A20 in Kidney Transplantation and Autoimmunity
Abstract
1. Introduction
2. Review
2.1. Molecular Interactions of A20
2.1.1. Structure of A20
2.1.2. A20 as Central Inhibitor of NFκB
2.1.3. A20 in Different Receptor Cascades
2.1.4. A20 Protects Cells from Death
2.1.5. A20 Effect on Different Cell Populations
2.1.6. A20 in Autoimmunity
2.1.7. A20 in Systemic Lupus Erythematosus (SLE)
2.1.8. A20 in Other Autoimmune Diseases
2.1.9. A20 in High-Glucose Environments
| Model | Result |
|---|---|
| Selective A20-deficient B cells in mice | Enhanced proliferation and excessive production of autoantibodies leading to glomerular deposits, similar to SLE [49,50] |
| Selective A20-deficient dendritic cells in mice | Activation and expansion of T cells leading to lymphocytic colitis, arthritis and enthesitis [69] |
| Selective A20-deficient myeloid cells in mice | All mice developed severe arthritis [72] |
| Enhanced A20 expression in mice with collagen-induced arthritis | Reduced tissue destruction [73] |
2.2. A20 in Transplant Biology
2.2.1. A20 in Delayed Graft Function
2.2.2. A20 in Acute and Chronic Rejection
| Model | Result |
|---|---|
| A20 overexpression in rat liver transplantation | Better allograft function, lower transplant fibrosis and longer survival [97] |
| Rat kidney transplantation | High A20 expression was associated with fewer infiltrates and less vascular inflammation [98] |
| A20-haploinsufficient aortic-transplant mice | Higher infiltration of CD3+ T cells and IFNγ-producing Th1 cells, fewer FoxP3+ T cells, more intima hyperplasia [99] |
| A20 overexpression in aortic-transplant mice | Lower Th1/Th17 infiltration, higher number of FoxP3+ T cells, less neointimal formation and arteriosclerosis [100] |
| Rat kidney transplant with chronic rejection | A20 expression was significantly higher when treated with MMF compared to CsA [104] |
3. Conclusions
3.1. Possible Roles of A20 in Kidney Autoimmunity
3.2. Possible Roles of A20 in Kidney Transplantation
3.3. Future Research into A20
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef]
- Evans, P.D.; Taal, M.W. Epidemiology and causes of chronic kidney disease. Medicine 2015, 43, 450–453. [Google Scholar] [CrossRef]
- Tonelli, M.; Wiebe, N.; Knoll, G.; Bello, A.; Browne, S.; Jadhav, D.; Klarenbach, S.; Gill, J. Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am. J. Transplant. 2011, 11, 2093–2109. [Google Scholar] [CrossRef]
- Li, M.T.; Ramakrishnan, A.; Yu, M.M.; Daniel, E.; Sandra, V.; Sanichar, N.; King, K.L.; Stevens, J.S.; Husain, S.A.; Mohan, S. Effects of Delayed Graft Function on Transplant Outcomes: A Meta-analysis. Transplant. Direct 2023, 9, e1433. [Google Scholar] [CrossRef]
- Tamargo, C.L.; Kant, S. Pathophysiology of Rejection in Kidney Transplantation. J. Clin. Med. 2023, 12, 4130. [Google Scholar] [CrossRef]
- Ma, A.; Malynn, B.A. A20: Linking a complex regulator of ubiquitylation to immunity and human disease. Nat. Rev. Immunol. 2012, 12, 774–785. [Google Scholar] [CrossRef]
- Majumdar, I.; Paul, J. The deubiquitinase A20 in immunopathology of autoimmune diseases. Autoimmunity 2014, 47, 307–319. [Google Scholar] [CrossRef]
- Dixit, V.M.; Green, S.; Sarma, V.; Holzman, L.B.; Wolf, F.W.; O’Rourke, K.; Ward, P.A.; Prochownik, E.V.; Marks, R.M. Tumor necrosis factor-alpha induction of novel gene products in human endothelial cells including a macrophage-specific chemotaxin. J. Biol. Chem. 1990, 265, 2973–2978. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.T.; Stroka, D.M.; Brostjan, C.; Palmetshofer, A.; Bach, F.H.; Ferran, C. A20 blocks endothelial cell activation through a NF-kappaB-dependent mechanism. J. Biol. Chem. 1996, 271, 18068–18073. [Google Scholar] [CrossRef] [PubMed]
- Priem, D.; van Loo, G.; Bertrand, M.J.M. A20 and Cell Death-driven Inflammation. Trends Immunol. 2020, 41, 421–435. [Google Scholar] [CrossRef]
- Catrysse, L.; Vereecke, L.; Beyaert, R.; van Loo, G. A20 in inflammation and autoimmunity. Trends Immunol. 2014, 35, 22–31. [Google Scholar] [CrossRef]
- Onuora, S. Experimental arthritis: Inflammasome-driven arthritis: A new model of RA? Nat. Rev. Rheumatol. 2014, 10, 445. [Google Scholar] [CrossRef]
- Karri, U.; Harasimowicz, M.; Tumba, M.C.; Schwartz, D.M. The Complexity of Being A20: From Biological Functions to Genetic Associations. J. Clin. Immunol. 2024, 44, 76. [Google Scholar] [CrossRef]
- Bosanac, I.; Wertz, I.E.; Pan, B.; Yu, C.; Kusam, S.; Lam, C.; Phu, L.; Phung, Q.; Maurer, B.; Arnott, D.; et al. Ubiquitin binding to A20 ZnF4 is required for modulation of NF-kappaB signaling. Mol. Cell 2010, 40, 548–557. [Google Scholar] [CrossRef]
- Bai, W.; Huo, S.; Li, J.; Shao, J. Advances in the Study of the Ubiquitin-Editing Enzyme A20. Front. Pharmacol. 2022, 13, 845262. [Google Scholar] [CrossRef]
- Wertz, I.E.; O’rourke, K.M.; Zhou, H.; Eby, M.; Aravind, L.; Seshagiri, S.; Wu, P.; Wiesmann, C.; Baker, R.; Boone, D.L.; et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 2004, 430, 694–699. [Google Scholar] [CrossRef]
- Martens, A.; Priem, D.; Hoste, E.; Vetters, J.; Rennen, S.; Catrysse, L.; Voet, S.; Deelen, L.; Sze, M.; Vikkula, H.; et al. Two distinct ubiquitin-binding motifs in A20 mediate its anti-inflammatory and cell-protective activities. Nat. Immunol. 2020, 21, 381–387. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-kappaB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Aggarwal, B.B. Transcription factor NF-kappaB: A sensor for smoke and stress signals. Ann. N. Y. Acad. Sci. 2005, 1056, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; Trzyna, W.C.; McClintock, D.S.; Schumacker, P.T. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J. Immunol. 2000, 165, 1013–1021. [Google Scholar] [CrossRef]
- Sun, S.C. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Kato, M.; Ogawa, S. Significance of A20 in B-cell lymphomas. [Rinsho Ketsueki] Jpn. J. Clin. Hematol. 2011, 52, 190–197. [Google Scholar]
- Shi, Y.; Wang, X.; Wang, J.; Wang, X.; Zhou, H.; Zhang, L. The dual roles of A20 in cancer. Cancer Lett. 2021, 511, 26–35. [Google Scholar] [CrossRef]
- Parvatiyar, K.; Harhaj, E.W. Regulation of inflammatory and antiviral signaling by A20. Microbes Infect. 2011, 13, 209–215. [Google Scholar] [CrossRef]
- Zilberman-Rudenko, J.; Shawver, L.M.; Wessel, A.W.; Luo, Y.; Pelletier, M.; Tsai, W.L.; Lee, Y.; Vonortas, S.; Cheng, L.; Ashwell, J.D.; et al. Recruitment of A20 by the C-terminal domain of NEMO suppresses NF-kappaB activation and autoinflammatory disease. Proc. Natl. Acad. Sci. USA 2016, 113, 1612–1617. [Google Scholar] [CrossRef]
- Vereecke, L.; Beyaert, R.; van Loo, G. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 2009, 30, 383–391. [Google Scholar] [CrossRef]
- Hutti, J.E.; Turk, B.E.; Asara, J.M.; Ma, A.; Cantley, L.C.; Abbott, D.W. IkappaB kinase beta phosphorylates the K63 deubiquitinase A20 to cause feedback inhibition of the NF-kappaB pathway. Mol. Cell. Biol. 2007, 27, 7451–7461. [Google Scholar] [CrossRef]
- Zammit, N.W.; Siggs, O.M.; Gray, P.E.; Horikawa, K.; Langley, D.B.; Walters, S.N.; Daley, S.R.; Loetsch, C.; Warren, J.; Yap, J.Y.; et al. Denisovan, modern human and mouse TNFAIP3 alleles tune A20 phosphorylation and immunity. Nat. Immunol. 2019, 20, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; He, X.; Huang, N.; Yu, J.; Shao, B. A20: A master regulator of arthritis. Arthritis Res. Ther. 2020, 22, 220. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liu, Y.; Li, Q.; Liao, L.; Sun, R.; Liu, X.; Jiang, M.; Hu, J. A20 regulates IL-1-induced tolerant production of CXC chemokines in human mesangial cells via inhibition of MAPK signaling. Sci. Rep. 2015, 5, 18007. [Google Scholar] [CrossRef]
- Duy, P.N.; Thuy, N.T.; Trang, B.K.; Giang, N.H.; Van, N.T.H.; Xuan, N.T. Regulation of NF-kappaB- and STAT1-mediated plasmacytoid dendritic cell functions by A20. PLoS ONE 2019, 14, e0222697. [Google Scholar] [CrossRef]
- Moll, H.P.; Lee, A.; Minussi, D.C.; da Silva, C.G.; Csizmadia, E.; Bhasin, M.; Ferran, C. A20 regulates atherogenic interferon (IFN)-gamma signaling in vascular cells by modulating basal IFNbeta levels. J. Biol. Chem. 2014, 289, 30912–30924. [Google Scholar] [CrossRef]
- Huyghe, J.; Priem, D.; Bertrand, M.J.M. Cell death checkpoints in the TNF pathway. Trends Immunol. 2023, 44, 628–643. [Google Scholar] [CrossRef]
- Jin, Z.; Li, Y.; Pitti, R.; Lawrence, D.; Pham, V.C.; Lill, J.R.; Ashkenazi, A. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell 2009, 137, 721–735. [Google Scholar] [CrossRef]
- Bellail, A.C.; Olson, J.J.; Yang, X.; Chen, Z.J.; Hao, C. A20 ubiquitin ligase-mediated polyubiquitination of RIP1 inhibits caspase-8 cleavage and TRAIL-induced apoptosis in glioblastoma. Cancer Discov. 2012, 2, 140–155. [Google Scholar] [CrossRef]
- Dong, B.; Lv, G.; Wang, Q.; Wang, G. Targeting A20 enhances TRAIL-induced apoptosis in hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2012, 418, 433–438. [Google Scholar] [CrossRef]
- Feoktistova, M.; Makarov, R.; Brenji, S.; Schneider, A.T.; Hooiveld, G.J.; Luedde, T.; Leverkus, M.; Yazdi, A.S.; Panayotova-Dimitrova, D. A20 Promotes Ripoptosome Formation and TNF-Induced Apoptosis via cIAPs Regulation and NIK Stabilization in Keratinocytes. Cells 2020, 9, 351. [Google Scholar] [CrossRef]
- Garcia-Carbonell, R.; Wong, J.; Kim, J.Y.; Close, L.A.; Boland, B.S.; Wong, T.L.; Harris, P.A.; Ho, S.B.; Das, S.; Ernst, P.B.; et al. Elevated A20 promotes TNF-induced and RIPK1-dependent intestinal epithelial cell death. Proc. Natl. Acad. Sci. USA 2018, 115, E9192–E9200. [Google Scholar] [CrossRef] [PubMed]
- Holgado, A.; Liu, Z.; Aidarova, A.; Mueller, C.; Haegman, M.; Driege, Y.; Kreike, M.; Scott, C.L.; Afonina, I.S.; Beyaert, R. A20 is a master switch of IL-33 signaling in macrophages and determines IL-33–induced lung immunity. J. Allergy Clin. Immunol. 2023, 152, 244–256.e4. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Ye, Y.; Gou, H.; Tang, H.; Zhou, Y.; Xu, X.; Xu, Y. A20 inhibits periodontal bone resorption and NLRP3-mediated M1 macrophage polarization. Exp. Cell Res. 2022, 418, 113264. [Google Scholar] [CrossRef]
- Pu, T.; Liu, W.; Wu, Y.; Zhao, Y. A20 functions as a negative regulator in macrophage for DSS-induced colitis. Int. Immunopharmacol. 2021, 97, 107804. [Google Scholar] [CrossRef]
- Polykratis, A.; Martens, A.; Eren, R.O.; Shirasaki, Y.; Yamagishi, M.; Yamaguchi, Y.; Uemura, S.; Miura, M.; Holzmann, B.; Kollias, G.; et al. A20 prevents inflammasome-dependent arthritis by inhibiting macrophage necroptosis through its ZnF7 ubiquitin-binding domain. Nat. Cell Biol. 2019, 21, 731–742. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Oshima, S.; Takahara, M.; Maeyashiki, C.; Nemoto, Y.; Kobayashi, M.; Nibe, Y.; Nozaki, K.; Nagaishi, T.; Okamoto, R.; et al. TNFAIP3 promotes survival of CD4 T cells by restricting MTOR and promoting autophagy. Autophagy 2015, 11, 1052–1062. [Google Scholar] [CrossRef]
- Giordano, M.; Roncagalli, R.; Bourdely, P.; Chasson, L.; Buferne, M.; Yamasaki, S.; Beyaert, R.; van Loo, G.; Auphan-Anezin, N.; Schmitt-Verhulst, A.-M.; et al. The tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20) imposes a brake on antitumor activity of CD8 T cells. Proc. Natl. Acad. Sci. USA 2014, 111, 11115–11120. [Google Scholar] [CrossRef]
- Razani, B.; Whang, M.I.; Kim, F.S.; Nakamura, M.C.; Sun, X.; Advincula, R.; Turnbaugh, J.A.; Pendse, M.; Tanbun, P.; Achacoso, P.; et al. Non-catalytic ubiquitin binding by A20 prevents psoriatic arthritis–like disease and inflammation. Nat. Immunol. 2020, 21, 422–433. [Google Scholar] [CrossRef]
- Rogers, N.M.; Zammit, N.; Nguyen-Ngo, D.; Souilmi, Y.; Minhas, N.; Meijles, D.N.; Self, E.; Walters, S.N.; Warren, J.; Cultrone, D.; et al. The impact of the cytoplasmic ubiquitin ligase TNFAIP3 gene variation on transcription factor NF-kappaB activation in acute kidney injury. Kidney Int. 2023, 103, 1105–1119. [Google Scholar] [CrossRef]
- Chu, Y.; Vahl, J.C.; Kumar, D.; Heger, K.; Bertossi, A.; Wójtowicz, E.; Soberon, V.; Schenten, D.; Mack, B.; Reutelshöfer, M.; et al. B cells lacking the tumor suppressor TNFAIP3/A20 display impaired differentiation and hyperactivation and cause inflammation and autoimmunity in aged mice. Blood 2011, 117, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Hövelmeyer, N.; Reissig, S.; Xuan, N.T.; Adams-Quack, P.; Lukas, D.; Nikolaev, A.; Schlüter, D.; Waisman, A. A20 deficiency in B cells enhances B-cell proliferation and results in the development of autoantibodies. Eur. J. Immunol. 2011, 41, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Tavares, R.M.; Turer, E.E.; Liu, C.L.; Advincula, R.; Scapini, P.; Rhee, L.; Barrera, J.; Lowell, C.A.; Utz, P.J.; Malynn, B.A.; et al. The ubiquitin modifying enzyme A20 restricts B cell survival and prevents autoimmunity. Immunity 2010, 33, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.G.; Boone, D.L.; Chai, S.; Libby, S.L.; Chien, M.; Lodolce, J.P.; Ma, A. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science 2000, 289, 2350–2354. [Google Scholar] [CrossRef] [PubMed]
- Verstrepen, L.; Verhelst, K.; van Loo, G.; Carpentier, I.; Ley, S.C.; Beyaert, R. Expression, biological activities and mechanisms of action of A20 (TNFAIP3). Biochem. Pharmacol. 2010, 80, 2009–2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Peng, L.-L.; Wang, Y.; Wang, J.-S.; Liu, J.; Liu, M.-M.; Hu, J.; Song, B.; Yang, H.-B. Roles of A20 in autoimmune diseases. Immunol. Res. 2016, 64, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.; Shah, S.; Altonsy, M.O.; Gerber, A.N. Glucocorticoid and cytokine crosstalk: Feedback, feedforward, and co-regulatory interactions determine repression or resistance. J. Biol. Chem. 2017, 292, 7163–7172. [Google Scholar] [CrossRef] [PubMed]
- Momtazi, G.; Lambrecht, B.N.; Naranjo, J.R.; Schock, B.C. Regulators of A20 (TNFAIP3): New drug-able targets in inflammation. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2019, 316, L456–L469. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, H.; Schwartz, D.M.; Stoffels, M.; Park, Y.H.; Zhang, Y.; Yang, D.; Demirkaya, E.; Takeuchi, M.; Tsai, W.L.; et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat. Genet. 2016, 48, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Aeschlimann, F.A.; Batu, E.D.; Canna, S.W.; Go, E.; Gül, A.; Hoffmann, P.; Leavis, H.L.; Ozen, S.; Schwartz, D.M.; Stone, D.L.; et al. A20 haploinsufficiency (HA20): Clinical phenotypes and disease course of patients with a newly recognised NF-kB-mediated autoinflammatory disease. Ann. Rheum. Dis. 2018, 77, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Musone, S.L.; Taylor, K.E.; Lu, T.T.; Nititham, J.; Ferreira, R.C.; Ortmann, W.; Shifrin, N.; Petri, M.A.; Kamboh, M.I.; Manzi, S.; et al. Multiple polymorphisms in the TNFAIP3 region are independently associated with systemic lupus erythematosus. Nat. Genet. 2008, 40, 1062–1064. [Google Scholar] [CrossRef]
- Rizk, M.M.; Elsayed, E.T.; ElKeraie, A.F.; Ramzy, I. Association of Tumor Necrosis Factor Alpha-Induced Protein 3 Interacting Protein 1 (TNIP1) Gene Polymorphism (rs7708392) with Lupus Nephritis in Egyptian Patients. Biochem. Genet. 2018, 56, 478–488. [Google Scholar] [CrossRef]
- Bates, J.S.; Lessard, C.J.; Leon, J.M.; Nguyen, T.; Battiest, L.J.; Rodgers, J.; Kaufman, K.M.; James, J.A.; Gilkeson, G.S.; Kelly, J.A.; et al. Meta-analysis and imputation identifies a 109 kb risk haplotype spanning TNFAIP3 associated with lupus nephritis and hematologic manifestations. Genes Immun. 2009, 10, 470–477. [Google Scholar] [CrossRef]
- Zhang, C.; Han, X.; Sun, L.; Yang, S.; Peng, J.; Chen, Y.; Jin, Y.; Xu, F.; Liu, Z.; Zhou, Q. Novel loss-of-function mutations in TNFAIP3 gene in patients with lupus nephritis. Clin. Kidney J. 2022, 15, 2027–2038. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Li, H.; Alateng, C.; Bai, Y.; Sun, Z.; Lv, X.; Wu, R. Single nucleotide polymorphisms of TNFAIP3 are associated with systemic lupus erythematosus in Han Chinese population. Int. J. Immunogenet. 2016, 43, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Villalvazo, P.; Carriazo, S.; Rojas-Rivera, J.; Ramos, A.M.; Ortiz, A.; Perez-Gomez, M.V. Gain-of-function TLR7 and loss-of-function A20 gene variants identify a novel pathway for Mendelian lupus and lupus nephritis. Clin. Kidney J. 2022, 15, 1973–1980. [Google Scholar] [CrossRef]
- Odqvist, L.; Jevnikar, Z.; Riise, R.; Öberg, L.; Rhedin, M.; Leonard, D.; Yrlid, L.; Jackson, S.; Mattsson, J.; Nanda, S.; et al. Genetic variations in A20 DUB domain provide a genetic link to citrullination and neutrophil extracellular traps in systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.; van Loo, G.; Waelput, W.; De Prijck, S.; Muskens, F.; Sze, M.; van Praet, J.; Branco-Madeira, F.; Janssens, S.; Reizis, B.; et al. The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity 2011, 35, 82–96. [Google Scholar] [CrossRef]
- Li, D.; Wang, L.; Fan, Y.; Song, L.; Guo, C.; Zhu, F.; Zhang, L.; Shi, Y. Down-regulation of A20 mRNA expression in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. J. Clin. Immunol. 2012, 32, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Qian, T.; Li, M.; Chen, F.; Chen, Y.; Hao, F. Aberrant Low Expression of A20 in Tumor Necrosis Factor-alpha-stimulated Monocytes Mediates Sustained NF-kappaB Inflammatory Response. Immunol. Investig. 2015, 44, 497–508. [Google Scholar] [CrossRef]
- Sun, L.; Zou, L.X.; Han, Y.C.; Zhu, D.D.; Chen, T.; Wang, J. Role of A20/TNFAIP3 deficiency in lupus nephritis in MRL/lpr mice. Clin. Exp. Nephrol. 2020, 24, 107–118. [Google Scholar] [CrossRef]
- Hammer, G.E.; Turer, E.E.; Taylor, K.E.; Fang, C.J.; Advincula, R.; Oshima, S.; Barrera, J.; Huang, E.J.; Hou, B.; Malynn, B.A.; et al. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat. Immunol. 2011, 12, 1184–1193. [Google Scholar] [CrossRef]
- Lee, Y.H.; Song, G.G. Associations between TNFAIP3 Polymorphisms and Rheumatoid Arthritis: A Systematic Review and Meta-Analysis Update with Trial Sequential Analysis. Public Health Genom. 2022, 25, 174–184. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, Y.; Tian, J.; Jiang, W.; Zhou, S.; Chen, J.; Xu, T.; Wu, M. Alterations and abnormal expression of A20 in peripheral monocyte subtypes in patients with rheumatoid arthritis. Clin. Rheumatol. 2021, 40, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Matmati, M.; Jacques, P.; Maelfait, J.; Verheugen, E.; Kool, M.; Sze, M.; Geboes, L.; Louagie, E.; Mc Guire, C.; Vereecke, L.; et al. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat. Genet. 2011, 43, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Hah, Y.; Lee, Y.; Jun, J.; Lim, H.; Kim, H.; Jeong, Y.; Hur, G.M.; Lee, S.Y.; Chung, M.J.; Park, J.; et al. A20 suppresses inflammatory responses and bone destruction in human fibroblast-like synoviocytes and in mice with collagen-induced arthritis. Arthritis Rheum. 2010, 62, 2313–2321. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, S.; Holle, J.U.; Müller, S.; Fricke, H.; Gross, W.L.; Epplen, J.T. A functionally relevant IRF5 haplotype is associated with reduced risk to Wegener’s granulomatosis. J. Mol. Med. 2010, 88, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Montúfar-Robles, I.; Soto, M.E.; Jiménez-Morales, S.; Gamboa, R.; Huesca-Gómez, C.; Ramírez-Bello, J. Polymorphisms in TNFAIP3, but not in STAT4, BANK1, BLK, and TNFSF4, are associated with susceptibility to Takayasu arteritis. Cell. Immunol. 2021, 365, 104375. [Google Scholar] [CrossRef]
- Dieudé, P.; Guedj, M.; Wipff, J.; Ruiz, B.; Riemekasten, G.; Matucci-Cerinic, M.; Melchers, I.; Hachulla, E.; Airo, P.; Diot, E.; et al. Association of the TNFAIP3 rs5029939 variant with systemic sclerosis in the European Caucasian population. Ann. Rheum. Dis. 2010, 69, 1958–1964. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.-I.; Yoon, H.-Y.; Lee, Y.-R.; Won, M.; Chung, M.J.; Park, J.-W.; Hur, G.M.; Lee, H.-K.; Park, B.-H. A20 attenuates allergic airway inflammation in mice. J. Immunol. 2009, 183, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Hoffjan, S.; Okur, A.; Epplen, J.T.; Wieczorek, S.; Chan, A.; Akkad, D.A. Association of TNFAIP3 and TNFRSF1A variation with multiple sclerosis in a German case-control cohort. Int. J. Immunogenet. 2015, 42, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Navone, N.; Perga, S.; Martire, S.; Berchialla, P.; Malucchi, S.; Bertolotto, A. Monocytes and CD4+ T cells contribution to the under-expression of NR4A2 and TNFAIP3 genes in patients with multiple sclerosis. J. Neuroimmunol. 2014, 272, 99–102. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, H.R.; Kwon, J.Y.; Jung, K.; Kim, S.Y.; Cho, K.H.; Choi, J.; Lee, H.H.; Lee, B.I.; Jue, D.M.; et al. A20 ameliorates inflammatory bowel disease in mice via inhibiting NF-kappaB and STAT3 activation. Immunol. Lett. 2018, 198, 44–51. [Google Scholar] [CrossRef]
- Hu, T.; Hu, W.; Ma, L.; Zeng, X.; Liu, J.; Cheng, B.; Yang, P.; Qiu, S.; Yang, G.; Chen, D.; et al. pVAX1-A20 alleviates colitis in mice by promoting regulatory T cells. Dig. Liver Dis. 2019, 51, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.E.C.; Rogier, E.W.; Arsenescu, R.I.; Flomenhoft, D.R.; Kurkjian, C.J.; Ellis, G.I.; Kaetzel, C.S. Correlation of Biomarker Expression in Colonic Mucosa with Disease Phenotype in Crohn’s Disease and Ulcerative Colitis. Dig. Dis. Sci. 2015, 60, 2976–2984. [Google Scholar] [CrossRef] [PubMed]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef]
- Shrikhande, G.V.; Scali, S.T.; da Silva, C.G.; Damrauer, S.M.; Csizmadia, E.; Putheti, P.; Matthey, M.; Arjoon, R.; Patel, R.; Siracuse, J.J.; et al. O-glycosylation regulates ubiquitination and degradation of the anti-inflammatory protein A20 to accelerate atherosclerosis in diabetic ApoE-null mice. PLoS ONE 2010, 5, e14240. [Google Scholar] [CrossRef] [PubMed]
- Angolano, C.; Kaczmarek, E.; Essayagh, S.; Daniel, S.; Choi, L.Y.; Tung, B.; Sauvage, G.; Lee, A.; Kipper, F.C.; Arvelo, M.B.; et al. A20/TNFAIP3 Increases ENOS Expression in an ERK5/KLF2-Dependent Manner to Support Endothelial Cell Health in the Face of Inflammation. Front. Cardiovasc. Med. 2021, 8, 651230. [Google Scholar] [CrossRef]
- Perico, N.; Cattaneo, D.; Sayegh, M.H.; Remuzzi, G. Delayed graft function in kidney transplantation. Lancet 2004, 364, 1814–1827. [Google Scholar] [CrossRef]
- Gueler, F.; Gwinner, W.; Schwarz, A.; Haller, H. Long-term effects of acute ischemia and reperfusion injury. Kidney Int. 2004, 66, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Braza, F.; Brouard, S.; Chadban, S.; Goldstein, D.R. Role of TLRs and DAMPs in allograft inflammation and transplant outcomes. Nat. Rev. Nephrol. 2016, 12, 281–290. [Google Scholar] [CrossRef]
- Smith, S.F.; Hosgood, S.A.; Nicholson, M.L. Ischemia-reperfusion injury in renal transplantation: 3 key signaling pathways in tubular epithelial cells. Kidney Int. 2019, 95, 50–56. [Google Scholar] [CrossRef]
- Lutz, J.; Luong, L.A.; Strobl, M.; Deng, M.; Huang, H.; Anton, M.; Zakkar, M.; Enesa, K.; Chaudhury, H.; Haskard, D.O.; et al. The A20 gene protects kidneys from ischaemia/reperfusion injury by suppressing pro-inflammatory activation. J. Mol. Med. 2008, 86, 1329–1339. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, X.; Ye, Q.; Yang, Y.; Chen, X. The transfection of A20 gene prevents kidney from ischemia reperfusion injury in rats. Mol. Med. Rep. 2017, 16, 1486–1492. [Google Scholar] [CrossRef]
- Shen, B.; Mei, M.; Ai, S.; Liao, X.; Li, N.; Xiang, S.; Wen, C.; Tao, Y.; Dai, H. TRPC6 inhibits renal tubular epithelial cell pyroptosis through regulating zinc influx and alleviates renal ischemia–reperfusion injury. FASEB J. 2022, 36, e22527. [Google Scholar] [CrossRef]
- Yang, Z.; Zhong, Z.; Li, M.; Xiong, Y.; Wang, Y.; Peng, G.; Ye, Q. Hypothermic machine perfusion increases A20 expression which protects renal cells against ischemia/reperfusion injury by suppressing inflammation, apoptosis and necroptosis. Int. J. Mol. Med. 2016, 38, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-L.; Lv, L.-L.; Tang, T.-T.; Wang, B.; Feng, Y.; Zhou, L.-T.; Cao, J.-Y.; Tang, R.-N.; Wu, M.; Liu, H.; et al. HIF-1alpha inducing exosomal microRNA-23a expression mediates the cross-talk between tubular epithelial cells and macrophages in tubulointerstitial inflammation. Kidney Int. 2019, 95, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Bodonyi-Kovacs, G.; Putheti, P.; Marino, M.; Avihingsanon, Y.; Uknis, M.E.; Monaco, A.P.; Strom, T.B.; Pavlakis, M. Gene expression profiling of the donor kidney at the time of transplantation predicts clinical outcomes 2 years after transplantation. Hum. Immunol. 2010, 71, 451–455. [Google Scholar] [CrossRef][Green Version]
- Daniel, S.; Patel, V.I.; Shrikhande, G.V.; Scali, S.T.; Ramsey, H.E.; Csizmadia, E.; Benhaga, N.; Fisher, M.D.; Arvelo, M.B.; Ferran, C. The universal NF-kappaB inhibitor a20 protects from transplant vasculopathy by differentially affecting apoptosis in endothelial and smooth muscle cells. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2006; Volume 38, pp. 3225–3227. [Google Scholar]
- Yang, J.; Xu, M.Q.; Yan, L.N.; Chen, X.B.; Liu, J. Zinc finger protein A20 protects rats against chronic liver allograft dysfunction. World J. Gastroenterol. 2012, 18, 3537–3550. [Google Scholar] [CrossRef] [PubMed]
- Kunter, U.; Floege, J.; Von Jürgensonn, A.S.; Stojanovic, T.; Merkel, S.; Gröne, H.J.; Ferran, C. Expression of A20 in the vessel wall of rat-kidney allografts correlates with protection from transplant arteriosclerosis. Transplantation 2003, 75, 3–9. [Google Scholar] [CrossRef]
- Moll, H.P.; Lee, A.; Peterson, C.R.; Cervantes, J.R.; Wojcik, B.M.; Parulkar, A.; Mele, A.; LoGerfo, P.J.; Siracuse, J.J.; Csizmadia, E.; et al. A20 Haploinsufficiency Aggravates Transplant Arteriosclerosis in Mouse Vascular Allografts: Implications for Clinical Transplantation. Transplantation 2016, 100, e106–e116. [Google Scholar] [CrossRef]
- Siracuse, J.J.; Fisher, M.D.; da Silva, C.G.; Peterson, C.R.; Csizmadia, E.; Moll, H.P.; Damrauer, S.M.; Studer, P.; Choi, L.Y.; Essayagh, S.; et al. A20-mediated modulation of inflammatory and immune responses in aortic allografts and development of transplant arteriosclerosis. Transplantation 2012, 93, 373–382. [Google Scholar] [CrossRef]
- Ahrens, H.E.; Petersen, B.; Ramackers, W.; Petkov, S.; Herrmann, D.; Hauschild-Quintern, J.; Lucas-Hahn, A.; Hassel, P.; Ziegler, M.; Baars, W.; et al. Kidneys from alpha1,3-Galactosyltransferase Knockout/Human Heme Oxygenase-1/Human A20 Transgenic Pigs Are Protected from Rejection during Ex Vivo Perfusion with Human Blood. Transplant. Direct 2015, 1, e23. [Google Scholar] [CrossRef]
- Avihingsanon, Y.; Ma, N.; Csizmadia, E.; Wang, C.; Pavlakis, M.; Giraldo, M.; Strom, T.B.; Soares, M.P.; Ferran, C. Expression of protective genes in human renal allografts: A regulatory response to injury associated with graft rejection. Transplantation 2002, 73, 1079–1085. [Google Scholar] [CrossRef]
- Avihingsanon, Y.; Ma, N.; Pavlakis, M.; Chon, J.W.; Uknis, M.E.; Monaco, A.P.; Ferran, C.; Stillman, I.; Schachter, A.D.; Mottley, C.; et al. On the intraoperative molecular status of renal allografts after vascular reperfusion and clinical outcomes. J. Am. Soc. Nephrol. 2005, 16, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Teng, D.; Lu, Y.; Gao, R.; Xin, Y.; Cao, G.; Li, X.; Wang, L.; Wang, J.; Li, Y. Conversion from cyclosporine to mycophenolate mofetil improves expression of A20 in the rat kidney allografts undergoing chronic rejection. Transplant. Proc. 2006, 38, 2164–2167. [Google Scholar] [CrossRef]
- Teng, D.; Lu, Y.; Gao, R.; Xin, Y.; Cao, G.; Li, X.; Wang, L.; Wang, J.; Li, Y. Comparison of rapamycin versus FK506 on expression of cytoprotective genes in the rat kidney allografts undergoing chronic allograft nephropathy. Transplant. Proc. 2006, 38, 2172–2175. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Ramesh, S.; Ming-hui, Z.; Ioannis, P.; Salmon, J.E.; Mohan, C. Lupus nephritis. Nat. Rev. Dis. Primers 2020, 6, 7. [Google Scholar] [PubMed]
- Obrișcă, B.; Sorohan, B.; Tuță, L.; Ismail, G. Advances in Lupus Nephritis Pathogenesis: From Bench to Bedside. Int. J. Mol. Sci. 2021, 22, 3766. [Google Scholar] [CrossRef] [PubMed]
- Rother, N.; van der Vlag, J. Disturbed T Cell Signaling and Altered Th17 and Regulatory T Cell Subsets in the Pathogenesis of Systemic Lupus Erythematosus. Front. Immunol. 2015, 6, 610. [Google Scholar] [CrossRef]
- Cornell, L.D.; Smith, R.N.; Colvin, R.B. Kidney transplantation: Mechanisms of rejection and acceptance. Annu. Rev. Pathol. 2008, 3, 189–220. [Google Scholar] [CrossRef]
- Kaewraemruaen, C.; Ritprajak, P.; Hirankarn, N. Dendritic cells as key players in systemic lupus erythematosus. Asian Pac. J. Allergy Immunol. 2020, 38, 225–232. [Google Scholar] [CrossRef]

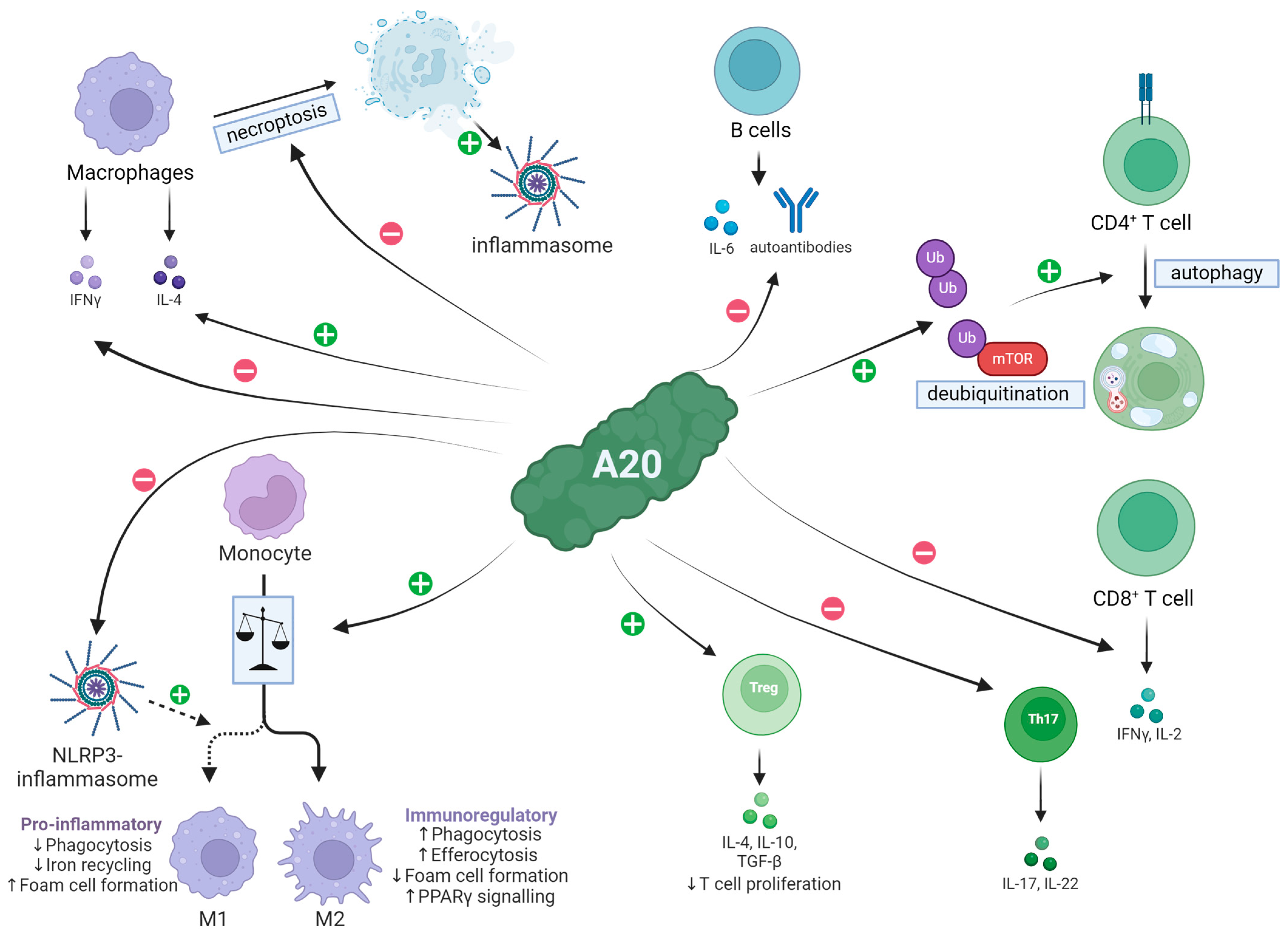
| Cell Population | Effect of A20 |
|---|---|
| Macrophages | Reduced IFN-γ production, increased IL-4 secretion [40]; suppresses M1 polarization and necroptosis [41,42,43] |
| CD4+ T cells | Promotion of autophagy and MTOR inhibition [44] |
| CD8+ T cells | Inhibition of IFN-γ and IL-2 secretion [45] |
| Th17 cells | Restricted proliferation; restraining activity [46] |
| FoxP3+ T cells | Increased influx in tissue [47] |
| B cells | Decreased proliferation and IL-6 expression; A20 deficiency causes autoantibody production [48,49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kommer, A.; Meineck, M.; Classen, P.; Weinmann-Menke, J. A20 in Kidney Transplantation and Autoimmunity. Int. J. Mol. Sci. 2024, 25, 6628. https://doi.org/10.3390/ijms25126628
Kommer A, Meineck M, Classen P, Weinmann-Menke J. A20 in Kidney Transplantation and Autoimmunity. International Journal of Molecular Sciences. 2024; 25(12):6628. https://doi.org/10.3390/ijms25126628
Chicago/Turabian StyleKommer, Andreas, Myriam Meineck, Paul Classen, and Julia Weinmann-Menke. 2024. "A20 in Kidney Transplantation and Autoimmunity" International Journal of Molecular Sciences 25, no. 12: 6628. https://doi.org/10.3390/ijms25126628
APA StyleKommer, A., Meineck, M., Classen, P., & Weinmann-Menke, J. (2024). A20 in Kidney Transplantation and Autoimmunity. International Journal of Molecular Sciences, 25(12), 6628. https://doi.org/10.3390/ijms25126628






