Local E-rhBMP-2/β-TCP Application Rescues Osteocyte Dendritic Integrity and Reduces Microstructural Damage in Alveolar Bone Post-Extraction in MRONJ-like Mouse Model
Abstract
1. Introduction
2. Results
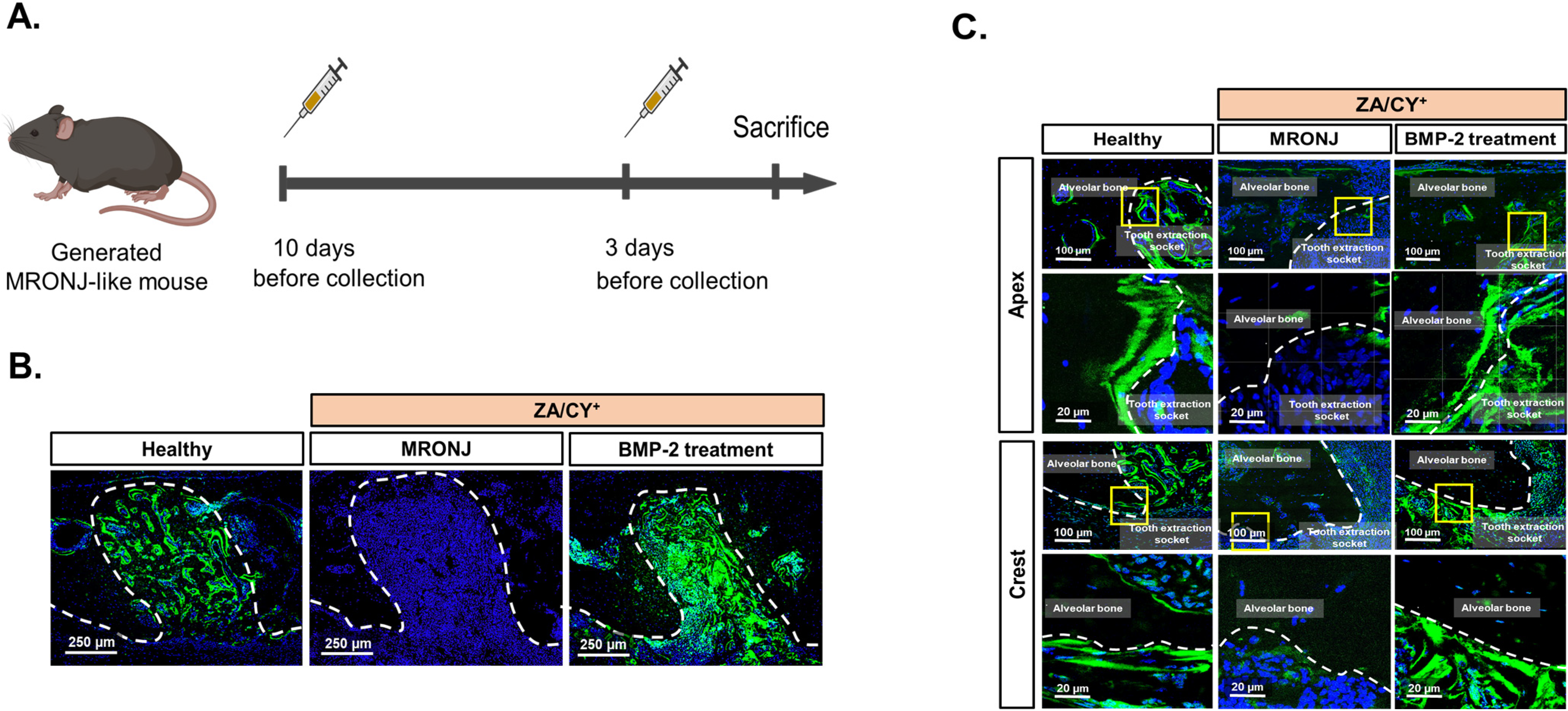
2.1. E-rhBMP-2/β-TCP Treatment Supports the Recovery of the MRONJ-Induced Degeneration of the Osteocyte Network
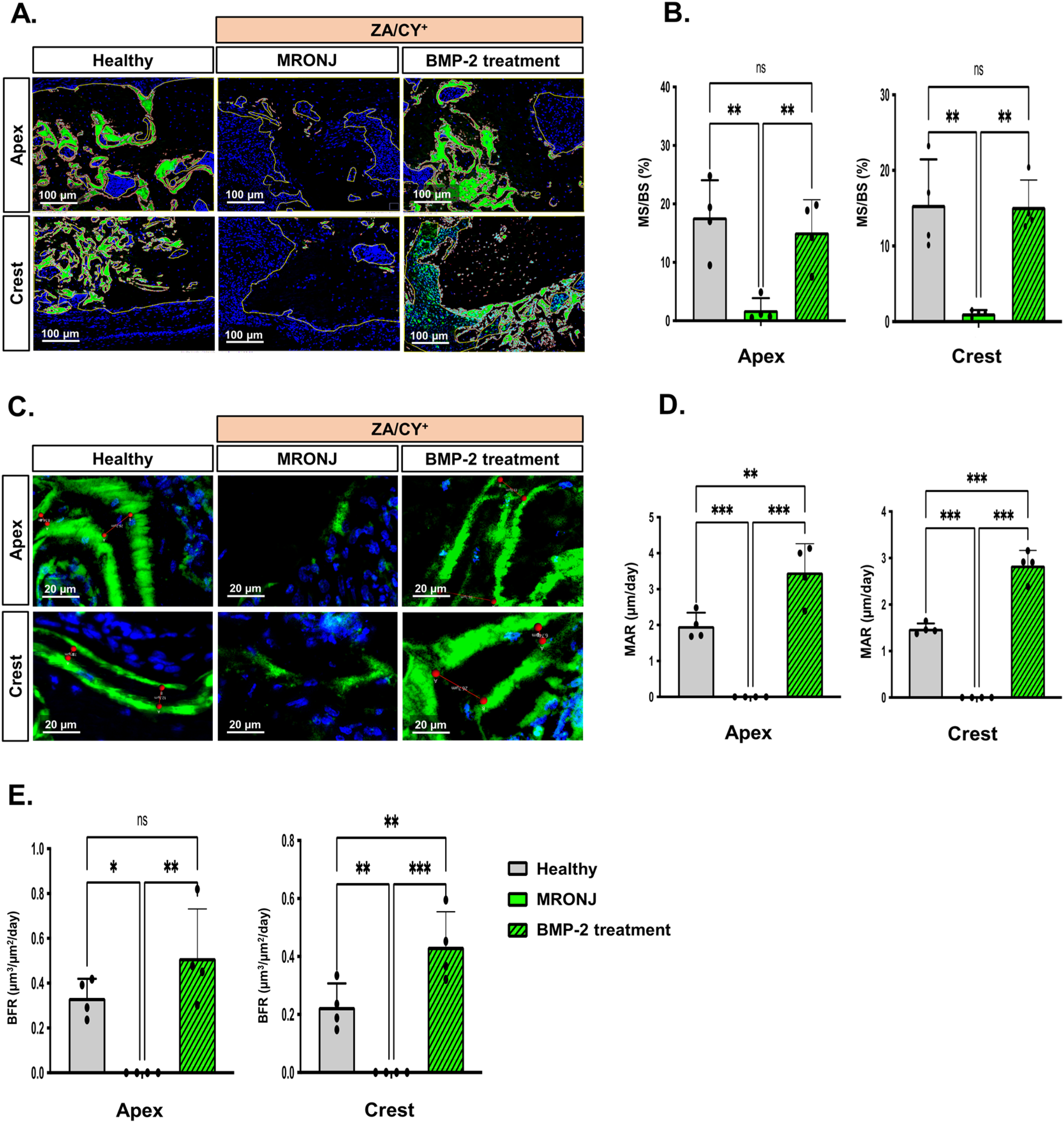
2.2. E-rhBMP-2/β-TCP Treatment Ameliorates MRONJ-Induced Suppression of Alveolar Bone Remodeling
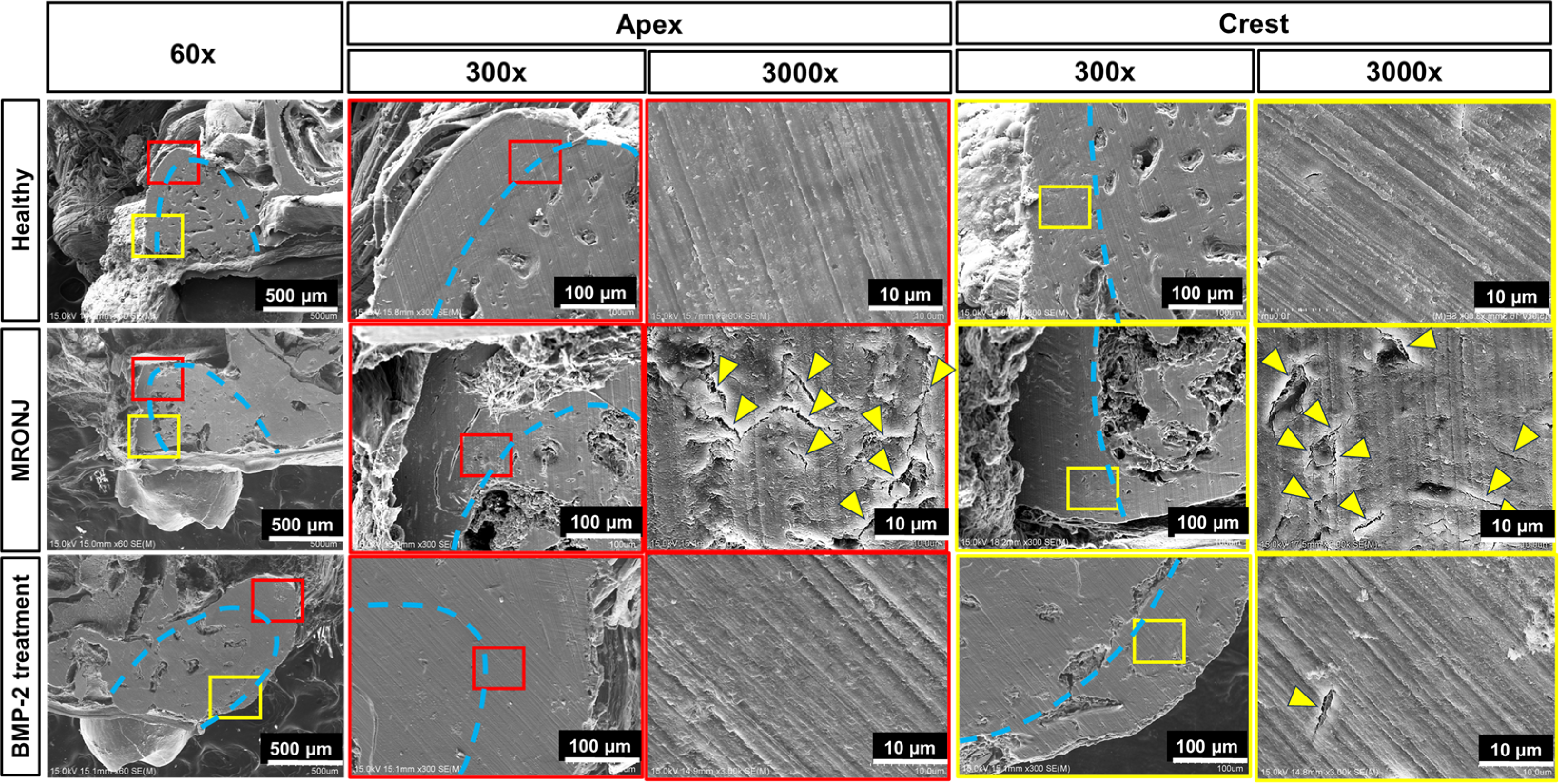
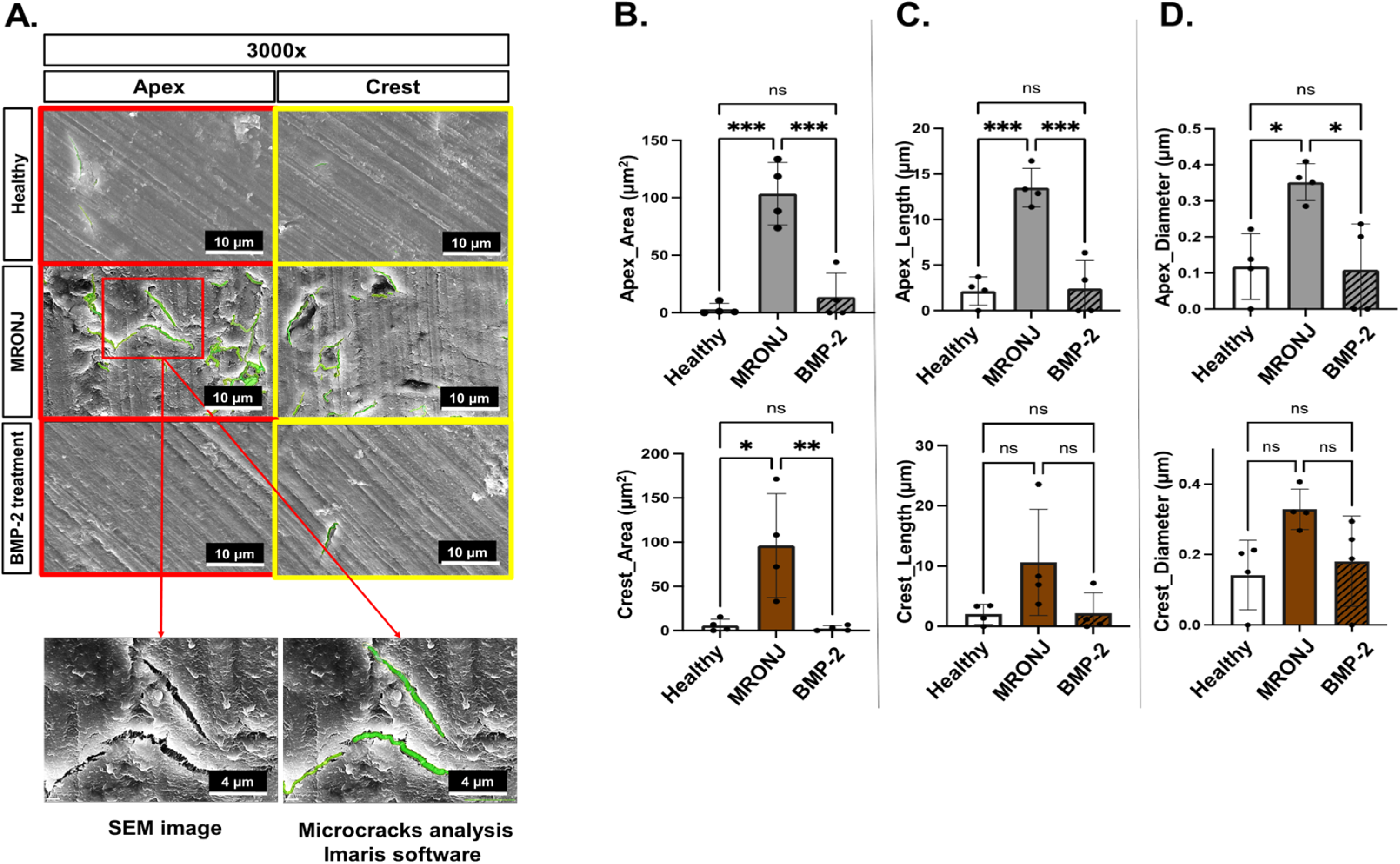
2.3. E-rhBMP-2/β-TCP Treatment Eliminates MRONJ-Induced Alveolar Bone Micro-Damage Accumulation
3. Discussion
4. Materials and Methods
4.1. BMP-2 Materials
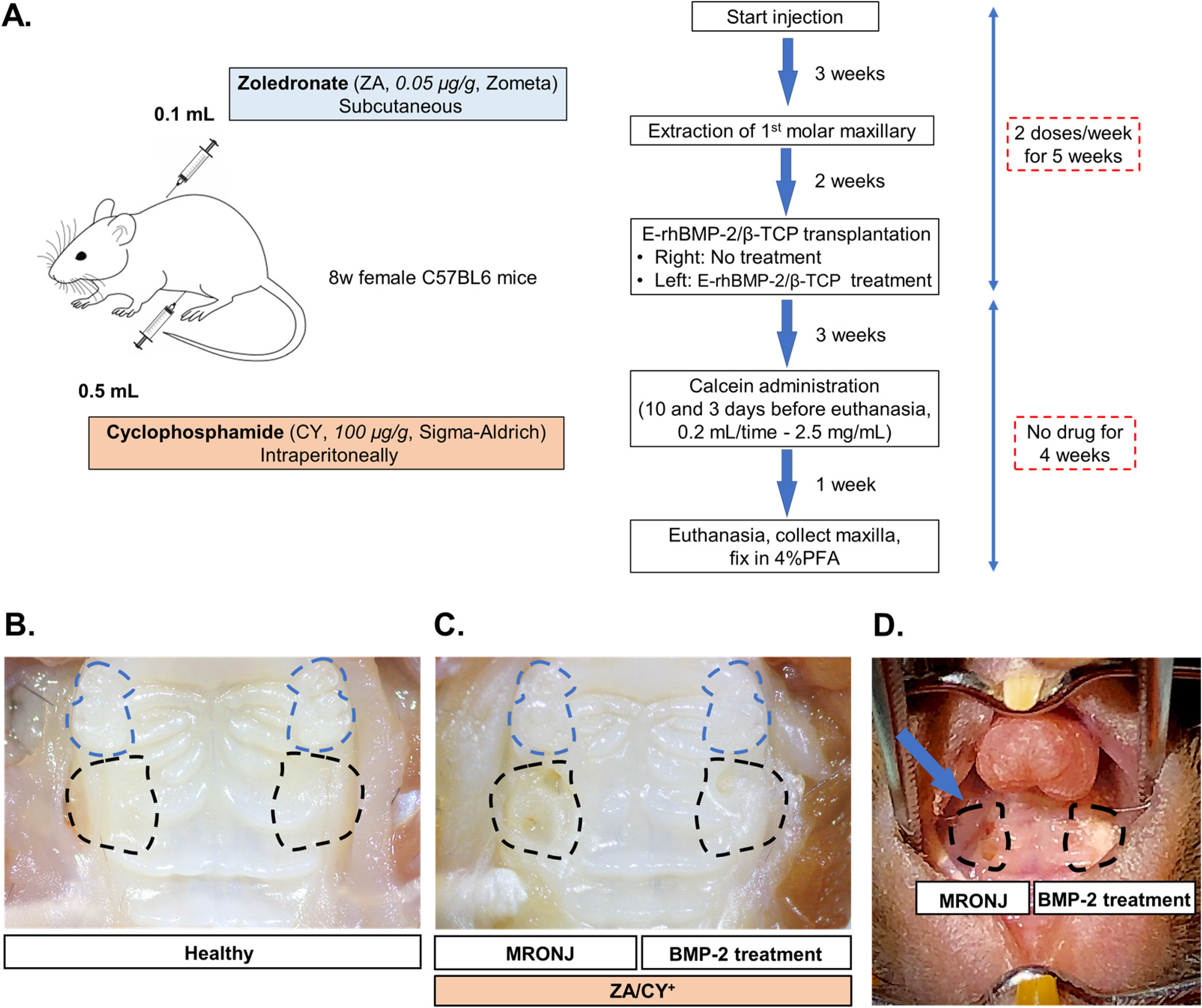
4.2. Generating the MRONJ-like Mouse Model
4.3. Calcein Double Bone Labeling Experiment
4.4. Osteocyte Dendrite Network Staining with Phalloidin
4.5. Bone Formation Analysis
4.6. Confocal Microscope Imaging
4.7. Scanning Electron Microscopy (SEM)
4.8. Image Processing Using Zen, ImageJ, and Imaris Software
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons, Position Paper on Medication-Related Osteonecrosis of the Jaw—2014 update. J. Oral. Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws—2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef]
- Tenore, G.; Mohsen, A.; Rossi, A.F.; Palaia, G.; Rocchetti, F.; Cassoni, A.; Valentini, V.; Ottolenghi, L.; Polimeni, A.; Romeo, U. Does medication-related osteonecrosis of the jaw influence the quality of life of cancer patients? Biomedicines 2020, 8, 95. [Google Scholar] [CrossRef]
- Miksad, R.A.; Lai, K.C.; Dodson, T.B.; Woo, S.B.; Treister, N.S.; Akinyemi, O.; Bihrle, M.; Maytal, G.; August, M.; Gazelle, G.S.; et al. Quality of life implications of bisphosphonate-associated osteonecrosis of the jaw. Oncologist 2011, 16, 121–132. [Google Scholar] [CrossRef]
- Sturrock, A.; Preshaw, P.M.; Hayes, C.; Wilkes, S. Perceptions and attitudes of patients towards medication-related osteonecrosis of the jaw (MRONJ): A qualitative study in England. BMJ Open 2019, 9, e024376. [Google Scholar] [CrossRef]
- Hughes, D.E.; Wright, K.R.; Uy, H.L.; Sasaki, A.; Yoneda, T.; Roodman, D.G.; Mundy, G.R.; Boyce, B.F. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J. Bone Miner. Res. 1995, 10, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.; Weber, M.; Creutzburg, K.; Möbius, P.; Preidl, R.; Amann, K.; Wehrhan, F. Osteoclast profile of medication-related osteonecrosis of the jaw secondary to bisphosphonate therapy: A comparison with osteoradionecrosis and osteomyelitis. J. Transl. Med. 2017, 15, 128. [Google Scholar] [CrossRef]
- Manzano-Moreno, F.J.; Ramos-Torrecillas, J.; Melguizo-Rodríguez, L.; Illescas-Montes, R.; Ruiz, C.; García-Martínez, O. Bisphosphonate modulation of the gene expression of different markers involved in osteoblast physiology: Possible implications in bisphosphonate-related osteonecrosis of the jaw. Int. J. Med. Sci. 2018, 15, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.R.; Andersen, T.L.; Chavassieux, P.; Roux, J.P.; Delaisse, J.M. Bisphosphonates impair the onset of bone formation at remodeling sites. Bone 2021, 145, 115850. [Google Scholar] [CrossRef] [PubMed]
- Burra, S.; Nicolella, D.P.; Francis, W.L.; Freitas, C.J.; Mueschke, N.J.; Poole, K.; Jiang, J.X. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc. Natl. Acad. Sci. USA 2010, 107, 13648–13653. [Google Scholar] [CrossRef]
- Choi, J.U.A.; Kijas, A.W.; Lauko, J.; Rowan, A.E. The Mechanosensory Role of Osteocytes and Implications for Bone Health and Disease States. Front. Cell Dev. Biol. 2022, 9, 770143. [Google Scholar] [CrossRef]
- Qin, L.; Liu, W.; Cao, H.; Xiao, G. Molecular mechanosensors in osteocytes. Bone Res. 2020, 8, 23. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Functions of the osteocyte network in the regulation of bone mass. Cell Tissue Res. 2013, 352, 191–198. [Google Scholar] [CrossRef]
- Kerschnitzki, M.; Kollmannsberger, P.; Burghammer, M.; Duda, G.N.; Weinkamer, R.; Wagermaier, W.; Fratzl, P. Architecture of the osteocyte network correlates with bone material quality. J. Bone Miner. Res. 2013, 28, 1837–1845. [Google Scholar] [CrossRef]
- Aguirre, J.I.; Castillo, E.J.; Kimmel, D.B. Biologic and pathologic aspects of osteocytes in the setting of medication-related osteonecrosis of the jaw (MRONJ). Bone 2021, 153, 116168. [Google Scholar] [CrossRef] [PubMed]
- Gkouveris, I.; Hadaya, D.; Elzakra, N.; Soundia, A.; Bezouglaia, O.; Dry, S.M.; Pirih, F.; Aghaloo, T.; Tetradis, S. Inhibition of HMGB1/RAGE Signaling Reduces the Incidence of Medication-Related Osteonecrosis of the Jaw (MRONJ) in Mice. J. Bone Miner. Res. 2022, 37, 1775–1786. [Google Scholar] [CrossRef]
- Tetradis, S.; Allen, M.R.; Ruggiero, S.L. Pathophysiology of Medication-Related Osteonecrosis of the Jaw-A Minireview. JBMR Plus. 2023, 7, e10785. [Google Scholar] [CrossRef]
- Fliefel, R.; Tröltzsch, M.; Kühnisch, J.; Ehrenfeld, M.; Otto, S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 568–585. [Google Scholar] [CrossRef]
- Hayashida, S.; Soutome, S.; Yamamoto, S.; Fujita, S.; Hasegawa, T.; Komori, T.; Kojima, Y.; Miyamoto, H.; Shibuya, Y.; Ueda, N.; et al. Evaluation of the Treatment Strategies for Medication-Related Osteonecrosis of the Jaws (MRONJ) and the Factors Affecting Treatment Outcome: A Multicenter Retrospective Study with Propensity Score Matching Analysis. J. Bone Miner. Res. 2017, 32, 2022–2029. [Google Scholar] [CrossRef]
- Kawahara, M.; Kuroshima, S.; Sawase, T. Clinical considerations for medication-related osteonecrosis of the jaw: A comprehensive literature review. Int. J. Implant Dent. 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Wall, A.; Board, T. Bone: Formation by autoinduction. In Classic Papers in Orthopaedics; Springer: London, UK, 2014; pp. 449–451. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel regulators of bone formation: Molecular clones and activities. Obstet. Gynecol. Surv. 1989, 44, 387–388. [Google Scholar] [CrossRef]
- Bragdon, B.; Moseychuk, O.; Saldanha, S.; King, D.; Julian, J.; Nohe, A. Bone Morphogenetic Proteins: A critical review. Cell. Signal. 2011, 23, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Li, Q.; Lin, X.; Hu, N.; Liao, J.Y.; Lin, L.B.; Zhao, C.; Hu, Z.M.; Liang, X.; Xu, W.; et al. BMP2 induces chondrogenic differentiation, osteogenic differentiation and endochondral ossification in stem cells. Cell Tissue Res. 2016, 366, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.C.; Brown, J.V.; Heirs, M.K.; Higgins, J.P.; Mannion, R.J.; Rodgers, M.A.; Stewart, L.A. Safety and Effectiveness of Recombinant Human Bone Morphogenetic Protein-2 for Spinal Fusion. Ann. Intern. Med. 2013, 158, 877. [Google Scholar] [CrossRef] [PubMed]
- Parajón, A.; Alimi, M.; Navarro-Ramirez, R.; Christos, P.; Torres-Campa, J.M.; Moriguchi, Y.; Lang, G.; Härtl, R. Minimally invasive transforaminal lumbar interbody fusion: Meta-analysis of the fusion rates. What is the optimal graft material? Clin. Neurosurg. 2017, 81, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Malham, G.M.; Louie, P.K.; Brazenor, G.A.; Mobbs, R.J.; Walsh, W.R.; Sethi, R.K. Recombinant human bone morphogenetic protein-2 in spine surgery: Recommendations for use and alternative bone substitutes—A narrative review. J. Spine Surg. 2022, 8, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Wikesjö, U.M.E.; Huang, Y.H.; Polimeni, G.; Qahash, M. Bone Morphogenetic Proteins: A Realistic Alternative to Bone Grafting for Alveolar Reconstruction. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 535–551. [Google Scholar] [CrossRef]
- Li, F.; Yu, F.; Liao, X.; Wu, C.; Wang, Y.; Li, C.; Lou, F.; Li, B.; Yin, B.; Wang, C.; et al. Efficacy of Recombinant Human BMP2 and PDGF-BB in Orofacial Bone Regeneration: A Systematic Review and Meta-analysis. Sci. Rep. 2019, 9, 8073. [Google Scholar] [CrossRef]
- Yano, K.; Hoshino, M.; Ohta, Y.; Manaka, T.; Naka, Y.; Imai, Y.; Sebald, W.; Takaoka, K. Osteoinductive capacity and heat stability of recombinant human bone morphogenetic protein-2 produced by Escherichia coli and dimerized by biochemical processing. J. Bone Miner. Metab. 2009, 27, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Jung, S.K.; Lee, J.; Chang, P.S.; Kang, S.H. Modulation of osteogenic differentiation by Escherichia coli-derived recombinant bone morphogenetic protein-2. AMB Express 2022, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Sonoyama, W.; Yamamoto, K.; Oida, Y.; Akiyama, K.; Shinkawa, S.; Nakajima, R.; Pham, H.T.; Hara, E.S.; Kuboki, T. Efficient bone formation in a swine socket lift model using Escherichia coli-derived recombinant human bone morphogenetic protein-2 adsorbed in β-tricalcium phosphate. Cells Tissues Organs 2014, 199, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Sonoyama, W.; Nema, K.; Hara, E.S.; Oida, Y.; Pham, H.T.; Yamamoto, K.; Hirota, K.; Sugama, K.; Sebald, W.; et al. Regeneration of calvarial defects with Escherichia coli-derived rhBMP-2 adsorbed in PLGA Membrane. Cells Tissues Organs 2014, 198, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Mikai, A.; Ono, M.; Tosa, I.; Nguyen, H.T.T.; Hara, E.S.; Nosho, S.; Kimura-Ono, A.; Nawachi, K.; Takarada, T.; Kuboki, T.; et al. BMP-2/Β-TCP local delivery for bone regeneration in MRONJ-like mouse model. Int. J. Mol. Sci. 2020, 21, 7028. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Aung, K.T.; Ono, M.; Mikai, A.; Dang, A.T.; Hara, E.S.; Tosa, I.; Ishibashi, K.; Ono-Kimura, A.; Nawachi, K.; et al. Suppression of bone necrosis around tooth extraction socket in an MRONJ-like mouse model by E-rhBMP-2 containing artificial bone graft administration. Int. J. Mol. Sci. 2021, 22, 12823. [Google Scholar] [CrossRef] [PubMed]
- Prideaux, M.; Findlay, D.M.; Atkins, G.J. Osteocytes: The master cells in bone remodelling. Curr. Opin. Pharmacol. 2016, 28, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Danova, N.A.; Colopy, S.A.; Radtke, C.L.; Kalscheur, V.L.; Markel, M.D.; Vanderby, R., Jr.; McCabe, R.P.; Escarcega, A.J.; Muir, P. Degradation of bone structural properties by accumulation and coalescence of microcracks. Bone 2003, 33, 197–205. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J.; Brennan, O.; Kennedy, O.D.; Lee, T.C. Microcracks in cortical bone: How do they affect bone biology? Curr. Osteoporos. Rep. 2005, 3, 39–45. [Google Scholar] [CrossRef]
- Hazenberg, J.G.; Hentunen, T.A.; Heino, T.J.; Kurata, K.; Lee, T.C.; Taylor, D. Microdamage detection and repair in bone: Fracture mechanics, histology, cell biology. Technol. Health Care 2009, 17, 67–75. [Google Scholar] [CrossRef]
- Bellido, T. Osteocyte-driven bone remodeling. Calcif. Tissue Int. 2014, 94, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Hoefert, S.; Schmitz, I.; Tannapfel, A.; Eufinger, H. Importance of microcracks in etiology of bisphosphonate-related osteonecrosis of the jaw: A possible pathogenetic model of symptomatic and non-symptomatic osteonecrosis of the jaw based on scanning electron microscopy findings. Clin. Oral Investig. 2010, 14, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Kurata, K.; Heino, T.J.; Higaki, H.; Väänänen, H.K. Bone marrow cell differentiation induced by mechanically damaged osteocytes in 3D gel-embedded culture. J. Bone Miner. Res. 2006, 21, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A.M. Targeted and nontargeted bone remodeling: Relationship to basic multicellular unit origination and progression. Bone 2002, 30, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Tami, A.E.; Nasser, P.; Verborgt, O.; Schaffler, M.B.; Knothe Tate, M.L. The role of interstitial fluid flow in the remodeling response to fatigue loading. J. Bone Miner. Res. 2002, 17, 2030–2037. [Google Scholar] [CrossRef]
- Verborgt, O.; Tatton, N.A.; Majeska, R.J.; Schaffler, M.B. Spatial distribution of Bax and Bcl-2 in osteocytes after bone fatigue: Complementary roles in bone remodeling regulation? J. Bone Miner. Res. 2002, 17, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-Hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Tiede-Lewis, L.A.M.; Xie, Y.; Hulbert, M.A.; Campos, R.; Dallas, M.R.; Dusevich, V.; Bonewald, L.F.; Dallas, S.L. Degeneration of the osteocyte network in the C57BL/6 mouse model of aging. Aging 2017, 9, 2187–2205. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Nojiri, H.; Saita, Y.; Morikawa, D.; Ozawa, Y.; Watanabe, K.; Koike, M.; Asou, Y.; Shirasawa, T.; Yokote, K.; et al. Mitochondrial superoxide in osteocytes perturbs canalicular networks in the setting of age-related osteoporosis. Sci. Rep. 2015, 5, 9148. [Google Scholar] [CrossRef] [PubMed]
- Knothe, M.L.; Adamson, J.R.; Tami, A.E.; Bauer, T.W. The osteocyte. Int. J. Biochem. Cell Biol. 2004, 36, 1–8. [Google Scholar] [CrossRef]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. 3), 131–139. [Google Scholar] [CrossRef]
- Kenkre, J.S.; Bassett, J.H.D. The bone remodeling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Lotz, E.M.; Lohmann, C.H.; Boyan, B.D.; Schwartz, Z. Bisphosphonates inhibit surface-mediated osteogenesis. J. Biomed. Mater. Res. A 2020, 108, 1774–1786. [Google Scholar] [CrossRef]
- Huja, S.S.; Fernandez, S.A.; Phillips, C.; Li, Y. Zoledronic acid decreases bone formation without causing osteocyte death in mice. Arch. Oral Biol. 2009, 54, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Howie, R.N.; Borke, J.L.; Kurago, Z.; Daoudi, A.; Cray, J.; Zakhary, I.E.; Brown, T.L.; Raley, J.N.; Tran, L.T.; Messer, R.; et al. A Model for Osteonecrosis of the Jaw with Zoledronate Treatment following Repeated Major Trauma. PLoS ONE 2015, 10, e0132520. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef]
- Zou, M.L.; Chen, Z.H.; Teng, Y.Y.; Liu, S.Y.; Jia, Y.; Zhang, K.W.; Sun, Z.L.; Wu, J.J.; Yuan, Z.D.; Feng, Y.; et al. The Smad Dependent TGF-β and BMP Signaling Pathway in Bone Remodeling and Therapies. Front. Mol. Biosci. 2021, 8, 593310. [Google Scholar] [CrossRef]
- Poundarik, A.A.; Vashishth, D. Multiscale imaging of bone microdamage. Connect. Tissue Res. 2015, 56, 87–98. [Google Scholar] [CrossRef]
- Dominguez, V.M.; Agnew, A.M. Microdamage as a Bone Quality Component: Practical Guidelines for the Two-Dimensional Analysis of Linear Microcracks in Human Cortical Bone. JBMR Plus 2019, 3, e10203. [Google Scholar] [CrossRef]
- Reilly, G.C.; Currey, J.D. The development of microcracking and failure in bone depends on the loading mode to which it is adapted. J. Exp. Biol. 1999, 202, 543–552. [Google Scholar] [CrossRef]
- Herman, B.C.; Cardoso, L.; Majeska, R.J.; Jepsen, K.J.; Schaffler, M.B. Activation of bone remodeling after fatigue: Differential response to linear microcracks and diffuse damage. Bone 2010, 47, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Baumann, M.J.; Case, E.D.; Irwin, R.K.; Meyer, S.E.; Pearson, C.S.; McCabe, L.R. Surface microcracks signal osteoblasts to regulate alignment and bone formation. Mater. Sci. Eng. C 2014, 44, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Toldrà, R.; Vasquez-Sancho, F.; Barroca, N.; Catalan, G. Investigation of The Cellular Response to Bone Fractures: Evidence for Flexoelectricity. Sci. Rep. 2020, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Landayan, M.E.; Lee, J.Y.; Tatad, J.C.; Kim, S.J.; Kim, M.R.; Cha, I.H. Role of microcracks in the pathogenesis of bisphosphonate-related osteonecrosis of the jaw. Clin. Oral Investig. 2016, 20, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mashiba, T.; Burr, D.B. Bisphosphonate treatment suppresses not only stochastic remodeling but also the targeted repair of microdamage. Calcif. Tissue Int. 2001, 69, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.Y.; Eo, M.Y.; Sodnom-Ish, B.; Mustakim, K.R.; Myoung, H.; Kim, S.M. Electron microscopic analysis of necrotic bone and failed implant surface in a patient with medication-related osteonecrosis of the jaw. Maxillofac. Plast. Reconstr. Surg. 2023, 45, 34. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D. Scaling effects in the fatigue strength of bones from different animals. J. Theor. Biol. 2000, 206, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, S.; O’Brien, F.J.; Lee, T.C. Microcracks in compact bone: A three-dimensional view. J. Anat. 2006, 209, 119–124. [Google Scholar] [CrossRef]
- Bonnet, N.; Lesclous, P.; Saffar, J.L.; Ferrari, S. Zoledronate Effects on Systemic and Jaw Osteopenias in Ovariectomized Periostin-Deficient Mice. PLoS ONE 2013, 8, e58726. [Google Scholar] [CrossRef]
- Lomba, P.M.; Fernández-Marchena, J.L.; Cazalla, I.; Valtierra, N.; Cáceres, I.; Ollé, A. An assessment of bone tool cleaning procedures in preparation for traceological analysis. Archaeol. Anthropol. Sci. 2022, 14, 95. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, A.T.; Ono, M.; Wang, Z.; Tosa, I.; Hara, E.S.; Mikai, A.; Kitagawa, W.; Yonezawa, T.; Kuboki, T.; Oohashi, T. Local E-rhBMP-2/β-TCP Application Rescues Osteocyte Dendritic Integrity and Reduces Microstructural Damage in Alveolar Bone Post-Extraction in MRONJ-like Mouse Model. Int. J. Mol. Sci. 2024, 25, 6648. https://doi.org/10.3390/ijms25126648
Dang AT, Ono M, Wang Z, Tosa I, Hara ES, Mikai A, Kitagawa W, Yonezawa T, Kuboki T, Oohashi T. Local E-rhBMP-2/β-TCP Application Rescues Osteocyte Dendritic Integrity and Reduces Microstructural Damage in Alveolar Bone Post-Extraction in MRONJ-like Mouse Model. International Journal of Molecular Sciences. 2024; 25(12):6648. https://doi.org/10.3390/ijms25126648
Chicago/Turabian StyleDang, Anh Tuan, Mitsuaki Ono, Ziyi Wang, Ikue Tosa, Emilio Satoshi Hara, Akihiro Mikai, Wakana Kitagawa, Tomoko Yonezawa, Takuo Kuboki, and Toshitaka Oohashi. 2024. "Local E-rhBMP-2/β-TCP Application Rescues Osteocyte Dendritic Integrity and Reduces Microstructural Damage in Alveolar Bone Post-Extraction in MRONJ-like Mouse Model" International Journal of Molecular Sciences 25, no. 12: 6648. https://doi.org/10.3390/ijms25126648
APA StyleDang, A. T., Ono, M., Wang, Z., Tosa, I., Hara, E. S., Mikai, A., Kitagawa, W., Yonezawa, T., Kuboki, T., & Oohashi, T. (2024). Local E-rhBMP-2/β-TCP Application Rescues Osteocyte Dendritic Integrity and Reduces Microstructural Damage in Alveolar Bone Post-Extraction in MRONJ-like Mouse Model. International Journal of Molecular Sciences, 25(12), 6648. https://doi.org/10.3390/ijms25126648





