Effects of 3-(4-Hydroxy-3-methoxyphenyl)propionic Acid on Enhancing Grip Strength and Inhibiting Protein Catabolism Induced by Exhaustive Exercise
Abstract
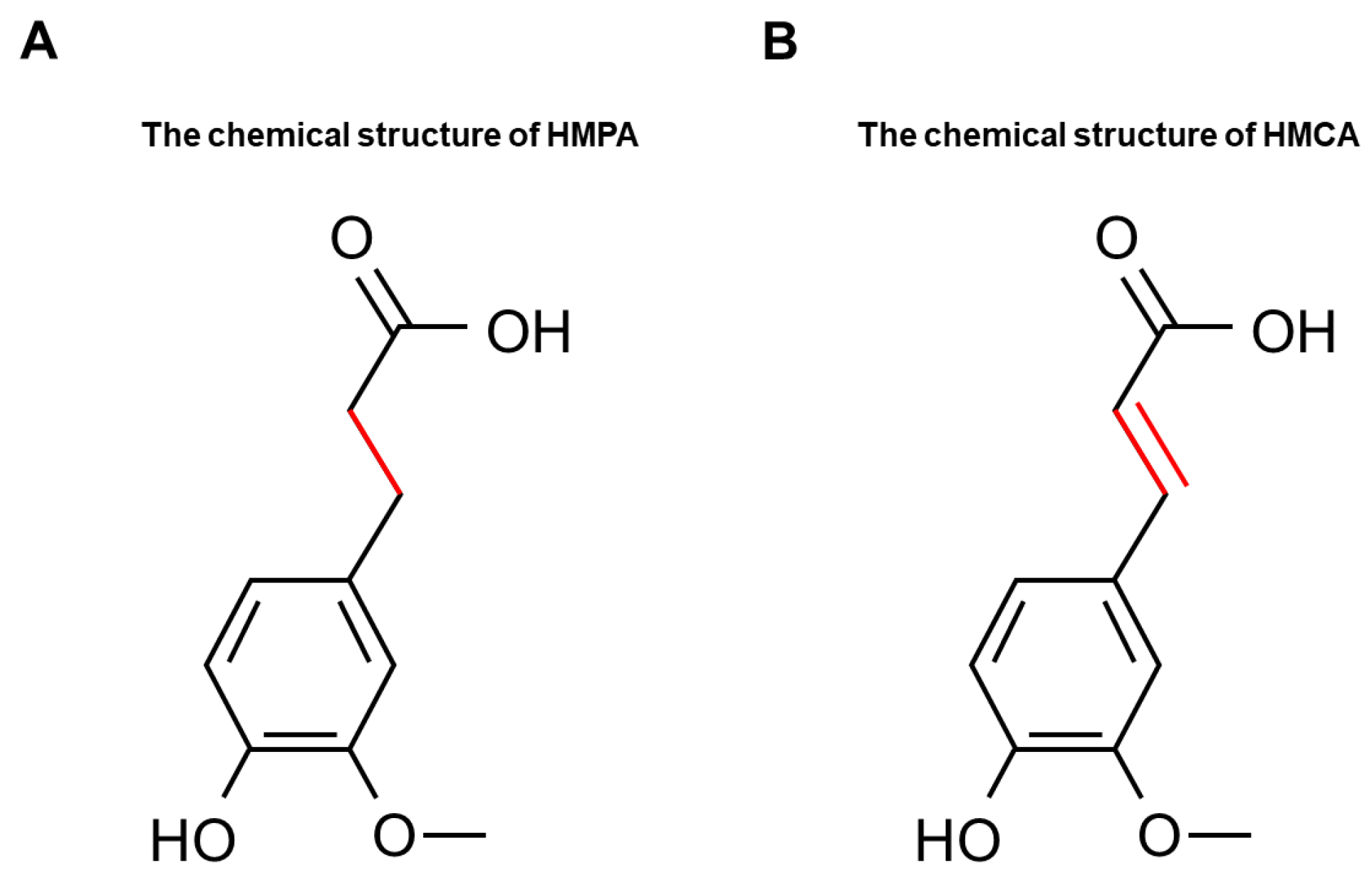
:1. Introduction
2. Results
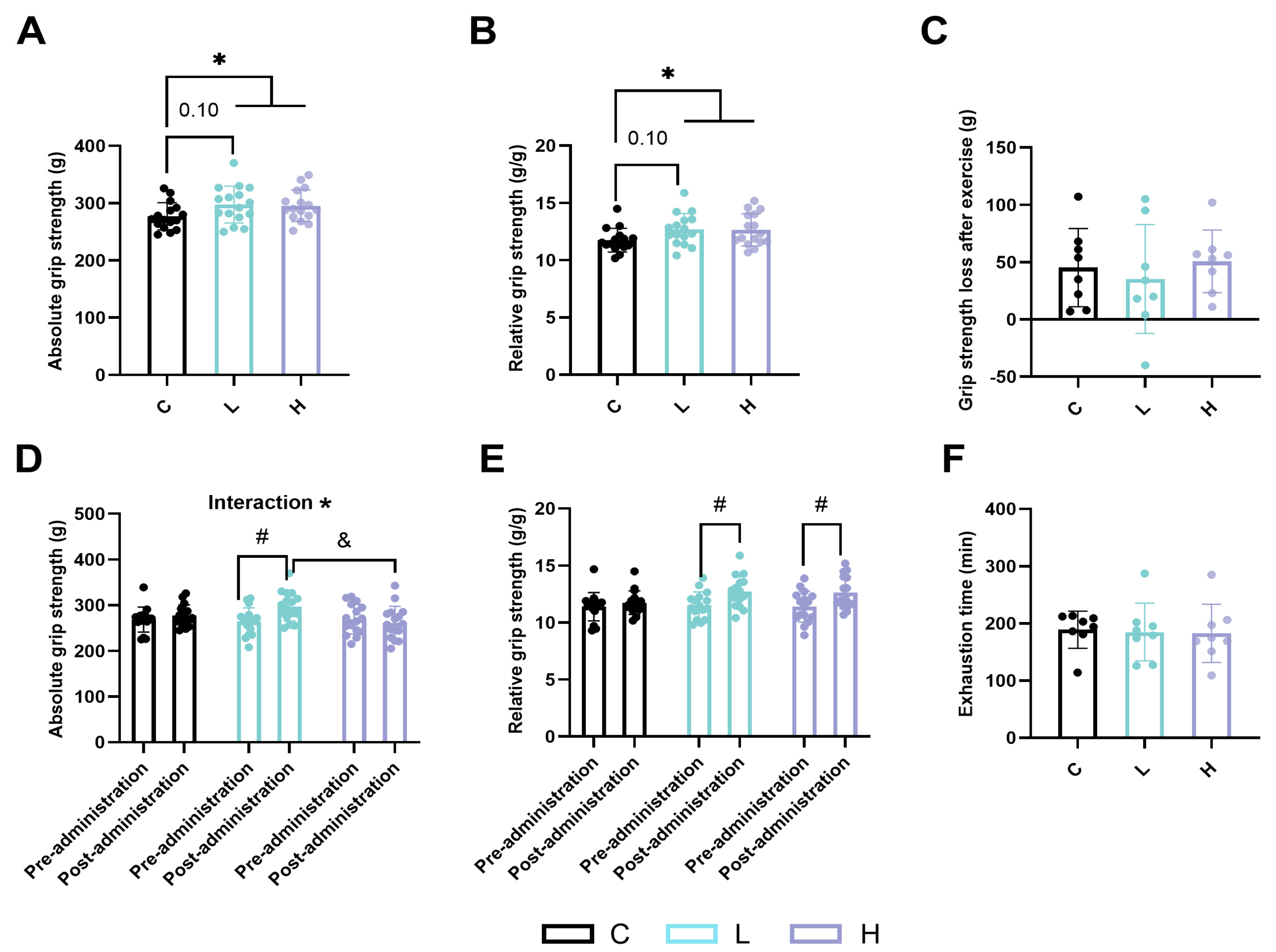
2.1. Effects of HMPA Administration on Skeletal Muscle Function in Mice
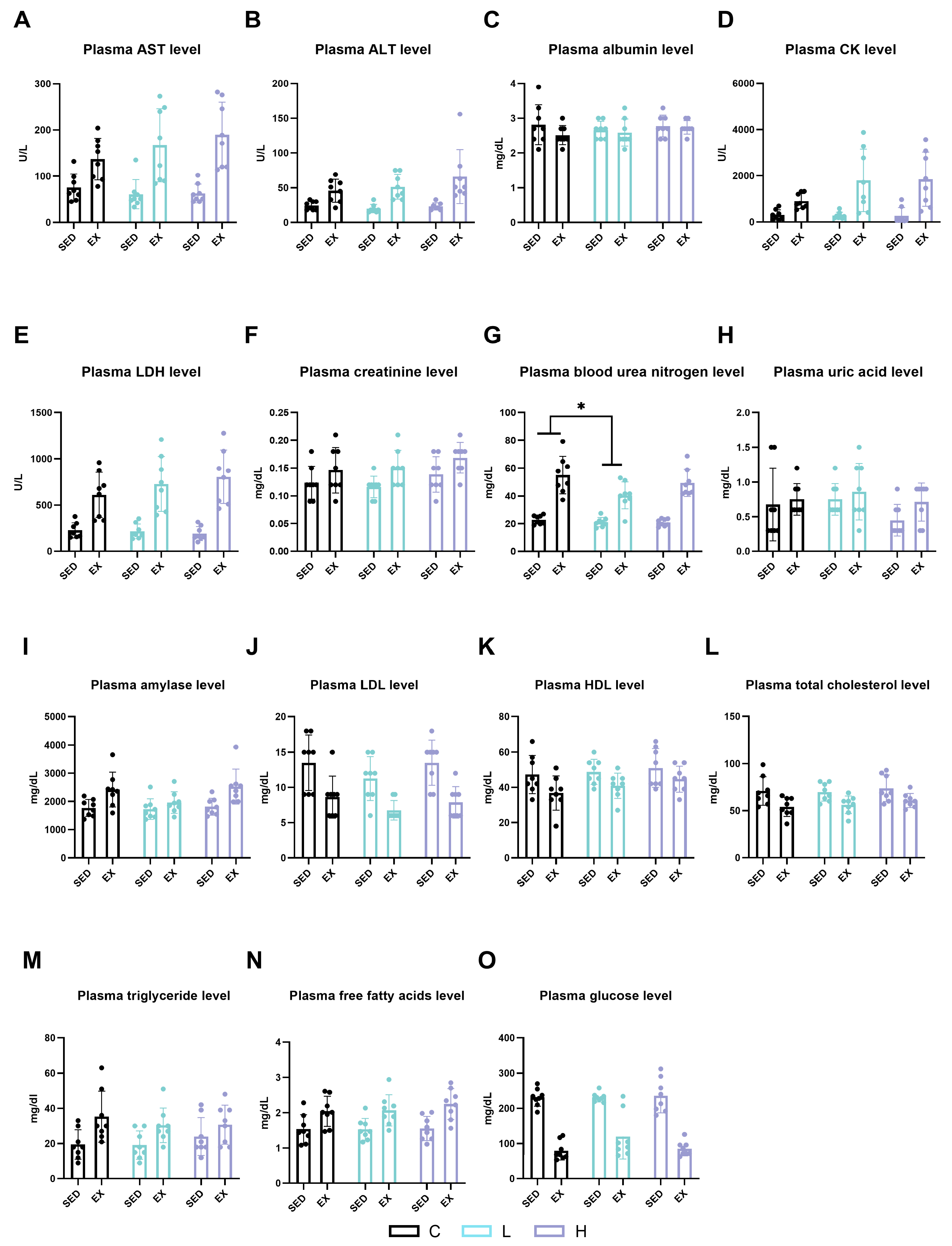
2.2. Effects of HMPA Administration on Visceral Function and Energy Metabolism Substances
2.3. Effect of HMPA Administration on Glucose Metabolism in Gastrocnemius
2.4. Effect of HMPA Administration on Glucose Metabolism in Liver
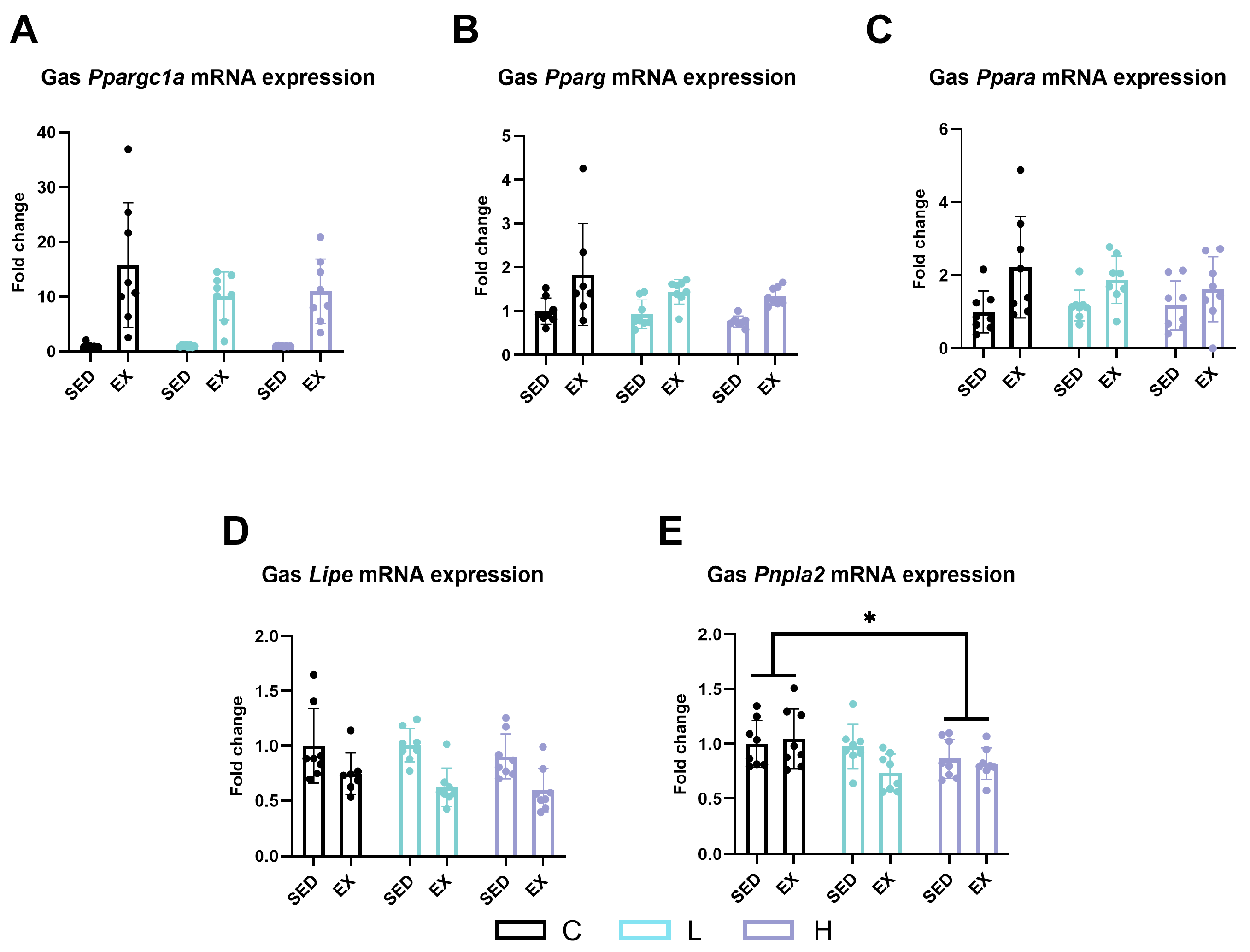
2.5. Effect of HMPA Administration on Lipid Metabolism in Gastrocnemius
2.6. Effect of HMPA Administration on Lipid Metabolism in Liver
2.7. Effect of HMPA Administration on Muscle Satellite Cell Proliferation and Differentiation
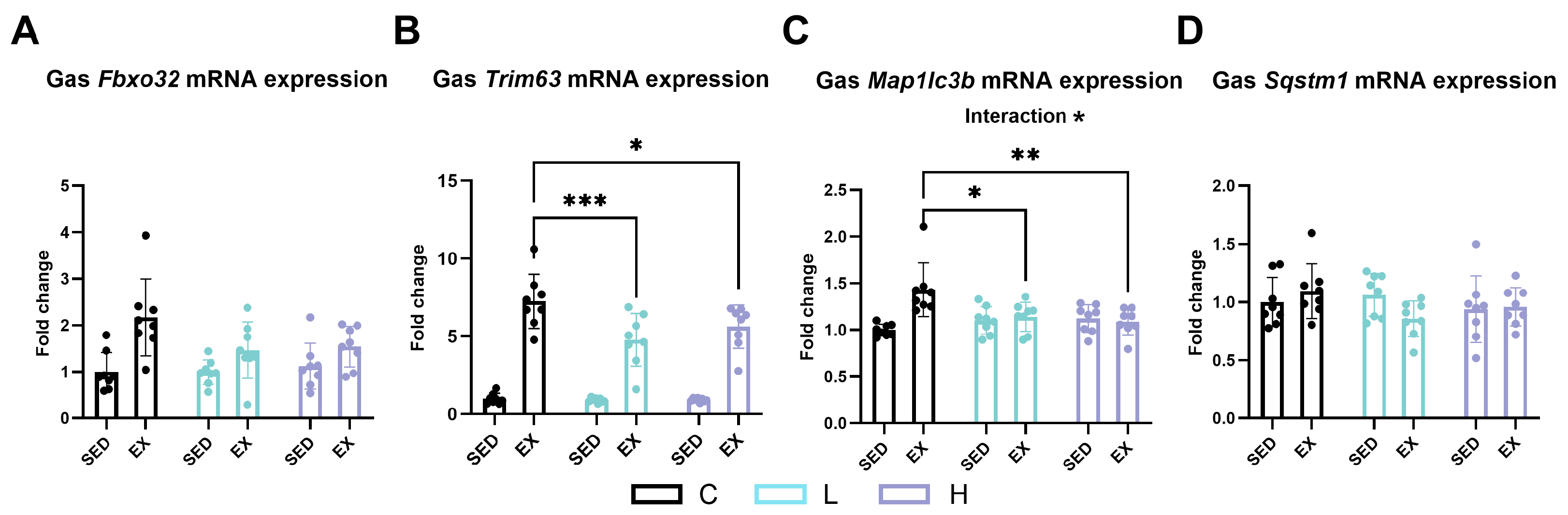
2.8. Effect of HMPA Administration on Muscle Ubiquitin–Proteasome and Autophagic Lysosomal Pathways
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. HMPA Administration
4.3. Functional Assessment of Skeletal Muscle
4.4. Assessment of Plasma Biochemical Markers
4.5. Quantitative RT-PCR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Periasamy, M.; Herrera, J.L.; Reis, F. Skeletal Muscle Thermogenesis and Its Role in Whole Body Energy Metabolism. Diabetes Metab. J. 2024, 41, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Mañas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.J.; Bergouignan, A.; Dempsey, P.C.; Roschel, H.; Owen, N.; Gualano, B.; Dunstan, D.W. Physiology of Sedentary Behavior. Physiol. Rev. 2023, 103, 2561–2622. [Google Scholar] [CrossRef] [PubMed]
- Rezuş, E.; Burlui, A.; Cardoneanu, A.; Rezuş, C.; Codreanu, C.; Pârvu, M.; Rusu-Zota, G.; Tamba, B.I. Inactivity and Skeletal Muscle Metabolism: A Vicious Cycle in Old Age. Int. J. Mol. Sci. 2020, 21, 592. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D.; Ferrando, A.A. Improving Muscle Mass: Response of Muscle Metabolism to Exercise, Nutrition and Anabolic Agents. Essays Biochem. 2008, 44, 85–98. [Google Scholar] [PubMed]
- McGee, S.L.; Hargreaves, M. Exercise Adaptations: Molecular Mechanisms and Potential Targets for Therapeutic Benefit. Nat. Rev. Endocrinol. 2020, 16, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S. Function of the Myogenic Regulatory Factors Myf5, MyoD, Myogenin and MRF4 in Skeletal Muscle, Satellite Cells and Regenerative Myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Atakan, M.M.; Kuang, J.; Hu, Y.; Bishop, D.J.; Yan, X. The Molecular Adaptive Responses of Skeletal Muscle to High-Intensity Exercise/Training and Hypoxia. Antioxidants 2020, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, H.; Song, S.; Liu, J.; Wang, Z.; Wang, J. Bioactive Components in Whole Grains for the Regulation of Skeletal Muscle Function. Foods 2022, 11, 2752. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Z.; Ling, D.; Li, J.; Wang, Y.; Shan, T. The Regulatory Role of Dietary Factors in Skeletal Muscle Development, Regeneration and Function. Crit. Rev. Food Sci. Nutr. 2022, 62, 764–782. [Google Scholar] [CrossRef]
- Tian, Y.; Xia, T.; Qiang, X.; Zhao, Y.; Li, S.; Wang, Y.; Zheng, Y.; Yu, J.; Wang, J.; Wang, M. Nutrition, Bioactive Components, and Hepatoprotective Activity of Fruit Vinegar Produced from Ningxia Wolfberry. Molecules 2022, 27, 4422. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Yeo, D.; Hong, J. Effect of Dihydroferulic Acid Obtained from Fermented Rice Bran Extract on Neuroprotection and Behavioral Recovery in an Ischemic Rat Model. Food Sci. Technol. 2020, 40, 475–481. [Google Scholar] [CrossRef]
- Ohue-Kitano, R.; Taira, S.; Watanabe, K.; Masujima, Y.; Kuboshima, T.; Miyamoto, J.; Nishitani, Y.; Kawakami, H.; Kuwahara, H.; Kimura, I. 3-(4-Hydroxy-3-methoxyphenyl)propionic Acid Produced from 4-Hydroxy-3-methoxycinnamic Acid by Gut Microbiota Improves Host Metabolic Condition in Diet-Induced Obese Mice. Nutrients 2019, 11, 1036. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A.R.; Spencer, J.P.; Kuhnle, G.; Hahn, U.; Rice-Evans, C.A. Novel Biomarkers of the Metabolism of Caffeic Acid Derivatives in Vivo. Free Radic. Biol. Med. 2001, 30, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Shimoji, Y.; Tamura, Y.; Nakamura, Y.; Nanda, K.; Nishidai, S.; Nishikawa, Y.; Ishihara, N.; Uenakai, K.; Ohigashi, H. Isolation and Identification of DPPH Radical Scavenging Compounds in Kurosu (Japanese Unpolished Rice Vinegar). J. Agric. Food Chem. 2002, 50, 6501–6503. [Google Scholar] [CrossRef]
- Chowdhury, S.; Ghosh, S.; Das, A.K.; Sil, P.C. Ferulic Acid Protects Hyperglycemia-Induced Kidney Damage by Regulating Oxidative Insult, Inflammation and Autophagy. Front. Pharmacol. 2019, 10, 27. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, P.; Zhao, P.; Wang, D.; Zhang, Y.; Wang, J.; Chen, L.; Guo, W.; Gao, H.; Jiao, Y. Pretreatment of Ferulic Acid Attenuates Inflammation and Oxidative Stress in a Rat Model of Lipopolysaccharide-Induced Acute Respiratory Distress Syndrome. Int. J. Immunopathol. Pharmacol. 2018, 32, 394632017750518. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Chen, H.; Yu, J.; Luo, Y.; Zheng, P.; He, J. Effects of Dietary Ferulic Acid Supplementation on Growth Performance and Skeletal Muscle Fiber Type Conversion in Weaned Piglets. J. Sci. Food Agric. 2021, 101, 5116–5123. [Google Scholar] [CrossRef]
- Wen, Y.; Ushio, H. Ferulic Acid Promotes Hypertrophic Growth of Fast Skeletal Muscle in Zebrafish Model. Nutrients 2017, 9, 1066. [Google Scholar] [CrossRef]
- You, Y.; Park, J.; Yoon, H.G.; Lee, Y.H.; Hwang, K.; Lee, J.; Kim, K.; Lee, K.W.; Shim, S.; Jun, W. Stimulatory Effects of Ferulic Acid on Endurance Exercise Capacity in Mice. Biosci. Biotechnol. Biochem. 2009, 73, 1392–1397. [Google Scholar] [CrossRef]
- You, Y.; Kim, K.; Yoon, H.G.; Lee, K.W.; Lee, J.; Chun, J.; Shin, D.H.; Park, J.; Jun, W. Chronic Effect of Ferulic Acid from Pseudosasa Japonica Leaves on Enhancing Exercise Activity in Mice. Phytother Res. 2010, 24, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, P.; Kumar, N.; Sharma, M.; Pruthi, V. Biomedical Applications of Ferulic Acid Encapsulated Electrospun Nanofibers. Biotechnol. Rep. (Amst). 2015, 8, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, A.; Varshosaz, J.; Fesharaki, M.; Farhang, A.; Jafari, S.M. Improving the Solubility and in Vitro Cytotoxicity (Anticancer Activity) of Ferulic Acid by Loading it into Cyclodextrin Nanosponges. Int. J. Nanomed. 2019, 14, 4589–4599. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Rui, Y.X.; Guo, S.D.; Luan, F.; Liu, R.; Zeng, N. Ferulic Acid: A Review of Its Pharmacology, Pharmacokinetics and Derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef] [PubMed]
- Baeza, G.; Bachmair, E.M.; Wood, S.; Mateos, R.; Bravo, L.; de Roos, B. The Colonic Metabolites Dihydrocaffeic Acid and Dihydroferulic Acid Are More Effective Inhibitors of in Vitro Platelet Activation Than Their Phenolic Precursors. Food Funct. 2017, 8, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Ohue-Kitano, R.; Masujima, Y.; Nishikawa, S.; Iwasa, M.; Nishitani, Y.; Kawakami, H.; Kuwahara, H.; Kimura, I. 3-(4-Hydroxy-3-methoxyphenyl)propionic Acid Contributes to Improved Hepatic Lipid Metabolism Via GPR41. Sci. Rep. 2023, 13, 21246. [Google Scholar] [CrossRef] [PubMed]
- Inamura, N.; Taniguchi, H.; Yoshida, S.; Nishioka, M.; Ishihara, K. A Comparative Observational Study of Carbohydrate Intake and Continuous Blood Glucose Levels in Relation to Performance in Ultramarathon. Sci. Rep. 2024, 14, 1089. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D. What Is the Evidence That Dietary Macronutrient Composition Influences Exercise Performance? A Narrative Review. Nutrients 2022, 14, 862. [Google Scholar] [CrossRef]
- Flis, D.J.; Dzik, K.; Kaczor, J.J.; Cieminski, K.; Halon-Golabek, M.; Antosiewicz, J.; Wieckowski, M.R.; Ziolkowski, W. Swim Training Modulates Mouse Skeletal Muscle Energy Metabolism and Ameliorates Reduction in Grip Strength in a Mouse Model of Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2019, 20, 233. [Google Scholar] [CrossRef]
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar]
- Zhang, X.; Yang, S.; Chen, J.; Su, Z. Unraveling the Regulation of Hepatic Gluconeogenesis. Front. Endocrinol. (Lausanne) 2019, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- van Hall, G. The Physiological Regulation of Skeletal Muscle Fatty Acid Supply and Oxidation During Moderate-Intensity Exercise. Sports Med. 2015, 45, S23–S32. [Google Scholar] [CrossRef] [PubMed]
- Dubé, J.J.; Sitnick, M.T.; Schoiswohl, G.; Wills, R.C.; Basantani, M.K.; Cai, L.; Pulinilkunnil, T.; Kershaw, E.E. Adipose Triglyceride Lipase Deletion from Adipocytes, but Not Skeletal Myocytes, Impairs Acute Exercise Performance in Mice. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E879–E890. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.; Etemad, S.; Yamamoto, M.; Goldhamer, D. MyoD-Expressing Progenitors Are Essential for Skeletal Myogenesis and Satellite Cell Development. Dev. Biol. 2015, 384, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, T.; Toyono, T.; Inoue, A.; Matsubara, T.; Kawamoto, T.; Kokabu, S. Factors Regulating or Regulated by Myogenic Regulatory Factors in Skeletal Muscle Stem Cells. Cells 2022, 11, 1493. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Legendre, N.P.; Biswas, A.A.; Lawton, A.; Yamamoto, S.; Tajbakhsh, S.; Kardon, G.; Goldhamer, D.J. Loss of MyoD and Myf5 in Skeletal Muscle Stem Cells Results in Altered Myogenic Programming and Failed Regeneration. Stem Cell Rep. 2018, 10, 956–969. [Google Scholar] [CrossRef]
- Ganassi, M.; Badodi, S.; Wanders, K.; Zammit, P.S.; Hughes, S.M. Myogenin Is an Essential Regulator of Adult Myofibre Growth and Muscle Stem Cell Homeostasis. eLife 2020, 9, e60445. [Google Scholar] [CrossRef]
- Kim, A.R.; Kim, K.M.; Byun, M.R.; Hwang, J.H.; Park, J.I.; Oh, H.T.; Jeong, M.G.; Hwang, E.S.; Hong, J.H. (-)-Epigallocatechin-3-gallate Stimulates Myogenic Differentiation Through TAZ Activation. Biochem. Biophys. Res. Commun. 2017, 486, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Dugdale, H.F.; Hughes, D.C.; Allan, R.; Deane, C.S.; Coxon, C.R.; Morton, J.P.; Stewart, C.E.; Sharples, A.P. The Role of Resveratrol on Skeletal Muscle Cell Differentiation and Myotube Hypertrophy During Glucose Restriction. Mol. Cell. Biochem. 2018, 444, 109–123. [Google Scholar] [CrossRef]
- Jang, Y.J.; Son, H.J.; Choi, Y.M.; Ahn, J.; Jung, C.H.; Ha, T.Y. Apigenin Enhances Skeletal Muscle Hypertrophy and Myoblast Differentiation by Regulating Prmt7. Oncotarget 2017, 8, 78300–78311. [Google Scholar] [CrossRef]
- Kim, A.R.; Kim, K.M.; Byun, M.R.; Hwang, J.H.; Park, J.I.; Oh, H.T.; Kim, H.K.; Jeong, M.G.; Hwang, E.S.; Hong, J.H. Catechins Activate Muscle Stem Cells by Myf5 Induction and Stimulate Muscle Regeneration. Biochem. Biophys. Res. Commun. 2017, 489, 142–148. [Google Scholar] [CrossRef]
- Lee, E.C.; Fragala, M.S.; Kavouras, S.A.; Queen, R.M.; Pryor, J.L.; Casa, D.J. Biomarkers in Sports and Exercise: Tracking Health, Performance, and Recovery in Athletes. J. Strength Cond. Res. 2017, 31, 2920–2937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zheng, J.; Qiu, J.; Wu, X.; Xu, Y.; Shen, W.; Sun, M. ALDH2 Restores Exhaustive Exercise-Induced Mitochondrial Dysfunction in Skeletal Muscle. Biochem. Biophys. Res. Commun. 2017, 485, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, H.; Yang, L.; Wang, K.; Chen, N.; Zhang, T.; Luo, J. A Review of Rehabilitation Benefits of Exercise Training Combined with Nutrition Supplement for Improving Protein Synthesis and Skeletal Muscle Strength in Patients With Cerebral Stroke. Nutrients 2022, 14, 4995. [Google Scholar] [CrossRef] [PubMed]
- Guimarães-Ferreira, L.; Cholewa, J.M.; Naimo, M.A.; Zhi, X.I.; Magagnin, D.; de Sá, R.B.; Streck, E.L.; Teixeira Tda, S.; Zanchi, N.E. Synergistic Effects of Resistance Training and Protein Intake: Practical Aspects. Nutrition 2014, 30, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, M. Roles of Ferulic Acid on Muscle Atrophy and Grip Strength in Diabetic Mice. Pak. J. Pharm. Sci. 2023, 36, 1025–1030. [Google Scholar]
- Liu, X.; Liu, M. Anti-Fatigue Effect of Ferulic Acid in Exercise Trained Mice. Acta Pol. Pharm.-Drug Res. 2023, 80, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Li, H.; Chen, X.; Chen, D.; Yu, B.; He, J.; Luo, Y.; Yan, H.; Zheng, P.; Yu, J.; et al. Dietary Ferulic Acid Increases Endurance Capacity by Promoting Skeletal Muscle Oxidative Phenotype, Mitochondrial Function, and Antioxidant Capacity. Mol. Nutr. Food Res. 2024, 68, e2200719. [Google Scholar] [CrossRef]
- Ma, S.; Yang, J.; Tominaga, T.; Liu, C.; Suzuki, K. A Low-Carbohydrate Ketogenic Diet and Treadmill Training Enhanced Fatty Acid Oxidation Capacity but Did Not Enhance Maximal Exercise Capacity in Mice. Nutrients 2021, 13, 611. [Google Scholar] [CrossRef]





| Gene | Forward | Reverse |
|---|---|---|
| Slc2a4 (Glut4) | GCTGGTGTGGTCAATACGGTCT | GCAGAGCCACGGTCATCAAGAT |
| Slc2a2 (Glut2) | CCTCATCATTGCTGGACGAAGTGT | CCTGAGTGTGGTTGGAGCGATCT |
| Slc16a1 (Mct1) | TCAGTGCAACGACCAGTGAAGT | CCGCAACCAGACAGACAACCA |
| Slc16a3 (Mct4) | ACGGGTTTCTCCTACGCCTTCC | AGAGCGGTCCTGTGCCATAGAG |
| Pfkfb1 | GCGGTGCCTTCTAGCATACTTCC | TCACAGCCTCCACATTCAGGTAGA |
| Gsk3b | TGGTGTGGATCAGTTGGTGGAA | TCCTGCTCCTGGTGAGTCCTT |
| Gys1 | CTGTCCTGTTCGGCTTCCTCAC | GGCAACCACATACGGCTTCTCT |
| Gys2 | GCTTCCGCTCTCCAGACGATTC | TGCCCAGGTATCTCCAGTCCAG |
| G6pc1 (G6Pase) | GTGGCAGTGGTCGGAGACT | ACGGGCGTTGTCCAAAC |
| Ppargc1a (Pgc-1alpha) | ACACAACCGCAGTCGCAACAT | GCAGTTCCAGAGAGTTCCACACTT |
| Ppara | GCGGGAAAGACCAGCAACAAC | CAGCAGTGGAAGAATCGGACCT |
| Pparg | GATGGAAGACCACTCGCATT | AACCATTGGGTCAGCTCTTG |
| Lipe (HSL) | GGAAGGACAGGACAG-CAAGGTACT | CGCCTCCGTGGATGTGAACAAC |
| Pnpla2 (Atgl) | TGTCCTTCACCATCCGCTTGTT | TGCTACCCGTCTGCTCTTTCAT |
| Cd36 | TGCGACATGATTAATGGCACAGAC | TCCGAACACAGCGTAGATAGACCT |
| Myf5 | CTCAGGAATGCCATCCGCTACA | CCGTCAGAGCAGTTGGAGGT |
| Myod1 | CGCAACGCCATCCGCTACAT | GCATCTGAGTCGCCACTGTAGT |
| Myog | GCAGGCTCAAGAAAGTGAATGA | TAGGCGCTCAATGTACTGGAT |
| Myf6 | CCTCAGCCTCCAGCAGTCTTCA | TTACTTCTCCACCACCTCCTCCAC |
| Trim63 (MuRF1) | CGACATCTTCCAGGCTGCGAAT | ATCACTTCATGGCGGCACGAG |
| Fbxo32 (ATROGIN1) | GCTGGATTGGAAGAAGATG | AGAGAATGTGGCAGTGTT |
| Map1lc3b (LC3b) | CCAAGCCTTCTTCCTCCT | CTCTCACTCTCGTACACTTC |
| Sqstm1 (p62) | CAGGCACAGAAGACAAGAG | CCGACTCCAAGGCTATCT |
| rps18 | TTCTGGCCAACGGTCTAGACAAC | CCAGTGGTCTTGGTGTGCTGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Y.; Huang, J.; Wang, S.; Awa, R.; Tagawa, T.; Zhang, Z.; Cao, T.; Kobori, H.; Suzuki, K. Effects of 3-(4-Hydroxy-3-methoxyphenyl)propionic Acid on Enhancing Grip Strength and Inhibiting Protein Catabolism Induced by Exhaustive Exercise. Int. J. Mol. Sci. 2024, 25, 6627. https://doi.org/10.3390/ijms25126627
Tong Y, Huang J, Wang S, Awa R, Tagawa T, Zhang Z, Cao T, Kobori H, Suzuki K. Effects of 3-(4-Hydroxy-3-methoxyphenyl)propionic Acid on Enhancing Grip Strength and Inhibiting Protein Catabolism Induced by Exhaustive Exercise. International Journal of Molecular Sciences. 2024; 25(12):6627. https://doi.org/10.3390/ijms25126627
Chicago/Turabian StyleTong, Yishan, Jiapeng Huang, Shuo Wang, Riyo Awa, Takashi Tagawa, Ziwei Zhang, Tiehan Cao, Haruki Kobori, and Katsuhiko Suzuki. 2024. "Effects of 3-(4-Hydroxy-3-methoxyphenyl)propionic Acid on Enhancing Grip Strength and Inhibiting Protein Catabolism Induced by Exhaustive Exercise" International Journal of Molecular Sciences 25, no. 12: 6627. https://doi.org/10.3390/ijms25126627







