Revealing the Mechanism of NLRP3 Inflammatory Pathway Activation through K+ Efflux Induced by PLO via Signal Point Mutations
Abstract
:1. Introduction
2. Results
2.1. Mutation, Expression, and Purification of the PLO Protein
2.2. Mutations in PLO Allow for Host Tolerance to rPLO
2.3. Mutations Weaken the Disruption of rPLO on Tight Junctions and K+ Efflux
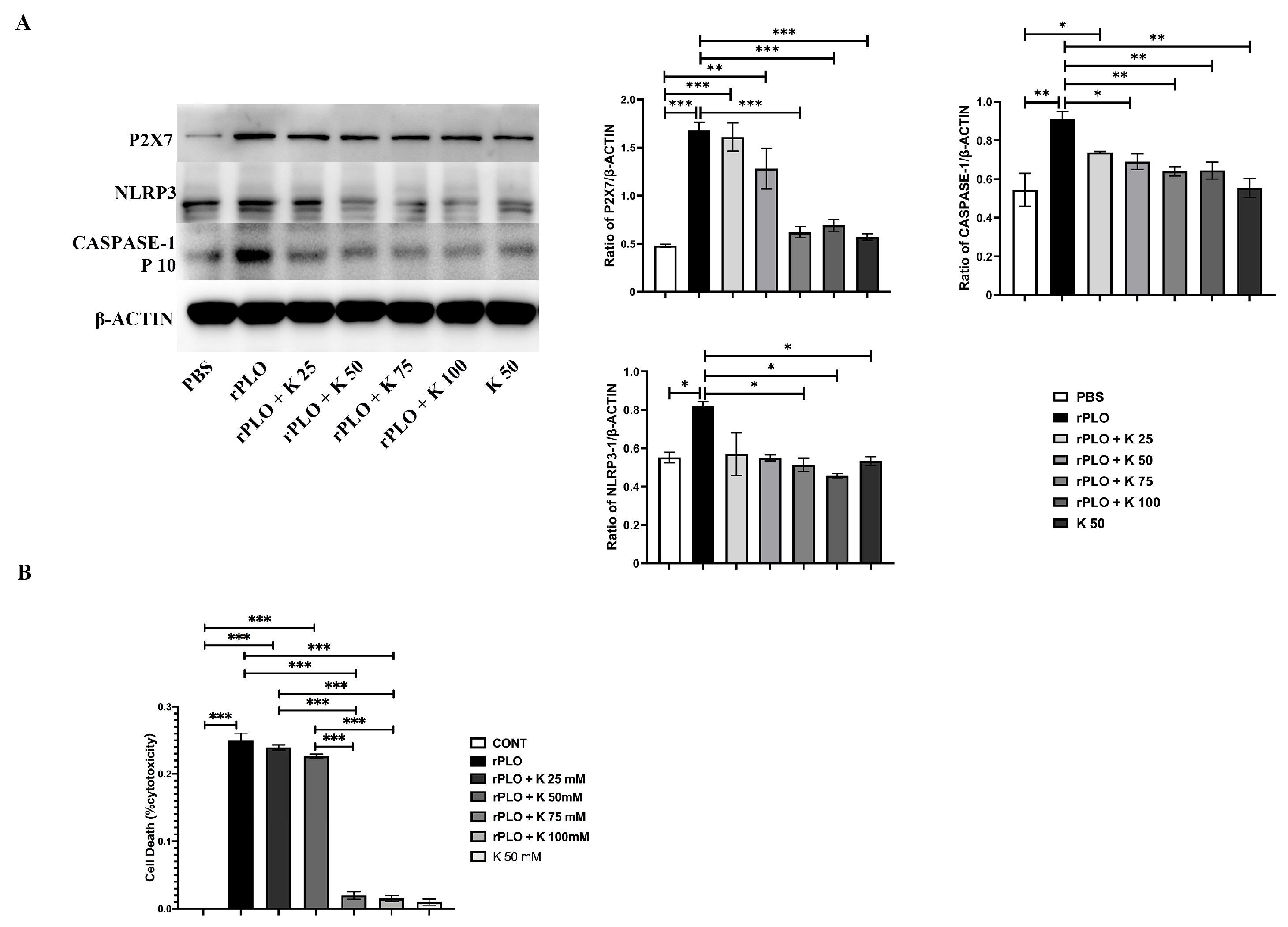
2.4. Mutations Alleviate the Activation of the NLRP3 Inflammatory Pathway
2.5. The Effect of K+ on rPLO Activation of the NLRP3 Inflammatory Pathway
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Plasmids, and Chemicals
4.2. Generation and Purification of Toxin
4.3. Biosecurity Statement
4.4. Animal Infection Experiment
4.5. Western Blotting
4.6. Immunofluorescence
4.7. Real-Time PCR
4.8. Cytotoxicity Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.L.; Liu, M.C.; Xu, J.; An, L.G.; Wang, J.F.; Zhu, Y.H. Uterine Microbiota of Dairy Cows With Clinical and Subclinical Endometritis. Front. Microbiol. 2018, 9, 2691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cai, Y.; Li, L.; Chen, C.; Zhao, H.; Zhang, Z.; Liu, Y.; Wang, Y.; Tian, C.; Liu, M. Effects of Luteolin on Biofilm of Trueperella pyogenes and Its Therapeutic Effect on Rat Endometritis. Int. J. Mol. Sci. 2022, 23, 14451. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Wang, H.; Ma, B.; Xu, L.; Wang, J.; Zhang, W. Identification of B-cell linear epitopes in domains 1–3 of pyolysin of Trueperella pyogenes using polyclonal antibodies. Vet. Microbiol. 2017, 210, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Liu, M.; Zhang, X.; Wang, H.; Yue, B. In vitro and in vivo expression of virulence genes in Trueperella pyogenes based on a mouse model. Vet. Microbiol. 2013, 163, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Jost, B.H.; Billington, S.J. Arcanobacterium pyogenes: Molecular pathogenesis of an animal opportunist. Antonie Van Leeuwenhoek 2005, 88, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Song, X.; Jing, J.; Zhao, K.; Shen, Y.; Zhang, X.; Yue, B. Chitosan-DNA nanoparticles enhanced the immunogenicity of multivalent DNA vaccination on mice against Trueperella pyogenes infection. J. Nanobiotechnology 2018, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, X.; Shan, Q.; Li, S.; Li, Y.; Chu, B.; Wang, J.; Zhu, Y. Single Point Mutation and Its Role in Specific Pathogenicity to Reveal the Mechanism of Related Protein Families. Microbiol. Spectr. 2022, 10, e0092322. [Google Scholar] [CrossRef] [PubMed]
- Jost, B.H.; Trinh, H.T.; Songer, J.G.; Billington, S.J. Immunization with genetic toxoids of the Arcanobacterium pyogenes cholesterol-dependent cytolysin, pyolysin, protects mice against infection. Infect. Immun. 2003, 71, 2966–2969. [Google Scholar] [CrossRef] [PubMed]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018, 17, 588–606. [Google Scholar] [CrossRef]
- Elliott, E.I.; Sutterwala, F.S. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol. Rev. 2015, 265, 35–52. [Google Scholar] [CrossRef]
- Prochnicki, T.; Mangan, M.S.; Latz, E. Recent insights into the molecular mechanisms of the NLRP3 inflammasome activation. F1000Research 2016, 5, 1469. [Google Scholar] [CrossRef] [PubMed]
- Greaney, A.J.; Leppla, S.H.; Moayeri, M. Bacterial Exotoxins and the Inflammasome. Front. Immunol. 2015, 6, 570. [Google Scholar] [CrossRef] [PubMed]
- Billington, S.J.; Jost, B.H.; Cuevas, W.A.; Bright, K.R.; Songer, J.G. The Arcanobacterium (Actinomyces) pyogenes hemolysin, pyolysin, is a novel member of the thiol-activated cytolysin family. J. Bacteriol. 1997, 179, 6100–6106. [Google Scholar] [CrossRef] [PubMed]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, S.T.; Jost, B.H.; Songer, J.G.; Billington, S.J. The gene encoding pyolysin, the pore-forming toxin of Arcanobacterium pyogenes, resides within a genomic islet flanked by essential genes. FEMS Microbiol. Lett. 2003, 225, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Jost, B.H.; Songer, J.G.; Billington, S.J. An Arcanobacterium (Actinomyces) pyogenes mutant deficient in production of the pore-forming cytolysin pyolysin has reduced virulence. Infect. Immun. 1999, 67, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Billington, S.J.; Songer, J.G.; Jost, B.H. Molecular characterization of the pore-forming toxin, pyolysin, a major virulence determinant of Arcanobacterium pyogenes. Vet. Microbiol. 2001, 82, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, S.T.; Jost, B.H.; Billington, S.J. Transcriptional regulation of pyolysin production in the animal pathogen, Arcanobacterium pyogenes. Vet. Microbiol. 2008, 132, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Kulma, M.; Kacprzyk-Stokowiec, A.; Kwiatkowska, K.; Traczyk, G.; Sobota, A.; Dadlez, M. R468A mutation in perfringolysin O destabilizes toxin structure and induces membrane fusion. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1075–1088. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Wang, B.; Zhang, Y.; Hu, Y.; Ma, B.; Wang, J. Replacing the 238th aspartic acid with an arginine impaired the oligomerization activity and inflammation-inducing property of pyolysin. Virulence 2018, 9, 1112–1125. [Google Scholar] [CrossRef]
- Liang, H.; Wang, B.; Wang, J.; Ma, B.; Zhang, W. Pyolysin of Trueperella pyogenes Induces Pyroptosis and IL-1beta Release in Murine Macrophages Through Potassium/NLRP3/Caspase-1/Gasdermin D Pathway. Front. Immunol. 2022, 13, 832458. [Google Scholar] [CrossRef]
- Johnson, B.B.; Heuck, A.P. Perfringolysin O structure and mechanism of pore formation as a paradigm for cholesterol-dependent cytolysins. Subcell. Biochem. 2014, 80, 63–81. [Google Scholar] [PubMed]
- Morton, C.J.; Sani, M.A.; Parker, M.W.; Separovic, F. Cholesterol-Dependent Cytolysins: Membrane and Protein Structural Requirements for Pore Formation. Chem. Rev. 2019, 119, 7721–7736. [Google Scholar] [CrossRef]
- Imaizumi, K.; Serizawa, A.; Hashimoto, N.; Kaidoh, T.; Takeuchi, S. Analysis of the functional domains of Arcanobacterium pyogenes pyolysin using monoclonal antibodies. Vet. Microbiol. 2001, 81, 235–242. [Google Scholar] [CrossRef]
- Heuck, A.P.; Moe, P.C.; Johnson, B.B. The cholesterol-dependent cytolysin family of gram-positive bacterial toxins. Subcell. Biochem. 2010, 51, 551–577. [Google Scholar] [PubMed]
- Billington, S.J.; Esmay, P.A.; Songer, J.G.; Jost, B.H. Identification and role in virulence of putative iron acquisition genes from Corynebacterium pseudotuberculosis. FEMS Microbiol. Lett. 2002, 208, 41–45. [Google Scholar] [CrossRef]
- Malley, R.; Henneke, P.; Morse, S.C.; Cieslewicz, M.J.; Lipsitch, M.; Thompson, C.M.; Kurt-Jones, E.; Paton, J.C.; Wessels, M.R.; Golenbock, D.T. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA 2003, 100, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Badgujar, D.C.; Anil, A.; Green, A.E.; Surve, M.V.; Madhavan, S.; Beckett, A.; Prior, I.A.; Godsora, B.K.; Patil, S.B.; More, P.K.; et al. Structural insights into loss of function of a pore forming toxin and its role in pneumococcal adaptation to an intracellular lifestyle. PLoS Pathog. 2020, 16, e1009016. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.; Mathur, A.; Xue, Y.; Liu, Y.; Tan, W.H.; Feng, S.; Pandey, A.; Ngo, C.; Hayward, J.A.; Atmosukarto, I.I.; et al. Bacillus cereus non-haemolytic enterotoxin activates the NLRP3 inflammasome. Nat. Commun. 2020, 11, 760. [Google Scholar] [CrossRef]
- Mathur, A.; Feng, S.; Hayward, J.A.; Ngo, C.; Fox, D.; Atmosukarto, I.I.; Price, J.D.; Schauer, K.; Martlbauer, E.; Robertson, A.A.B.; et al. A multicomponent toxin from Bacillus cereus incites inflammation and shapes host outcome via the NLRP3 inflammasome. Nat. Microbiol. 2019, 4, 362–374. [Google Scholar] [CrossRef]
- Shan, Q.; Liu, N.; Wang, X.; Zhu, Y.; Yin, J.; Wang, J. Lactobacillus rhamnosus GR-1 attenuates foodborne Bacillus cereus-induced NLRP3 inflammasome activity in bovine mammary epithelial cells by protecting intercellular tight junctions. J. Anim. Sci. Biotechnol. 2022, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Amos, M.R.; Healey, G.D.; Goldstone, R.J.; Mahan, S.M.; Duvel, A.; Schuberth, H.J.; Sandra, O.; Zieger, P.; Dieuzy-Labaye, I.; Smith, D.G.; et al. Differential endometrial cell sensitivity to a cholesterol-dependent cytolysin links Trueperella pyogenes to uterine disease in cattle. Biol. Reprod. 2014, 90, 54. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Juarbe, N.; Gilley, R.P.; Hinojosa, C.A.; Bradley, K.M.; Kamei, A.; Gao, G.; Dube, P.H.; Bergman, M.A.; Orihuela, C.J. Pore-Forming Toxins Induce Macrophage Necroptosis during Acute Bacterial Pneumonia. PLoS Pathog. 2015, 11, e1005337. [Google Scholar] [CrossRef] [PubMed]
- Pokrajac, L.; Baik, C.; Harris, J.R.; Sarraf, N.S.; Palmer, M. Partial oligomerization of pyolysin induced by a disulfide-tethered mutant. Biochem. Cell Biol. 2012, 90, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Hu, Y.; Bao, J.; Xiao, Y.; Zhang, Y.; Yang, L.; Wang, J.; Zhang, W. Isoleucine 61 is important for the hemolytic activity of pyolysin of Trueperella pyogenes. Vet. Microbiol. 2016, 182, 196–201. [Google Scholar] [CrossRef]
- Liu, N.; Wang, X.; Shan, Q.; Xu, L.; Li, Y.; Chu, B.; Yang, L.; Wang, J.; Zhu, Y. Lactobacillus rhamnosus Ameliorates Multi-Drug-Resistant Bacillus cereus-Induced Cell Damage through Inhibition of NLRP3 Inflammasomes and Apoptosis in Bovine Endometritis. Microorganisms 2022, 10, 137. [Google Scholar] [CrossRef]


| Primer Name | Direction | Sequence (5′-3′) |
|---|---|---|
| P2X7 | F | AGCCTGTTATCAGCTCCGTG |
| R | CCTAACTTCGTCACCCCACC | |
| KNCK6 | F | CCTGGATGCCTTCGTGGAG |
| R | AAGCGTGCTGGCGAAGAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, Q.; Ma, W.; Li, B.; Li, Q.; Wang, X.; Li, Y.; Wang, J.; Zhu, Y.; Liu, N. Revealing the Mechanism of NLRP3 Inflammatory Pathway Activation through K+ Efflux Induced by PLO via Signal Point Mutations. Int. J. Mol. Sci. 2024, 25, 6703. https://doi.org/10.3390/ijms25126703
Shan Q, Ma W, Li B, Li Q, Wang X, Li Y, Wang J, Zhu Y, Liu N. Revealing the Mechanism of NLRP3 Inflammatory Pathway Activation through K+ Efflux Induced by PLO via Signal Point Mutations. International Journal of Molecular Sciences. 2024; 25(12):6703. https://doi.org/10.3390/ijms25126703
Chicago/Turabian StyleShan, Qiang, Wenbo Ma, Bolin Li, Qian Li, Xue Wang, Yanan Li, Jiufeng Wang, Yaohong Zhu, and Ning Liu. 2024. "Revealing the Mechanism of NLRP3 Inflammatory Pathway Activation through K+ Efflux Induced by PLO via Signal Point Mutations" International Journal of Molecular Sciences 25, no. 12: 6703. https://doi.org/10.3390/ijms25126703





