Leukemic Stem Cells and Hematological Malignancies
Abstract
1. Introduction
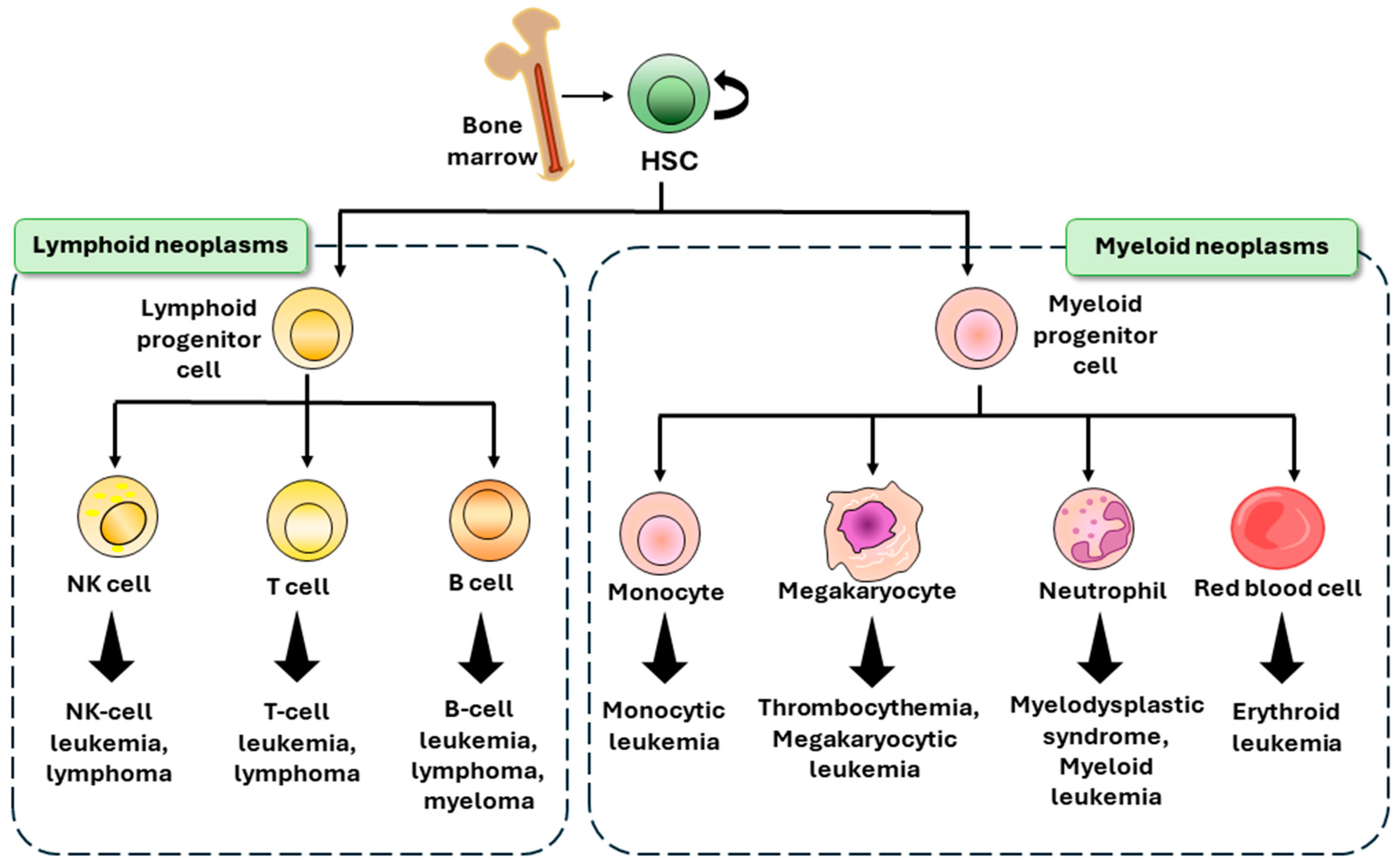
2. Hematological Malignancies
2.1. Leukemia
2.2. Lymphoma
2.3. Multiple Myeloma
3. LSCs and Phenotype
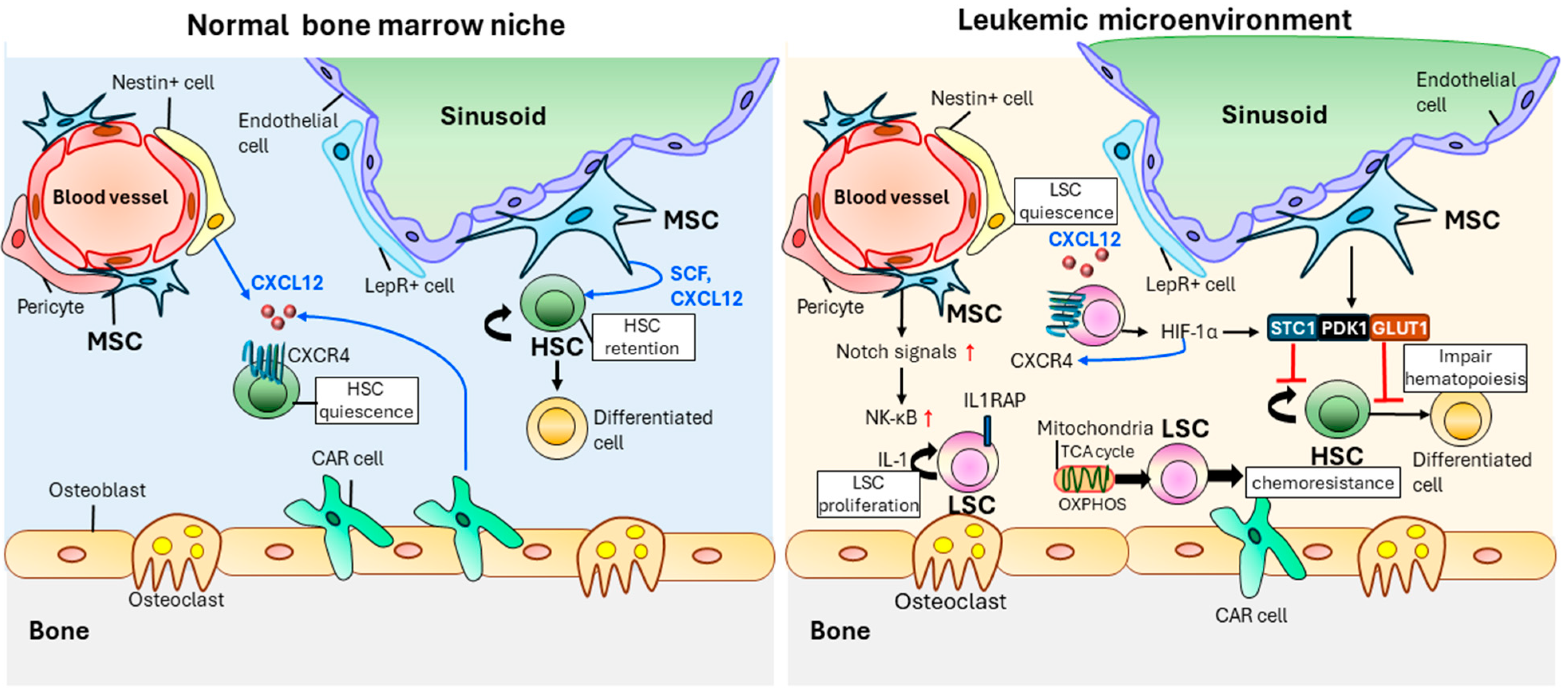
4. Effect of the Hematopoietic Microenvironment on LSCs
5. Role of LSCs in Leukemogenesis and Relapse
6. Clinical Applications of LSCs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dzierzak, E.; Bigas, A. Blood development: Hematopoietic stem cell dependence and independence. Cell Stem Cell 2018, 22, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.C.; Igarashi, K.J.; Nakauchi, H. Haematopoietic stem cell self-renewal in vivo and ex vivo. Nat. Rev. Genet. 2020, 21, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.B.; Scadden, D.T. The hematopoietic stem cell in its place. Nat. Immunol. 2006, 7, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Gray, T.F.; Temel, J.S.; El-Jawahri, A. Illness and prognostic understanding in patients with hematologic malignancies. Blood Rev. 2021, 45, 100692. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Shilatifard, A. Epigenetics of hematopoiesis and hematological malignancies. Genes Dev. 2016, 30, 2021–2041. [Google Scholar] [CrossRef] [PubMed]
- Wade, J.C. Viral infections in patients with hematological malignancies. ASH Educ. Program Book 2006, 2006, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Shimony, S.; Stahl, M.; Stone, R.M. Acute myeloid leukemia: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2023, 98, 502–526. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Filho, A.; Piñeros, M.; Ferlay, J.; Soerjomataram, I.; Monnereau, A.; Bray, F. Epidemiological patterns of leukaemia in 184 countries: A population-based study. Lancet Haematol. 2018, 5, e14–e24. [Google Scholar] [CrossRef] [PubMed]
- Hoelzer, D.; Gökbuget, N. Acute lymphocytic leukemia in adults. In Hematology; Churchill Livingston: New York, NY, USA, 2003. [Google Scholar]
- Rozman, C.; Montserrat, E. Chronic lymphocytic leukemia. N. Engl. J. Med. 1995, 333, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Küppers, R.; Engert, A.; Hansmann, M.-L. Hodgkin lymphoma. J. Clin. Investig. 2012, 122, 3439–3447. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J.O.; Gascoyne, R.D.; Lunning, M.A.; Cavalli, F. Non-hodgkin lymphoma. Lancet 2017, 390, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Stelmach, P.; Trumpp, A. Leukemic stem cells and therapy resistance in acute myeloid leukemia. Haematologica 2023, 108, 353. [Google Scholar] [CrossRef] [PubMed]
- Palani, H.K.; Ganesan, S.; Balasundaram, N.; Venkatraman, A.; Korula, A.; Abraham, A.; George, B.; Mathews, V. Ablation of Wnt signaling in bone marrow stromal cells overcomes microenvironment-mediated drug resistance in acute myeloid leukemia. Sci. Rep. 2024, 14, 8404. [Google Scholar] [CrossRef] [PubMed]
- Bütow, M.; Testaquadra, F.J.; Baumeister, J.; Maié, T.; Chatain, N.; Jaquet, T.; Tillmann, S.; Crysandt, M.; Costa, I.G.; Brümmendorf, T.H. Targeting cytokine-induced leukemic stem cell persistence in chronic myeloid leukemia by IKK2-inhibition. Haematologica 2023, 108, 1179. [Google Scholar] [CrossRef] [PubMed]
- Klement, L.; Drube, J. The interplay of FLT3 and CXCR4 in acute myeloid leukemia: An ongoing debate. Front. Oncol. 2023, 13, 1258679. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, C.S.; Saw, J.; Boyle, J.A.; Haigh, K.; Litalien, V.; McCalmont, H.; Evans, K.; Lock, R.B.; Jane, S.M.; Haigh, J.J. STAT5 activation promotes progression and chemotherapy resistance in early T-cell precursor acute lymphoblastic leukemia. Blood J. Am. Soc. Hematol. 2023, 142, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Hope, K.J.; Jin, L.; Dick, J.E. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat. Immunol. 2004, 5, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Kitamura, H.; Hijikata, A.; Tomizawa-Murasawa, M.; Tanaka, S.; Takagi, S.; Uchida, N.; Suzuki, N.; Sone, A.; Najima, Y. Identification of therapeutic targets for quiescent, chemotherapy-resistant human leukemia stem cells. Sci. Transl. Med. 2010, 2, 17ra19. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Hope, K.J.; Zhai, Q.; Smadja-Joffe, F.; Dick, J.E. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat. Med. 2006, 12, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Buss, E.C.; Ho, A.D. Leukemia stem cells. Int. J. Cancer 2011, 129, 2328–2336. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ii, M.; Kamei, N.; Alev, C.; Kwon, S.-M.; Kawamoto, A.; Akimaru, H.; Masuda, H.; Sawa, Y.; Asahara, T. CD34+ cells represent highly functional endothelial progenitor cells in murine bone marrow. PLoS ONE 2011, 6, e20219. [Google Scholar] [PubMed]
- Lund, F.E.; Cockayne, D.A.; Randall, T.D.; Solvason, N.; Schuber, F.; Howard, M.C. CD38: A new paradigm in lymphocyte activation and signal transduction. Immunol. Rev. 1998, 161, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Sakoda, T.; Kikushige, Y.; Miyamoto, T.; Irifune, H.; Harada, T.; Hatakeyama, K.; Kunisaki, Y.; Kato, K.; Akashi, K. TIM-3 signaling hijacks the canonical Wnt/β-catenin pathway to maintain cancer stemness in acute myeloid leukemia. Blood Adv. 2023, 7, 2053–2065. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, A.; Frenette, P.S. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 2014, 20, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mohammad, K.S.; Pelus, L.M. CXCR4 expression in the bone marrow microenvironment is required for hematopoietic stem and progenitor cell maintenance and early hematopoietic regeneration after myeloablation. Stem Cells 2020, 38, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Peña-Martínez, P.; Agarwal, P.; Rodriguez-Zabala, M.; Chapellier, M.; Högberg, C.; Eriksson, M.; Yudovich, D.; Shah, M.; Ehinger, M. CXCR4 signaling has a CXCL12-independent essential role in murine MLL-AF9-driven acute myeloid leukemia. Cell Rep. 2020, 31, 107684. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Isringhausen, S.; Li, H.; Paterson, A.J.; He, J.; Gomariz, Á.; Nagasawa, T.; Nombela-Arrieta, C.; Bhatia, R. Mesenchymal niche-specific expression of Cxcl12 controls quiescence of treatment-resistant leukemia stem cells. Cell Stem Cell 2019, 24, 769–784.e6. [Google Scholar] [CrossRef] [PubMed]
- Skinner, A.M.; O’Neill, S.L.; Grompe, M.; Kurre, P. CXCR4 induction in hematopoietic progenitor cells from Fanca−/−,-c−/−, and-d2−/− mice. Exp. Hematol. 2008, 36, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Labbaye, C.; Castelli, G.; Pelosi, E. Oxidative stress and hypoxia in normal and leukemic stem cells. Exp. Hematol. 2016, 44, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Chand, R.; Chandra, H.; Chandra, S.; Verma, S.K. Role of microvessel density and vascular endothelial growth factor in angiogenesis of hematological malignancies. Bone Marrow Res. 2016, 2016, 5043483. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.; Choy, M.; Alitalo, K.; Rafii, S. Vascular endothelial growth factor (VEGF)–C signaling through FLT-4 (VEGFR-3) mediates leukemic cell proliferation, survival, and resistance to chemotherapy. Blood J. Am. Soc. Hematol. 2002, 99, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Giles, F.J. The vascular endothelial growth factor (VEGF) signaling pathway: A therapeutic target in patients with hematologic malignancies. Oncologist 2001, 6, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Tallman, M.S.; Gilliland, D.G.; Rowe, J.M. Drug therapy for acute myeloid leukemia. Blood 2005, 106, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Vetrie, D.; Helgason, G.V.; Copland, M. The leukaemia stem cell: Similarities, differences and clinical prospects in CML and AML. Nat. Rev. Cancer 2020, 20, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, B.C.; Chan, S.M.; Daver, N.G.; Jonas, B.A.; Pollyea, D.A. Optimizing survival outcomes with post-remission therapy in acute myeloid leukemia. Am. J. Hematol. 2019, 94, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Riether, C.; Schürch, C.; Ochsenbein, A. Regulation of hematopoietic and leukemic stem cells by the immune system. Cell Death Differ. 2015, 22, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, D.G.; Jordan, C.T.; Felix, C.A. The molecular basis of leukemia. ASH Educ. Program Book 2004, 2004, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Munir, A.H.; Khan, M.I. Pattern of basic hematological parameters in acute and chronic leukemias. J. Med. Sci. 2019, 27, 125–129. [Google Scholar]
- Arber, D.A.; Campo, E.; Jaffe, E.S. Advances in the classification of myeloid and lymphoid neoplasms. Virchows Arch. 2023, 482, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Mohty, M. Acute lymphoblastic leukaemia. Lancet 2020, 395, 1146–1162. [Google Scholar] [CrossRef] [PubMed]
- Maino, E.; Sancetta, R.; Viero, P.; Imbergamo, S.; Scattolin, A.M.; Vespignani, M.; Bassan, R. Current and future management of Ph/BCR-ABL positive ALL. Expert Rev. Anticancer Ther. 2014, 14, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.S.; McLaughlin, J.; Timmons, M.; Pendergast, A.M.; Ben-Neriah, Y.; Dow, L.W.; Crist, W.; Rovera, G.; Smith, S.D.; Witte, O.N. Expression of a distinctive BCR-ABL oncogene in Ph1-positive acute lymphocytic leukemia (ALL). Science 1988, 239, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Mullighan, C.G.; Collins-Underwood, J.R.; Phillips, L.A.; Loudin, M.G.; Liu, W.; Zhang, J.; Ma, J.; Coustan-Smith, E.; Harvey, R.C.; Willman, C.L. Rearrangement of CRLF2 in B-progenitor–and Down syndrome–associated acute lymphoblastic leukemia. Nat. Genet. 2009, 41, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Terwilliger, T.; Abdul-Hay, M. Acute lymphoblastic leukemia: A comprehensive review and 2017 update. Blood Cancer J. 2017, 7, e577. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Rangel, F.A.; Chávez-Valencia, V.; Gómez-Guijosa, M.Á.; Cortes-Penagos, C. Acute myeloid leukemia—Genetic alterations and their clinical prognosis. Int. J. Hematol.-Oncol. Stem Cell Res. 2017, 11, 328. [Google Scholar] [PubMed]
- Hackl, H.; Astanina, K.; Wieser, R. Molecular and genetic alterations associated with therapy resistance and relapse of acute myeloid leukemia. J. Hematol. Oncol. 2017, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, A.; Vormoor, J.; Hanenberg, H.; Wang, J.C.; Bhatia, M.; Lapidot, T.; Moritz, T.; Murdoch, B.; Xiao, X.L.; Kato, I. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: Implications for gene therapy. Nat. Med. 1996, 2, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, T.; Pflumio, F.; Doedens, M.; Murdoch, B.; Williams, D.E.; Dick, J.E. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science 1992, 255, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood J. Am. Soc. Hematol. 2018, 131, 2745–2760. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, F.K.; Forconi, F.; Kipps, T.J. Exploring the pathways to chronic lymphocytic leukemia. Blood 2021, 138, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Puente, X.S.; Pinyol, M.; Quesada, V.; Conde, L.; Ordóñez, G.R.; Villamor, N.; Escaramis, G.; Jares, P.; Beà, S.; González-Díaz, M. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature 2011, 475, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Varghese, A.M.; Sood, N.; Chiattone, C.; Akinola, N.O.; Huang, X.; Gale, R.P. Ethnic and geographic diversity of chronic lymphocytic leukaemia. Leukemia 2021, 35, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Puiggros, A.; Blanco, G.; Espinet, B. Genetic abnormalities in chronic lymphocytic leukemia: Where we are and where we go. BioMed Res. Int. 2014, 2014, 435983. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Pavlovsky, C.; Saußele, S. Chronic myeloid leukaemia. Lancet 2021, 398, 1914–1926. [Google Scholar] [CrossRef] [PubMed]
- Pane, F.; Intrieri, M.; Quintarelli, C.; Izzo, B.; Muccioli, G.C.; Salvatore, F. BCR/ABL genes and leukemic phenotype: From molecular mechanisms to clinical correlations. Oncogene 2002, 21, 8652–8667. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Basso, I.N.; Kim, D.D.H. Target spectrum of the BCR-ABL tyrosine kinase inhibitors in chronic myeloid leukemia. Int. J. Hematol. 2021, 113, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Küppers, R. The biology of Hodgkin’s lymphoma. Nat. Rev. Cancer 2009, 9, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Townsend, W.; Linch, D. Hodgkin’s lymphoma in adults. Lancet 2012, 380, 836–847. [Google Scholar] [CrossRef] [PubMed]
- de Leval, L.; Jaffe, E.S. Lymphoma classification. Cancer J. 2020, 26, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Dima, D.; Omar, N.; Ogbue, O.; Ahmed, S. Advances in the treatment of Hodgkin lymphoma: Current and future approaches. Front. Oncol. 2023, 13, 1067289. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.D.; Lilly, S.; Jones, K.L. Lymphoma: Diagnosis and treatment. Am. Fam. Physician 2020, 101, 34–41. [Google Scholar] [PubMed]
- Crombie, J.; LaCasce, A. The treatment of Burkitt lymphoma in adults. Blood J. Am. Soc. Hematol. 2021, 137, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Opat, S.; Simpson, D.; Cull, G.; Munoz, J.; Phillips, T.J.; Kim, W.S.; Rule, S.; Atwal, S.K.; Wei, R. Zanubrutinib for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv. 2021, 5, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.; Foureau, D.M.; Atrash, S.; Voorhees, P.M.; Usmani, S.Z. Extramedullary multiple myeloma. Leukemia 2020, 34, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Shan, H.; Liu, M.; Liu, J.; Zhang, Z.; Xu, X.; Liu, Y.; Xu, H.; Lei, H.; Yu, M. Directly targeting c-Myc contributes to the anti-multiple myeloma effect of anlotinib. Cell Death Dis. 2021, 12, 396. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, F.; Jones, R.J.; Singh, R.K.; Zou, J.; Kuiatse, I.; Berkova, Z.; Wang, H.; Lee, H.C.; Hong, S.; Dick, L. Activating KRAS, NRAS, and BRAF mutants enhance proteasome capacity and reduce endoplasmic reticulum stress in multiple myeloma. Proc. Natl. Acad. Sci. USA 2020, 117, 20004–20014. [Google Scholar] [CrossRef] [PubMed]
- Perroud, C.; Thurian, D.; Andres, M.; Künzi, A.; Wiedemann, G.; Zeerleder, S.; Bacher, U.; Pabst, T.; Banz, Y.; Porret, N. Effect of MAPK activation via mutations in NRAS, KRAS and BRAF on clinical outcome in newly diagnosed multiple myeloma. Hematol. Oncol. 2023, 41, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Huff, C.A.; Matsui, W. Multiple myeloma cancer stem cells. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 2895. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Otero, P.; van de Donk, N.W.; Pillarisetti, K.; Cornax, I.; Vishwamitra, D.; Gray, K.; Hilder, B.; Tolbert, J.; Renaud, T.; Masterson, T. GPRC5D as a novel target for the treatment of multiple myeloma: A narrative review. Blood Cancer J. 2024, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Eppert, K.; Takenaka, K.; Lechman, E.R.; Waldron, L.; Nilsson, B.; Van Galen, P.; Metzeler, K.H.; Poeppl, A.; Ling, V.; Beyene, J. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat. Med. 2011, 17, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Zeijlemaker, W.; Grob, T.; Meijer, R.; Hanekamp, D.; Kelder, A.; Carbaat-Ham, J.C.; Oussoren-Brockhoff, Y.J.; Snel, A.N.; Veldhuizen, D.; Scholten, W.J. CD34+ CD38− leukemic stem cell frequency to predict outcome in acute myeloid leukemia. Leukemia 2019, 33, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Taussig, D.C.; Vargaftig, J.; Miraki-Moud, F.; Griessinger, E.; Sharrock, K.; Luke, T.; Lillington, D.; Oakervee, H.; Cavenagh, J.; Agrawal, S.G. Leukemia-initiating cells from some acute myeloid leukemia patients with mutated nucleophosmin reside in the CD34− fraction. Blood J. Am. Soc. Hematol. 2010, 115, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Moshaver, B.; Kelder, A.; Westra, G.; van Rhenen, A.; Ossenkoppele, G.J.; Zweegman, S.; Schuurhuis, G.J. Identification of a Small Subpopulation of Candidate Leukemia Initiating Cells within the Side Population (SP) of Patients with Acute Myeloid Leukemia. Blood 2007, 110, 4120. [Google Scholar] [CrossRef]
- Taussig, D.C.; Miraki-Moud, F.; Anjos-Afonso, F.; Pearce, D.J.; Allen, K.; Ridler, C.; Lillington, D.; Oakervee, H.; Cavenagh, J.; Agrawal, S.G. Anti-CD38 antibody–mediated clearance of human repopulating cells masks the heterogeneity of leukemia-initiating cells. Blood J. Am. Soc. Hematol. 2008, 112, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Blair, A.; Hogge, D.; Sutherland, H. Most acute myeloid leukemia progenitor cells with long-term proliferative ability in vitro and in vivo have the phenotype CD34+/CD71−/HLA-DR−. Blood J. Am. Soc. Hematol. 1998, 92, 4325–4335. [Google Scholar]
- Hosen, N.; Park, C.Y.; Tatsumi, N.; Oji, Y.; Sugiyama, H.; Gramatzki, M.; Krensky, A.M.; Weissman, I.L. CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2007, 104, 11008–11013. [Google Scholar] [CrossRef]
- Vergez, F.; Nicolau-Travers, M.-L.; Bertoli, S.; Rieu, J.-B.; Tavitian, S.; Bories, P.; Luquet, I.; De Mas, V.; Largeaud, L.; Sarry, A. CD34+ CD38− CD123+ leukemic stem cell frequency predicts outcome in older acute myeloid leukemia patients treated by intensive chemotherapy but not hypomethylating agents. Cancers 2020, 12, 1174. [Google Scholar] [CrossRef]
- Pabst, C.; Bergeron, A.; Lavallée, V.-P.; Yeh, J.; Gendron, P.; Norddahl, G.L.; Krosl, J.; Boivin, I.; Deneault, E.; Simard, J. GPR56 identifies primary human acute myeloid leukemia cells with high repopulating potential in vivo. Blood J. Am. Soc. Hematol. 2016, 127, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.; Barreyro, L.; Todorova, T.I.; Taylor, S.J.; Antony-Debré, I.; Narayanagari, S.-R.; Carvajal, L.A.; Leite, J.; Piperdi, Z.; Pendurti, G. IL1RAP potentiates multiple oncogenic signaling pathways in AML. J. Exp. Med. 2018, 215, 1709–1727. [Google Scholar] [CrossRef] [PubMed]
- Kikushige, Y.; Miyamoto, T. Identification of TIM-3 as a leukemic stem cell surface molecule in primary acute myeloid leukemia. Oncology 2015, 89 (Suppl. S1), 28–32. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, B.; Perkins, B.; Karantanos, T.; Levis, M.; Ghiaur, G.; Smith, B.D.; Jones, R.J. Expression of putative leukemia stem cell targets in genetically-defined acute myeloid leukemia subtypes. Leuk. Res. 2020, 99, 106477. [Google Scholar] [CrossRef] [PubMed]
- Kollet, O.; Canaani, J.; Kalinkovich, A.; Lapidot, T. Regulatory cross talks of bone cells, hematopoietic stem cells and the nervous system maintain hematopoiesis. Inflamm. Allergy-Drug Targets 2012, 11, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Xiao, W.; Abdel-Wahab, O. Diagnosis and classification of hematologic malignancies on the basis of genetics. Blood J. Am. Soc. Hematol. 2017, 130, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.R.; Schatoff, E.; Abdel-Wahab, O. Epigenetic alterations in hematopoietic malignancies. Int. J. Hematol. 2012, 96, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Tabe, Y.; Konopleva, M. Advances in understanding the leukaemia microenvironment. Br. J. Haematol. 2014, 164, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.D.; Copland, M. The bone marrow immune microenvironment in CML: Treatment responses, treatment-free remission, and therapeutic vulnerabilities. Curr. Hematol. Malig. Rep. 2023, 18, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Eiring, A.M.; Khorashad, J.S.; Anderson, D.J.; Yu, F.; Redwine, H.M.; Mason, C.C.; Reynolds, K.R.; Clair, P.M.; Gantz, K.C.; Zhang, T.Y. β-Catenin is required for intrinsic but not extrinsic BCR-ABL1 kinase-independent resistance to tyrosine kinase inhibitors in chronic myeloid leukemia. Leukemia 2015, 29, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Gurska, L.M.; Ames, K.; Gritsman, K. Signaling pathways in leukemic stem cells. Leuk. Stem Cells Hematol. Malig. 2019, 1143, 1–39. [Google Scholar]
- Hu, J.; Feng, M.; Liu, Z.-L.; Liu, Y.; Huang, Z.-L.; Li, H.; Feng, W.-L. Potential role of Wnt/β-catenin signaling in blastic transformation of chronic myeloid leukemia: Cross talk between β-catenin and BCR-ABL. Tumor Biol. 2016, 37, 15859–15872. [Google Scholar] [CrossRef] [PubMed]
- Duchartre, Y.; Kim, Y.-M.; Kahn, M. The Wnt signaling pathway in cancer. Crit. Rev. Oncol./Hematol. 2016, 99, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.W.; Wang, Y.J.; Lo Celso, C.; Ragu, C.; Bullinger, L.; Sykes, S.M.; Ferraro, F.; Shterental, S.; Lin, C.P.; Gilliland, D.G. Differential niche and Wnt requirements during acute myeloid leukemia progression. Blood J. Am. Soc. Hematol. 2011, 118, 2849–2856. [Google Scholar] [CrossRef] [PubMed]
- Santaguida, M.; Schepers, K.; King, B.; Sabnis, A.J.; Forsberg, E.C.; Attema, J.L.; Braun, B.S.; Passegué, E. JunB protects against myeloid malignancies by limiting hematopoietic stem cell proliferation and differentiation without affecting self-renewal. Cancer Cell 2009, 15, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.S.; Fulzele, K.; Catic, A.; Sun, C.C.; Dombkowski, D.; Hurley, M.P.; Lezeau, S.; Attar, E.; Wu, J.Y.; Lin, H.Y. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat. Med. 2013, 19, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Berndt, J.D.; Aoyagi, A.; Yang, P.; Anastas, J.N.; Tang, L.; Moon, R.T. Mindbomb 1, an E3 ubiquitin ligase, forms a complex with RYK to activate Wnt/β-catenin signaling. J. Cell Biol. 2011, 12, 626. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-W.; Koo, B.-K.; Jeong, H.-W.; Yoon, M.-J.; Song, R.; Shin, J.; Jeong, D.-C.; Kim, S.-H.; Kong, Y.-Y. Defective Notch activation in microenvironment leads to myeloproliferative disease. Blood J. Am. Soc. Hematol. 2008, 112, 4628–4638. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.P.; Schmidt-Arras, D. Novel approaches to target mutant FLT3 leukaemia. Cancers 2020, 12, 2806. [Google Scholar] [CrossRef]
- Mead, A.J.; Neo, W.H.; Barkas, N.; Matsuoka, S.; Giustacchini, A.; Facchini, R.; Thongjuea, S.; Jamieson, L.; Booth, C.A.; Fordham, N. Niche-mediated depletion of the normal hematopoietic stem cell reservoir by Flt3-ITD–induced myeloproliferation. J. Exp. Med. 2017, 214, 2005–2021. [Google Scholar] [CrossRef]
- Green, A.S.; Maciel, T.T.; Hospital, M.-A.; Yin, C.; Mazed, F.; Townsend, E.C.; Pilorge, S.; Lambert, M.; Paubelle, E.; Jacquel, A. Pim kinases modulate resistance to FLT3 tyrosine kinase inhibitors in FLT3-ITD acute myeloid leukemia. Sci. Adv. 2015, 1, e1500221. [Google Scholar] [CrossRef] [PubMed]
- Czardybon, W.; Windak, R.; Gołas, A.; Gałęzowski, M.; Sabiniarz, A.; Dolata, I.; Salwińska, M.; Guzik, P.; Zawadzka, M.; Gabor-Worwa, E. A novel, dual pan-PIM/FLT3 inhibitor SEL24 exhibits broad therapeutic potential in acute myeloid leukemia. Oncotarget 2018, 9, 16917. [Google Scholar] [CrossRef] [PubMed]
- Onish, C.; Mori-Kimachi, S.; Hirade, T.; Abe, M.; Taketani, T.; Suzumiya, J.; Sugimoto, T.; Yamaguchi, S.; Kapur, R.; Fukuda, S. Internal tandem duplication mutations in FLT3 gene augment chemotaxis to Cxcl12 protein by blocking the down-regulation of the Rho-associated kinase via the Cxcl12/Cxcr4 signaling axis. J. Biol. Chem. 2014, 289, 31053–31065. [Google Scholar] [CrossRef] [PubMed]
- Rupec, R.A.; Jundt, F.; Rebholz, B.; Eckelt, B.; Herzinger, T.; Flaig, M.J.; Moosmann, S.; Plewig, G.; Dörken, B.; Förster, I. Stroma-mediated dysregulation of myelopoiesis in mice lacking IκBα. Immunity 2005, 22, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Frietsch, J.J.; Kastner, C.; Grunewald, T.G.; Schweigel, H.; Nollau, P.; Ziermann, J.; Clement, J.H.; La Rosée, P.; Hochhaus, A.; Butt, E. LASP1 is a novel BCR-ABL substrate and a phosphorylation-dependent binding partner of CRKL in chronic myeloid leukemia. Oncotarget 2014, 5, 5257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, B.; Ho, Y.W.; Huang, Q.; Maeda, T.; Lin, A.; Lee, S.-u.; Hair, A.; Holyoake, T.L.; Huettner, C.; Bhatia, R. Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia. Cancer Cell 2012, 21, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Wang, H.; Li, L.; Ma, X.; Chen, Y.; Zhou, H.; Luo, Y.; Xiao, Y.; Liu, L. miR-34c-5p promotes eradication of acute myeloid leukemia stem cells by inducing senescence through selective RAB27B targeting to inhibit exosome shedding. Leukemia 2018, 32, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wen, J.; Li, Q.; Peng, D.; Liao, C.; Ma, X.; Wang, M.; Niu, J.; Wang, D.; Li, Y. RAB27B-regulated exosomes mediate LSC maintenance via resistance to senescence and crosstalk with the microenvironment. Leukemia 2024, 38, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Ptasinska, A.; Assi, S.A.; Martinez-Soria, N.; Imperato, M.R.; Piper, J.; Cauchy, P.; Pickin, A.; James, S.R.; Hoogenkamp, M.; Williamson, D. Identification of a dynamic core transcriptional network in t (8; 21) AML that regulates differentiation block and self-renewal. Cell Rep. 2014, 8, 1974–1988. [Google Scholar] [CrossRef]
- Kellaway, S.G.; Potluri, S.; Keane, P.; Blair, H.J.; Ames, L.; Worker, A.; Chin, P.S.; Ptasinska, A.; Derevyanko, P.K.; Adamo, A. Leukemic stem cells activate lineage inappropriate signalling pathways to promote their growth. Nat. Commun. 2024, 15, 1359. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.-A.; Chou, W.-C.; Lin, L.-I.; Tang, J.-L.; Tseng, M.-H.; Huang, C.-F.; Yao, M.; Chen, C.-Y.; Tsay, W.; Tien, H.-F. Expression of angiopoietins and vascular endothelial growth factors and their clinical significance in acute myeloid leukemia. Leuk. Res. 2008, 32, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.C.R.; Dias, S. Internal and external autocrine VEGF/KDR loops regulate survival of subsets of acute leukemia through distinct signaling pathways. Blood 2004, 103, 3883–3889. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.-H.; Ku, C.-C.; Johansson, G.; Chen, M.-W.; Hsiao, M.; Su, J.-L.; Inoue, H.; Hua, K.-T.; Wei, L.-H.; Kuo, M.-L. Vascular endothelial growth factor-C (VEGF-C) promotes angiogenesis by induction of COX-2 in leukemic cells via the VEGF-R3/JNK/AP-1 pathway. Carcinogenesis 2009, 30, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Avanzini, M.; Bernardo, M.; Novara, F.; Mantelli, M.; Poletto, V.; Villani, L.; Lenta, E.; Ingo, D.; Achille, V.; Bonetti, E. Functional and genetic aberrations of in vitro-cultured marrow-derived mesenchymal stromal cells of patients with classical Philadelphia-negative myeloproliferative neoplasms. Leukemia 2014, 28, 1742–1745. [Google Scholar] [CrossRef] [PubMed]
- Mehrpouri, M. The contributory roles of the CXCL12/CXCR4/CXCR7 axis in normal and malignant hematopoiesis: A possible therapeutic target in hematologic malignancies. Eur. J. Pharmacol. 2022, 920, 174831. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Kan, C.; Wong, M.; Sun, M.; Liu, Y.; Yang, F.; Wang, S.; Zheng, H. Regulation of malignant myeloid leukemia by mesenchymal stem cells. Front. Cell Dev. Biol. 2022, 10, 857045. [Google Scholar] [CrossRef] [PubMed]
- Tabe, Y.; Konopleva, M. Leukemia stem cells microenvironment. Stem Cell Microenviron. Beyond 2017, 1041, 19–32. [Google Scholar]
- Waclawiczek, A.; Hamilton, A.; Rouault-Pierre, K.; Abarrategi, A.; Albornoz, M.G.; Miraki-Moud, F.; Bah, N.; Gribben, J.; Fitzgibbon, J.; Taussig, D. Mesenchymal niche remodeling impairs hematopoiesis via stanniocalcin 1 in acute myeloid leukemia. J. Clin. Investig. 2020, 130, 3038–3050. [Google Scholar] [CrossRef] [PubMed]
- Frolova, O.; Samudio, I.; Benito, J.M.; Jacamo, R.; Kornblau, S.M.; Markovic, A.; Schober, W.; Lu, H.; Qiu, Y.H.; Buglio, D. Regulation of HIF-1α signaling and chemoresistance in acute lymphocytic leukemia under hypoxic conditions of the bone marrow microenvironment. Cancer Biol. Ther. 2012, 13, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Liou, A.; Delgado-Martin, C.; Teachey, D.T.; Hermiston, M.L. The CXCR4/CXCL12 Axis Mediates Chemotaxis, Survival, and Chemoresistance in T-Cell Acute Lymphoblastic Leukemia; American Society of Hematology: Washington, DC, USA, 2014. [Google Scholar]
- Pillozzi, S.; Bernini, A.; Spiga, O.; Lelli, B.; Petroni, G.; Bracci, L.; Niccolai, N.; Arcangeli, A. Peptides and small molecules blocking the CXCR4/CXCL12 axis overcome bone marrow-induced chemoresistance in acute leukemias. Oncol. Rep. 2019, 41, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Rosati, E.; Sabatini, R.; Rampino, G.; Tabilio, A.; Di Ianni, M.; Fettucciari, K.; Bartoli, A.; Coaccioli, S.; Screpanti, I.; Marconi, P. Constitutively activated Notch signaling is involved in survival and apoptosis resistance of B-CLL cells. Blood J. Am. Soc. Hematol. 2009, 113, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Takam Kamga, P.; Bazzoni, R.; Dal Collo, G.; Cassaro, A.; Tanasi, I.; Russignan, A.; Tecchio, C.; Krampera, M. The role of notch and wnt signaling in MSC communication in normal and leukemic bone marrow niche. Front. Cell Dev. Biol. 2021, 8, 599276. [Google Scholar] [CrossRef] [PubMed]
- Binder, S.; Luciano, M.; Horejs-Hoeck, J. The cytokine network in acute myeloid leukemia (AML): A focus on pro-and anti-inflammatory mediators. Cytokine Growth Factor Rev. 2018, 43, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.; Edwards, D.K.; Eide, C.A.; Newell, L.; Traer, E.; Medeiros, B.C.; Pollyea, D.A.; Deininger, M.W.; Collins, R.H.; Tyner, J.W. Identification of interleukin-1 by functional screening as a key mediator of cellular expansion and disease progression in acute myeloid leukemia. Cell Rep. 2017, 18, 3204–3218. [Google Scholar] [CrossRef] [PubMed]
- Moschoi, R.; Imbert, V.; Nebout, M.; Chiche, J.; Mary, D.; Prebet, T.; Saland, E.; Castellano, R.; Pouyet, L.; Collette, Y. Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood J. Am. Soc. Hematol. 2016, 128, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Forte, D.; García-Fernández, M.; Sanchez-Aguilera, A.; Stavropoulou, V.; Fielding, C.; Martín-Pérez, D.; López, J.A.; Costa, A.S.; Tronci, L.; Nikitopoulou, E. Bone marrow mesenchymal stem cells support acute myeloid leukemia bioenergetics and enhance antioxidant defense and escape from chemotherapy. Cell Metab. 2020, 32, 829–843.e9. [Google Scholar] [CrossRef] [PubMed]
- Kamga, P.T.; Bassi, G.; Cassaro, A.; Midolo, M.; Di Trapani, M.; Gatti, A.; Carusone, R.; Resci, F.; Perbellini, O.; Gottardi, M. Notch signalling drives bone marrow stromal cell-mediated chemoresistance in acute myeloid leukemia. Oncotarget 2016, 7, 21713. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.Z.; Mak, P.Y.; Chen, Y.; Mak, D.H.; Mu, H.; Jacamo, R.; Ruvolo, V.; Arold, S.T.; Ladbury, J.E.; Burks, J.K. Anti-apoptotic ARC protein confers chemoresistance by controlling leukemia-microenvironment interactions through a NFκB/IL1β signaling network. Oncotarget 2016, 7, 20054. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, S.; Haque, T.; Gritsman, K. Inflammatory signaling pathways in preleukemic and leukemic stem cells. Front. Oncol. 2017, 7, 265. [Google Scholar] [CrossRef]
- Morikawa, T.; Takubo, K. Hypoxia regulates the hematopoietic stem cell niche. Pflügers Arch.-Eur. J. Physiol. 2016, 468, 13–22. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, Y.; Luo, X.; Hu, Q. Oxidative resistance of leukemic stem cells and oxidative damage to hematopoietic stem cells under pro-oxidative therapy. Cell Death Dis. 2020, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Schepers, K.; Campbell, T.B.; Passegué, E. Normal and leukemic stem cell niches: Insights and therapeutic opportunities. Cell Stem Cell 2015, 16, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Fahy, L.; Calvo, J.; Chabi, S.; Renou, L.; Le Maout, C.; Poglio, S.; Leblanc, T.; Petit, A.; Baruchel, A.; Ballerini, P. Hypoxia favors chemoresistance in T-ALL through an HIF1α-mediated mTORC1 inhibition loop. Blood Adv. 2021, 5, 513–526. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, E.; Zeinabad, H.A.; Szegezdi, E. Hematopoietic versus leukemic stem cell quiescence: Challenges and therapeutic opportunities. Blood Rev. 2021, 50, 100850. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Gerhard, B.; Hogge, D.E. Detection, isolation, and stimulation of quiescent primitive leukemic progenitor cells from patients with acute myeloid leukemia (AML). Blood J. Am. Soc. Hematol. 2003, 101, 3142–3149. [Google Scholar] [CrossRef] [PubMed]
- Lagadinou, E.D.; Sach, A.; Callahan, K.; Rossi, R.M.; Neering, S.J.; Minhajuddin, M.; Ashton, J.M.; Pei, S.; Grose, V.; O’Dwyer, K.M. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell 2013, 12, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Grønningsæter, I.S.; Reikvam, H.; Aasebø, E.; Bartaula-Brevik, S.; Tvedt, T.H.; Bruserud, Ø.; Hatfield, K.J. Targeting cellular metabolism in acute myeloid leukemia and the role of patient heterogeneity. Cells 2020, 9, 1155. [Google Scholar] [CrossRef]
- Xie, C.; Zhou, H.; Qin, D.; Zheng, H.; Tang, Y.; Li, W.; Zhou, J.; Liu, L.; Yu, X.; Duan, H. Bcl-2 inhibition combined with PPARα activation synergistically targets leukemic stem cell-like cells in acute myeloid leukemia. Cell Death Dis. 2023, 14, 573. [Google Scholar] [CrossRef] [PubMed]
- Sadovnik, I.; Herrmann, H.; Blatt, K.; Eisenwort, G.; Mueller, N.; Stefanzl, G.; Hoermann, G.; Herndlhofer, S.; Bauer, K.; Peter, B. Evaluation of cell surface markers and targets in leukemic stem cells (LSC) reveals distinct expression profiles, unique drug effects, and specific checkpoint regulation in AML LSC and CML LSC. Blood 2016, 128, 4234. [Google Scholar] [CrossRef]
- Khan, N.; Hills, R.K.; Virgo, P.; Couzens, S.; Clark, N.; Gilkes, A.; Richardson, P.; Knapper, S.; Grimwade, D.; Russell, N.H. Expression of CD33 is a predictive factor for effect of gemtuzumab ozogamicin at different doses in adult acute myeloid leukaemia. Leukemia 2017, 31, 1059–1068. [Google Scholar] [CrossRef]
- El Khawanky, N.; Hughes, A.; Yu, W.; Myburgh, R.; Matschulla, T.; Taromi, S.; Aumann, K.; Clarson, J.; Vinnakota, J.M.; Shoumariyeh, K. Demethylating therapy increases anti-CD123 CAR T cell cytotoxicity against acute myeloid leukemia. Nat. Commun. 2021, 12, 6436. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, N.; Rinne, M.L.; Sun, H.; Stein, A.M. Sabatolimab (MBG453) model-informed drug development for dose selection in patients with myelodysplastic syndrome/acute myeloid leukemia and solid tumors. CPT Pharmacomet. Syst. Pharmacol. 2023, 12, 1653–1665. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Chen, H.; Li, W.; Huang, T.; Zhang, W.; Ling, W.; Lai, P.; Wang, Y.; Geng, S. C-type lectin-like molecule-1 as a biomarker for diagnosis and prognosis in acute myeloid leukemia: A preliminary study. BioMed Res. Int. 2021, 2021, 6643948. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-P.; Liu, B.Y.; Zheng, Q.; Panuganti, S.; Chen, R.; Zhu, J.; Mishra, M.; Huang, J.; Dao-Pick, T.; Roy, S. CLT030, a leukemic stem cell–targeting CLL1 antibody-drug conjugate for treatment of acute myeloid leukemia. Blood Adv. 2018, 2, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Laborda, E.; Mazagova, M.; Shao, S.; Wang, X.; Quirino, H.; Woods, A.K.; Hampton, E.N.; Rodgers, D.T.; Kim, C.H.; Schultz, P.G. Development of a chimeric antigen receptor targeting C-type lectin-like molecule-1 for human acute myeloid leukemia. Int. J. Mol. Sci. 2017, 18, 2259. [Google Scholar] [CrossRef] [PubMed]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A. International phase 3 study of azacitidine vs. conventional care regimens in older patients with newly diagnosed AML with> 30% blasts. Blood J. Am. Soc. Hematol. 2015, 126, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Craddock, C.; Quek, L.; Goardon, N.; Freeman, S.; Siddique, S.; Raghavan, M.; Aztberger, A.; Schuh, A.; Grimwade, D.; Ivey, A. Azacitidine fails to eradicate leukemic stem/progenitor cell populations in patients with acute myeloid leukemia and myelodysplasia. Leukemia 2013, 27, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, H.K.; Jaekel, N.; Niederwieser, D. The role of hypomethylating agents in the treatment of elderly patients with AML. J. Geriatr. Oncol. 2014, 5, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Riether, C.; Pabst, T.; Höpner, S.; Bacher, U.; Hinterbrandner, M.; Banz, Y.; Müller, R.; Manz, M.G.; Gharib, W.H.; Francisco, D. Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nat. Med. 2020, 26, 1459–1467. [Google Scholar] [CrossRef]
- Nolte, M.A.; Van Olffen, R.W.; Van Gisbergen, K.P.; Van Lier, R.A. Timing and tuning of CD27–CD70 interactions: The impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol. Rev. 2009, 229, 216–231. [Google Scholar] [CrossRef]
- Wilson, A.; Trumpp, A. Bone-marrow haematopoietic-stem-cell niches. Nat. Rev. Immunol. 2006, 6, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Calvi, L.M.; Link, D.C. The hematopoietic stem cell niche in homeostasis and disease. Blood J. Am. Soc. Hematol. 2015, 126, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, M.A. A historical perspective on the development of the cytarabine (7 days) and daunorubicin (3 days) treatment regimen for acute myelogenous leukemia: 2013 the 40th anniversary of 7 + 3. Blood Cells Mol. Dis. 2013, 50, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Eide, C.A.; Zabriskie, M.S.; Stevens, S.L.S.; Antelope, O.; Vellore, N.A.; Than, H.; Schultz, A.R.; Clair, P.; Bowler, A.D.; Pomicter, A.D. Combining the allosteric inhibitor asciminib with ponatinib suppresses emergence of and restores efficacy against highly resistant BCR-ABL1 mutants. Cancer Cell 2019, 36, 431–443.e5. [Google Scholar] [CrossRef]
- Riether, C.; Gschwend, T.; Huguenin, A.-L.; Schürch, C.; Ochsenbein, A. Blocking programmed cell death 1 in combination with adoptive cytotoxic T-cell transfer eradicates chronic myelogenous leukemia stem cells. Leukemia 2015, 29, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Yen, K.; Travins, J.; Wang, F.; David, M.D.; Artin, E.; Straley, K.; Padyana, A.; Gross, S.; DeLaBarre, B.; Tobin, E. AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 mutations. Cancer Discov. 2017, 7, 478–493. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Stein, E.M.; de Botton, S.; Roboz, G.J.; Altman, J.K.; Mims, A.S.; Swords, R.; Collins, R.H.; Mannis, G.N.; Pollyea, D.A. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N. Engl. J. Med. 2018, 378, 2386–2398. [Google Scholar] [CrossRef] [PubMed]
- Pollyea, D.A.; Stevens, B.M.; Jones, C.L.; Winters, A.; Pei, S.; Minhajuddin, M.; D’Alessandro, A.; Culp-Hill, R.; Riemondy, K.A.; Gillen, A.E. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat. Med. 2018, 24, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gill, S. CAR-T cell persistence in the treatment of leukemia and lymphoma. Leuk. Lymphoma 2021, 62, 2587–2599. [Google Scholar] [CrossRef]
- Sheykhhasan, M.; Manoochehri, H.; Dama, P. Use of CAR T-cell for acute lymphoblastic leukemia (ALL) treatment: A review study. Cancer Gene Ther. 2022, 29, 1080–1096. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.-S.; Kim, B.S.; Yoon, S.; Oh, S.-O.; Lee, D. Leukemic Stem Cells and Hematological Malignancies. Int. J. Mol. Sci. 2024, 25, 6639. https://doi.org/10.3390/ijms25126639
Choi H-S, Kim BS, Yoon S, Oh S-O, Lee D. Leukemic Stem Cells and Hematological Malignancies. International Journal of Molecular Sciences. 2024; 25(12):6639. https://doi.org/10.3390/ijms25126639
Chicago/Turabian StyleChoi, Hee-Seon, Byoung Soo Kim, Sik Yoon, Sae-Ock Oh, and Dongjun Lee. 2024. "Leukemic Stem Cells and Hematological Malignancies" International Journal of Molecular Sciences 25, no. 12: 6639. https://doi.org/10.3390/ijms25126639
APA StyleChoi, H.-S., Kim, B. S., Yoon, S., Oh, S.-O., & Lee, D. (2024). Leukemic Stem Cells and Hematological Malignancies. International Journal of Molecular Sciences, 25(12), 6639. https://doi.org/10.3390/ijms25126639







