Genome-Wide Comparative Analysis of the Cytochrome P450 Monooxygenase Family in 19 Aphid Species and Their Expression Analysis in 4 Cereal Crop Aphids
Abstract
1. Introduction
2. Results
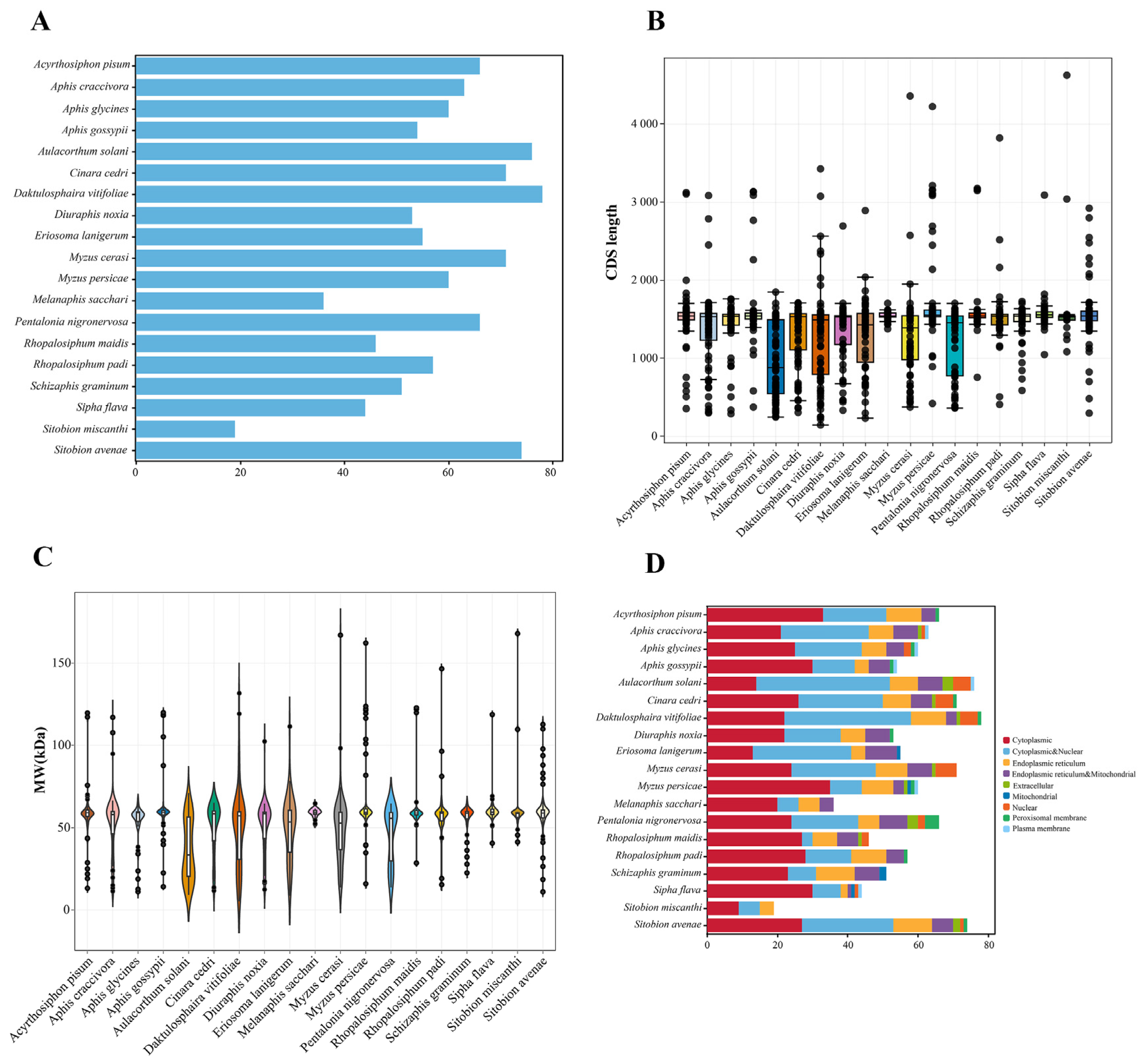
2.1. Genome-Wide Identification of CYP450 Genes in 19 Aphid Species
2.2. Chromosome Localizations of CYP450 Genes among 6 Cereal Crop Aphid Species
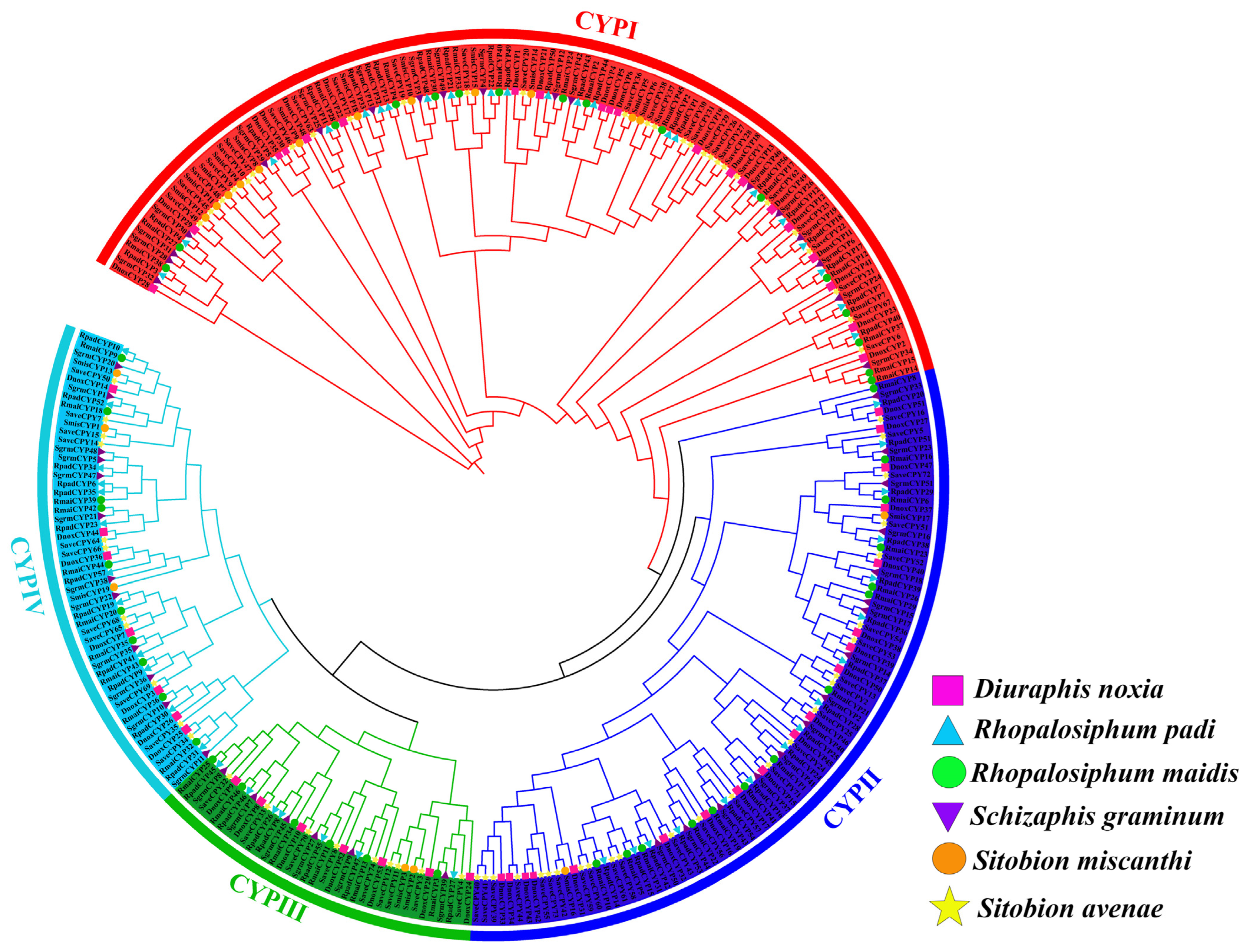
2.3. Phylogenetic Relationship Analysis of CYP450 Genes among Six Cereal Crop Aphids
2.4. Gene Structure and Conserved Motif Analysis of CYP450 Genes among Six Cereal Crop Aphids
2.5. Gene Duplication and Synteny Analysis of CYP450 Genes among Six Cereal Crop Aphid Species
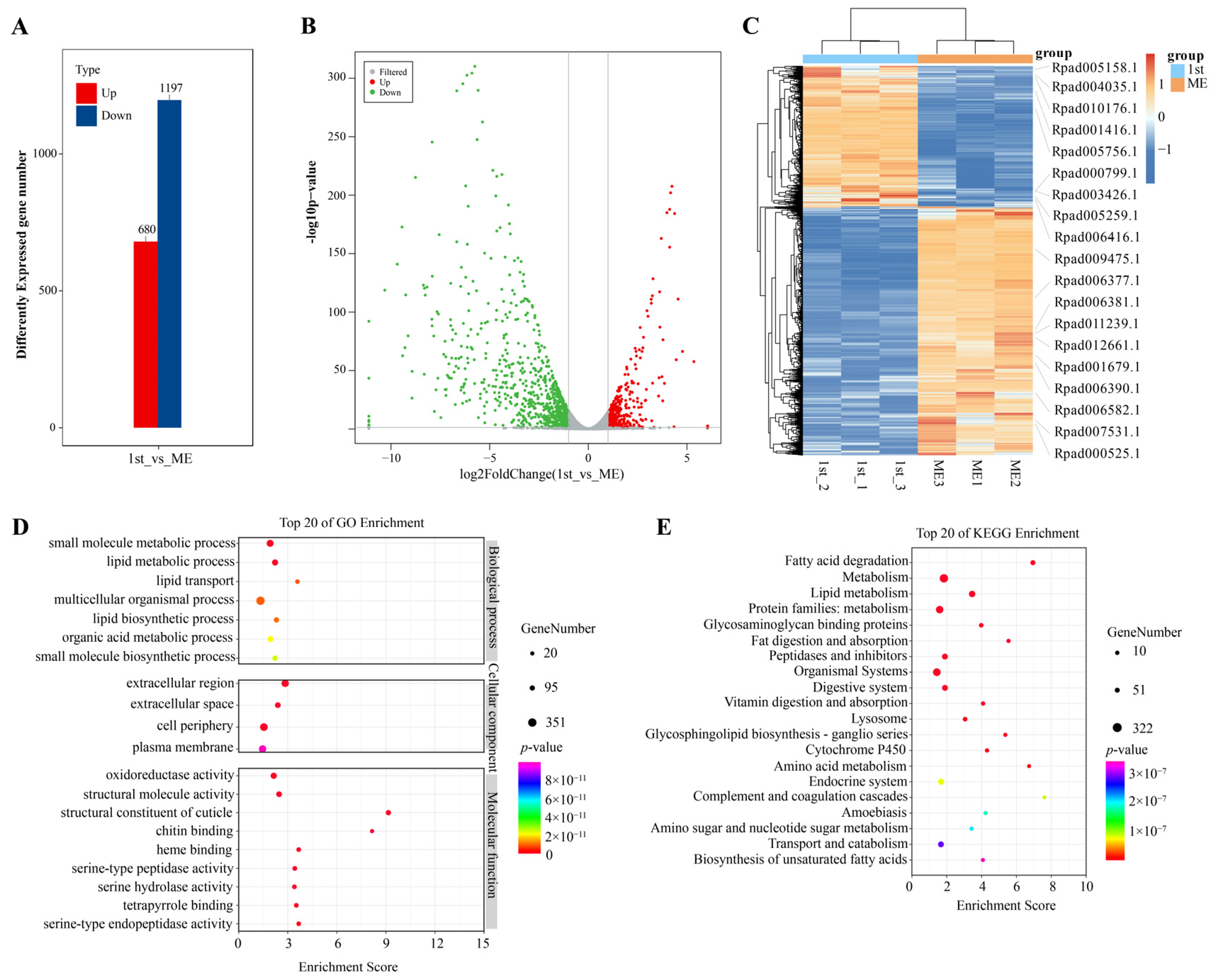
2.6. Transcriptomic Analysis of Rhopalosiphum Padi at Different Developmental Stages
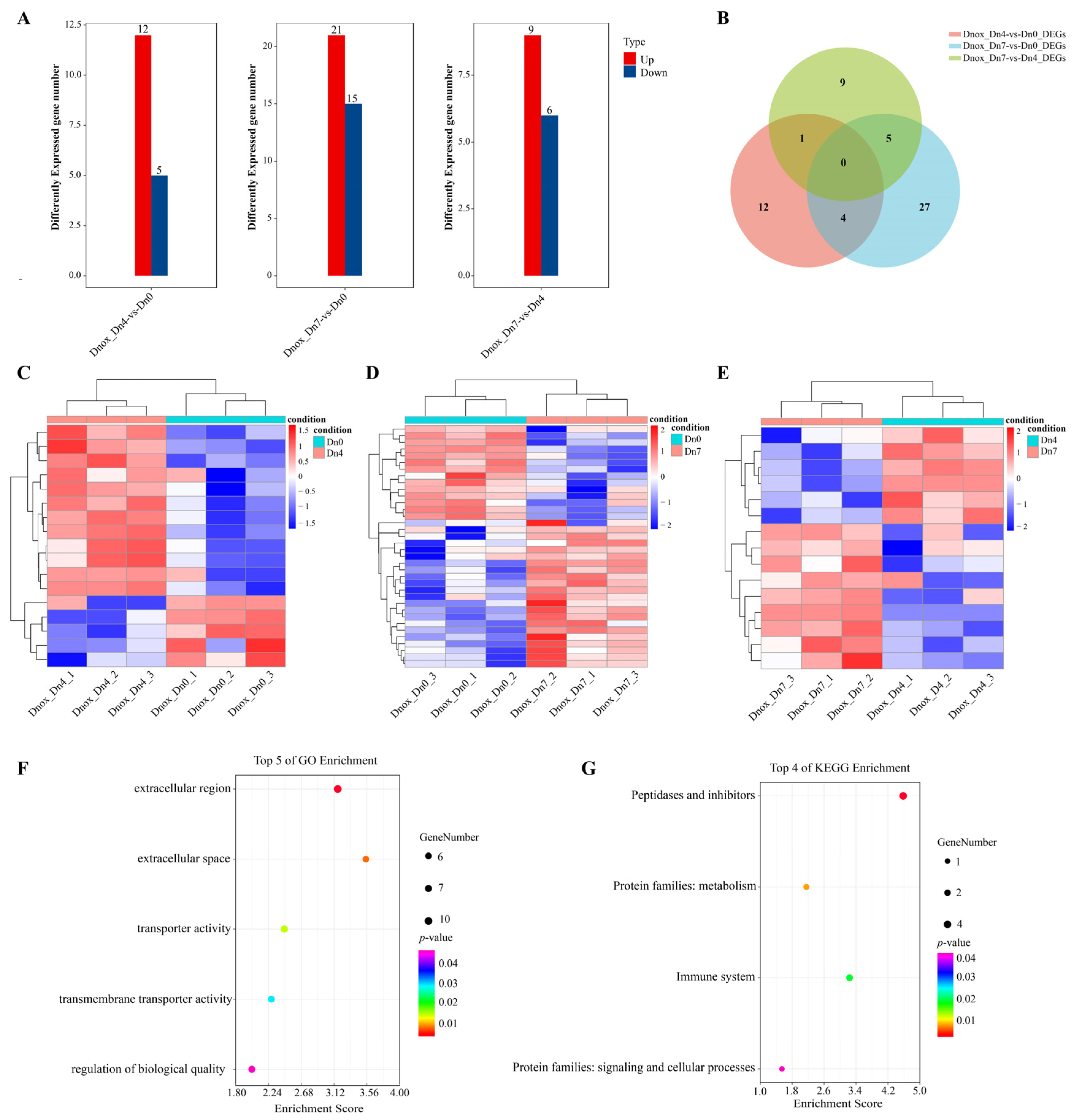
2.7. Transcriptomic Analysis of Diuraphis Noxia Fed on Wheat Plants Containing Dn0, Dn4, or Dn7
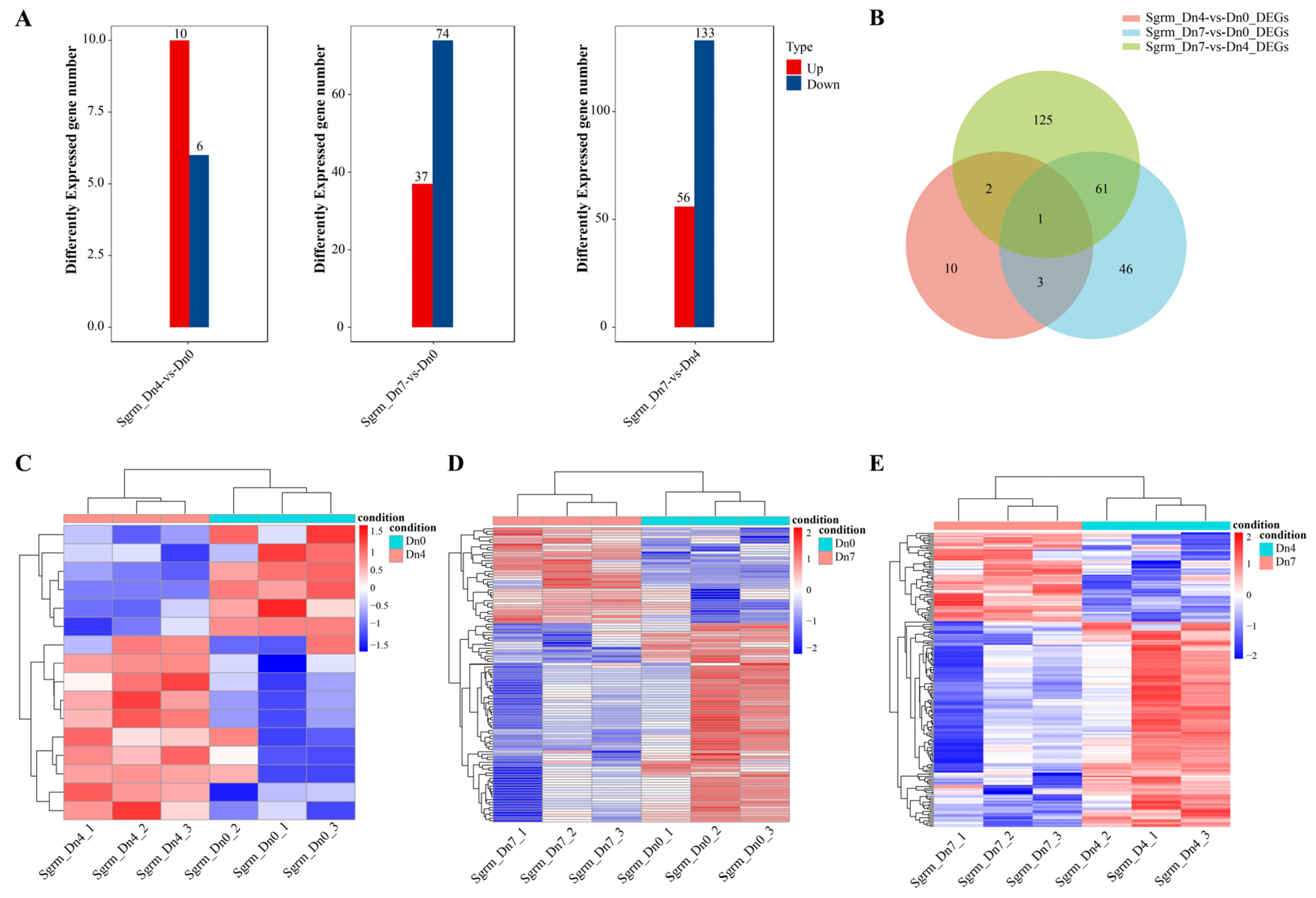
2.8. Transcriptomic Analysis of Schizaphis Graminum Fed on Wheat Plants Dn0, Dn4, or Dn7
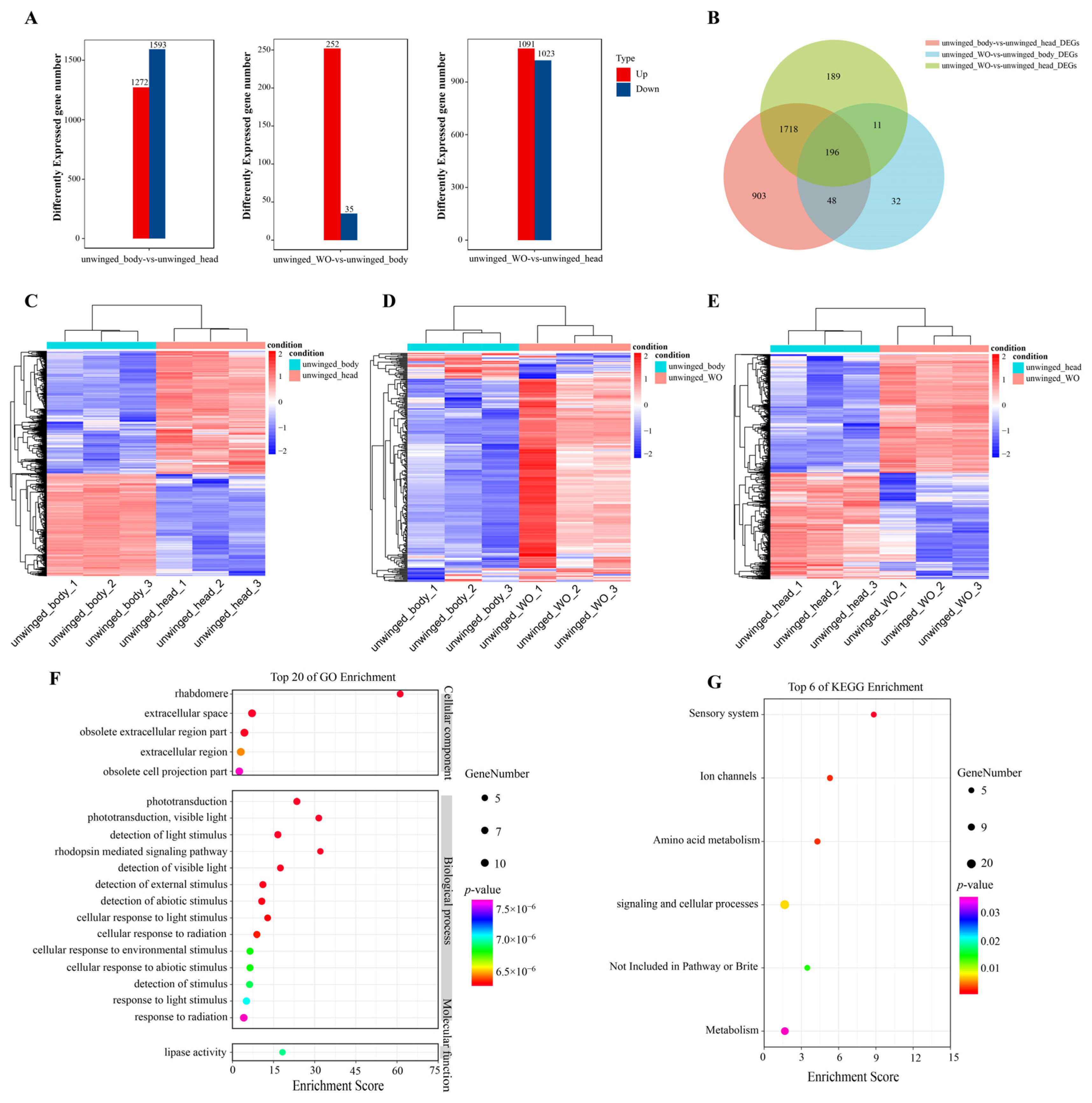
2.9. Transcriptomic Analysis of Winged and Unwinged Sitobion Avenae Aphids
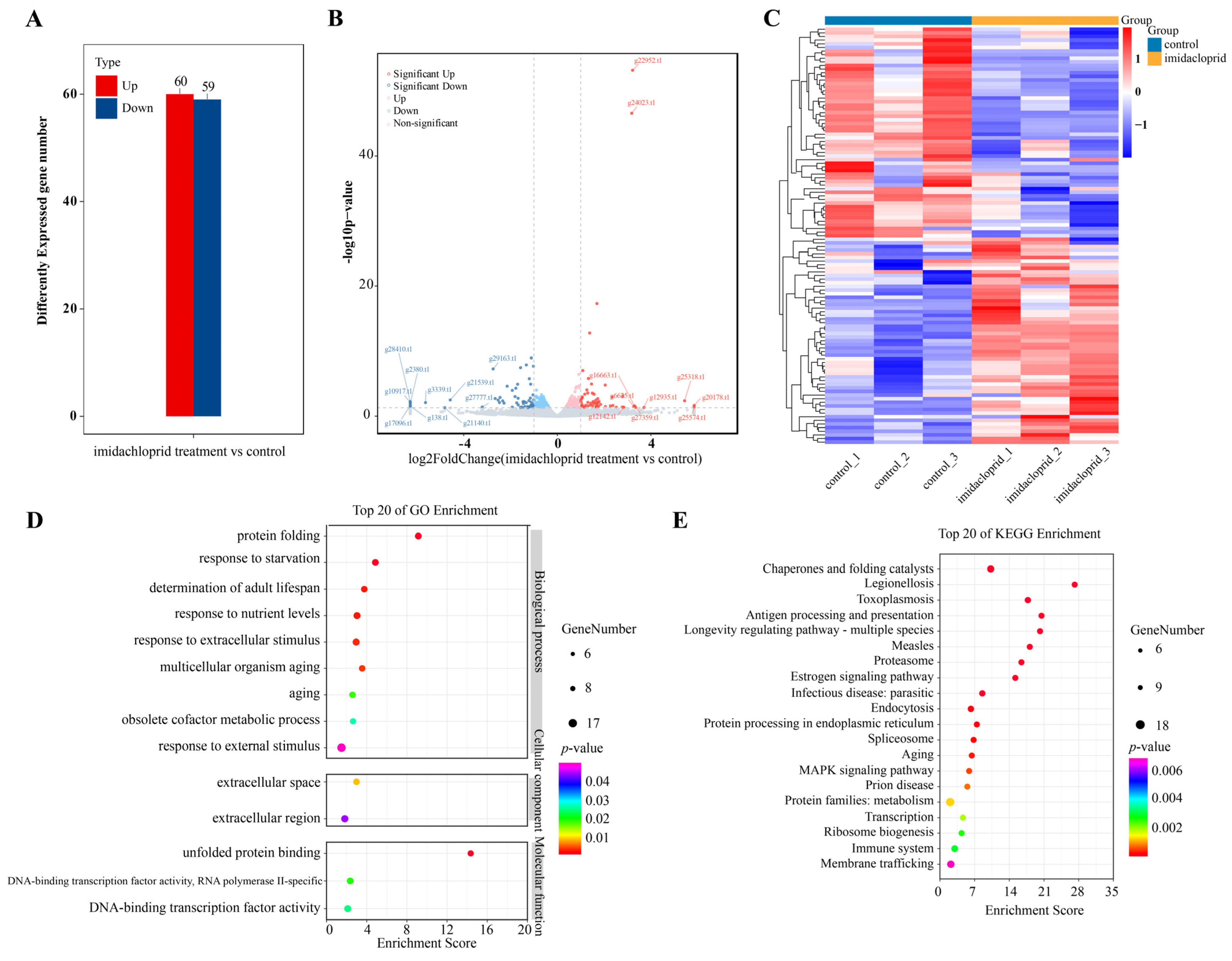
2.10. Transcriptomic Analysis of Sitobion Avenae Aphids Fed on Wheat Treated with Imidacloprid
3. Discussion
4. Materials and Methods
4.1. Genome-Wide Identification of CYP450 Genes in 19 Aphid Species
4.2. Phylogenetic Relationship, Gene Structure and Conserved Motif Analysis of CYP450 Genes among Six Cereal Crop Aphid Species
4.3. Gene Duplication and Synteny Analysis of CYP450 Genes among Six Cereal Crop Aphid Species
4.4. RNA-seq Data Analysis of Four Cereal Crop Aphid Species
4.5. Differential Expression Analysis among Four Cereal Crop Aphids
4.6. GO Enrichment and KEGG Pathway Enrichment of DEGs among Four Cereal Crop Aphids
4.7. Expression Patterns of Differential Expressed CYP450 Genes among Four Cereal Crop Aphids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathers, T.C.; Mugford, S.T.; Hogenhout, S.A.; Tripathi, L. Genome sequence of the banana aphid, Pentalonia nigronervosa Coquerel (Hemiptera: Aphididae) and its symbionts. G3 (Bethesda) 2020, 10, 4315–4321. [Google Scholar] [CrossRef] [PubMed]
- Sims, C.; Birkett, M.A.; Oldham, N.J.; Stockman, R.A.; Withall, D.M. Pea aphid odorant-binding protein ApisOBP6 discriminates between aphid sex pheromone components, aphid alarm pheromone and a host plant volatile. Insect Biochem. Mol. Biol. 2023, 162, 104026. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Duan, A.; Zhang, C.; Zhang, Y.; Wang, A.; Xue, C.; Wang, H.; Zhao, M.; Zhang, J. Overexpression of ATP-binding cassette transporters ABCG10, ABCH3 and ABCH4 in Aphis craccivora (Koch) facilitates its tolerance to imidacloprid. Pestic. Biochem. Physiol. 2022, 186, 105170. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fan, Y.; Zhang, W.; Tian, Z.; Liu, J.; Zhao, K. Soybean aphid, Aphis glycines (Hemiptera: Aphididae), developmental and reproductive capacity on white clover, Trifolium repens (Rosales: Leguminosae), in northeast China. Appl. Entomol. Zool. 2017, 52, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J. Influence of Lecanicillium attenuatum on the development and reproduction of the cotton aphid Aphis gossypii. BioControl 2007, 52, 789–799. [Google Scholar] [CrossRef]
- Shi, X.B.; Jiang, L.L.; Wang, H.Y.; Qiao, K.; Wang, D.; Wang, K.Y. Toxicities and sublethal effects of seven neonicotinoid insecticides on survival, growth and reproduction of imidacloprid-resistant cotton aphid Aphis gossypii. Pest Manag. Sci. 2011, 67, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.Y.; Kim, E.Y.; Ahn, J.J.; Kim, Y.; Kang, S.; Jung, J.K. Development, reproduction, and life table parameters of the foxglove aphid, Aulacorthum solani Kaltenbach (Hemiptera: Aphididae), on soybean at constant temperatures. Insects 2020, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, G.; Zhou, C.; Yin, S. Influence of temperature on the development and reproduction of Cinara cedri (Hemiptera: Aphidoidea: Lachninae). Bull. Entomol. Res. 2021, 111, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Gao, G.; Wei, J. Potential global distribution of Daktulosphaira vitifoliae under climate change based on MaxEnt. Insects 2021, 12, 347. [Google Scholar] [CrossRef]
- Alins, G.; Lordan, J.; Rodríguez-Gasol, N.; Arnó, J.; Peñalver-Cruz, A. Earwig Releases Provide Accumulative Biological Control of the Woolly Apple Aphid over the Years. Insects 2023, 14, 890. [Google Scholar] [CrossRef]
- Pekarcik, A.J.; Jacobson, A.L. Evaluating sugarcane aphid, Melanaphis sacchari (Hemiptera: Aphididae), population dynamics, feeding injury, and grain yield among commercial sorghum varieties in Alabama. J. Econ. Entomol. 2021, 114, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Van Emden, H.F.; Harrington, R. Aphids as Crop Pests; CAB International: Wallingford, UK, 2017. [Google Scholar]
- Salinas-Sánchez, D.O.; Flores-Franco, G.; Avilés-Montes, D.; Valladares-Cisneros, M.G.; Arias-Ataide, D.M.; Mendoza-Catalán, M.Á.; Sotelo-Leyva, C. Bioactivity of a linoleic acid-rich fraction of Ricinus communis L. (Euphorbiaceae) leaves against the yellow sugarcane aphid, Sipha flava (Hemiptera: Aphididae). J. Food. Prot. 2021, 84, 1524–1527. [Google Scholar] [CrossRef]
- Thorpe, P.; Escudero-Martinez, C.M.; Eves-van den Akker, S.; Bos, J.I.B. Transcriptional changes in the aphid species Myzus cerasi under different host and environmental conditions. Insect Mol. Biol. 2020, 29, 271–282. [Google Scholar] [CrossRef]
- Liu, X.L.; Yang, X.F.; Wang, C.Y.; Wang, Y.J.; Zhang, H.; Ji, W.Q. Molecular mapping of resistance gene to English grain aphid (Sitobion avenae F.) in Triticum durum wheat line C273. Theor. Appl. Genet. 2012, 124, 287–293. [Google Scholar] [CrossRef]
- Xin, J.J.; Shang, Q.L.; Desneux, N.; Gao, X.W. Genetic diversity of Sitobion avenae (Homoptera: Aphididae) populations from different geographic regions in China. PLoS ONE 2014, 9, e109349. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.S.; Liu, X.F.; Thieme, T.; Zhang, G.S.; Liu, T.X.; Zhao, H.Y. Testing the fecundity advantage hypothesis with Sitobion avenae, Rhopalosiphum padi, and Schizaphis graminum (Hemiptera: Aphididae) feeding on ten wheat accessions. Sci. Rep. 2015, 5, 18549. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shakir, S.; Bigham, M.; Richter, A.; Fei, Z.; Jander, G. Genome sequence of the corn leaf aphid (Rhopalosiphum maidis Fitch). GigaScience 2019, 8, giz033. [Google Scholar] [CrossRef]
- Nicolis, V.F.; Burger, N.F.V.; Botha, A.M. Whole-body transcriptome mining for candidate effectors from Diuraphis noxia. BMC Genom. 2022, 23, 493. [Google Scholar] [CrossRef]
- Feyereisen, R. Evolution of insect P450. Biochem. Soc. Trans. 2006, 34, 1252–1255. [Google Scholar] [CrossRef] [PubMed]
- Iga, M.; Kataoka, H. Recent studies on insect hormone metabolic pathways mediated by cytochrome P450 enzymes. Biol. Pharm. Bull. 2012, 35, 838–843. [Google Scholar] [CrossRef]
- Herrero, Ó.; Aquilino, M.; Sánchez-Argüello, P.; Planelló, R. The BPA-substitute bisphenol S alters the transcription of genes related to endocrine, stress response and biotransformation pathways in the aquatic midge Chironomus riparius (Diptera, Chironomidae). PLoS ONE 2018, 13, e0193387. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Keeling, C.I.; Bohlmann, J. Cytochromes P450 preferentially expressed in antennae of the mountain pine beetle. J. Chem. Ecol. 2019, 45, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Fotoukkiaii, S.M.; Wybouw, N.; Kurlovs, A.H.; Leeuwen, T.V. High-resolution genetic mapping reveals cis-regulatory and copy number variation in loci associated with cytochrome P450-mediated detoxification in a generalist arthropod pest. PLoS Genet. 2021, 17, e1009422. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Choi, B.; Park, W.R.; Kim, Y.J.; Kim, B.E.; Mun, S.; Kim, D.Y. Nuclear receptor HR96 up-regulates cytochrome P450 for insecticide detoxification in Tribolium castaneum. Pest. Manag. Sci. 2022, 78, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Moural, T.W.; Nelson, D.R.; Palli, S.R. A specialist herbivore pest adaptation to xenobiotics through up-regulation of multiple cytochrome P450s. Sci. Rep. 2016, 6, 20421. [Google Scholar] [CrossRef] [PubMed]
- David, J.P.; Boyer, S.; Mesneau, A.; Ball, A.; Ranson, H.; Dauphin-Villemant, C. Involvement of cytochrome P450 monooxygenases in the response of mosquito larvae to dietary plant xenobiotics. Insect Biochem. Mol. Biol. 2006, 36, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, G.; Hilliou, F. Resistance evolution in Drosophila: The case of CYP6G1. Pest Manag. Sci. 2017, 73, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Karunker, I.; Benting, J.; Lueke, B.; Ponge, T.; Nauen, R.; Roditakis, E.; Vontas, J.; Gorman, K.; Denholm, I.; Morin, S. Over expression of cytochrome P450 CYP6CM1 is associated with high resistance to imidacloprid in the B and Q biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Biochem. Mol. Biol. 2008, 38, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wen, S.; Ding, Y.; Gao, X.; Chen, X.; Yan, K.; Yang, F.; Pan, Y.; Shang, Q. Functional validation of the roles of Cytochrome P450s in tolerance to thiamethoxam and imidacloprid in a field population of Aphis gossypii. J. Agric. Food Chem. 2022, 70, 14339–14351. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Zhang, H.; Shan, T.; Shi, X.; Gao, X. The overexpression of three cytochrome P450 genes CYP6CY14, CYP6CY22 and CYP6UN1 contributed to metabolic resistance to dinotefuran in melon/cotton aphid, Aphis gossypii Glover. Pestic. Biochem. Physiol. 2020, 167, 104601. [Google Scholar] [CrossRef]
- Ji, R.; Lei, J.; Chen, I.W.; Sang, W.; Yang, S.; Fang, J.; Zhu-Salzman, K. Cytochrome P450s CYP380C6 and CYP380C9 in green peach aphid facilitate its adaptation to indole glucosinolate-mediated plant defense. Pest Manag. Sci. 2021, 77, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Hu, J.; Yang, J.; Yin, C.; Du, T.; Huang, M.; Fu, B.; Gong, P.; Liang, J.; Liu, S.; et al. Cytochrome P450 CYP6DB3 was involved in thiamethoxam and imidacloprid resistance in Bemisia tabaci Q (Hemiptera: Aleyrodidae). Pestic. Biochem. Physiol. 2023, 194, 105468. [Google Scholar] [CrossRef]
- Wang, R.L.; Zhu-Salzman, K.; Baerson, S.R.; Xin, X.W.; Li, J.; Su, Y.J.; Zeng, R.S. Identification of a novel cytochrome P450 CYP321B1 gene from tobacco cutworm (Spodoptera litura) and RNA interference to evaluate its role in commonly used insecticides. Insect Sci. 2017, 24, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Z.; Zhou, C.; Ali, A.; Ali, S.; Wu, J. RNA interference in cytochrome P450 monooxygenase (CYP) gene results in reduced insecticide resistance in Megalurothrips usitatus Bagnall. Front. Physiol. 2023, 14, 1130389. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liao, M.; Yang, Q.; Xiao, J.; Hu, Z.; Zhou, L.; Cao, H. Transcriptome profiling reveals differential gene expression of detoxification enzymes in Sitophilus zeamais responding to terpinen-4-ol fumigation. Pestic. Biochem. Physiol. 2018, 149, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Zheng, H.; Tao, Y.; Zhang, Y.; Chu, D. Genome-wide identification and expression analysis of the Cytochrome P450 gene Family in Bemisia tabaci MED and their roles in the insecticide resistance. Int. J. Mol. Sci. 2023, 24, 5899. [Google Scholar] [CrossRef] [PubMed]
- Khumalo, M.J.; Nzuza, N.; Padayachee, T.; Chen, W.; Yu, J.H.; Nelson, D.R.; Syed, K. Comprehensive analyses of Cytochrome P450 monooxygenases and secondary metabolite biosynthetic gene clusters in cyanobacteria. Int. J. Mol. Sci. 2020, 21, 656. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lee, M.K.; Jefcoate, C.; Kim, S.C.; Chen, F.; Yu, J.H. Fungal cytochrome p450 monooxygenases: Their distribution, structure, functions, family expansion, and evolutionary origin. Genome Biol. Evol. 2014, 6, 1620–1634. [Google Scholar] [CrossRef]
- Wei, K.; Chen, H. Global identification, structural analysis and expression characterization of cytochrome P450 monooxygenase superfamily in rice. BMC Genom. 2018, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Vasav, A.P.; Barvkar, V.T. Phylogenomic analysis of cytochrome P450 multigene family and their differential expression analysis in Solanum lycopersicum L. suggested tissue specific promoters. BMC Genom. 2019, 20, 116. [Google Scholar] [CrossRef]
- Li, Y.; Wei, K. Comparative functional genomics analysis of cytochrome P450 gene superfamily in wheat and maize. BMC Plant Biol. 2020, 20, 93. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Jiang, J.; Du, Q.; Luo, L.; Li, X.; Xie, X. Cytochrome P450 superfamily: Evolutionary and functional divergence in sorghum (Sorghum bicolor) stress resistance. J. Agric. Food Chem. 2021, 69, 10952–10961. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Panwar, R.; Rustagi, A.; Chakraborty, A.; Roy, A.; Singh, I.K.; Singh, A. Comprehensive and evolutionary analysis of Spodoptera litura-inducible Cytochrome P450 monooxygenase gene family in Glycine max elucidate their role in defense. Front. Plant Sci. 2023, 14, 1221526. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Li, X. Genome-wide analysis of the P450 gene family in tea plant (Camellia sinensis) reveals functional diversity in abiotic stress. BMC Genom. 2023, 24, 535. [Google Scholar] [CrossRef] [PubMed]
- Tijet, N.; Helvig, C.; Feyereisen, R. The cytochrome P450 gene superfamily in Drosophila melanogaster: Annotation, intron-exon organization and phylogeny. Gene 2001, 262, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Zhu, Y.; Duan, J.; Yu, Q.; Zhang, G.; Wan, F.; Xiang, Z.H. Genome-wide analysis of cytochrome P450 monooxygenase genes in the silkworm, Bombyx mori. Gene 2011, 480, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Tang, W.; He, W.; Ma, X.; Vasseur, L.; Baxter, S.W.; Yang, G.; Huang, S.; Song, F.; You, M. Characterization and expression of the cytochrome P450 gene family in diamondback moth, Plutella xylostella (L.). Sci. Rep. 2015, 5, 8952. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.L.; Luo, Z.X.; Li, J.L.; Cai, X.M.; Bian, L.; Xiu, C.L.; Li, Z.Q.; Chen, Z.M.; Zhang, L.W. Identification of cytochrome P450, odorant-binding protein, and chemosensory protein genes involved in Type II sex pheromone biosynthesis and transportation in the tea pest, Scopula subpunctaria. Pestic. Biochem. Physiol. 2020, 169, 104650. [Google Scholar] [CrossRef]
- Gupta, M.; Singh, S.; Kaur, G.; Pandher, S.; Kaur, N.; Goel, N.; Kaur, R.; Rathore, P. Transcriptome analysis unravels RNAi pathways genes and putative expansion of CYP450 gene family in cotton leafhopper Amrasca biguttula (Ishida). Mol. Biol. Rep. 2021, 48, 4383–4396. [Google Scholar] [CrossRef]
- Darragh, K.; Nelson, D.R.; Ramírez, S.R. The birth-and-death evolution of Cytochrome P450 genes in bees. Genome Biol. Evol. 2021, 13, evab261. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, H.; Wang, Q.; Huang, X. Genome-Wide Identification and Expression Pattern of Cytochrome P450 Genes in the Social Aphid Pseudoregma bambucicola. Insects 2023, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Guo, X.; Liu, S.; Li, S.; Zhang, J.; Xue, S.; Tang, Q.; Zhang, K.; Li, R. Cytochrome P450 gene CYP6BQ8 mediates terpinen-4-ol susceptibility in the red flour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Bull. Entomol. Res. 2023, 113, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, H.; Xie, Z.; Li, D.; Yin, C.; Luo, B.; Yan, R.; Sun, W.; Liu, H. Identification and functional characterization of CYP4D2 putatively associated with β-Cypermethrin detoxification in Phortica okadai. Genes 2022, 13, 2338. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.T.; Staehelin, C.; Elzaki, M.E.A.; Hafeez, M.; Luo, Y.S.; Wang, R.L. Functional analysis of CYP6AE68, a cytochrome P450 gene associated with indoxacarb resistance in Spodoptera litura (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2021, 178, 104946. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, R.; Zhu, B.; Gao, X.; Liang, P. Overexpression of cytochrome P450 CYP6BG1 may contribute to chlorantraniliprole resistance in Plutella xylostella (L.). Pest Manag. Sci. 2018, 74, 1386–1393. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, X.; Wang, C.; Wang, L.; Zhang, K.; Li, D.; Ji, J.; Niu, L.; Luo, J.; Cui, J. Silencing of Cytochrome P450 Gene AgoCYP6CY19 Reduces the Tolerance to Host Plant in Cotton- and Cucumber-Specialized Aphids, Aphis gossypii. J. Agric. Food Chem. 2022, 70, 12408–12417. [Google Scholar] [CrossRef] [PubMed]
- Byrne, S.; Schughart, M.; Carolan, J.C.; Gaffney, M.; Thorpe, P.; Malloch, G.; Wilkinson, T.; McNamara, L. Genome sequence of the English grain aphid, Sitobion avenae and its endosymbiont Buchnera aphidicola. G3 (Bethesda) 2022, 12, jkab418. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. Meme suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Quick, J.S.; Stromberger, J.A.; Clayshulte, S.; Clifford, B.; Johnson, J.J.; Peairs, F.B.; Rudolph, J.B.; Lorenz, K. Registration of ‘Yumar’ wheat. Crop Sci. 2001, 41, 1363–1364. [Google Scholar] [CrossRef]
- Marais, G.F.; Horn, M.; Torr, F. Intergeneric transfer (rye to wheat) of a gene(s) for Russian wheat aphid resistance. Plant Breed. 1994, 113, 265–271. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Hao, W.; Wang, H.; Deng, P.; Li, T.; Wang, C.; Zhao, J.; Chen, C.; Ji, W.; Liu, X. Genome-Wide Comparative Analysis of the Cytochrome P450 Monooxygenase Family in 19 Aphid Species and Their Expression Analysis in 4 Cereal Crop Aphids. Int. J. Mol. Sci. 2024, 25, 6668. https://doi.org/10.3390/ijms25126668
Wang Z, Hao W, Wang H, Deng P, Li T, Wang C, Zhao J, Chen C, Ji W, Liu X. Genome-Wide Comparative Analysis of the Cytochrome P450 Monooxygenase Family in 19 Aphid Species and Their Expression Analysis in 4 Cereal Crop Aphids. International Journal of Molecular Sciences. 2024; 25(12):6668. https://doi.org/10.3390/ijms25126668
Chicago/Turabian StyleWang, Zhenyu, Weixi Hao, Hao Wang, Pingchuan Deng, Tingdong Li, Changyou Wang, Jixin Zhao, Chunhuan Chen, Wanquan Ji, and Xinlun Liu. 2024. "Genome-Wide Comparative Analysis of the Cytochrome P450 Monooxygenase Family in 19 Aphid Species and Their Expression Analysis in 4 Cereal Crop Aphids" International Journal of Molecular Sciences 25, no. 12: 6668. https://doi.org/10.3390/ijms25126668
APA StyleWang, Z., Hao, W., Wang, H., Deng, P., Li, T., Wang, C., Zhao, J., Chen, C., Ji, W., & Liu, X. (2024). Genome-Wide Comparative Analysis of the Cytochrome P450 Monooxygenase Family in 19 Aphid Species and Their Expression Analysis in 4 Cereal Crop Aphids. International Journal of Molecular Sciences, 25(12), 6668. https://doi.org/10.3390/ijms25126668




