef]
- WHO. W.H.O. Standard for Honey; International Food Standards: Rome, Italy, 1981. [Google Scholar]
- Perez, C.; Jimeno, M.F. MANEJO Y ALTERACIONES DE LA MIEL; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1985; Num 13/85 1–16. LS.B.N.: 84-341-0500.4. [Google Scholar]
- Boletín Oficial del Estado. Real Decreto 1049/2003, de 1 de agosto, por el que se Aprueba la Norma de Calidad Relativa a la Miel; Ministerio de la Presidencia, Justicia y Relaciones con las Cortes: Madrid, Spain, 2003. [Google Scholar]
- Molan, P.C. The antibacterial activity of honey. Bee World 1992, 73, 5–28. [Google Scholar] [CrossRef]
- Szabó, R.T.; Mézes, M.; Szalai, T.; Zajácz, E.; Weber, M. Colour identification of honey and methodical development of its instrumental measuring. Columella J. Agric. Environ. Sci. 2016, 3, 29–36. [Google Scholar] [CrossRef]
- Starowicz, M.; Ostaszyk, A.; Zieliński, H. The relationship between the browning index, total phenolics, color, and antioxidant activity of polish-originated honey samples. Foods 2021, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Gośliński, M.; Nowak, D.; Kłębukowska, L. Antioxidant properties and antimicrobial activity of manuka honey versus Polish honeys. J. Food Sci. Technol. 2020, 57, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Can, Z.; Yildiz, O.; Sahin, H.; Akyuz Turumtay, E.; Silici, S.; Kolayli, S. An investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Ramos, A.; Gonçalves, M.M.; Bernardo, M.; Mendes, B. Antioxidant activity, quality parameters and mineral content of Portuguese monofloral honeys. J. Food Compos. Anal. 2013, 30, 130–138. [Google Scholar] [CrossRef]
- Osés, S.M.; Pascual-Maté, A.; de la Fuente, D.; de Pablo, A.; Fernández-Muiño, M.A.; Sancho, M.T. Comparison of methods to determine antibacterial activity of honeys against Staphylococcus aureus. NJAS-Wagening. J. Life Sci. 2016, 78, 29–33. [Google Scholar] [CrossRef]
- Grecka, K.; Kús, P.M.; Worobo, R.W.; Szweda, P. Study of the Anti-Staphylococcal Potential of Honeys Produced in Northern Poland. Molecules 2018, 23, 260. [Google Scholar] [CrossRef] [PubMed]
- Gkoutzouvelidou, M.; Panos, G.; Xanthou, M.N.; Papachristoforou, A.; Giaouris, E. Comparing the antimicrobial actions of greek honeys from the island of lemnos and manuka honey from new zealand against clinically important bacteria. Foods 2021, 10, 1402. [Google Scholar] [CrossRef]
- Cocinschi, V.V. Efectos Terapéuticos de la Miel de Abeja Tópica en el Tratamiento de Heridas. Revisión Bibliográfica; Universitat Rovira i Virgili: Tarragona, Spain, 2018. [Google Scholar]
- Molan, P.C. The evidence supporting the use of honey as a wound dressing. Int. J. Low. Extrem. Wounds 2006, 5, 40–54. [Google Scholar] [CrossRef]
- Basualdo, C.; Sgroy, V.; Finola, M.S.; Marioli, J.M. Comparison of the antibacterial activity of honey from different provenance against bacteria usually isolated from skin wounds. Vet. Microbiol. 2007, 124, 375–381. [Google Scholar] [CrossRef]
- Lobit, P.; Soing, P.; Génard, M.; Habib, R. Theoretical analysis of relationships between composition, ph, and titratable acidity of peach fruit. J. Plant Nutr. 2002, 25, 2775–2792. [Google Scholar] [CrossRef]
- Chataway, H.D. The determination of moisture in honey. Can. J. Res. 1932, 6, 532–547. [Google Scholar] [CrossRef]
- Chataway, H.D. Honey tables, showing the relationship between various hydrometer scales and refractive index to moisture content and weight per gallon of honey. Can. Bee J. 1935, 43, 215. [Google Scholar]
- Periago Castón, M.J.; Navarro González, I.; Alaminos, A.B.; Elvira Torales, L.I.; García Alonso, F.J. Paramétros de calidad en mieles de diferentes orígenes botánicos producidas en la alpujarra granadina. An. Vet. Murcia 2016, 32, 59–71. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 114–158. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16. [Google Scholar] [CrossRef]
- McLoone, P.; Zhumbayeva, A.; Yunussova, S.; Kaliyev, Y.; Yevstafeva, L.; Verrall, S.; Sungurtas, J.; Austin, C.; Allwood, J.W.; McDougall, G.J. Identification of components in Kazakhstan honeys that correlate with antimicrobial activity against wound and skin infecting microorganisms. BMC Complement. Med. Ther. 2021, 21, 300. [Google Scholar] [CrossRef]
- Green, K.J.; Dods, K.; Hammer, K.A. Development and validation of a new microplate assay that utilises optical density to quantify the antibacterial activity of honeys including Jarrah, Marri and Manuka. PLoS ONE 2020, 15, e0243246. [Google Scholar] [CrossRef]
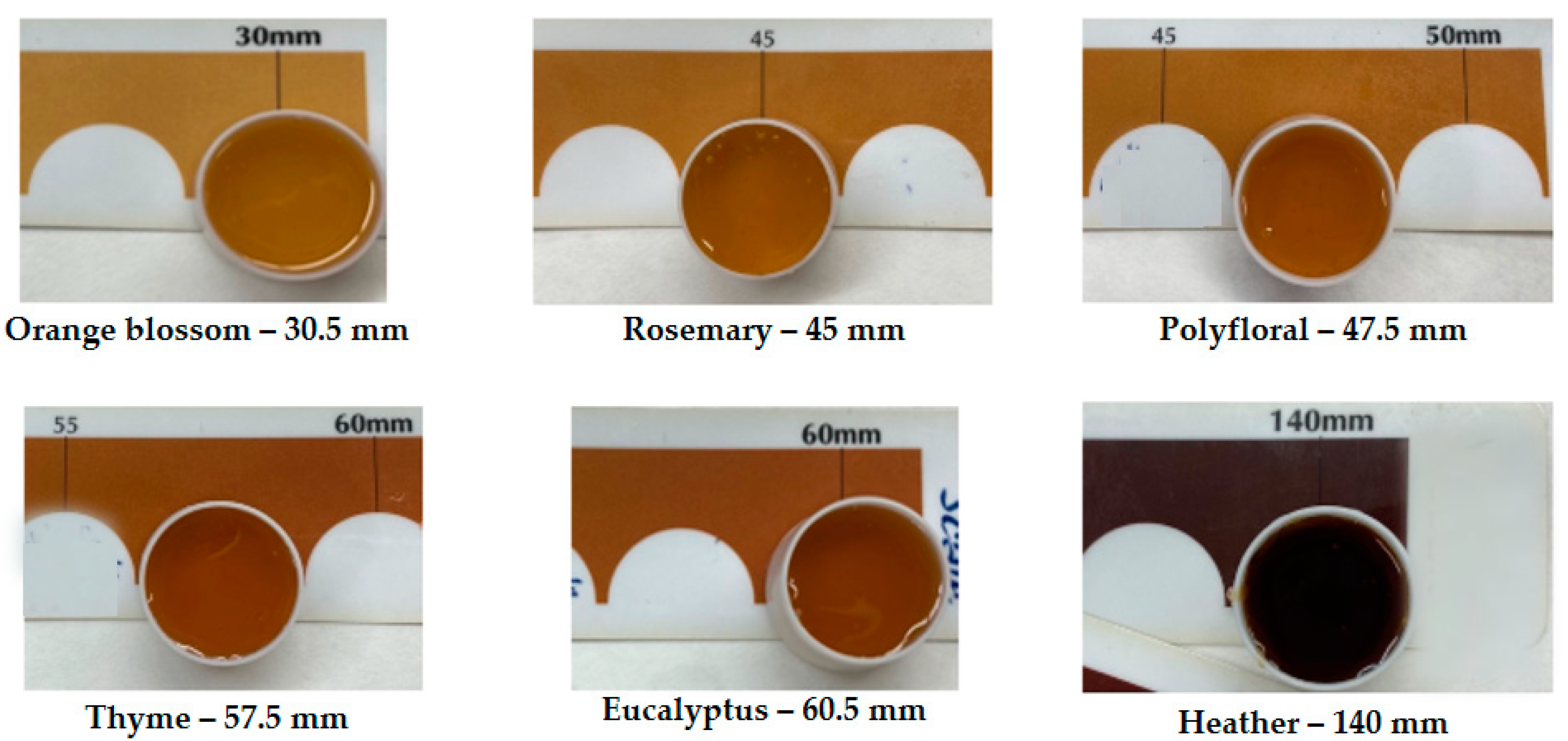
| Honey Sample | pH | Acidity | Moisture | Sugar Concentration |
|---|---|---|---|---|
| Orange blossom | 3.9 ± 0.3 d | 15.5 ± 0.7 c | 17.5 ± 0.1 b | 80.7 ± 1.0 |
| Polyfloral | 4.0 ± 0.0 c | 22.8 ± 2.5 b | 21.6 ± 0.9 a | 82.6 ± 0.0 |
| Eucalyptus | 4.2 ± 0.0 b | 22.8 ± 0.4 b | 16.0 ± 0.3 b | 82.1 ± 0.1 |
| Rosemary | 4.1 ± 0.0 b | 16.0 ± 0.7 c | 16.1 ± 0.1 b | 82.1 ± 0.1 |
| Thyme | 4.0 ± 0.0 c | 25.8 ± 0.4 b | 16.4 ± 0.3 b | 81.9 ± 0.2 |
| Heather | 4.4 ± 0.0 a | 44.0 ± 1.4 a | 16.2 ± 0.0 b | 81.9 ± 0.0 |
| Honey Sample | L* | a* | b* | C* | H* |
|---|---|---|---|---|---|
| Orange blossom | 34.7 ± 0.0 a | 2.1 ± 0.0 d | 7.0 ± 0.0 a | 7.3 ± 0.0 a | 73.7 ± 0.1 a |
| Polyfloral | 33.2 ± 0.8 cd | 3.2 ± 0.1 a | 6.0 ± 0.1 c | 6.8 ± 0.1 b | 61.9 ± 0.3 d |
| Eucalyptus | 32.7 ± 0.0 d | 3.0 ± 0.1 b | 4.3 ± 0.1 e | 5.2 ± 0.1 c | 55.0 ± 0.2 f |
| Rosemary | 34.3 ± 0.1 ab | 2.4 ± 0.0 c | 6.5 ± 0.0 b | 6.9 ± 0.0 b | 70.0 ± 0.3 b |
| Thyme | 33.1 ± 0.0 bc | 3.2 ± 0.0 a | 4.9 ± 0.0 d | 2.7 ± 0.2 d | 57.0 ± 0.1 e |
| Heather | 31.3 ± 0.0 e | 0.8 ± 0.0 e | 1.9 ± 0.0 f | 2.1 ± 0.0 e | 67.6 ± 0.2 c |
| Honey Sample | TPC | Antioxidant Capacity |
|---|---|---|
| Orange blossom | 334.8 ± 14.5 e | 191.5 ± 6.7 b |
| Polyfloral | 394.0 ± 8.4 d | 334.4 ± 13.8 b |
| Eucalyptus | 439.6 ± 6.9 c | 413.0 ± 21.6 b |
| Rosemary | 315.9 ± 6.2 e | 245.3 ± 17.2 b |
| Thyme | 651.8 ± 2.2 b | 789.5 ± 68.7 a |
| Heather | 737.7 ± 22.9 a | 702.2 ± 9.4 a |
| Honey Sample | MIC | MBC |
|---|---|---|
| Orange blossom | 10.6 ± 2.4 a | 28.0 ± 2.1 a |
| Polyfloral | 7.8 ± 1.4 a | 26.8 ± 1.5 a |
| Eucalyptus | 8.6 ± 1.1 a | 30.6 ± 4.3 a |
| Thyme | 0.1 ± 0.0 b | 16.2 ± 0.5 b |
| Heather | 2.4 ± 1.0 b | 12.4 ± 0.8 b |
| Honey Sample | 1.25 | 2.5 | 5 | 6.25 | 7.5 | 10 | 15 | 20 |
|---|---|---|---|---|---|---|---|---|
| Orange blossom | X | X | X | X | X | |||
| Polyfloral | X | X | X | X | X | |||
| Eucalyptus | X | X | X | X | X | |||
| Rosemary | X | X | X | X | X | |||
| Thyme | X | X | X | X | X | |||
| Heather | X | X | X | X | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Gómez, V.; San Mateo, M.; Sánchez-Martínez, L.; Periago, M.J. Antibacterial Effect of Spanish Honeys of Different Botanical Origins against Staphylococcus epidermidis. Int. J. Mol. Sci. 2024, 25, 6590. https://doi.org/10.3390/ijms25126590
Núñez-Gómez V, San Mateo M, Sánchez-Martínez L, Periago MJ. Antibacterial Effect of Spanish Honeys of Different Botanical Origins against Staphylococcus epidermidis. International Journal of Molecular Sciences. 2024; 25(12):6590. https://doi.org/10.3390/ijms25126590
Chicago/Turabian StyleNúñez-Gómez, Vanesa, Marta San Mateo, Lorena Sánchez-Martínez, and María Jesús Periago. 2024. "Antibacterial Effect of Spanish Honeys of Different Botanical Origins against Staphylococcus epidermidis" International Journal of Molecular Sciences 25, no. 12: 6590. https://doi.org/10.3390/ijms25126590
APA StyleNúñez-Gómez, V., San Mateo, M., Sánchez-Martínez, L., & Periago, M. J. (2024). Antibacterial Effect of Spanish Honeys of Different Botanical Origins against Staphylococcus epidermidis. International Journal of Molecular Sciences, 25(12), 6590. https://doi.org/10.3390/ijms25126590







