Orchestrating the Impact of KIR/HLA Interactions on Kidney Transplant
Abstract
1. Introduction
2. Results
- KIR2DL1/HLA-C2: The survival curves overlap significantly, with a p-value of 0.81, suggesting no substantial difference in survival probabilities between compatible and incompatible pairings.
- KIR2DL3/HLA-C1: Both groups show similar survival probabilities with a p-value of 0.69, indicating minimal impact of compatibility on survival outcomes.
- KIR3DL1/HLA-Bw4: The curves are closely aligned with a p-value of 0.086, suggesting a slight, non-significant advantage for compatible pairings.
- KIR3DL2/HLA-A3/A11: This graph shows a distinct separation between compatible and incompatible groups, with compatibility associated with better survival outcomes (p-value = 0.23).
- KIR2DL2/HLA-C1: The survival probabilities are similar for both groups, with a p-value of 0.35, indicating that compatibility does not significantly affect survival.
- KIR2DS5/HLA-C2: There is a notable divergence in survival curves after 20 months, with incompatible pairings showing decreased survival, highlighted by a significant p-value of 0.0012.
- KIR2DS1/HLA-C2: There are minimal differences in survival probabilities between the groups, with a p-value of 0.76.
- KIR2DS2/HLA-C1: The survival curves are closely aligned, indicating negligible impact of compatibility, with a p-value of 0.39.
- KIR2DS3/HLA-C1: There are similar survival probabilities for both matches and mismatches, with a p-value of 0.87.
- KIR2DS4/HLA-C2: The curves show no significant differences, with a p-value of 0.78.
- KIR2DS4/HLA-A03/A11: Both groups exhibit similar survival probabilities, with a p-value of 0.65.
- KIR3DS1/HLA-Bw4: The curves are closely aligned with a p-value of 0.7, indicating that compatibility does not significantly affect survival.
3. Discussion
3.1. KIR Genotype Frequencies and Transplant Outcomes
3.2. Impact of KIR/HLA Matching
4. Materials and Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chadban, S.J.; Ahn, C.; Axelrod, D.A.; Foster, B.J.; Kasiske, B.L.; Kher, V.; Kumar, D.; Oberbauer, R.; Pascual, J.; Pilmore, H.L.; et al. KDIGO Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation 2020, 104, S11–S103. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, M.; Cressman, S.; Edwards, L.; Shechter, S.; Doyle-Waters, M.M.; Keown, P.; Sapir-Pichhadze, R.; Bryan, S. A Systematic Review of Kidney Transplantation Decision Modelling Studies. Appl. Health Econ. Health Policy 2023, 21, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Iancu Loga, L.I.; Dican, L.; Chiorean, A.D.; Chelaru, V.F.; Elec, F.I.; Catana, C.S.; Marta, M.M.; Lucaciu, R.L.; Hangan, A.C.; Bondor, C.I.; et al. Association between Human Leukocyte Antigen and End-Stage Renal Disease in Patients from Transylvania, Romania. Int. J. Mol. Sci. 2023, 24, 13383. [Google Scholar] [CrossRef] [PubMed]
- Callemeyn, J.; Lamarthée, B.; Koenig, A.; Koshy, P.; Thaunat, O.; Naesens, M. Allorecognition and the spectrum of kidney transplant rejection. Kidney Int. 2022, 101, 692–710. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Zheng, X.; Mathew, J.M.; Gallon, L.; Leventhal, J.R.; Zhang, Z.J. Tackling Chronic Kidney Transplant Rejection: Challenges and Promises. Front. Immunol. 2021, 12, 661643. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.F.; Fribourg, M.; Yuki, Y.; Park, Y.H.; Martin, M.; Kelly, G.; Lee, B.; Miguel de Real, R.; Lee, R.; Geanon, D.; et al. Understanding the heterogeneity of alloreactive natural killer cell function in kidney transplantation. bioRxiv 2023, bioRxiv:2023.09.01.555962. [Google Scholar] [CrossRef]
- Senev, A.; Tambur, A.R.; Kosmoliaptsis, V.; Copley, H.C.; García-Sánchez, C.; Usenko, C.; Ildstad, S.T.; Leventhal, J.R. HLA molecular mismatches and induced donor-specific tolerance in combined living donor kidney and hematopoietic stem cell transplantation. Front. Immunol. 2024, 15, 1377535. [Google Scholar] [CrossRef] [PubMed]
- Duygu, B.; Olieslagers, T.I.; Groeneweg, M.; Voorter, C.E.M.; Wieten, L. HLA Class I Molecules as Immune Checkpoints for NK Cell Alloreactivity and Anti-Viral Immunity in Kidney Transplantation. Front. Immunol. 2021, 12, 680480. [Google Scholar] [CrossRef] [PubMed]
- Dęborska-Materkowska, D.; Perkowska-Ptasinska, A.; Sadowska-Jakubowicz, A.; Pazik, J.; Serwańska-Świętek, M.; Mikołajczyk, N.; Świder, R.; Nowak, J.; Durlik, M. Antiviral prophylaxis, male sex, and killer immunoglobulin-like receptor KIR2DL3 as markers for stratifying the risk of BK polyomavirus-associated nephropathy in kidney transplant recipients. Pol. Arch. Intern. Med. 2023, 133, 16331. [Google Scholar] [CrossRef]
- Pollock, N.R.; Harrison, G.F.; Norman, P.J. Immunogenomics of Killer Cell Immunoglobulin-Like Receptor (KIR) and HLA Class I: Coevolution and Consequences for Human Health. J. Allergy Clin. Immunol. Pract. 2022, 10, 1763–1775. [Google Scholar] [CrossRef]
- Hanvesakul, R.; Kubal, C.; Moore, J.; Neil, D.; Cook, M.; Ball, S.; Briggs, D.; Moss, P.; Cockwell, P. KIR and HLA-C interactionspromote differential dendritic cell maturation and is a major determinant of graft failure following kidney transplantation. PLoS ONE 2011, 6, e23631. [Google Scholar] [CrossRef]
- Yu, L.X.; Xiao, F.; Xiao, L.L.; Luo, M.; Fu, S.J.; Wang, Y.B.; Miao, Y. KIR/HLA ligand matching and acute rejection after kidney transplantation. Nan Fang Yi Ke Da Xue Xue Bao 2010, 30, 288–291. [Google Scholar] [PubMed]
- Laperrousaz, S.; Tiercy, S.; Villard, J.; Ferrari-Lacraz, S. HLA and non-HLA polymorphisms in renal transplantation. Swiss Med. Wkly. 2012, 142, w13668. [Google Scholar] [CrossRef] [PubMed]
- Loga, L.; Dican, L.; Matei, H.V.; Mărunțelu, I.; Constantinescu, I. Relevant biomarkers of kidney allograft rejection. J. Med. Life 2022, 15, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Vică, M.L.; Matei, H.V.; Bondor, C.I.; Nicula, G.Z.; Siserman, C.V.; Loga, L.; Dican, L. HLA Polymorphisms and Haplotype Diversity in Transylvania, Romania. J. Immunol. Res. 2019, 2019, 1342762. [Google Scholar] [CrossRef] [PubMed]
- Zamir, M.R.; Shahi, A.; Salehi, S.; Amirzargar, A. Natural killer cells and killer cell immunoglobulin-like receptors in solid organ transplantation: Protectors or opponents? Transplant. Rev. (Orlando) 2022, 36, 100723. [Google Scholar] [CrossRef] [PubMed]
- Rajalingam, R. Killer cell immunoglobulin-like receptors influence the innate and adaptive immune responses. Iran. J. Immunol. 2007, 4, 61–78. [Google Scholar] [PubMed]
- Kreijveld, E.; van der Meer, A.; Tijssen, H.J.; Hilbrands, L.B.; Joosten, I. KIR gene and KIR ligand analysis to predict graft rejection after renal transplantation. Transplantation 2007, 84, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Kunert, K.; Seiler, M.; Mashreghi, M.F.; Klippert, K.; Schönemann, C.; Neumann, K.; Pratschke, J.; Reinke, P.; Volk, H.D.; Kotsch, K. KIR/HLA ligand incompatibility in kidney transplantation. Transplantation 2007, 84, 1527–1533. [Google Scholar] [CrossRef]
- van Bergen, J.; Thompson, A.; Haasnoot, G.W.; Roodnat, J.I.; de Fijter, J.W.; Claas, F.H.; Koning, F.; Doxiadis, I.I. KIR-ligand mismatches are associated with reduced long-term graft survival in HLA-compatible kidney transplantation. Am. J. Transplant. 2011, 11, 1959–1964. [Google Scholar] [CrossRef]
- Dębska-Zielkowska, J.; Moszkowska, G.; Zieliński, M.; Zielińska, H.; Dukat-Mazurek, A.; Trzonkowski, P.; Stefańska, K. KIR Receptors as Key Regulators of NK Cells Activity in Health and Disease. Cells 2021, 10, 1777. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Mytilineos, J.; Scherer, S.; Laux, G.; Middleton, D.; Opelz, G. Analysis of KIR ligand incompatibility in human renal transplantation. Transplantation 2005, 80, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Unterrainer, C.; Fiedler, G.; Döhler, B.; Scherer, S.; Ruhenstroth, A.; Adamek, M.; Middleton, D.; Opelz, G. No impact of KIR-ligand mismatch on allograft outcome in HLA-compatible kidney transplantation. Am. J. Transplant. 2013, 13, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Rajalingam, R.; Gebel, H.M. KIR-HLA mismatching in human renal allograft transplantation: Emergence of a new concept. Am. J. Transplant. 2011, 11, 1771–1772. [Google Scholar] [CrossRef] [PubMed]
- Jafari, D.; Nafar, M.; Yekaninejad, M.S.; Abdolvahabi, R.; Lesan Pezeshki, M.; Razaghi, E.; Amirzargar, A.A. Investigation of Killer Immunoglobulin-like Receptor (KIR) and HLA Genotypes to Predict the Occurrence of Acute Allograft Rejection after Kidney Transplantation. Iran. J. Allergy Asthma Immunol. 2017, 16, 245–255. [Google Scholar] [PubMed]
- Nowak, I.; Magott-Procelewska, M.; Kowal, A.; Miazga, M.; Wagner, M.; Niepiekło-Miniewska, W.; Kamińska, M.; Wiśniewski, A.; Majorczyk, E.; Klinger, M.; et al. Killer immunoglobulin-like receptor (KIR) and HLA genotypes affect the outcome of allogeneic kidney transplantation. PLoS ONE 2012, 7, e44718. [Google Scholar] [CrossRef] [PubMed]
- La Manna, G.; Corsini, S.; Iannelli, S.; Cappuccilli, M.L.; Comai, G.; Iorio, M.; Todeschini, P.; Carretta, E.; Scolari, M.P.; Bontadini, A.; et al. Influence of the immunogenetic KIR and HLA systems on long-term renal transplant outcome. Ann. Transplant. 2013, 18, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Legaz, I.; López-Álvarez, M.R.; Campillo, J.A.; Moya-Quiles, M.R.; Bolarín, J.M.; de la Peña, J.; Salgado, G.; Gimeno, L.; García-Alonso, A.M.; Muro, M.; et al. KIR gene mismatching and KIR/C ligands in liver transplantation: Consequences for short-term liver allograft injury. Transplantation 2013, 95, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Callemeyn, J.; Senev, A.; Coemans, M.; Lerut, E.; Sprangers, B.; Kuypers, D.; Koenig, A.; Thaunat, O.; Emonds, M.P.; Naesens, M. Missing Self-Induced Microvascular Rejection of Kidney Allografts: A Population-Based Study. J. Am. Soc. Nephrol. 2021, 32, 2070–2082. [Google Scholar] [CrossRef]
- López-Botet, M.; Vilches, C.; Redondo-Pachón, D.; Muntasell, A.; Pupuleku, A.; Yélamos, J.; Pascual, J.; Crespo, M. Dual Role of Natural Killer Cells on Graft Rejection and Control of Cytomegalovirus Infection in Renal Transplantation. Front. Immunol. 2017, 8, 166. [Google Scholar] [CrossRef]
- McQueen, K.L.; Parham, P. Variable receptors controlling activation and inhibition of NK cells. Curr. Opin. Immunol. 2002, 14, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Rajalingam, R. Overview of the killer cell immunoglobulin-like receptor system. Methods Mol. Biol. 2012, 882, 391–414. [Google Scholar] [CrossRef] [PubMed]
- Posit Team. RStudio: Integrated Development Environment for R [Internet]; Posit Software, PBC: Boston, MA, USA, 2023; Available online: http://www.posit.co/ (accessed on 6 March 2023).
- Therneau, T. A Package for Survival Analysis in R [Internet]. 2023. Available online: https://CRAN.R-project.org/package=survival (accessed on 8 April 2023).



| Patients | SGF | CR | p Value; OR (a) or x1 − x2 (b) (95% CI) | |
|---|---|---|---|---|
| Kidney transplant patients | 84 | 68 | 16 | - |
| Patient age, years (median, IQR) | 46 (32–55) | 48 (37–56) | 29 (23–40) | 0.001; 19 (b) (−20.59 to −5.35) |
| Male patients, n (%) | 50 (59.52) | 38 (45.2) | 12 (14.3) | 0.93; 0.94 (a) (0.25 to 3.49) |
| Female patients, n (%) | 34 (40.48) | 30 (35.71) | 4 (4.76) | 0.16; 2.37 (a) (0.693 to 8.09) |
| DD, n (%) | 68 (81.0) | 56 (66.7) | 12 (14.3) | 0.223; 2.12 (a) (0.622 to 7.24) |
| LRD, n (%) | 16 (19.0) | 11 (13.0) | 5 (6.0) | 0.86; 4.25 (a) (−0.475 to 1.98) |
| Patient/donor HLA compatibility (mean ± SD) | ||||
| Class I (HLA-A, -B) mismatch (0–4) | 2.97 ± 0.99 | 3.01 ± 1.02 | 2.81 ± 0.83 | 0.412; 0.2 (b) (−0.70 to 0.29) |
| 0Class II (HLA-DR) mismatch (0–2) | 0.83 ± 0.83 | 0.72 ± 0.86 | 1.31 ± 0.47 | 0.001; −0.59 (b) (0.27 to 0.91) |
| PRA, n (%) | ||||
| PRA% Class I > 50% | 6 (7.14) | - | 6 (7.14) | |
| PRA% Class II > 50% | 10 (11.90) | - | 10 (11.90) | |
| Transplantation outcome | ||||
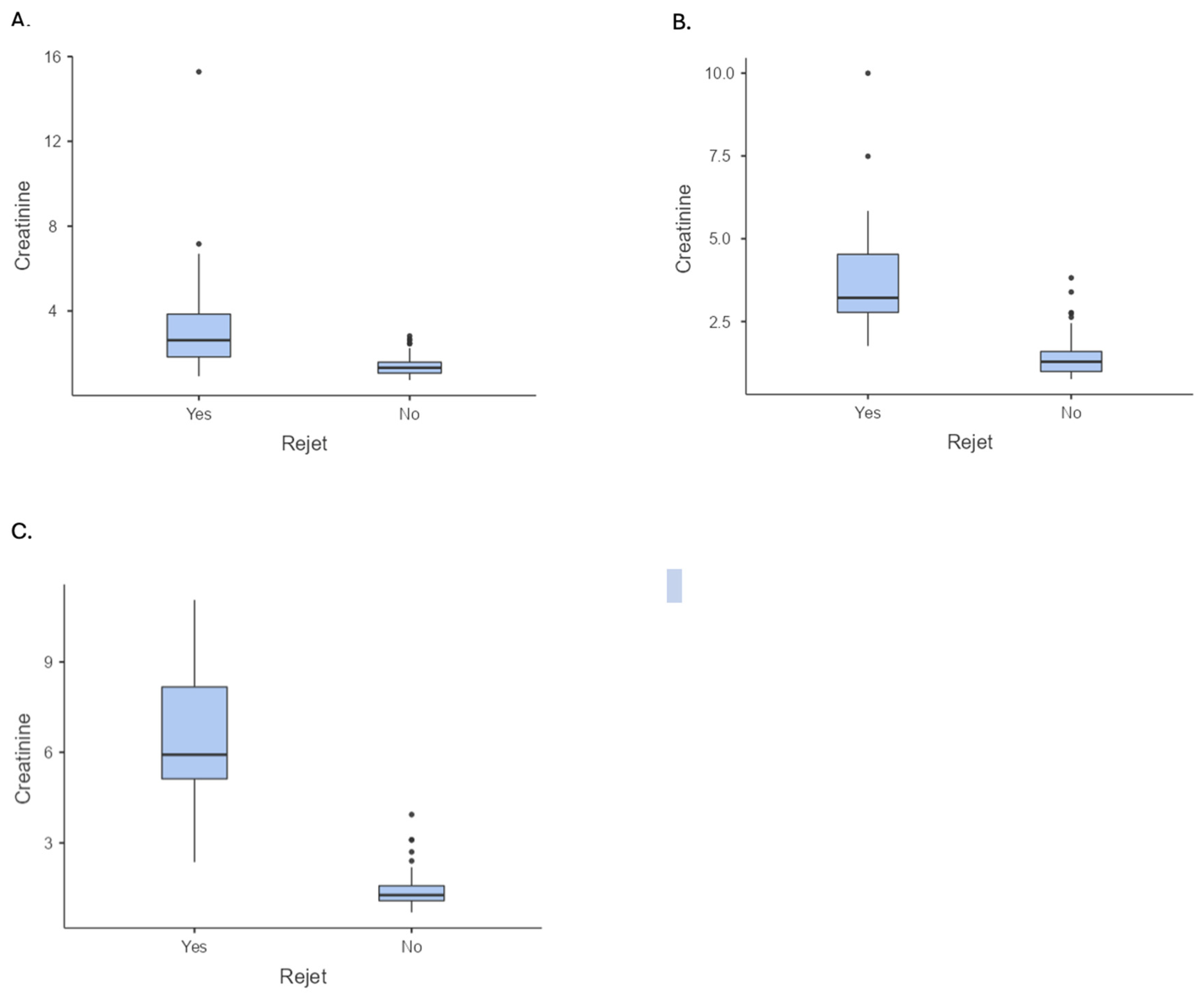
| Serum creatinine 1 year (mean ± SD) | 1.85 ± 1.83 | 1.39 ± 0.47 | 3.81 ± 3.41 | 0.001; −2.42 (b) (1.45 to 2.44) |
| Serum creatinine 3 years (mean ± SD) | 1.91 ± 1.49 | 1.41 ± 0.59 | 4.03 ± 2.10 | 0.001; −2.62 (b) (1.58 to 2.23) |
| Serum creatinine 5 years (mean ± SD) | 2.34 ± 2.23 | 1.43 ± 0.59 | 6.50 ± 2.16 | 0.001; −5.07 (b) (1.85 to 2.83) |
| 97 Controls n (%) | 84 Patients n (%) | p Value | 68 SGF n (%) | 16 CR n (%) | p Value | OR (95% CI) | |
|---|---|---|---|---|---|---|---|
| Patient inhibitory KIR genes | |||||||
| 2DL1 | 94 (96.91) | 74 (89.28) | 0.193 | 59 (86.76) | 15 (93.75) | 0.430 | 0.44 (0.05 to 3.72) |
| 2DL2 | 49 (50.52) | 50 (59.52) | 0.268 | 41 (60.29) | 9 (56.25) | 0.766 | 0.85 (0.28 to 2.55) |
| 2DL3 | 85 (87.63) | 70 (83.33) | 0.233 | 55 (80.88) | 15 (93.75) | 0.214 | 0.28 (0.03 to 2.33) |
| 2DL4 | 72 (74.23) | 64 (76.19) | 0.315 | 51 (75) | 13 (81.25) | 0.597 | 0.69 (0.18 to 2.72) |
| 2DL5 | 42 (43.30) | 48 (57.14) | 0.365 | 39 (57.35) | 9 (56.25) | 0.936 | 1.05 (0.35 to 3.14) |
| 3DL1 | 84 (86.60) | 77 (91.66) | 0.151 | 65 (95.58) | 12 (75) | 0.007 | 0.14 (0.03 to 0.70) |
| 3DL2 | 95 (97.94) | 84 (100) | 0.961 | 68 (100) | 16 (100) | 1 | - |
| 3DL3 | 94 (96.91) | 84 (100) | 0.945 | 68 (100) | 16 (100) | 1 | - |
| Patient activating KIR genes | |||||||
| 2DS1 | 36 (37.11) | 33 (39.28) | 0.339 | 26 (38.23) | 7 (43.75) | 0.684 | 0.80 (0.26 to 2.40) |
| 2DS2 | 50 (51.55) | 41 (48.81) | 0.390 | 33 (48.52) | 8 (48.52) | 0.915 | 0.83 (0.27 to 2.53) |
| 2DS3 | 32 (32.99) | 33 (39.28) | 0.424 | 26 (38.23) | 7 (43.75) | 0.684 | 0.80 (0.26 to 2.40) |
| 2DS4 | 28 (28.87) | 25 (29.76) | 0.466 | 21 (30.88) | 4 (25.00) | 0.643 | 1.34 (0.39 to 4.65) |
| 2DS5 | 31 (31.96) | 35 (41.66) | 0.517 | 22 (32.35) | 13 (81.25) | 0.001 | 2.17 (0.56 to 8.41) |
| 84 Patients n (%) | 68 SGF n (%) | 16 CR n (%) | p Value | OR (95% CI) | |
|---|---|---|---|---|---|
| Recipient activating KIR genes/donor HLA ligands | |||||
| 2DS1/HLA-C2 | 66 (78.57) | 54 (79.41) | 12 (75.00) | 0.698 | 0.78 (0.22 to 2.78) |
| 2DS4/HLA-A*03/A*11 | 28 (33.33) | 25 (36.76) | 3 (18.75) | 0.169 | 0.40 (0.10 to 1.53) |
| 3DS1/HLA-Bw4 | 51 (60.71) | 44 (64.70) | 7 (43.75) | 0.122 | 0.42 (0.14 to 1.28) |
| 2DS2/HLA-C1 | 28 (33.33) | 24 (35.29) | 4 (25.00) | 0.432 | 0.61 (0.17 to 2.10) |
| 2DS3/HLA-C1 | 23 (33.82) | 17 (25.00) | 6(37.50) | 0.999 | 1.00 (0.28 to 3.52) |
| 2DS4/HLA-C2 | 21 (25.00) | 19 (27.94) | 2 (12.50) | 0.812 | 0.86 (0.24 to 3.00) |
| 2DS5/HLA-C2 | 28 (33.33) | 17 (25.00) | 9(56.25) | 0.001 | 6.60 (2.01 to 21.7) |
| Recipient inhibitory KIR genes/donor HLA ligands | |||||
| 2DL1/HLA-C2 | 66 (78.57) | 54 (79.41) | 12 (75.00) | 0.698 | 0.78 (0.22 to 2.78) |
| 2DL2/HLA-C1 | 49 (58.33) | 42 (61.76) | 7 (43.75) | 0.188 | 0.48 (0.16 to 1.45) |
| 2DL3/HLA-C1 | 49 (58.33) | 42 (61.76) | 7 (43.75) | 0.188 | 0.48 (0.16 to 1.45) |
| 3DL1/HLA-Bw4 | 51 (60.71) | 44 (64.70) | 7 (43.75) | 0.122 | 0.42 (0.14 to 1.28) |
| 3DL2/HLA-A*03/A*11 | 28 (33.33) | 25 (36.76) | 3 (18.75) | 0.169 | 0.40 (0.10 to 1.53) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loga, L.-I.I.; Suharoschi, R.; Elec, F.I.; Chiorean, A.D.; Elec, A.D.; Muntean, A.M.; Suciu, M.D.; Antal, O.; Toth, A.T.; Lucaciu, R.L.; et al. Orchestrating the Impact of KIR/HLA Interactions on Kidney Transplant. Int. J. Mol. Sci. 2024, 25, 8228. https://doi.org/10.3390/ijms25158228
Loga L-II, Suharoschi R, Elec FI, Chiorean AD, Elec AD, Muntean AM, Suciu MD, Antal O, Toth AT, Lucaciu RL, et al. Orchestrating the Impact of KIR/HLA Interactions on Kidney Transplant. International Journal of Molecular Sciences. 2024; 25(15):8228. https://doi.org/10.3390/ijms25158228
Chicago/Turabian StyleLoga, Luminița-Ioana Iancu, Ramona Suharoschi, Florin Ioan Elec, Alin Dan Chiorean, Alina Daciana Elec, Adriana Milena Muntean, Mihai Domnuțiu Suciu, Oana Antal, Andreea Teodora Toth, Roxana Liana Lucaciu, and et al. 2024. "Orchestrating the Impact of KIR/HLA Interactions on Kidney Transplant" International Journal of Molecular Sciences 25, no. 15: 8228. https://doi.org/10.3390/ijms25158228
APA StyleLoga, L.-I. I., Suharoschi, R., Elec, F. I., Chiorean, A. D., Elec, A. D., Muntean, A. M., Suciu, M. D., Antal, O., Toth, A. T., Lucaciu, R. L., Hangan, A. C., Drugan, T., Matei, H. V., & Dican, L. (2024). Orchestrating the Impact of KIR/HLA Interactions on Kidney Transplant. International Journal of Molecular Sciences, 25(15), 8228. https://doi.org/10.3390/ijms25158228






.jpg)



