Exploring the Potential Role of Oligodendrocyte-Associated PIP4K2A in Alzheimer’s Disease Complicated with Type 2 Diabetes Mellitus via Multi-Omic Analysis
Abstract
:1. Introduction
2. Results
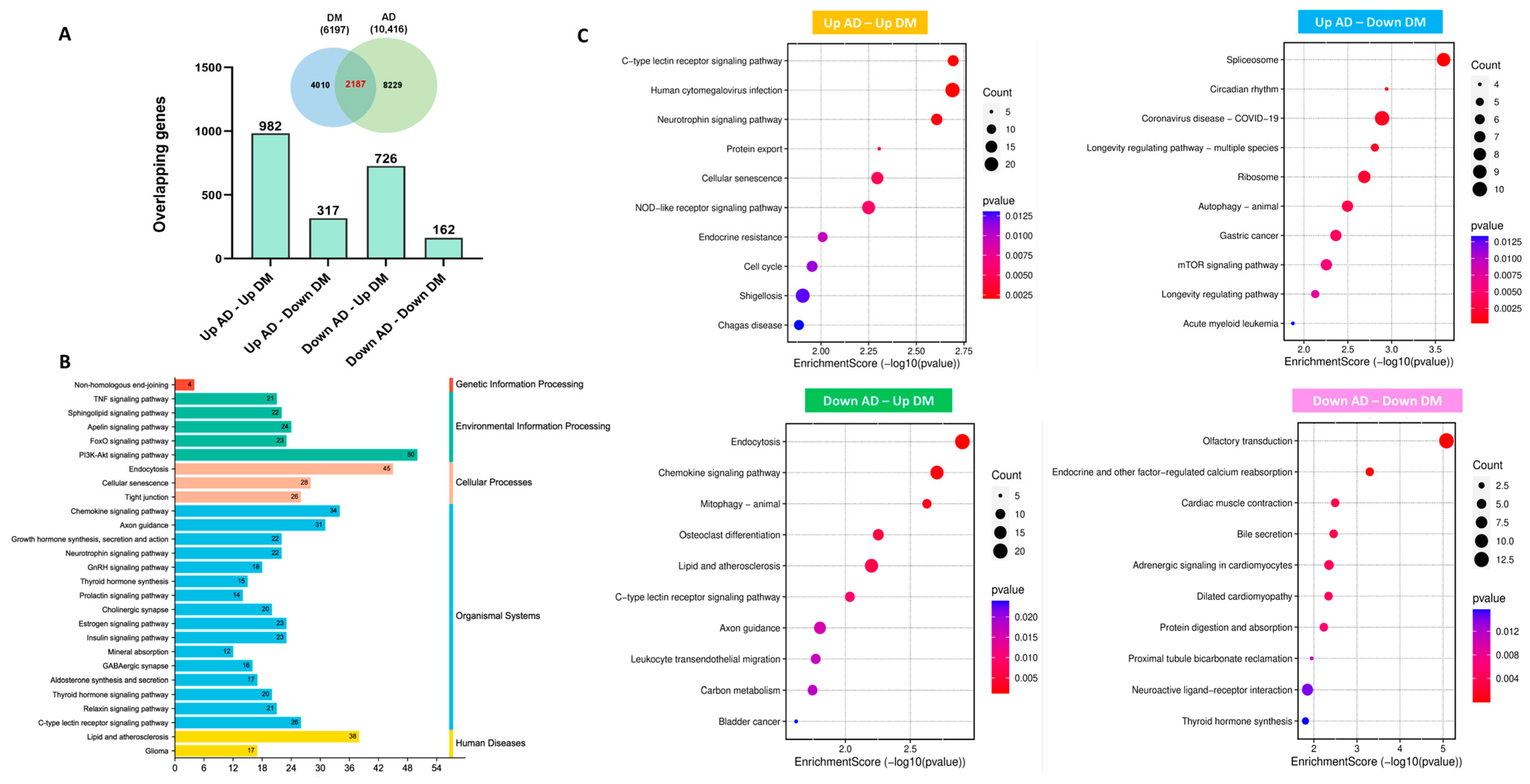
2.1. Transcriptional Profiling of Hub Genes in AD and T2DM
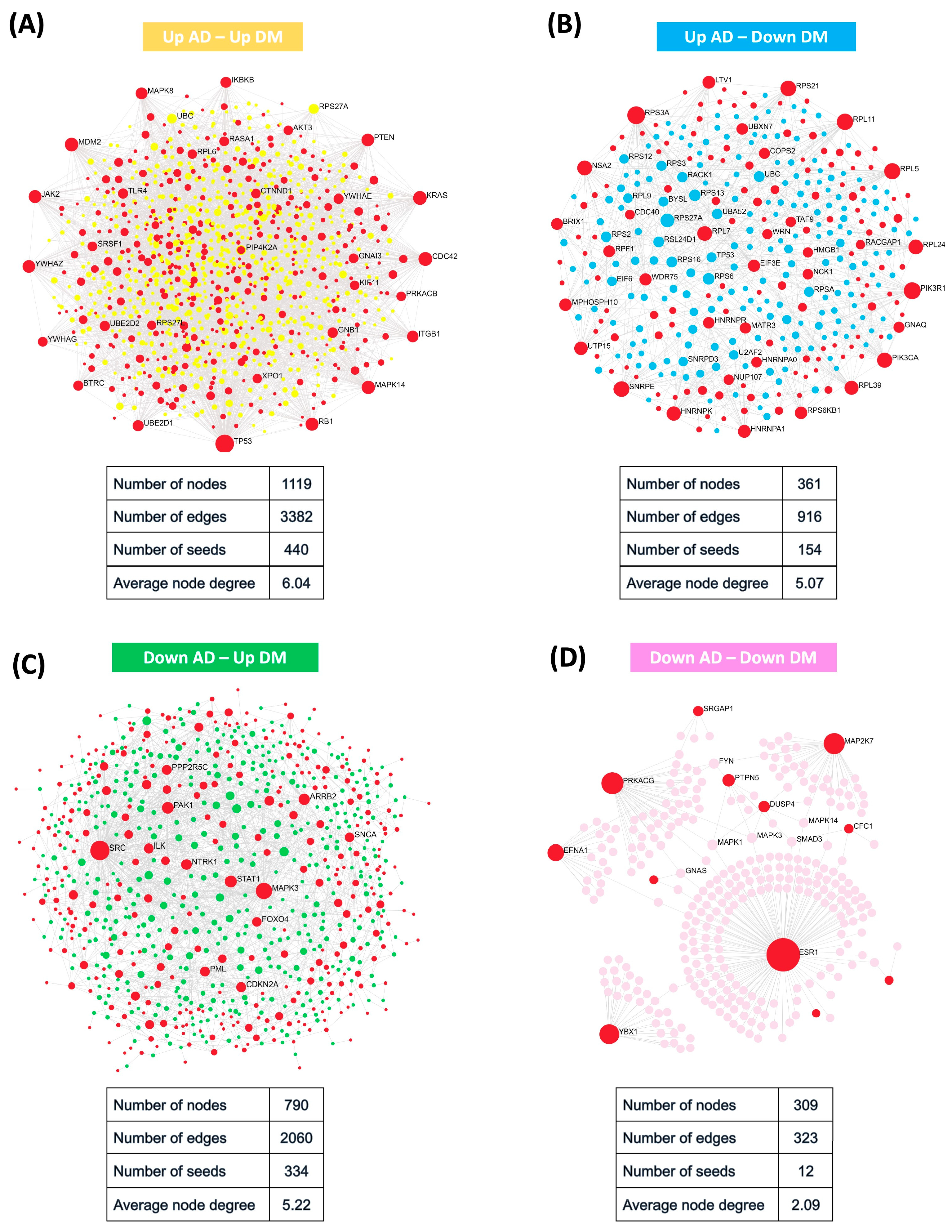
2.2. Protein–Protein Interaction Network Uncovered Distinct Biological Patterns
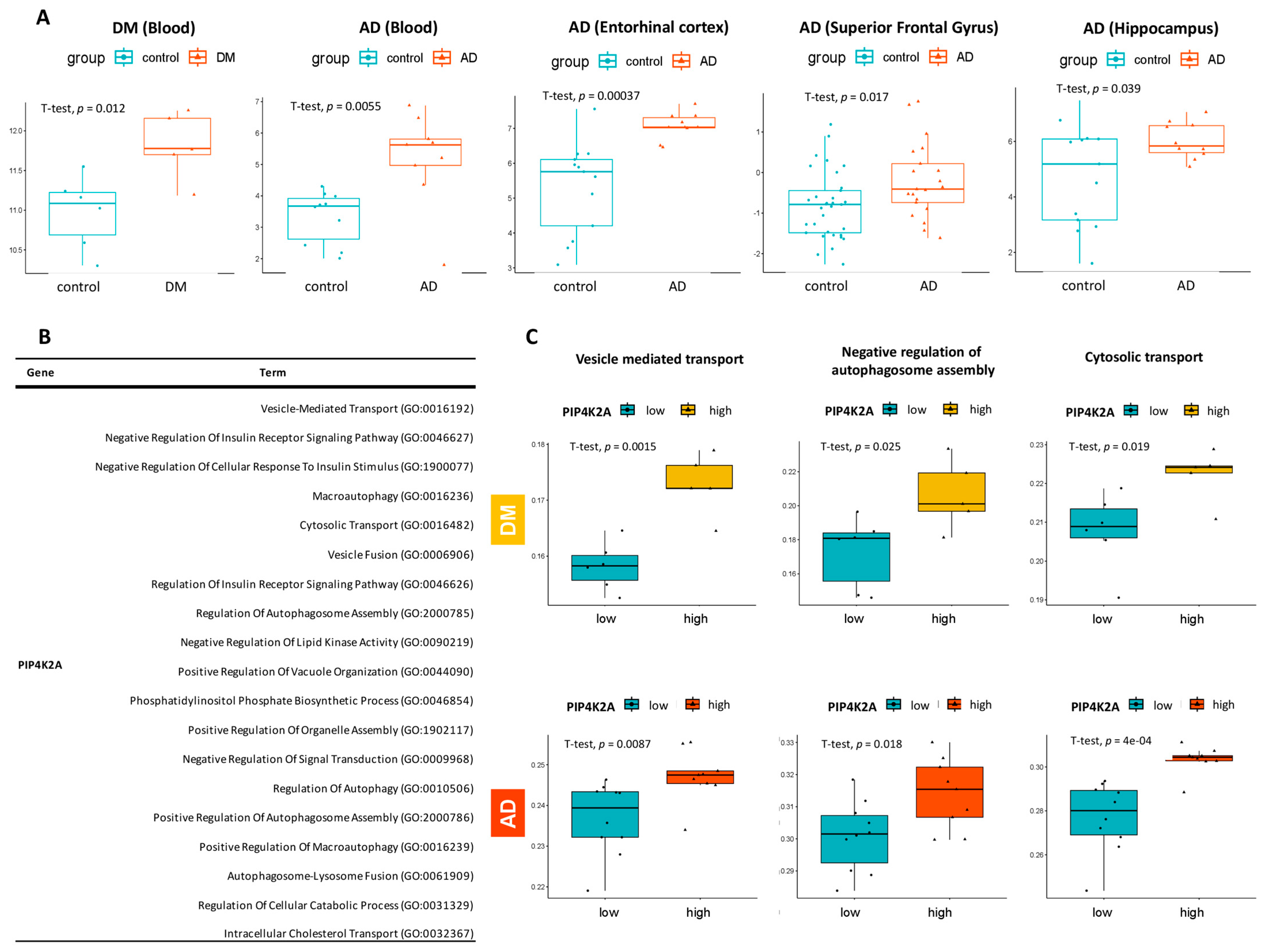
2.3. Identification of AD-Predictive Significance Genes across Multiple Datasets
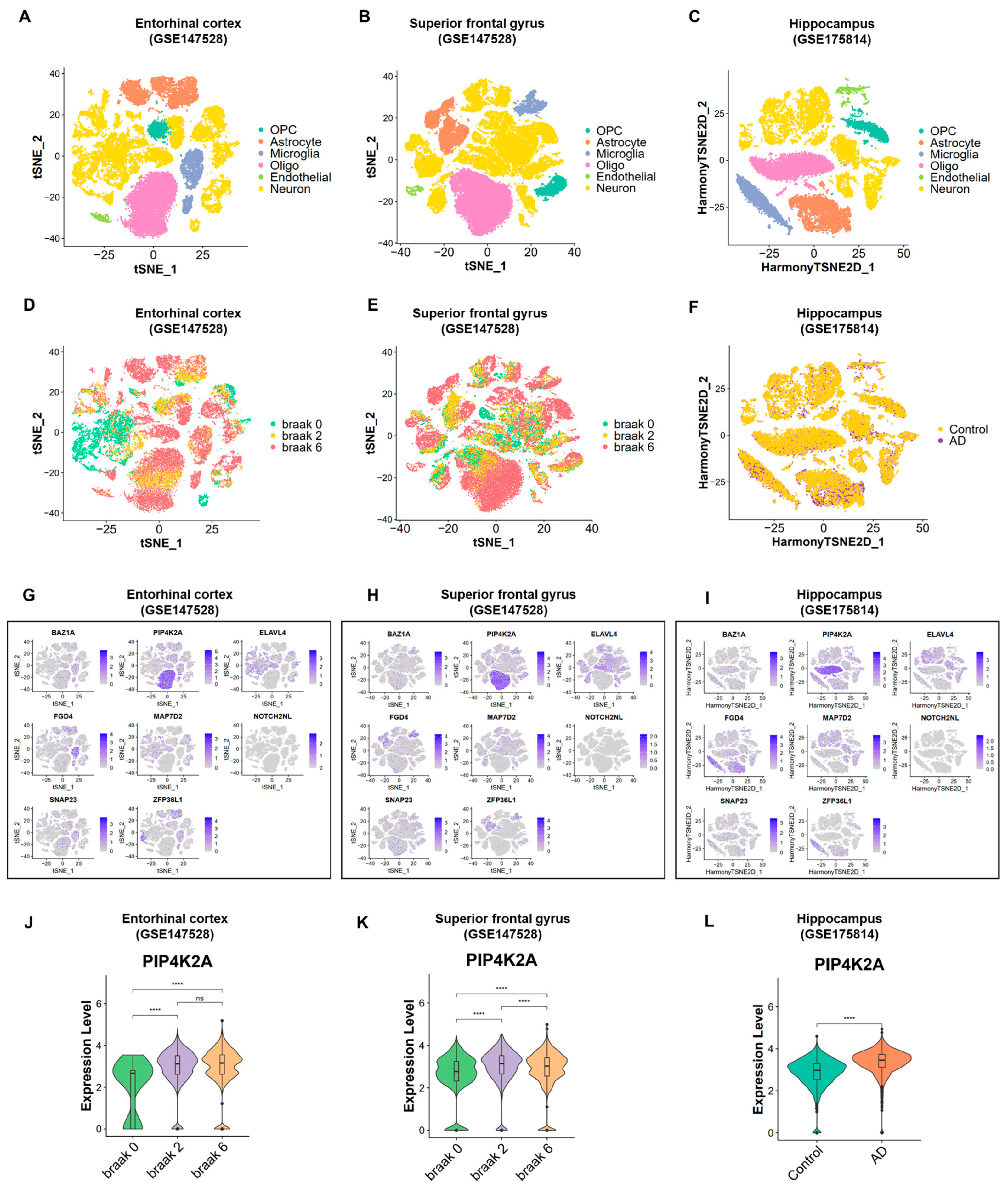
2.4. Transcriptional Diversity in AD and Control Samples Revealed by Single-Cell Human Brain Region Atlas
2.5. Exploration of PIP4K2A Expression Level in Multi-Cohorts and Its Key Biological Pathways
2.6. Validation of PIP4K2A Encoded Protein Level through Western Blotting
3. Discussion
4. Materials and Methods
4.1. Data Collection
4.2. Data Preprocessing
4.3. Differential Gene Expression Analysis
4.4. Identification of Commonly Dysregulated Genes in AD and T2DM and The Relevant Signaling Pathways
4.5. Protein–Protein Interaction Network Analysis
4.6. AUC-Based Validation and Predictive Modeling of the Common Hub Genes
4.7. Single-Cell RNA Sequencing Analysis
4.8. Analysis of Key Biological Pathways Related to The Target Gene
4.9. Human Subjects for Validation Experiment
4.10. Western Blotting
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [Google Scholar] [CrossRef]
- Wong, W. Economic burden of Alzheimer disease and managed care considerations. Am. J. Manag. Care 2020, 26, S177–S183. [Google Scholar] [CrossRef] [PubMed]
- Gunes, S.; Aizawa, Y.; Sugashi, T.; Sugimoto, M.; Rodrigues, P.P. Biomarkers for Alzheimer’s Disease in the Current State: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 4962. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. What causes alzheimer’s disease? Folia Neuropathol. 2013, 51, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.D.; Springer, D.A.; Burg, M.B.; Boehm, M.; Dmitrieva, N.I. Suboptimal hydration remodels metabolism, promotes degenerative diseases, and shortens life. JCI Insight 2019, 4, e130949. [Google Scholar] [CrossRef]
- Procaccini, C.; Santopaolo, M.; Faicchia, D.; Colamatteo, A.; Formisano, L.; de Candia, P.; Galgani, M.; De Rosa, V.; Matarese, G. Role of metabolism in neurodegenerative disorders. Metabolism 2016, 65, 1376–1390. [Google Scholar] [CrossRef]
- Sanotra, M.R.; Kao, S.H.; Lee, C.K.; Hsu, C.H.; Huang, W.C.; Chang, T.C.; Tu, F.Y.; Hsu, I.U.; Lin, Y.F. Acrolein adducts and responding autoantibodies correlate with metabolic disturbance in Alzheimer’s disease. Alzheimers Res. Ther. 2023, 15, 115. [Google Scholar] [CrossRef]
- Renuka Sanotra, M.; Huang, W.C.; Silver, S.; Lin, C.Y.; Chang, T.C.; Nguyen, D.P.Q.; Lee, C.K.; Kao, S.H.; Chang-Cheng Shieh, J.; Lin, Y.F. Serum levels of 4-hydroxynonenal adducts and responding autoantibodies correlate with the pathogenesis from hyperglycemia to Alzheimer’s disease. Clin. Biochem. 2022, 101, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Sheu, J.J.; Tsai, I.S.; Wang, S.T.; Yang, L.Y.; Hsu, I.U.; Chang, H.W.; Lee, H.M.; Kao, S.H.; Lee, C.K.; et al. Elevated IgM against Nepsilon-(Carboxyethyl)lysine-modified Apolipoprotein A1 peptide 141-147 in Taiwanese with Alzheimer’s disease. Clin. Biochem. 2018, 56, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qu, F.; Yu, X.; Yang, S.; Zhao, B.; Chen, Y.; Li, P.; Zhang, Z.; Zhang, J.; Han, X.; et al. Cortical lipid metabolic pathway alteration of early Alzheimer’s disease and candidate drugs screen. Eur. J. Med. Res. 2024, 29, 199. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.J.; Yang, L.Y.; Sanotra, M.R.; Wang, S.T.; Lu, H.T.; Kam, R.S.Y.; Hsu, I.U.; Kao, S.H.; Lee, C.K.; Shieh, J.C.; et al. Reduction of AHI1 in the serum of Taiwanese with probable Alzheimer’s disease. Clin. Biochem. 2020, 76, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ting, L.L.; Lu, H.T.; Yen, S.F.; Ngo, T.H.; Tu, F.Y.; Tsai, I.S.; Tsai, Y.H.; Chang, F.Y.; Li, X.J.; Li, S.; et al. Expression of AHI1 Rescues Amyloidogenic Pathology in Alzheimer’s Disease Model Cells. Mol. Neurobiol. 2019, 56, 7572–7582. [Google Scholar] [CrossRef]
- Athanasaki, A.; Melanis, K.; Tsantzali, I.; Stefanou, M.I.; Ntymenou, S.; Paraskevas, S.G.; Kalamatianos, T.; Boutati, E.; Lambadiari, V.; Voumvourakis, K.I.; et al. Type 2 Diabetes Mellitus as a Risk Factor for Alzheimer’s Disease: Review and Meta-Analysis. Biomedicines 2022, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.A.; Bottero, V.; Potashkin, J.A. Transcriptomic and Network Analysis Highlight the Association of Diabetes at Different Stages of Alzheimer’s Disease. Front. Neurosci. 2019, 13, 1273. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.B. Comorbidity Genes of Alzheimer’s Disease and Type 2 Diabetes Associated with Memory and Cognitive Function. Int. J. Mol. Sci. 2024, 25, 2211. [Google Scholar] [CrossRef]
- Michailidis, M.; Moraitou, D.; Tata, D.A.; Kalinderi, K.; Papamitsou, T.; Papaliagkas, V. Alzheimer’s disease as type 3 diabetes: Common pathophysiological mechanisms between Alzheimer’s disease and type 2 diabetes. Int. J. Mol. Sci. 2022, 23, 2687. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Di Domenico, F.; Barone, E. Elevated risk of type 2 diabetes for development of Alzheimer disease: A key role for oxidative stress in brain. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 1693–1706. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mudher, A. Alzheimer’s disease and type 2 diabetes: A critical assessment of the shared pathological traits. Front. Neurosci. 2018, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Akomolafe, A.; Beiser, A.; Meigs, J.B.; Au, R.; Green, R.C.; Farrer, L.A.; Wolf, P.A.; Seshadri, S. Diabetes mellitus and risk of developing Alzheimer disease: Results from the Framingham Study. Arch. Neurol. 2006, 63, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Hanyu, H. Diabetes-Related Dementia. In Diabetes Mellitus: A Risk Factor for Alzheimer’s Disease; Nakabeppu, Y., Ninomiya, T., Eds.; Springer: Singapore, 2019; pp. 147–160. [Google Scholar]
- Yang, A.; Du, L.; Gao, W.; Liu, B.; Chen, Y.; Wang, Y.; Liu, X.; Lv, K.; Zhang, W.; Xia, H. Associations of cortical iron accumulation with cognition and cerebral atrophy in Alzheimer’s disease. Quant. Imaging Med. Surg. 2022, 12, 4570. [Google Scholar] [CrossRef] [PubMed]
- Bottero, V.; Powers, D.; Yalamanchi, A.; Quinn, J.P.; Potashkin, J.A. Key disease mechanisms linked to Alzheimer’s disease in the entorhinal cortex. Int. J. Mol. Sci. 2021, 22, 3915. [Google Scholar] [CrossRef] [PubMed]
- Salta, E.; Lazarov, O.; Fitzsimons, C.P.; Tanzi, R.; Lucassen, P.J.; Choi, S.H. Adult hippocampal neurogenesis in Alzheimer’s disease: A roadmap to clinical relevance. Cell Stem Cell 2023, 30, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Frühbeis, C.; Kuo-Elsner, W.P.; Müller, C.; Barth, K.; Peris, L.; Tenzer, S.; Möbius, W.; Werner, H.B.; Nave, K.-A.; Fröhlich, D.; et al. Oligodendrocytes support axonal transport and maintenance via exosome secretion. PLoS Biol. 2020, 18, e3000621. [Google Scholar] [CrossRef] [PubMed]
- Salimi, L.; Seyedaghamiri, F.; Karimipour, M.; Mobarak, H.; Mardi, N.; Taghavi, M.; Rahbarghazi, R. Physiological and pathological consequences of exosomes at the blood–brain-barrier interface. Cell Commun. Signal. 2023, 21, 118. [Google Scholar] [CrossRef]
- De Felice, F.G.; Ferreira, S.T. Inflammation, Defective Insulin Signaling, and Mitochondrial Dysfunction as Common Molecular Denominators Connecting Type 2 Diabetes to Alzheimer Disease. Diabetes 2014, 63, 2262–2272. [Google Scholar] [CrossRef] [PubMed]
- Tumminia, A.; Vinciguerra, F.; Parisi, M.; Frittitta, L. Type 2 Diabetes Mellitus and Alzheimer’s Disease: Role of Insulin Signalling and Therapeutic Implications. Int. J. Mol. Sci. 2018, 19, 3306. [Google Scholar] [CrossRef] [PubMed]
- Llorente, A.; Arora, G.K.; Grenier, S.F.; Emerling, B.M. PIP kinases: A versatile family that demands further therapeutic attention. Adv. Biol. Regul. 2023, 87, 100939. [Google Scholar] [CrossRef]
- Bulley, S.J.; Clarke, J.H.; Droubi, A.; Giudici, M.-L.; Irvine, R.F. Exploring phosphatidylinositol 5-phosphate 4-kinase function. Adv. Biol. Regul. 2015, 57, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Ravi, A.; Palamiuc, L.; Emerling, B.M. Crucial players for inter-organelle communication: PI5P4Ks and their lipid product PI-4, 5-P2 come to the surface. Front. Cell Dev. Biol. 2022, 9, 791758. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, M.R.; Goncalves, M.D.; Loughran, R.M.; Possik, E.; Vijayaraghavan, T.; Yang, A.; Pauli, C.; Ravi, A.; Verma, A.; Yang, Z. Phosphatidylinositol-5-phosphate 4-kinases regulate cellular lipid metabolism by facilitating autophagy. Mol. Cell 2018, 70, 531–544.e9. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.G.; Paddock, M.N.; Lundquist, M.R.; Sun, J.Y.; Mashadova, O.; Amadiume, S.; Bumpus, T.W.; Hodakoski, C.; Hopkins, B.D.; Fine, M. PIP4Ks suppress insulin signaling through a catalytic-independent mechanism. Cell Rep. 2019, 27, 1991–2001.e1995. [Google Scholar] [CrossRef] [PubMed]
- Mandal, K. Review of PIP2 in Cellular Signaling, Functions and Diseases. Int. J. Mol. Sci. 2020, 21, 8342. [Google Scholar] [CrossRef]
- Ramasubbu, K.; Devi Rajeswari, V. Impairment of insulin signaling pathway PI3K/Akt/mTOR and insulin resistance induced AGEs on diabetes mellitus and neurodegenerative diseases: A perspective review. Mol. Cell. Biochem. 2023, 478, 1307–1324. [Google Scholar] [CrossRef] [PubMed]
- Goel, P.; Chakrabarti, S.; Goel, K.; Bhutani, K.; Chopra, T.; Bali, S. Neuronal cell death mechanisms in Alzheimer’s disease: An insight. Front. Mol. Neurosci. 2022, 15, 937133. [Google Scholar] [CrossRef]
- Shin, Y.J.; Sa, J.K.; Lee, Y.; Kim, D.; Chang, N.; Cho, H.J.; Son, M.; Oh, M.Y.T.; Shin, K.; Lee, J.-K.; et al. PIP4K2A as a negative regulator of PI3K in PTEN-deficient glioblastoma. J. Exp. Med. 2019, 216, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, K.; Miller, M.; Chludzinski, A.; Herrup, K.; Zagorski, M.; Lamb, B.T. The PI3K-Akt-mTOR pathway regulates Aβ oligomer induced neuronal cell cycle events. Mol. Neurodegener. 2009, 4, 14. [Google Scholar] [CrossRef]
- Zeng, Y.; Cao, S.; Li, N.; Tang, J.; Lin, G. Identification of key lipid metabolism-related genes in Alzheimer’s disease. Lipids Health Dis. 2023, 22, 155. [Google Scholar] [CrossRef]
- Kotob, S.E. Review Article: An Overview of Cellular Signal Transduction Pathway. Biomed. J. Sci. Tech. Res. 2021, 38, 30215–30229. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, P. Role of insulin receptor substance-1 modulating PI3K/Akt insulin signaling pathway in Alzheimer’s disease. 3 Biotech 2021, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Arancio, O. PIP2: A new key player in Alzheimer’s disease. Cellscience 2008, 5, 44. [Google Scholar] [PubMed]
- Cremona, O.; Di Paolo, G.; Wenk, M.R.; Lüthi, A.; Kim, W.T.; Takei, K.; Daniell, L.; Nemoto, Y.; Shears, S.B.; Flavell, R.A. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell 1999, 99, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.; Kim, J. Autophagy: An essential degradation program for cellular homeostasis and life. Cells 2018, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Stachowiak, A.; Mamun, A.A.; Tzvetkov, N.T.; Takeda, S.; Atanasov, A.G.; Bergantin, L.B.; Abdel-Daim, M.M.; Stankiewicz, A.M. Autophagy and Alzheimer’s disease: From molecular mechanisms to therapeutic implications. Front. Aging Neurosci. 2018, 10, 04. [Google Scholar] [CrossRef] [PubMed]
- Caberlotto, L.; Nguyen, T.-P.; Lauria, M.; Priami, C.; Rimondini, R.; Maioli, S.; Cedazo-Minguez, A.; Sita, G.; Morroni, F.; Corsi, M. Cross-disease analysis of Alzheimer’s disease and type-2 Diabetes highlights the role of autophagy in the pathophysiology of two highly comorbid diseases. Sci. Rep. 2019, 9, 3965. [Google Scholar] [CrossRef]
- Liang, C. Negative regulation of autophagy. Cell Death Differ. 2010, 17, 1807–1815. [Google Scholar] [CrossRef]
- Vicinanza, M.; Korolchuk, V.I.; Ashkenazi, A.; Puri, C.; Menzies, F.M.; Clarke, J.H.; Rubinsztein, D.C. PI (5) P regulates autophagosome biogenesis. Mol. Cell 2015, 57, 219–234. [Google Scholar] [CrossRef]
- Yang, J.-S.; Lu, C.-C.; Kuo, S.-C.; Hsu, Y.-M.; Tsai, S.-C.; Chen, S.-Y.; Chen, Y.-T.; Lin, Y.-J.; Huang, Y.-C.; Chen, C.-J. Autophagy and its link to type II diabetes mellitus. Biomedicine 2017, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Mukhopadhyay, M.; Bhattacharyya, M.; Karmakar, P. Is autophagy associated with diabetes mellitus and its complications? A review. EXCLI J. 2018, 17, 709. [Google Scholar] [PubMed]
- Drummond, E.; Pires, G.; MacMurray, C.; Askenazi, M.; Nayak, S.; Bourdon, M.; Safar, J.; Ueberheide, B.; Wisniewski, T. Phosphorylated tau interactome in the human Alzheimer’s disease brain. Brain 2020, 143, 2803–2817. [Google Scholar] [CrossRef] [PubMed]
- Aber, E.R.; Griffey, C.J.; Davies, T.; Li, A.M.; Yang, Y.J.; Croce, K.R.; Goldman, J.E.; Grutzendler, J.; Canman, J.C.; Yamamoto, A. Oligodendroglial macroautophagy is essential for myelin sheath turnover to prevent neurodegeneration and death. Cell Rep. 2022, 41, 111480. [Google Scholar] [CrossRef] [PubMed]
- Belgrad, J.; De Pace, R.; Fields, R.D. Autophagy in myelinating glia. J. Neurosci. 2020, 40, 256–266. [Google Scholar] [CrossRef]
- Yang, G.Z.; Yang, M.; Lim, Y.; Lu, J.J.; Wang, T.H.; Qi, J.G.; Zhong, J.H.; Zhou, X.F. Huntingtin associated protein 1 regulates trafficking of the amyloid precursor protein and modulates amyloid beta levels in neurons. J. Neurochem. 2012, 122, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, G.; Reyna, S.M.; Dunlap, M.; Van Der Kant, R.; Callender, J.A.; Young, J.E.; Roberts, E.A.; Goldstein, L.S. Defective transcytosis of APP and lipoproteins in human iPSC-derived neurons with familial Alzheimer’s disease mutations. Cell Rep. 2016, 17, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Maitre, M.; Jeltsch-David, H.; Okechukwu, N.G.; Klein, C.; Patte-Mensah, C.; Mensah-Nyagan, A.-G. Myelin in Alzheimer’s disease: Culprit or bystander? Acta Neuropathol. Commun. 2023, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Depp, C.; Sun, T.; Sasmita, A.O.; Spieth, L.; Berghoff, S.A.; Nazarenko, T.; Overhoff, K.; Steixner-Kumar, A.A.; Subramanian, S.; Arinrad, S. Myelin dysfunction drives amyloid-β deposition in models of Alzheimer’s disease. Nature 2023, 618, 349–357. [Google Scholar] [CrossRef]
- Cui, L.; Li, H.; Xi, Y.; Hu, Q.; Liu, H.; Fan, J.; Xiang, Y.; Zhang, X.; Shui, W.; Lai, Y. Vesicle trafficking and vesicle fusion: Mechanisms, biological functions, and their implications for potential disease therapy. Mol. Biomed. 2022, 3, 29. [Google Scholar] [CrossRef]
- Naughton, B.J.; Duncan, F.J.; Murrey, D.A.; Meadows, A.S.; Newsom, D.E.; Stoicea, N.; White, P.; Scharre, D.W.; Mccarty, D.M.; Fu, H.J.; et al. Blood genome-wide transcriptional profiles reflect broad molecular impairments and strong blood-brain links in Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 43, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhou, W.-H.; Cai, J.-J.; Feng, M.; Zhou, M.; Hu, S.-P.; Xu, J.; Ji, L.-D. Gene expression profiling identifies downregulation of the neurotrophin-MAPK signaling pathway in female diabetic peripheral neuropathy patients. J. Diabetes Res. 2017, 2017, 8103904. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, N.C.; Coleman, P.D.; Cribbs, D.H.; Rogers, J.; Gillen, D.L.; Cotman, C.W. Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.S.; Dunckley, T.; Beach, T.G.; Grover, A.; Mastroeni, D.; Walker, D.G.; Caselli, R.J.; Kukull, W.A.; McKeel, D.; Morris, J.C.; et al. Gene expression profiles in anatomically and functionally distinct regions of the normal aged human brain. Physiol. Genom. 2007, 28, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Leng, K.; Li, E.; Eser, R.; Piergies, A.; Sit, R.; Tan, M.; Neff, N.; Li, S.H.; Rodriguez, R.D.; Suemoto, C.K.; et al. Molecular characterization of selectively vulnerable neurons in Alzheimer’s disease. Nat. Neurosci. 2021, 24, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Soreq, L.; Bird, H.; Mohamed, W.; Hardy, J. Single-cell RNA sequencing analysis of human Alzheimer’s disease brain samples reveals neuronal and glial specific cells differential expression. PLoS ONE 2023, 18, e0277630. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.K. Limma: Linear models for microarray data. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor; Springer: Berlin/Heidelberg, Germany, 2005; pp. 397–420. [Google Scholar]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.J.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene set knowledge discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef]
- Zhou, G.; Soufan, O.; Ewald, J.; Hancock, R.E.; Basu, N.; Xia, J.J. NetworkAnalyst 3.0: A visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019, 47, W234–W241. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M.J.B.b. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 1–8. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, J.; Hou, Y.; Bekris, L.; Leverenz, J.B.; Pieper, A.A.; Cummings, J.; Cheng, F.J.A.S.; Research, D.T.; Interventions, C. The Alzheimer’s Cell Atlas (TACA): A single-cell molecular map for translational therapeutics accelerator in Alzheimer’s disease. Alzheimers Dement. 2022, 8, e12350. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-W.; Duan, T.-T.; Chu, J.-Y.; Pan, S.-Y.; Zeng, Y.; Hu, F.-F.J.F.i.A.N. SCAD-Brain: A public database of single cell RNA-seq data in human and mouse brains with Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1157792. [Google Scholar] [CrossRef] [PubMed]
- Hänzelmann, S.; Castelo, R.; Guinney, J.J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G. DSM-IV. Diagnostic and Statistical Manual of Mental Disorders, 4th edn. By the American Psychiatric Association. (Pp. 886;£ 34.95.) APA: Washington, DC. 1994. Psychol. Med. 1996, 26, 651–652. [Google Scholar] [CrossRef]




| Gene | DM—Blood | AD—Blood | AD—Entorhinal Cortex | AD—Superior Frontal Gyrus | AD—Hippocampus |
|---|---|---|---|---|---|
| BAZ1A | 0.97 | 0.88 | 0.82 | 0.89 | 0.95 |
| FGD4 | 0.83 | 0.89 | 0.87 | 0.88 | 0.91 |
| NOTCH2NL | 0.90 | 0.98 | 0.90 | 0.82 | 0.82 |
| PIP4K2A | 0.90 | 0.89 | 0.93 | 0.71 | 0.78 |
| SNAP23 | 0.93 | 0.96 | 0.83 | 0.89 | 0.76 |
| ZFP36L1 | 0.93 | 0.96 | 0.85 | 0.84 | 0.80 |
| ELAVL4 | 0.87 | 0.73 | 0.89 | 0.79 | 0.83 |
| MAP7D2 | 0.90 | 0.92 | 0.74 | 0.71 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.P.Q.; Jallow, A.W.; Lin, Y.-F.; Lin, Y.-F. Exploring the Potential Role of Oligodendrocyte-Associated PIP4K2A in Alzheimer’s Disease Complicated with Type 2 Diabetes Mellitus via Multi-Omic Analysis. Int. J. Mol. Sci. 2024, 25, 6640. https://doi.org/10.3390/ijms25126640
Nguyen DPQ, Jallow AW, Lin Y-F, Lin Y-F. Exploring the Potential Role of Oligodendrocyte-Associated PIP4K2A in Alzheimer’s Disease Complicated with Type 2 Diabetes Mellitus via Multi-Omic Analysis. International Journal of Molecular Sciences. 2024; 25(12):6640. https://doi.org/10.3390/ijms25126640
Chicago/Turabian StyleNguyen, Doan Phuong Quy, Amadou Wurry Jallow, Yi-Fang Lin, and Yung-Feng Lin. 2024. "Exploring the Potential Role of Oligodendrocyte-Associated PIP4K2A in Alzheimer’s Disease Complicated with Type 2 Diabetes Mellitus via Multi-Omic Analysis" International Journal of Molecular Sciences 25, no. 12: 6640. https://doi.org/10.3390/ijms25126640
APA StyleNguyen, D. P. Q., Jallow, A. W., Lin, Y.-F., & Lin, Y.-F. (2024). Exploring the Potential Role of Oligodendrocyte-Associated PIP4K2A in Alzheimer’s Disease Complicated with Type 2 Diabetes Mellitus via Multi-Omic Analysis. International Journal of Molecular Sciences, 25(12), 6640. https://doi.org/10.3390/ijms25126640







