From Gut Microbiota to Brain Waves: The Potential of the Microbiome and EEG as Biomarkers for Cognitive Impairment
Abstract
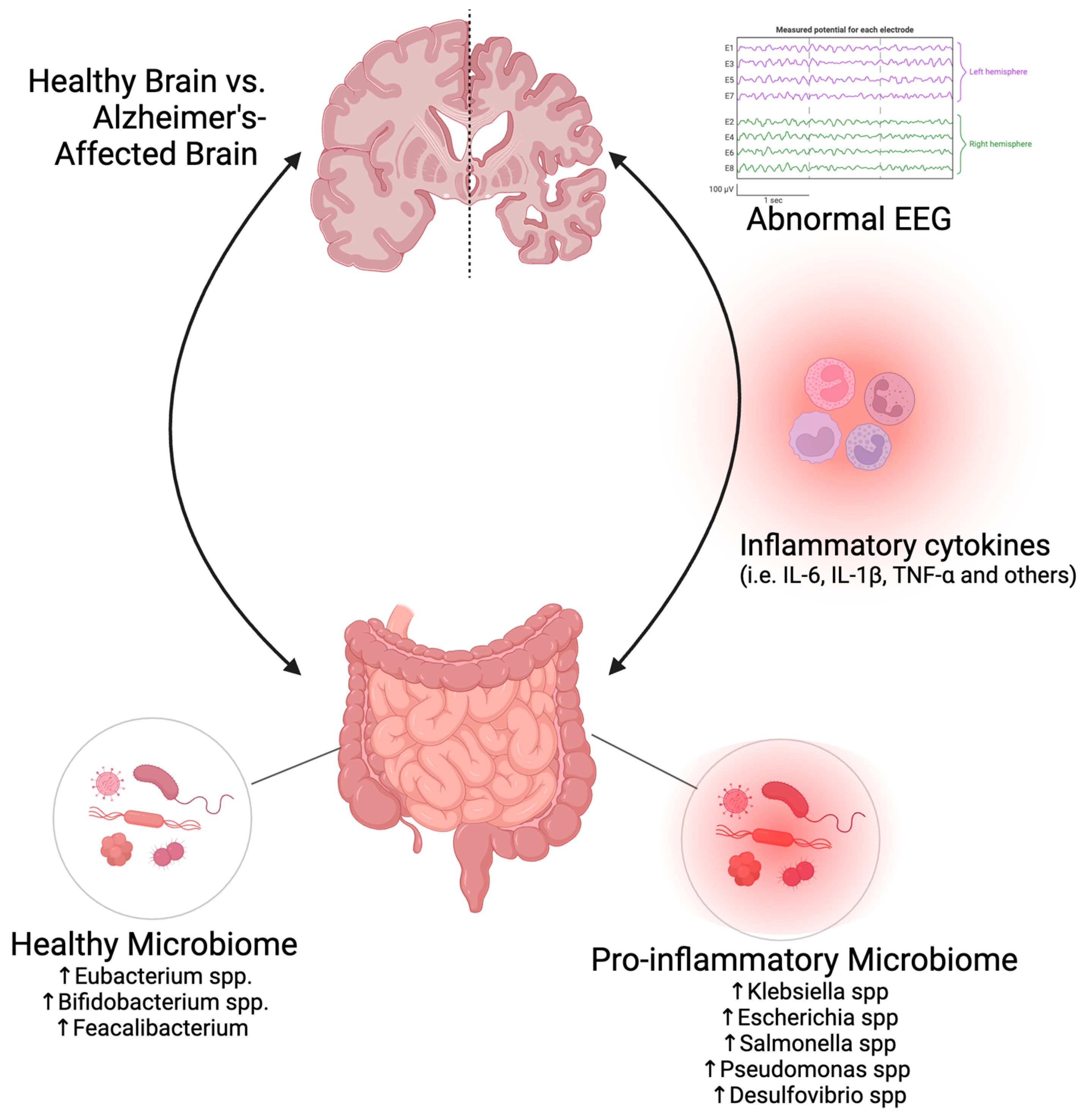
:1. Introduction
2. Cognitive Function in Aging and AD
3. The Role of the Microbiome in Aging and AD
4. EEG as a Biomarker in AD
5. Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, A.; Sidhu, J.; Goyal, A.; Tsao, J.W. Alzheimer Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Harada, C.N.; Natelson Love, M.C.; Triebel, K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef]
- Kamal, F.; Morrison, C.; Maranzano, J.; Zeighami, Y.; Dadar, M. Topographical differences in white matter hyperintensity burden and cognition in aging, MCI, and AD. Geroscience 2023, 45, 1–16. [Google Scholar] [CrossRef]
- Marshall, G.A.; Amariglio, R.E.; Sperling, R.A.; Rentz, D.M. Activities of daily living: Where do they fit in the diagnosis of Alzheimer’s disease? Neurodegener. Dis. Manag. 2012, 2, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Cassani, R.; Estarellas, M.; San-Martin, R.; Fraga, F.J.; Falk, T.H. Systematic Review on Resting-State EEG for Alzheimer’s Disease Diagnosis and Progression Assessment. Dis. Markers 2018, 2018, 5174815. [Google Scholar] [CrossRef]
- Morozova, A.; Zorkina, Y.; Abramova, O.; Pavlova, O.; Pavlov, K.; Soloveva, K.; Volkova, M.; Alekseeva, P.; Andryshchenko, A.; Kostyuk, G.; et al. Neurobiological Highlights of Cognitive Impairment in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 1217. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.-Q.; Shen, L.-L.; Li, W.-W.; Fu, X.; Zeng, F.; Gui, L.; Lü, Y.; Cai, M.; Zhu, C.; Tan, Y.-L.; et al. Gut Microbiota is Altered in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 63, 1337–1346. [Google Scholar] [CrossRef]
- Ling, Z.; Zhu, M.; Liu, X.; Shao, L.; Cheng, Y.; Yan, X.; Jiang, R.; Wu, S. Fecal Fungal Dysbiosis in Chinese Patients with Alzheimer’s Disease. Front. Cell Dev. Biol. 2020, 8, 631460. [Google Scholar] [CrossRef]
- Hopfner, F.; Künstner, A.; Müller, S.H.; Künzel, S.; Zeuner, K.E.; Margraf, N.G.; Deuschl, G.; Baines, J.F.; Kuhlenbäumer, G. Gut microbiota in Parkinson disease in a northern German cohort. Brain Res. 2017, 1667, 41–45. [Google Scholar] [CrossRef]
- Hasegawa, S.; Goto, S.; Tsuji, H.; Okuno, T.; Asahara, T.; Nomoto, K.; Shibata, A.; Fujisawa, Y.; Minato, T.; Okamoto, A.; et al. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS ONE 2015, 10, e0142164. [Google Scholar] [CrossRef]
- Wasser, C.I.; Mercieca, E.C.; Kong, G.; Hannan, A.J.; McKeown, S.J.; Glikmann-Johnston, Y.; Stout, J.C. Gut dysbiosis in Huntington’s disease: Associations among gut microbiota, cognitive performance and clinical outcomes. Brain Commun. 2020, 2, fcaa110. [Google Scholar] [CrossRef]
- Du, G.; Dong, W.; Yang, Q.; Yu, X.; Ma, J.; Gu, W.; Huang, Y. Altered Gut Microbiota Related to Inflammatory Responses in Patients With Huntington’s Disease. Front. Immunol. 2020, 11, 603594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhuang, Z.; Zhang, G.; Huang, T.; Fan, D. Assessment of bidirectional relationships between 98 genera of the human gut microbiota and amyotrophic lateral sclerosis: A 2-sample Mendelian randomization study. BMC Neurol. 2022, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Hertzberg, V.S.; Singh, H.; Fournier, C.N.; Moustafa, A.; Polak, M.; Kuelbs, C.A.; Torralba, M.G.; Tansey, M.G.; Nelson, K.E.; Glass, J.D. Gut microbiome differences between amyotrophic lateral sclerosis patients and spouse controls. Amyotroph. Lateral Scler. Front. Degener. 2022, 23, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Cosorich, I.; Dalla-Costa, G.; Sorini, C.; Ferrarese, R.; Messina, M.J.; Dolpady, J.; Radice, E.; Mariani, A.; Testoni, P.A.; Canducci, F.; et al. High frequency of intestinal T(H)17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci. Adv. 2017, 3, e1700492. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Battistini, C.; Sun, J. A Gut Feeling in Amyotrophic Lateral Sclerosis: Microbiome of Mice and Men. Front. Cell. Infect. Microbiol. 2022, 12, 839526. [Google Scholar] [CrossRef]
- Chen, C.; Liao, J.; Xia, Y.; Liu, X.; Jones, R.; Haran, J.; McCormick, B.; Sampson, T.R.; Alam, A.; Ye, K. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 2022, 71, 2233–2252. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Baumann, R.; Gao, X.; Mendoza, M.; Singh, S.; Sand, I.K.; Xia, Z.; Cox, L.M.; Chitnis, T.; Yoon, H.; et al. Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course. Cell 2022, 185, 3467–3486.e3416. [Google Scholar] [CrossRef]
- Seo, D.O.; Holtzman, D.M. Current understanding of the Alzheimer’s disease-associated microbiome and therapeutic strategies. Exp. Mol. Med. 2024, 56, 86–94. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Jin, F. Gut-Brain Psychology: Rethinking Psychology from the Microbiota-Gut-Brain Axis. Front. Integr. Neurosci. 2018, 12, 33. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Chandra, S.; Sisodia, S.S.; Vassar, R.J. The gut microbiome in Alzheimer’s disease: What we know and what remains to be explored. Mol. Neurodegener. 2023, 18, 9. [Google Scholar] [CrossRef]
- Coradduzza, D.; Sedda, S.; Cruciani, S.; De Miglio, M.R.; Ventura, C.; Nivoli, A.; Maioli, M. Age-Related Cognitive Decline, Focus on Microbiome: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 13680. [Google Scholar] [CrossRef]
- Boehme, M.; Guzzetta, K.E.; Wasén, C.; Cox, L.M. The gut microbiota is an emerging target for improving brain health during ageing. Gut Microbiome 2023, 4, e2. [Google Scholar] [CrossRef]
- Chen, L.; Xu, X.; Wu, X.; Cao, H.; Li, X.; Hou, Z.; Wang, B.; Liu, J.; Ji, X.; Zhang, P.; et al. A comparison of the composition and functions of the oral and gut microbiotas in Alzheimer’s patients. Front. Cell Infect. Microbiol. 2022, 12, 942460. [Google Scholar] [CrossRef]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Ma, J.; Piao, X.; Mahfuz, S.; Long, S.; Wang, J. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim. Nutr. 2022, 9, 159–174. [Google Scholar] [CrossRef]
- Vemuri, R.; Gundamaraju, R.; Shastri, M.D.; Shukla, S.D.; Kalpurath, K.; Ball, M.; Tristram, S.; Shankar, E.M.; Ahuja, K.; Eri, R. Gut Microbial Changes, Interactions, and Their Implications on Human Lifecycle: An Ageing Perspective. Biomed. Res. Int. 2018, 2018, 4178607. [Google Scholar] [CrossRef] [PubMed]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The Role of Gut Microbiota in Intestinal Inflammation with Respect to Diet and Extrinsic Stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Al-Nusaif, M.; Ding, C.; Zhao, L.; Dong, C. The potential of the gut microbiome for identifying Alzheimer’s disease diagnostic biomarkers and future therapies. Front. Neurosci. 2023, 17, 1130730. [Google Scholar] [CrossRef]
- Meghdadi, A.H.; Stevanovic Karic, M.; McConnell, M.; Rupp, G.; Richard, C.; Hamilton, J.; Salat, D.; Berka, C. Resting state EEG biomarkers of cognitive decline associated with Alzheimer’s disease and mild cognitive impairment. PLoS ONE 2021, 16, e0244180. [Google Scholar] [CrossRef]
- Newson, J.J.; Thiagarajan, T.C. EEG Frequency Bands in Psychiatric Disorders: A Review of Resting State Studies. Front. Hum. Neurosci. 2018, 12, 521. [Google Scholar] [CrossRef]
- Mason, L.M.; Barry, R.J.; Clarke, A.R. Age-related changes in the EEG in an eyes-open condition: I. Normal development. Int. J. Psychophysiol. 2022, 172, 40–45. [Google Scholar] [CrossRef]
- Knyazeva, M.G.; Barzegaran, E.; Vildavski, V.Y.; Demonet, J.F. Aging of human alpha rhythm. Neurobiol. Aging 2018, 69, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Cesnaite, E.; Steinfath, P.; Jamshidi Idaji, M.; Stephani, T.; Kumral, D.; Haufe, S.; Sander, C.; Hensch, T.; Hegerl, U.; Riedel-Heller, S.; et al. Alterations in rhythmic and non-rhythmic resting-state EEG activity and their link to cognition in older age. Neuroimage 2023, 268, 119810. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Liu, F.; Nummenmaa, A.; Hämäläinen, M.; Dickerson, B.C.; Purdon, P.L. Age-Related EEG Power Reductions Cannot Be Explained by Changes of the Conductivity Distribution in the Head Due to Brain Atrophy. Front. Aging Neurosci. 2021, 13, 632310. [Google Scholar] [CrossRef]
- Pappalettera, C.; Cacciotti, A.; Nucci, L.; Miraglia, F.; Rossini, P.M.; Vecchio, F. Approximate entropy analysis across electroencephalographic rhythmic frequency bands during physiological aging of human brain. Geroscience 2023, 45, 1131–1145. [Google Scholar] [CrossRef]
- Baker, M.; Akrofi, K.; Schiffer, R.; Boyle, M.W. EEG Patterns in Mild Cognitive Impairment (MCI) Patients. Open Neuroimag. J. 2008, 2, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Del Percio, C.; Lopez, S.; Noce, G.; Lizio, R.; Tucci, F.; Soricelli, A.; Ferri, R.; Nobili, F.; Arnaldi, D.; Famà, F.; et al. What a Single Electroencephalographic (EEG) Channel Can Tell us About Alzheimer’s Disease Patients With Mild Cognitive Impairment. Clin. EEG Neurosci. 2023, 54, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Takahashi, R.; Suzuki, Y.; Pascual-Marqui, R.D.; Kito, Y.; Hikida, S.; Maruyama, K.; Hata, M.; Ishii, R.; Iwase, M.; et al. EEG resting-state networks in Alzheimer’s disease associated with clinical symptoms. Sci. Rep. 2023, 13, 3964. [Google Scholar] [CrossRef]
- Andrade, S.M.; da Silva-Sauer, L.; de Carvalho, C.D.; de Araújo, E.L.M.; Lima, E.O.; Fernandes, F.M.L.; Moreira, K.; Camilo, M.E.; Andrade, L.; Borges, D.T.; et al. Identifying biomarkers for tDCS treatment response in Alzheimer’s disease patients: A machine learning approach using resting-state EEG classification. Front. Hum. Neurosci. 2023, 17, 1234168. [Google Scholar] [CrossRef]
- Mitsukura, Y.; Sumali, B.; Watanabe, H.; Ikaga, T.; Nishimura, T. Frontotemporal EEG as potential biomarker for early MCI: A case-control study. BMC Psychiatry 2022, 22, 289. [Google Scholar] [CrossRef]
- Wijaya, A.; Setiawan, N.A.; Ahmad, A.H.; Zakaria, R.; Othman, Z. Electroencephalography and mild cognitive impairment research: A scoping review and bibliometric analysis (ScoRBA). AIMS Neurosci. 2023, 10, 154–171. [Google Scholar] [CrossRef]
- D’Atri, A.; Scarpelli, S.; Gorgoni, M.; Truglia, I.; Lauri, G.; Cordone, S.; Ferrara, M.; Marra, C.; Rossini, P.M.; De Gennaro, L. EEG alterations during wake and sleep in mild cognitive impairment and Alzheimer’s disease. iScience 2021, 24, 102386. [Google Scholar] [CrossRef]
- Flores-Sandoval, A.A.; Davila-Pérez, P.; Buss, S.S.; Donohoe, K.; O’Connor, M.; Shafi, M.M.; Pascual-Leone, A.; Benwell, C.S.Y.; Fried, P.J. Spectral power ratio as a measure of EEG changes in mild cognitive impairment due to Alzheimer’s disease: A case-control study. Neurobiol. Aging 2023, 130, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Azami, H.; Zrenner, C.; Brooks, H.; Zomorrodi, R.; Blumberger, D.M.; Fischer, C.E.; Flint, A.; Herrmann, N.; Kumar, S.; Lanctôt, K.; et al. Beta to theta power ratio in EEG periodic components as a potential biomarker in mild cognitive impairment and Alzheimer’s dementia. Alzheimer’s Res. Ther. 2023, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, B.; Liu, H.; Wu, W.; Sun, J.; Fang, W.; Jiang, R.; Hu, Y.; Jin, C.; Wei, X.; et al. Diagnosis of Alzheimer’s disease via resting-state EEG: Integration of spectrum, complexity, and synchronization signal features. Front. Aging Neurosci. 2023, 15, 1288295. [Google Scholar] [CrossRef] [PubMed]
- Houmani, N.; Vialatte, F.; Gallego-Jutglà, E.; Dreyfus, G.; Nguyen-Michel, V.H.; Mariani, J.; Kinugawa, K. Diagnosis of Alzheimer’s disease with Electroencephalography in a differential framework. PLoS ONE 2018, 13, e0193607. [Google Scholar] [CrossRef]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4586–4591. [Google Scholar] [CrossRef]
- Engedal, K.; Barca, M.L.; Hogh, P.; Bo Andersen, B.; Winther Dombernowsky, N.; Naik, M.; Gudmundsson, T.E.; Oksengaard, A.R.; Wahlund, L.O.; Snaedal, J. The Power of EEG to Predict Conversion from Mild Cognitive Impairment and Subjective Cognitive Decline to Dementia. Dement. Geriatr. Cogn. Disord. 2020, 49, 38–47. [Google Scholar] [CrossRef]
- Temsumrit, N. Can aging population affect economic growth through the channel of government spending? Heliyon 2023, 9, e19521. [Google Scholar] [CrossRef]
- Dunning, L.; Ty, D.; Shah, P.; McDermott, M. Awareness and Perceptions of “Age-Friendly”: Analyzing Survey Results from Voices in the United States. Geriatrics 2023, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [CrossRef] [PubMed]
- Koca, E.; Taskapilioglu, O.; Bakar, M. Caregiver Burden in Different Stages of Alzheimer’s Disease. Noro Psikiyatr. Ars. 2017, 54, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Hurd, M.D.; Martorell, P.; Delavande, A.; Mullen, K.J.; Langa, K.M. Monetary costs of dementia in the United States. N. Engl. J. Med. 2013, 368, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Yang, K.; He, B.; Du, W.; Cai, Y.; Han, Y. Combination of gut microbiota and plasma amyloid-beta as a potential index for identifying preclinical Alzheimer’s disease: A cross-sectional analysis from the SILCODE study. Alzheimer’s Res. Ther. 2022, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, S.; Houdayer, E.; Spina, A.; Nocera, G.; Alemanno, F. Quantitative EEG for early differential diagnosis of dementia with Lewy bodies. Front. Psychol. 2023, 14, 1150540. [Google Scholar] [CrossRef] [PubMed]
- Murman, D.L. The Impact of Age on Cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, W.R.; Newton, B.W. Neuroanatomy, Prefrontal Cortex. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2018. [Google Scholar]
- Chayer, C.; Freedman, M. Frontal lobe functions. Curr. Neurol. Neurosci. Rep. 2001, 1, 547–552. [Google Scholar] [CrossRef]
- Terry, R.D.; Katzman, R. Life span and synapses: Will there be a primary senile dementia? Neurobiol. Aging 2001, 22, 347–348, discussion 353–344. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-beta and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T.; et al. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimer’s Dement. 2023, 19, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Amariglio, R.E.; Buckley, R.F.; van der Flier, W.M.; Han, Y.; Molinuevo, J.L.; Rabin, L.; Rentz, D.M.; Rodriguez-Gomez, O.; Saykin, A.J.; et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020, 19, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Shigemizu, D.; Akiyama, S.; Higaki, S.; Sugimoto, T.; Sakurai, T.; Boroevich, K.A.; Sharma, A.; Tsunoda, T.; Ochiya, T.; Niida, S.; et al. Prognosis prediction model for conversion from mild cognitive impairment to Alzheimer’s disease created by integrative analysis of multi-omics data. Alzheimer’s Res. Ther. 2020, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qian, X.; Zhang, Y.; Su, W.; Huang, Y.; Wang, X.; Chen, X.; Zhao, E.; Han, L.; Ma, Y. Prediction Models for Conversion From Mild Cognitive Impairment to Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2022, 14, 840386. [Google Scholar] [CrossRef] [PubMed]
- Koenig, L.N.; Day, G.S.; Salter, A.; Keefe, S.; Marple, L.M.; Long, J.; LaMontagne, P.; Massoumzadeh, P.; Snider, B.J.; Kanthamneni, M.; et al. Select Atrophied Regions in Alzheimer disease (SARA): An improved volumetric model for identifying Alzheimer disease dementia. NeuroImage Clin. 2020, 26, 102248. [Google Scholar] [CrossRef] [PubMed]
- Kaštelan, S.; Braš, M.; Pjevač, N.; Bakija, I.; Tomić, Z.; Pjevač Keleminić, N.; Gverović Antunica, A. Tear Biomarkers and Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 13429. [Google Scholar] [CrossRef] [PubMed]
- Padda, I.S.; Parmar, M. Aducanumab. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Padala, S.P.; Newhouse, P.A. Blood-based biomarkers in Alzheimer’s disease: A mini-review. Metab. Brain Dis. 2023, 38, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Askarova, S.; Umbayev, B.; Masoud, A.R.; Kaiyrlykyzy, A.; Safarova, Y.; Tsoy, A.; Olzhayev, F.; Kushugulova, A. The Links Between the Gut Microbiome, Aging, Modern Lifestyle and Alzheimer’s Disease. Front. Cell Infect. Microbiol. 2020, 10, 104. [Google Scholar] [CrossRef]
- Bowland, G.B.; Weyrich, L.S. The Oral-Microbiome-Brain Axis and Neuropsychiatric Disorders: An Anthropological Perspective. Front. Psychiatry 2022, 13, 810008. [Google Scholar] [CrossRef]
- Wan, J.; Fan, H. Oral Microbiome and Alzheimer’s Disease. Microorganisms 2023, 11, 2550. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Chen, T.; Paster, B.J. A practical guide to the oral microbiome and its relation to health and disease. Oral Dis. 2017, 23, 276–286. [Google Scholar] [CrossRef]
- Tuganbaev, T.; Yoshida, K.; Honda, K. The effects of oral microbiota on health. Science 2022, 376, 934–936. [Google Scholar] [CrossRef]
- Maitre, Y.; Mahalli, R.; Micheneau, P.; Delpierre, A.; Amador, G.; Denis, F. Evidence and Therapeutic Perspectives in the Relationship between the Oral Microbiome and Alzheimer’s Disease: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 11157. [Google Scholar] [CrossRef]
- Da, D.; Zhao, Q.; Zhang, H.; Wu, W.; Zeng, X.; Liang, X.; Jiang, Y.; Xiao, Z.; Yu, J.; Ding, S.; et al. Oral microbiome in older adults with mild cognitive impairment. J. Oral. Microbiol. 2023, 15, 2173544. [Google Scholar] [CrossRef]
- Liu, X.X.; Jiao, B.; Liao, X.X.; Guo, L.N.; Yuan, Z.H.; Wang, X.; Xiao, X.W.; Zhang, X.Y.; Tang, B.S.; Shen, L. Analysis of Salivary Microbiome in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 72, 633–640. [Google Scholar] [CrossRef]
- Raja, M.; Ummer, F.; Dhivakar, C.P. Aggregatibacter actinomycetemcomitans—A tooth killer? J. Clin. Diagn. Res. 2014, 8, Ze13–Ze16. [Google Scholar] [CrossRef] [PubMed]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Alsegiani, A.S.; Shah, Z.A. The influence of gut microbiota alteration on age-related neuroinflammation and cognitive decline. Neural Regen. Res. 2022, 17, 2407–2412. [Google Scholar] [CrossRef]
- Brooks, C.N.; Wight, M.E.; Azeez, O.E.; Bleich, R.M.; Zwetsloot, K.A. Growing old together: What we know about the influence of diet and exercise on the aging host’s gut microbiome. Front. Sports Act. Living 2023, 5, 1168731. [Google Scholar] [CrossRef]
- Cooke, M.B.; Catchlove, S.; Tooley, K.L. Examining the Influence of the Human Gut Microbiota on Cognition and Stress: A Systematic Review of the Literature. Nutrients 2022, 14, 4623. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Bashir, Y.; Khan, A.U. The interplay between the gut-brain axis and the microbiome: A perspective on psychiatric and neurodegenerative disorders. Front. Neurosci. 2022, 16, 1030694. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Dwivedi, R.; Bansal, M.; Tripathi, M.; Dada, R. Role of Gut Microbiota in Neurological Disorders and Its Therapeutic Significance. J. Clin. Med. 2023, 12, 1650. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Wang, Z.; Xie, G.; Liu, M.; Yuan, B.; Chai, H.; Wang, W.; Cheng, P. Implications of Gut Microbiota in Neurodegenerative Diseases. Front. Immunol. 2022, 13, 785644. [Google Scholar] [CrossRef] [PubMed]
- Rutsch, A.; Kantsjo, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Caballero, M.; Warming, H.; Walker, R.; Holmes, C.; Cruickshank, G.; Patel, B. Vagus Nerve Stimulation as a Potential Therapy in Early Alzheimer’s Disease: A Review. Front. Hum. Neurosci. 2022, 16, 866434. [Google Scholar] [CrossRef]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases. J. Inflamm. Res. 2022, 15, 6213–6230. [Google Scholar] [CrossRef]
- Hattori, N.; Yamashiro, Y. The Gut-Brain Axis. Ann. Nutr. Metab. 2021, 77 (Suppl. S2), 1–3. [Google Scholar] [CrossRef]
- Kowalski, K.; Mulak, A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Neurogastroenterol. Motil. 2019, 25, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Razazan, A.; Nagpal, R.; Jain, S.; Wang, B.; Mishra, S.P.; Wang, S.; Justice, J.; Ding, J.; McClain, D.A.; et al. Metformin Reduces Aging-Related Leaky Gut and Improves Cognitive Function by Beneficially Modulating Gut Microbiome/Goblet Cell/Mucin Axis. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, e9–e21. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Wang, S.; Nagpal, R.; Wang, B.; Jain, S.; Razazan, A.; Mishra, S.P.; Zhu, X.; Wang, Z.; Kavanagh, K.; et al. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight 2020, 5, e132055. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut microbiome and aging: Physiological and mechanistic insights. Nutr. Healthy Aging 2018, 4, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Marotta, F.; Haghshenas, L.; Yadav, H. Treating Leaky Syndrome in the Over 65s: Progress and Challenges. Clin. Interv. Aging 2023, 18, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Obrenovich, M.E.M. Leaky Gut, Leaky Brain? Microorganisms 2018, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Zotova, E.; Nicoll, J.A.; Kalaria, R.; Holmes, C.; Boche, D. Inflammation in Alzheimer’s disease: Relevance to pathogenesis and therapy. Alzheimer’s Res. Ther. 2010, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.E.; Kahana, D.D.; Ghuman, S.; Wilson, H.P.; Wilson, J.; Kim, S.C.J.; Lagishetty, V.; Jacobs, J.P.; Sinha-Hikim, A.P.; Friedman, T.C. Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome. Front. Endocrinol. 2021, 12, 667066. [Google Scholar] [CrossRef]
- Janakiraman, M.; Krishnamoorthy, G. Emerging Role of Diet and Microbiota Interactions in Neuroinflammation. Front. Immunol. 2018, 9, 2067. [Google Scholar] [CrossRef]
- Kinare, S.G.; Dave, K.M.; Sivaraman, A. Chronic obstructive pulmonary disease in urban environment of Bombay. Indian J. Med. Res. 1988, 87, 262–269. [Google Scholar] [PubMed]
- Sheng, C.; Lin, L.; Lin, H.; Wang, X.; Han, Y.; Liu, S.L. Altered Gut Microbiota in Adults with Subjective Cognitive Decline: The SILCODE Study. J. Alzheimer’s Dis. 2021, 82, 513–526. [Google Scholar] [CrossRef]
- Miri, S.; Yeo, J.; Abubaker, S.; Hammami, R. Neuromicrobiology, an emerging neurometabolic facet of the gut microbiome? Front. Microbiol. 2023, 14, 1098412. [Google Scholar] [CrossRef]
- Wiley, N.C.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Stanton, C. Production of Psychoactive Metabolites by Gut Bacteria. Mod. Trends Psychiatry 2021, 32, 74–99. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Friedland, R.P. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J. Alzheimer’s Dis. 2015, 45, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, S.; Shin, S.J.; Park, Y.H.; Nam, Y.; Kim, C.W.; Lee, K.W.; Kim, S.M.; Jung, I.D.; Yang, H.D.; et al. Gram-negative bacteria and their lipopolysaccharides in Alzheimer’s disease: Pathologic roles and therapeutic implications. Transl. Neurodegener. 2021, 10, 49. [Google Scholar] [CrossRef]
- Perry, V.H.; Holmes, C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 2014, 10, 217–224. [Google Scholar] [CrossRef]
- Swer, N.M.; Venkidesh, B.S.; Murali, T.S.; Mumbrekar, K.D. Gut microbiota-derived metabolites and their importance in neurological disorders. Mol. Biol. Rep. 2023, 50, 1663–1675. [Google Scholar] [CrossRef]
- Choi, H.; Mook-Jung, I. Functional effects of gut microbiota-derived metabolites in Alzheimer’s disease. Curr. Opin. Neurobiol. 2023, 81, 102730. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.H.; Xie, R.Y.; Liu, X.L.; Chen, S.D.; Tang, H.D. Mechanisms of Short-Chain Fatty Acids Derived from Gut Microbiota in Alzheimer’s Disease. Aging Dis. 2022, 13, 1252–1266. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Gut Microbiota and its Metabolites: Bridge of Dietary Nutrients and Alzheimer’s Disease. Adv. Nutr. 2023, 14, 819–839. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, K.R.; Han, J.H.; Singh, A.P.; Thomas, S.P.; Bendlin, B.B.; Denu, J.M.; Yu, J.J.; Rey, F.E.; Ulland, T.K. Trimethylamine N-Oxide Reduces Neurite Density and Plaque Intensity in a Murine Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 90, 585–597. [Google Scholar] [CrossRef]
- Hasavci, D.; Blank, T. Age-dependent effects of gut microbiota metabolites on brain resident macrophages. Front. Cell Neurosci. 2022, 16, 944526. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Cross, P.J.; Dobson, R.C.J.; Adams, L.E.; Savka, M.A.; Hudson, A.O. A Three-Ring Circus: Metabolism of the Three Proteogenic Aromatic Amino Acids and Their Role in the Health of Plants and Animals. Front. Mol. Biosci. 2018, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Savonije, K.; Weaver, D.F. The Role of Tryptophan Metabolism in Alzheimer’s Disease. Brain Sci. 2023, 13, 292. [Google Scholar] [CrossRef]
- Parker, D.C.; Kraus, W.E.; Whitson, H.E.; Kraus, V.B.; Smith, P.J.; Cohen, H.J.; Pieper, C.F.; Faldowski, R.A.; Hall, K.S.; Huebner, J.L.; et al. Tryptophan Metabolism and Neurodegeneration: Longitudinal Associations of Kynurenine Pathway Metabolites with Cognitive Performance and Plasma Alzheimer’s Disease and Related Dementias Biomarkers in the Duke Physical Performance Across the LifeSpan Study. J. Alzheimer’s Dis. 2023, 91, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, W.; Hu, X.; Yu, X.; Guo, C.; Jin, X.; Wang, W. Targeting the blood-brain barrier to delay aging-accompanied neurological diseases by modulating gut microbiota, circadian rhythms, and their interplays. Acta Pharm. Sin. B 2023, 13, 4667–4687. [Google Scholar] [CrossRef]
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B.; et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10, 124. [Google Scholar] [CrossRef]
- Buawangpong, N.; Pinyopornpanish, K.; Siri-Angkul, N.; Chattipakorn, N.; Chattipakorn, S.C. The role of trimethylamine-N-Oxide in the development of Alzheimer’s disease. J. Cell Physiol. 2022, 237, 1661–1685. [Google Scholar] [CrossRef] [PubMed]
- Allaband, C.; McDonald, D.; Vazquez-Baeza, Y.; Minich, J.J.; Tripathi, A.; Brenner, D.A.; Loomba, R.; Smarr, L.; Sandborn, W.J.; Schnabl, B.; et al. Microbiome 101: Studying, Analyzing, and Interpreting Gut Microbiome Data for Clinicians. Clin. Gastroenterol. Hepatol. 2019, 17, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Martinek, R.; Ladrova, M.; Sidikova, M.; Jaros, R.; Behbehani, K.; Kahankova, R.; Kawala-Sterniuk, A. Advanced Bioelectrical Signal Processing Methods: Past, Present and Future Approach-Part II: Brain Signals. Sensors 2021, 21, 6343. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.A.; Cuevas, K. Using EEG to Study Cognitive Development: Issues and Practices. J. Cogn. Dev. 2012, 13, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Shida-Tokeshi, J.; Vanderbilt, D.L.; Smith, B.A. Electroencephalography power and coherence changes with age and motor skill development across the first half year of life. PLoS ONE 2018, 13, e0190276. [Google Scholar] [CrossRef] [PubMed]
- Traikapi, A.; Konstantinou, N. Gamma Oscillations in Alzheimer’s Disease and Their Potential Therapeutic Role. Front. Syst. Neurosci. 2021, 15, 782399. [Google Scholar] [CrossRef]
- Baik, K.; Jung, J.H.; Jeong, S.H.; Chung, S.J.; Yoo, H.S.; Lee, P.H.; Sohn, Y.H.; Kang, S.W.; Ye, B.S. Implication of EEG theta/alpha and theta/beta ratio in Alzheimer’s and Lewy body disease. Sci. Rep. 2022, 12, 18706. [Google Scholar] [CrossRef] [PubMed]
- Musaeus, C.S.; Engedal, K.; Høgh, P.; Jelic, V.; Mørup, M.; Naik, M.; Oeksengaard, A.R.; Snaedal, J.; Wahlund, L.O.; Waldemar, G.; et al. EEG Theta Power Is an Early Marker of Cognitive Decline in Dementia due to Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Monllor, P.; Cervera-Ferri, A.; Lloret, M.A.; Esteve, D.; Lopez, B.; Leon, J.L.; Lloret, A. Electroencephalography as a Non-Invasive Biomarker of Alzheimer’s Disease: A Forgotten Candidate to Substitute CSF Molecules? Int. J. Mol. Sci. 2021, 22, 10889. [Google Scholar] [CrossRef]
- Özbek, Y.; Fide, E.; Yener, G.G. Resting-state EEG alpha/theta power ratio discriminates early-onset Alzheimer’s disease from healthy controls. Clin. Neurophysiol. 2021, 132, 2019–2031. [Google Scholar] [CrossRef]
- Al-Nuaimi, A.H.; Blūma, M.; Al-Juboori, S.S.; Eke, C.S.; Jammeh, E.; Sun, L.; Ifeachor, E. Robust EEG Based Biomarkers to Detect Alzheimer’s Disease. Brain Sci. 2021, 11, 1026. [Google Scholar] [CrossRef] [PubMed]
- Jiao, B.; Li, R.; Zhou, H.; Qing, K.; Liu, H.; Pan, H.; Lei, Y.; Fu, W.; Wang, X.; Xiao, X.; et al. Neural biomarker diagnosis and prediction to mild cognitive impairment and Alzheimer’s disease using EEG technology. Alzheimer’s Res. Ther. 2023, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Rosales, I.; Brunetti, E.; Montefusco-Siegmund, R.; Madariaga, S.; Hafelin, R.; Ponce, D.P.; Behrens, M.I.; Maldonado, P.E.; Paula-Lima, A. Visual-spatial processing impairment in the occipital-frontal connectivity network at early stages of Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1097577. [Google Scholar] [CrossRef] [PubMed]
- Lejko, N.; Larabi, D.I.; Herrmann, C.S.; Aleman, A.; Ćurčić-Blake, B. Alpha Power and Functional Connectivity in Cognitive Decline: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2020, 78, 1047–1088. [Google Scholar] [CrossRef] [PubMed]
- Byron, N.; Semenova, A.; Sakata, S. Mutual Interactions between Brain States and Alzheimer’s Disease Pathology: A Focus on Gamma and Slow Oscillations. Biology 2021, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Murty, D.; Manikandan, K.; Kumar, W.S.; Ramesh, R.G.; Purokayastha, S.; Javali, M.; Rao, N.P.; Ray, S. Gamma oscillations weaken with age in healthy elderly in human EEG. Neuroimage 2020, 215, 116826. [Google Scholar] [CrossRef] [PubMed]
- Stothart, G.; Smith, L.J.; Milton, A.; Coulthard, E. A passive and objective measure of recognition memory in Alzheimer’s disease using Fastball memory assessment. Brain 2021, 144, 2812–2825. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Sebastianelli, L.; Versace, V.; Saltuari, L.; Lochner, P.; Frey, V.; Golaszewski, S.; Brigo, F.; Trinka, E.; Höller, Y. Usefulness of EEG Techniques in Distinguishing Frontotemporal Dementia from Alzheimer’s Disease and Other Dementias. Dis. Markers 2018, 2018, 6581490. [Google Scholar] [CrossRef]
- Musa, G.; Slachevsky, A.; Muñoz-Neira, C.; Méndez-Orellana, C.; Villagra, R.; González-Billault, C.; Ibáñez, A.; Hornberger, M.; Lillo, P. Alzheimer’s Disease or Behavioral Variant Frontotemporal Dementia? Review of Key Points Toward an Accurate Clinical and Neuropsychological Diagnosis. J. Alzheimer’s Dis. 2020, 73, 833–848. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, A.; Yu, J.; Wang, P.; Bi, Y.; Xue, S.; Zhang, J.; Guo, H.; Zhang, W. The effect of aperiodic components in distinguishing Alzheimer’s disease from frontotemporal dementia. Geroscience 2024, 46, 751–768. [Google Scholar] [CrossRef]
- Olğun, Y.; Aksoy Poyraz, C.; Bozluolçay, M.; Poyraz, B. Quantitative EEG in the Differential Diagnosis of Dementia Subtypes. J. Geriatr. Psychiatry Neurol. 2024; in press. [Google Scholar] [CrossRef]
| Brain Wave | Brain Activity | Frequency (Hz) | Change Observed in Aging | Change Observed in MCI | Change Observed in AD |
|---|---|---|---|---|---|
| Delta | Deep sleep | 0.5 to 4 | Decrease [31] | Increase [31] | Increase [39] |
| Theta | Initial stage of sleep, deeply relaxed | 4 to 8 | Decrease [31] | Increase [31] | Increase [39] |
| Alpha | Relaxed and attentive | 8 to 13 | Slight decrease [31] | Slight decrease [31] | Significant decrease [39] |
| Beta | Active, anxiety-dominant | 13 to 30 | No change [31] | No change [31] | Decrease [39] |
| Gamma | High cognitive function, concentration | 30 to 80 | No change [31] | No change [31] | Change observed [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krothapalli, M.; Buddendorff, L.; Yadav, H.; Schilaty, N.D.; Jain, S. From Gut Microbiota to Brain Waves: The Potential of the Microbiome and EEG as Biomarkers for Cognitive Impairment. Int. J. Mol. Sci. 2024, 25, 6678. https://doi.org/10.3390/ijms25126678
Krothapalli M, Buddendorff L, Yadav H, Schilaty ND, Jain S. From Gut Microbiota to Brain Waves: The Potential of the Microbiome and EEG as Biomarkers for Cognitive Impairment. International Journal of Molecular Sciences. 2024; 25(12):6678. https://doi.org/10.3390/ijms25126678
Chicago/Turabian StyleKrothapalli, Mahathi, Lauren Buddendorff, Hariom Yadav, Nathan D. Schilaty, and Shalini Jain. 2024. "From Gut Microbiota to Brain Waves: The Potential of the Microbiome and EEG as Biomarkers for Cognitive Impairment" International Journal of Molecular Sciences 25, no. 12: 6678. https://doi.org/10.3390/ijms25126678
APA StyleKrothapalli, M., Buddendorff, L., Yadav, H., Schilaty, N. D., & Jain, S. (2024). From Gut Microbiota to Brain Waves: The Potential of the Microbiome and EEG as Biomarkers for Cognitive Impairment. International Journal of Molecular Sciences, 25(12), 6678. https://doi.org/10.3390/ijms25126678








