Exploring Novel Biomarkers for an Acute Coronary Syndrome Diagnosis Utilizing Plasma Metabolomics
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics of the ACS and HC Groups
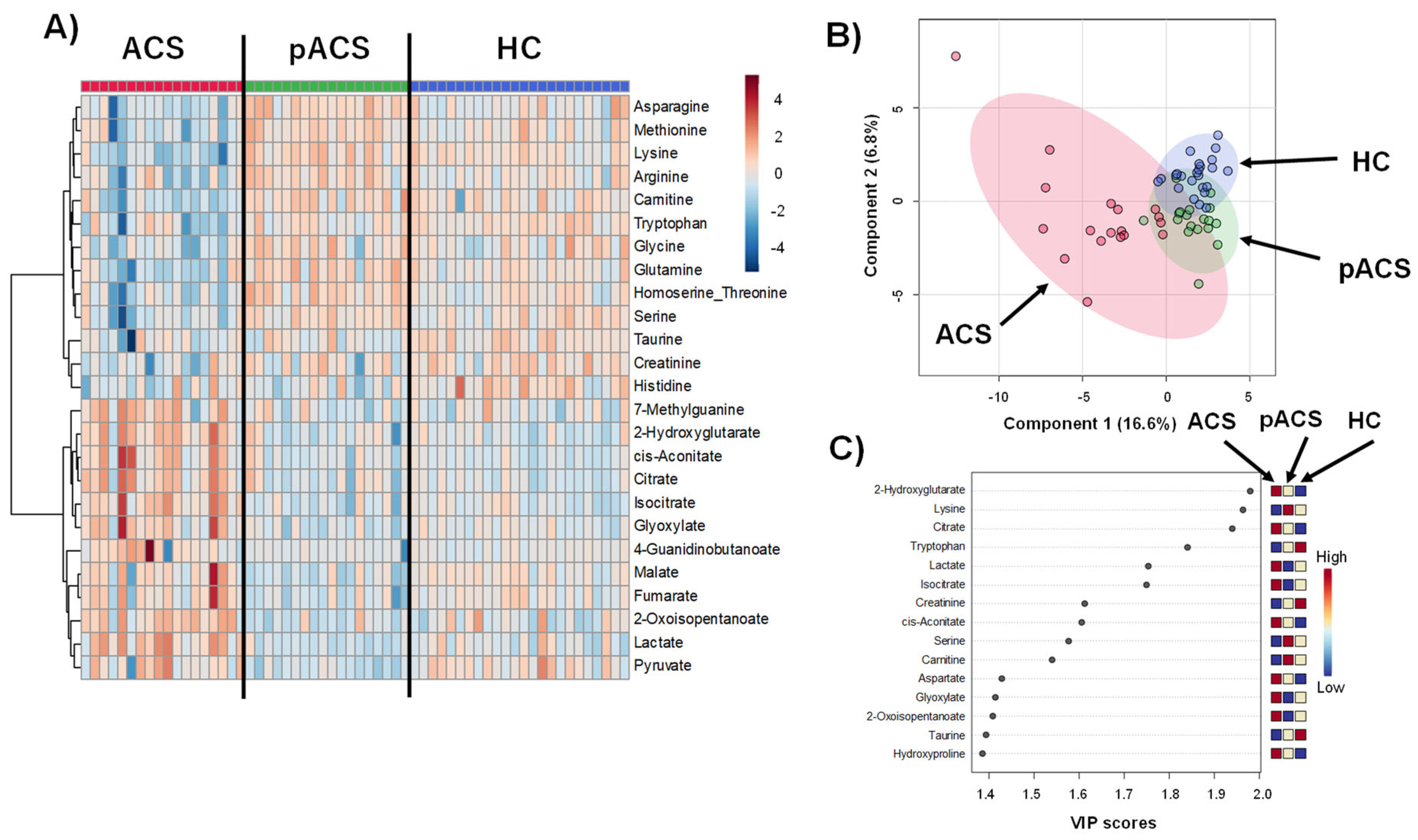
2.2. The Comparison of the Metabolomic Profiles among the ACS Patients and HC Controls
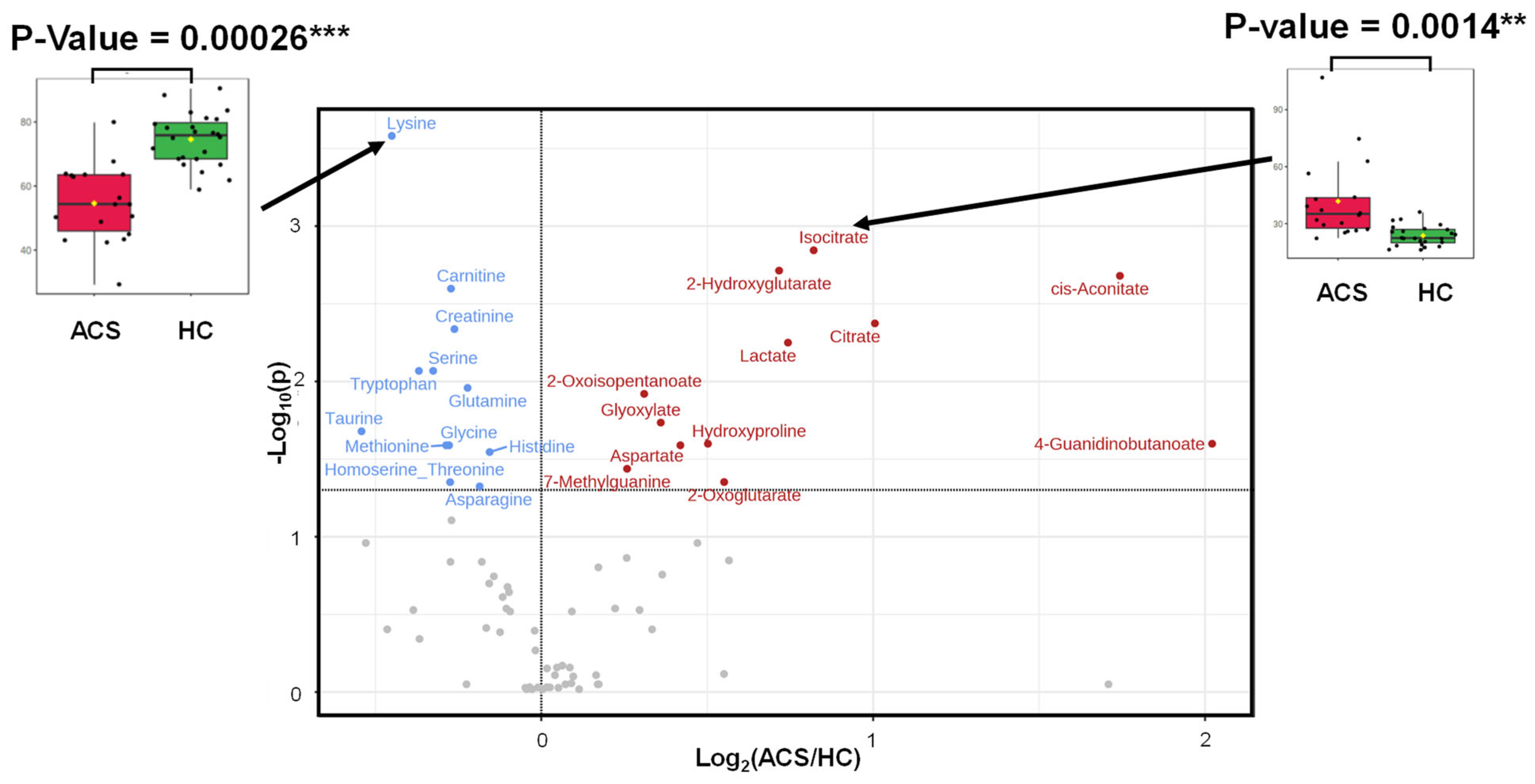
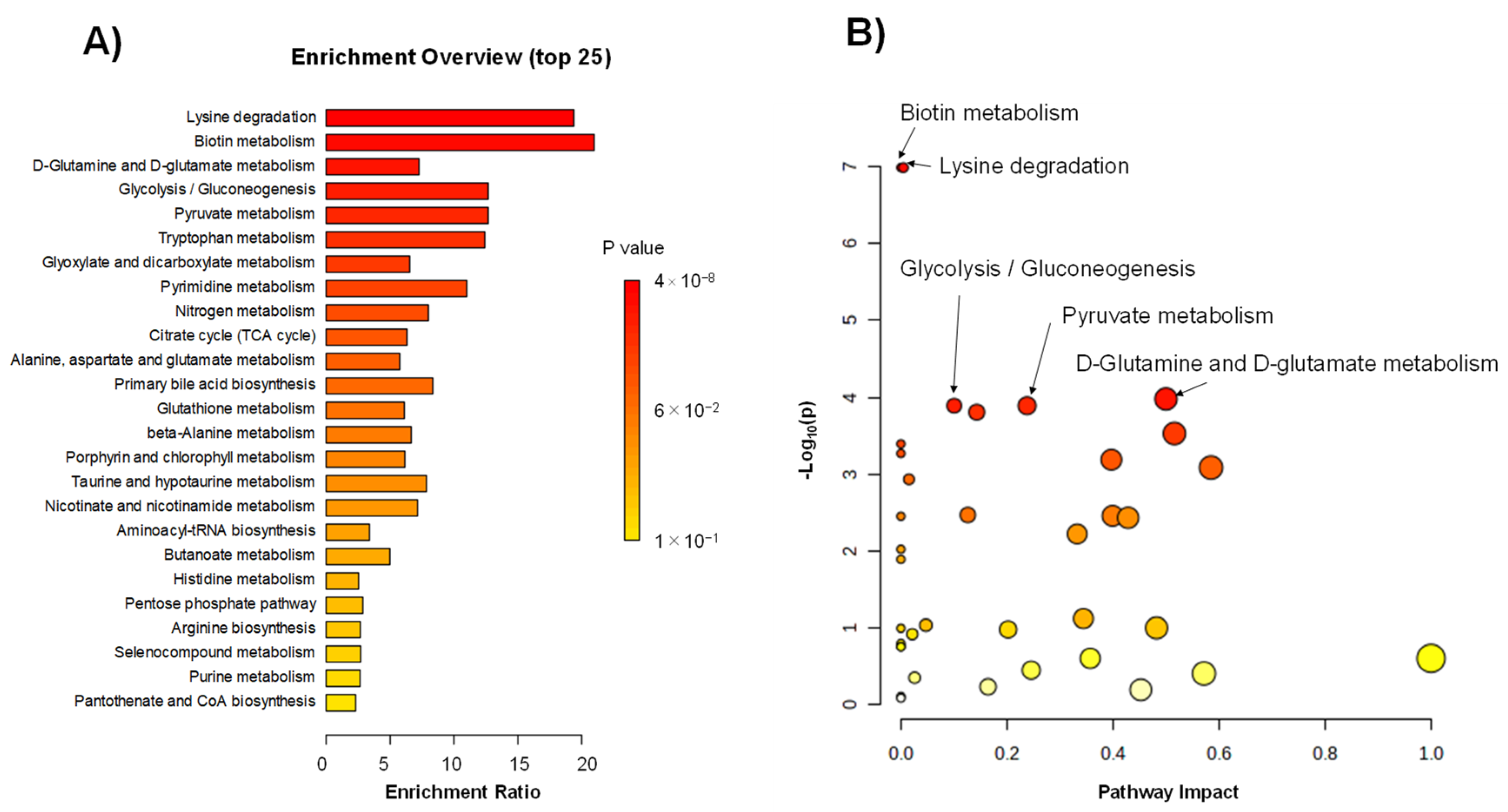
2.3. Differences in Metabolites between the ACS and HC Groups
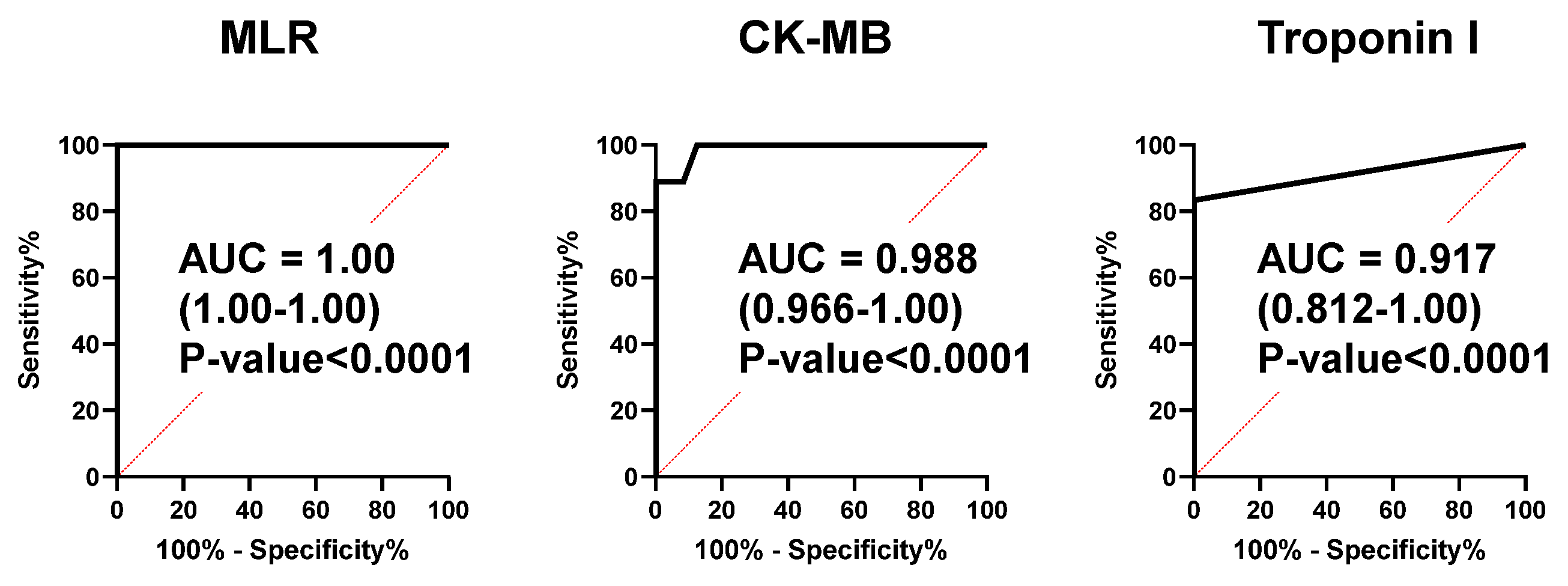
2.4. An MLR Model as a Novel Biomarker for the ACS Diagnosis
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Metabolomic Analyses
4.3. Data Analyses
4.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Surendran, A.; Atefi, N.; Zhang, H.; Aliani, M.; Ravandi, A. Defining acute coronary syndrome through metabolomics. Metabolites 2021, 11, 685. [Google Scholar] [CrossRef]
- Boersma, E.; Mercado, N.; Poldermans, D.; Gardien, M.; Vos, J.; Simoons, M.L. Acute myocardial infarction. Lancet 2003, 361, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Naghavi, M.; Mensah, G.A.; Murray, C.J. Demographic and Epidemiologic Drivers of Global cardiovascular mortality. N. Engl. J. Med. 2015, 372, 1333–1341. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Lopes, R.D.; Harrington, R.A. Diagnosis and treatment of acute coronary syndromes. JAMA 2022, 327, 662–675. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, V.S.; Jarolim, P. How to Interpret elevated cardiac troponin levels. Circulation 2011, 124, 2350–2354. [Google Scholar] [CrossRef]
- Tan, W.C.J.; Inoue, K.; Abdel Wareth, L.; Giannitsis, E.; Kasim, S.; Shiozaki, M.; Aw, T.C.; Cheng, F.; Dung, H.T.; Li, Y.-H.; et al. The Asia-Pacific Society of Cardiology (APSC) expert committee consensus recommendations for assessment of suspected acute coronary syndrome using high-sensitivity cardiac troponin T in the emergency department. Circ. J. 2020, 84, 136–143. [Google Scholar] [CrossRef]
- Feng, L.; Yang, J.; Liu, W.; Wang, Q.; Wang, H.; Shi, L.; Fu, L.; Xu, Q.; Wang, B.; Li, T. Lipid Biomarkers in Acute Myocardial Infarction before and after Percutaneous Coronary Intervention by Lipidomics Analysis. Med. Sci. Monit. 2018, 24, 4175–4182. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Tian, Q.; Gu, S.; Xie, D.; Cai, Y.; Wang, Z.; Chu, H.; Qiu, S.; Tang, S.; Zhang, A. Metabolomics for clinical biomarker discovery and therapeutic target identification. Molecules 2024, 29, 2198. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, T.; Nicholls, S.J. Perspectives on the success of plasma lipidomics in cardiovascular drug discovery and future challenges. Expert. Opin. Drug Discov. 2024, 19, 281–290. [Google Scholar] [CrossRef]
- Lin, X.; Qiao, L.; Liu, H.; Bao, M.; Deng, H.; Jia, L.; Wen, X.; Deng, F.; Wan, P.; Lyu, Y.; et al. An untargeted metabolomics study of cardiac pathology damage in rats caused by low selenium diet alone or in combination with T-2 toxin. Food Chem. Toxicol. 2024, 23, 114759. [Google Scholar] [CrossRef]
- McGarrah, R.W.; Crown, S.B.; Zhang, G.-F.; Shah, S.H.; Newgard, C.B. Cardiovascular metabolomics. Circ. Res. 2018, 122, 1238–1258. [Google Scholar] [CrossRef] [PubMed]
- Laborde, C.M.; Mourino-Alvarez, L.; Posada-Ayala, M.; Alvarez-Llamas, G.; Serranillos-Reus, M.G.; Moreu, J.; Vivanco, F.; Padial, L.R.; Barderas, M.G. Plasma metabolomics reveals a potential panel of biomarkers for early diagnosis in acute coronary syndrome. Metabolomics 2014, 10, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Würtz, P.; Havulinna, A.S.; Soininen, P.; Tynkkynen, T.; Prieto-Merino, D.; Tillin, T.; Ghorbani, A.; Artati, A.; Wang, Q.; Tiainen, M.; et al. Metabolite profiling and cardiovascular event risk. Circulation 2015, 131, 774–785. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Liu, E.; Morrow, D.A.; Heller, E.; McCarroll, R.; Wiegand, R.; Berriz, G.F.; Roth, F.P.; Gerszten, R.E. Metabolomic identification of novel biomarkers of myocardial ischemia. Circulation 2005, 112, 3868–3875. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Wei, R.; Liu, E.; Yang, E.; Shi, X.; Martinovic, M.; Farrell, L.; Asnani, A.; Cyrille, M.; Ramanathan, A.; et al. Metabolite profiling of blood from individuals undergoing planned myocardial infarction reveals early markers of myocardial injury. J. Clin. Investig. 2008, 118, 3503–3512. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, M.; García, A.; Tuñón, J.; García-Martínez, D.; Angulo, S.; Martin-Ventura, J.; Blanco-Colio, L.M.; Almeida, P.; Egido, J.; Barbas, C. Plasma fingerprinting with GC-MS in acute coronary syndrome. Anal. Bioanal. Chem. 2009, 394, 1517–1524. [Google Scholar] [CrossRef]
- Li, X.; Yan, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.-X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef] [PubMed]
- Ussher, J.R.; Elmariah, S.; Gerszten, R.E.; Dyck, J.R.B. The emerging role of metabolomics in the diagnosis and prognosis of cardiovascular disease. J. Am. Coll. Cardiol. 2016, 68, 2850–2870. [Google Scholar] [CrossRef]
- Vichova, T.; Motovska, Z. Oxidative stress: Predictive marker for coronary artery disease. Exp. Clin. Cardiol. 2013, 18, e88–e91. [Google Scholar] [PubMed]
- Kumar, S.H.S.; Anandan, R. Biochemical studies on the cardioprotective effect of glutamine on tissue antioxidant defense system in isoprenaline-induced myocardial infarction in rats. J. Clin. Biochem. Nutr. 2007, 40, 49–55. [Google Scholar] [CrossRef]
- Eggrs, K.M.; Hammarsten, O.; Lindahl, B. Differences between high-sensitivity cardiac troponin T and I in stable populations: Underlying causes and clinical implications. Clin. Chem. Lab. Med. 2023, 61, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.E.; Farag, M.A.; Holvoet, P.; Hanafi, R.S.; Gad, M.Z. A comparative metabolomics approach reveals early biomarkers for metabolic response to acute myocardial infarction. Sci. Rep. 2016, 6, 36359. [Google Scholar] [CrossRef] [PubMed]
- Pouralijan Amiri, M.; Khoshkam, M.; Salek, R.M.; Madadi, R.; Faghanzadeh Ganji, G.; Ramazani, A. Metabolomics in early detection and prognosis of acute coronary syndrome. Clin. Chim. Acta 2019, 495, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Melhem, N.J.; Taleb, S. Tryptophan: From diet to cardiovascular diseases. Int. J. Mol. Sci. 2021, 22, 9904. [Google Scholar] [CrossRef]
- Deidda, M.; Piras, C.; Binaghi, G.; Congia, D.; Pani, A.; Boi, A.; Sanna, F.; Rossi, A.; Loi, B.; Dessalvi, C.C.; et al. Metabolomic fingerprint of coronary blood in STEMI patients depends on the ischemic time and inflammatory state. Sci. Rep. 2019, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Kakoki, M.; Kim, H.S.; Arendshorst, W.J.; Mattson, D.L.; Mattson, D.L. L-Arginine uptake affects nitric oxide production and blood flow in the renal medulla. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1478–R1485. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Kaneko, F.T.; Zheng, S.; Comhair, S.A.A.; Janocha, A.J.; Goggans, T.; Thunnissen, F.B.J.M.; Farver, C.; Hazen, S.L.; Jennings, C.; et al. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J. 2004, 18, 1746–1748. [Google Scholar] [CrossRef]
- Cui, Y.; Hao, K.; Takahashi, J.; Miyata, S.; Shindo, T.; Nishimiya, K.; Kikuchi, Y.; Tsuburaya, R.; Matsumoto, Y.; Ito, K.; et al. Age-specific trends in the incidence and in-hospital mortality of acute myocardial infarction over 30 years in Japan—Report from the Miyagi AMI Registry study. Circ. J. 2017, 81, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef]
- Shibata, M.; Namiki, A.; Yoshitake, K.; Sakai, K.; Yamaguchi, K.; Onuma, Y.; Watanabe, N.; Kondo, T.; Kimura, M.; Miyashita, M.; et al. Overwork and acute coronary syndrome patients-related changes in plasma metabolomics. JJOMT 2019, 67, 406–415. Available online: www.jsomt.jp/journal/pdf/067050406.pdf (accessed on 8 June 2024).
- Matsumoto, T.; Fukuzawa, M.; Itoi, T.; Sugimoto, M.; Aizawa, Y.; Sunamura, M.; Kawai, T.; Nemoto, D.; Shinohara, H.; Muramatsu, T.; et al. Targeted metabolomic profiling of plasma samples in gastric cancer by liquid chromatography-mass spectrometry. Digestion 2023, 104, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Fuse, S.; Sugimoto, M.; Kurosawa, Y.; Kuroiwa, M.; Aita, Y.; Tomita, A.; Yamaguchi, E.; Tanaka, R.; Endo, T.; Kime, R.; et al. Relationships between plasma lipidomic profiles and brown adipose tissue density in humans. Int. J. Obes. 2020, 44, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]

| Item | ACS (n = 18) | HC (n = 24) | p-Value |
|---|---|---|---|
| Age (years) | 49.6 ± 6.6 | 49.6 ± 7.9 | NS |
| Body mass index (kg/m2) | 23.2 ± 6.3 | 24.0 ± 2.8 | NS |
| Blood pressure | |||
| Systolic(mmHg) | 147.4 ± 32.8 | 117.0 ± 15.1 | 0.001 |
| Diastolic(mmHg) | 102.7 ± 21.3 | 70 ± 11.5 | <0.001 |
| Heart rate (beats/min) | 70.7 ± 21.9 | 58 ± 9.3 | 0.03 |
| Ejectionfraction (%) | 53 ± 8.9 | ||
| Comorbidity | |||
| Hypertension (%) | 5.6 | 4.2 | NS |
| Diabetes mellitus (%) | 0 | 0 | NS |
| Dyslipidemia (%) | 5.6 | 4.2 | NS |
| Hyperuricemia (%) | 5.6 | 4.2 | NS |
| Current smoker (%) | 83.3 | 20.8 | <0.001 |
| Medication | |||
| ACE/ARB (%) | 0 | 4.2 | NS |
| Calcium-channel blocker (%) | 5.6 | 4.2 | NS |
| Beta-blocker (%) | 5.6 | 0 | NS |
| Statins (%) | 5.6 | 4.2 | NS |
| Xanthine oxidase inhibitor (%) | 5.6 | 4.2 | NS |
| Laboratory data | |||
| WBC (cells/µL) | 9922.2 ± 3499.0 | 5525.0 ± 1347.9 | <0.001 |
| Total cholesterol (mmol/L) | 4.9 ± 0.8 | 5.5 ± 0.8 | 0.032 |
| LDL-C (mmol/L) | 3.1 ± 0.7 | 3.5 ± 0.7 | NS |
| HDL-C (mmol/L) | 1.2 ± 0.3 | 1.6 ± 0.3 | <0.001 |
| Triglycerides (mmol/L) | 5.2 ± 5.3 | 3.1 ± 1.4 | NS |
| AST (IU/L) | 36.3 ± 27.7 | 24.1 ± 6.7 | NS |
| ALT (IU/L) | 33.7 ± 36.3 | 33.5 ± 20.8 | NS |
| LDH (IU/L) | 208.2 ± 91.3 | 175.0 ± 20.1 | NS |
| γGT (IU/L) | 35.9 ± 20.4 | 44.4 ± 28.2 | NS |
| Sodium (mmol/L) | 140.3 ± 1.8 | 141.6 ± 1.8 | 0.022 |
| Potassium (mmol/L) | 3.8 ± 0.4 | 4.2 ± 0.3 | <0.001 |
| Urea nitrogen (mmol/L) | 5.3 ± 1.5 | 5.1 ± 1.8 | NS |
| Serum creatinine (µmol/L) | 79.6 ± 17.7 | 79.6 ± 8.8 | NS |
| Uric acid (µmol/L) | 362.8 ± 113 | 345 ± 71.4 | NS |
| Creatine kinase (IU/L) | 204.3 ± 155.7 | 114.6 ± 43.3 | 0.028 |
| Creatine kinase-MB (IU/L) | 19.2 ± 18.1 | ||
| hs-cTn (pg/mL) | 875 ± 1602 | ||
| hs-CRP (mg/dL) | 0.41 ± 1.21 | 0.08 ± 0.10 | NS |
| Blood sugar (mmol/L) | 153.5 ± 69.1 | 100.3 ± 7.8 | 0.006 |
| HbA1c (%) | 6.4 ± 2.2 | 5.5 ± 0.3 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibata, M.; Sugimoto, M.; Watanabe, N.; Namiki, A. Exploring Novel Biomarkers for an Acute Coronary Syndrome Diagnosis Utilizing Plasma Metabolomics. Int. J. Mol. Sci. 2024, 25, 6674. https://doi.org/10.3390/ijms25126674
Shibata M, Sugimoto M, Watanabe N, Namiki A. Exploring Novel Biomarkers for an Acute Coronary Syndrome Diagnosis Utilizing Plasma Metabolomics. International Journal of Molecular Sciences. 2024; 25(12):6674. https://doi.org/10.3390/ijms25126674
Chicago/Turabian StyleShibata, Masayuki, Masahiro Sugimoto, Norikazu Watanabe, and Atsuo Namiki. 2024. "Exploring Novel Biomarkers for an Acute Coronary Syndrome Diagnosis Utilizing Plasma Metabolomics" International Journal of Molecular Sciences 25, no. 12: 6674. https://doi.org/10.3390/ijms25126674
APA StyleShibata, M., Sugimoto, M., Watanabe, N., & Namiki, A. (2024). Exploring Novel Biomarkers for an Acute Coronary Syndrome Diagnosis Utilizing Plasma Metabolomics. International Journal of Molecular Sciences, 25(12), 6674. https://doi.org/10.3390/ijms25126674







