Changes in Telomere Length in Leukocytes and Leukemic Cells after Ultrashort Electron Beam Radiation
Abstract
1. Introduction
2. Results
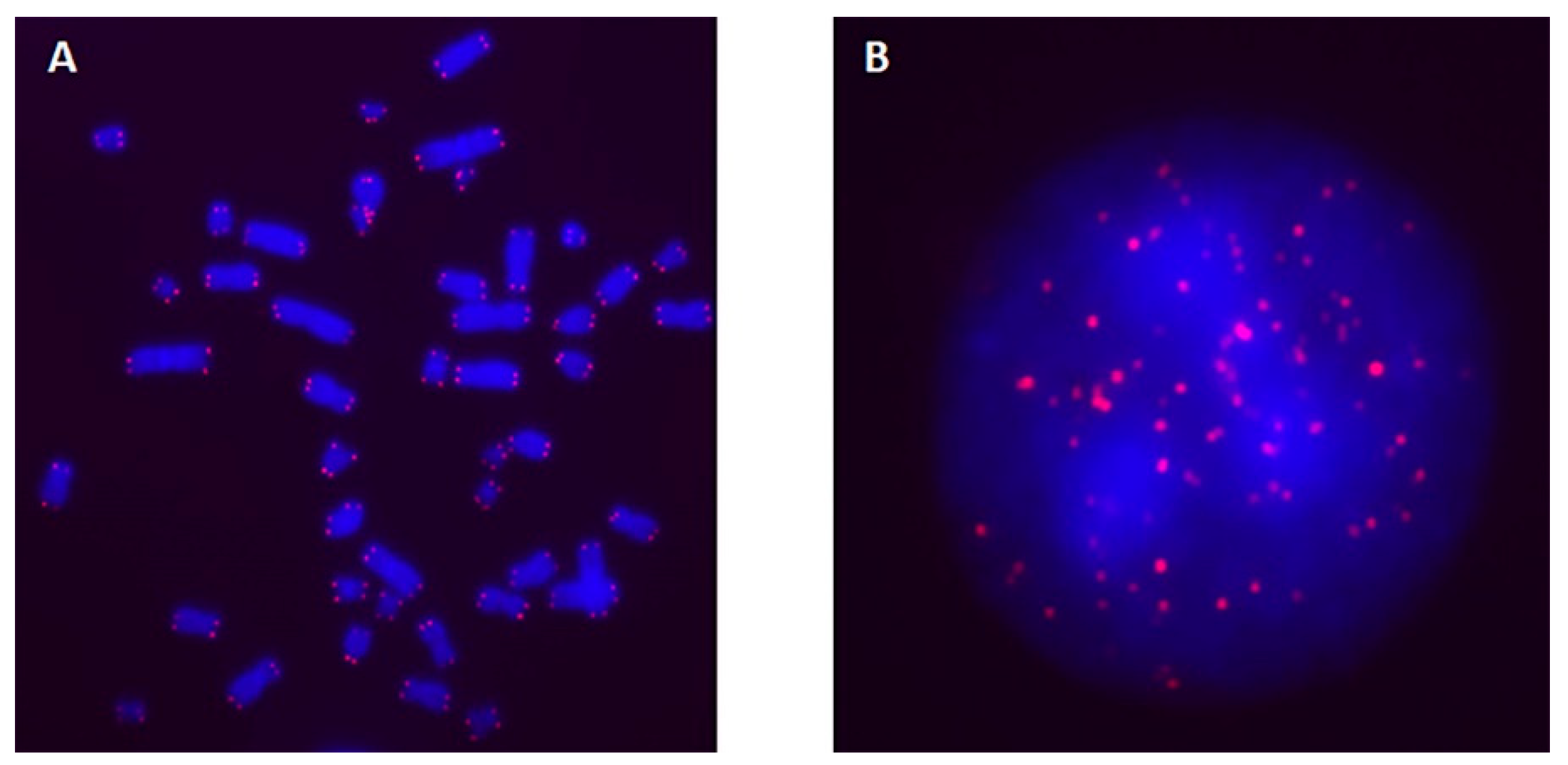
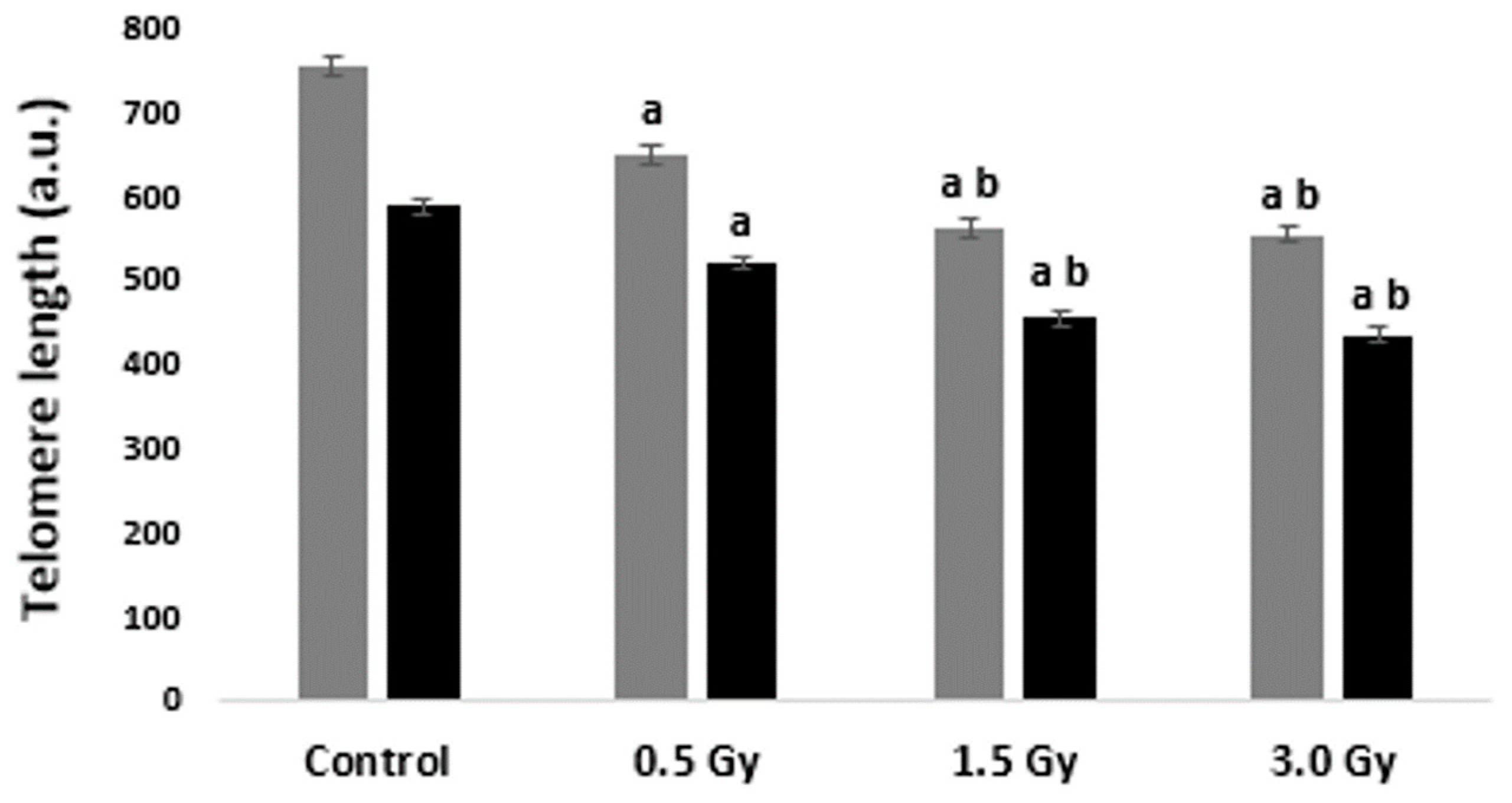
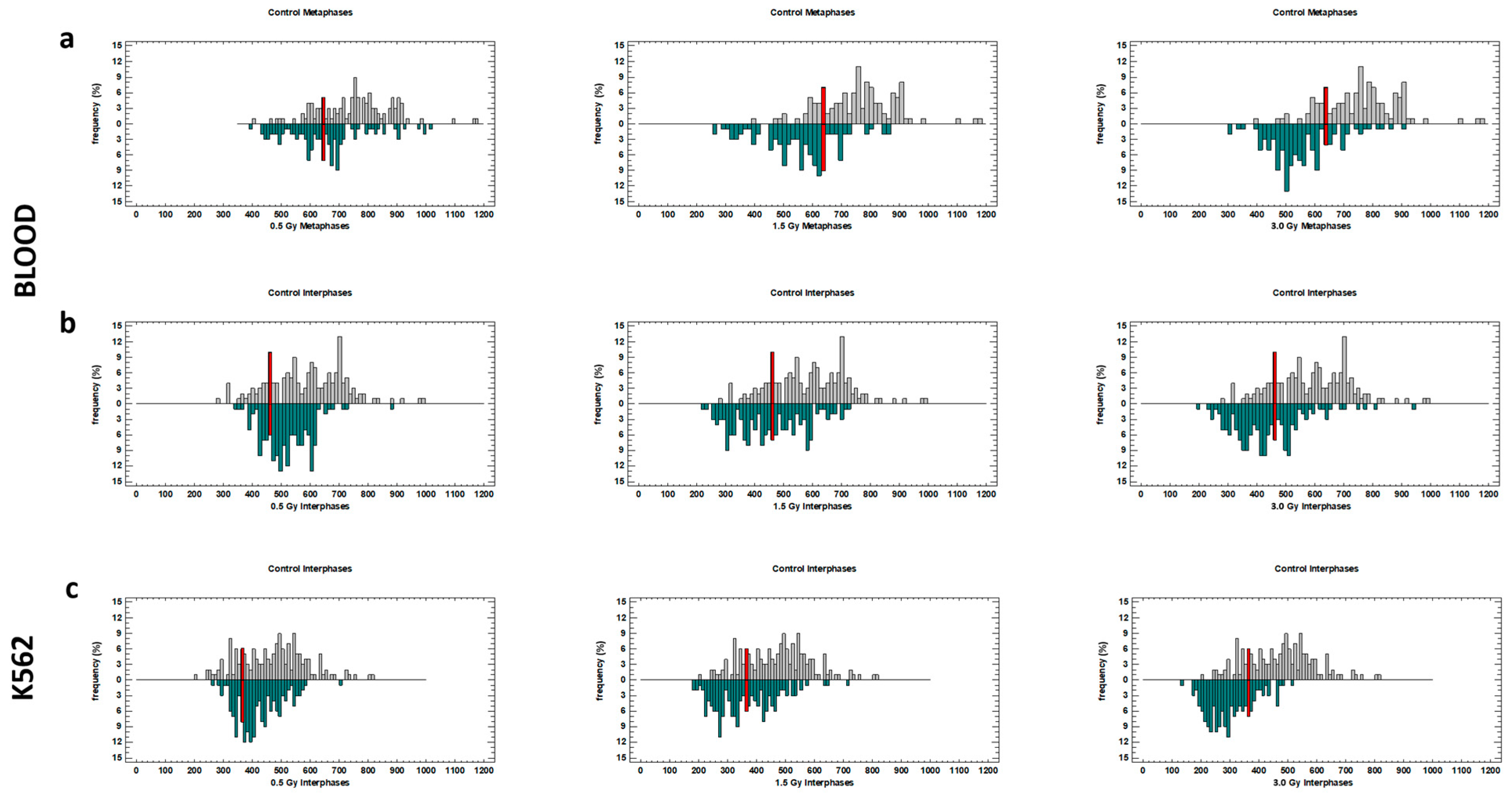
2.1. TL in Interphase Nuclei and Metaphase Chromosomes of Blood Cells Irradiated with Laser-Generated Electrons
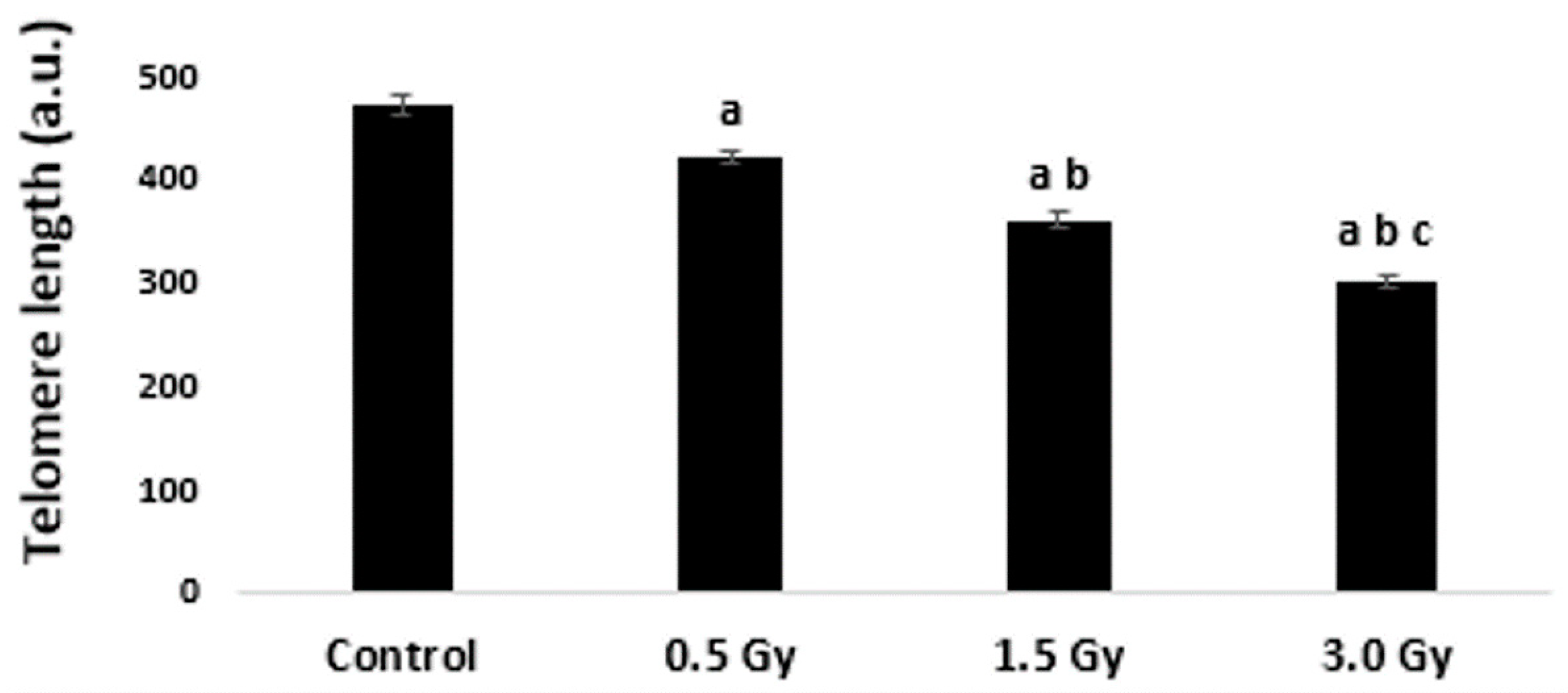
2.2. TL in Interphase Nuclei of K562 Cells Irradiated with Laser-Generated Electrons
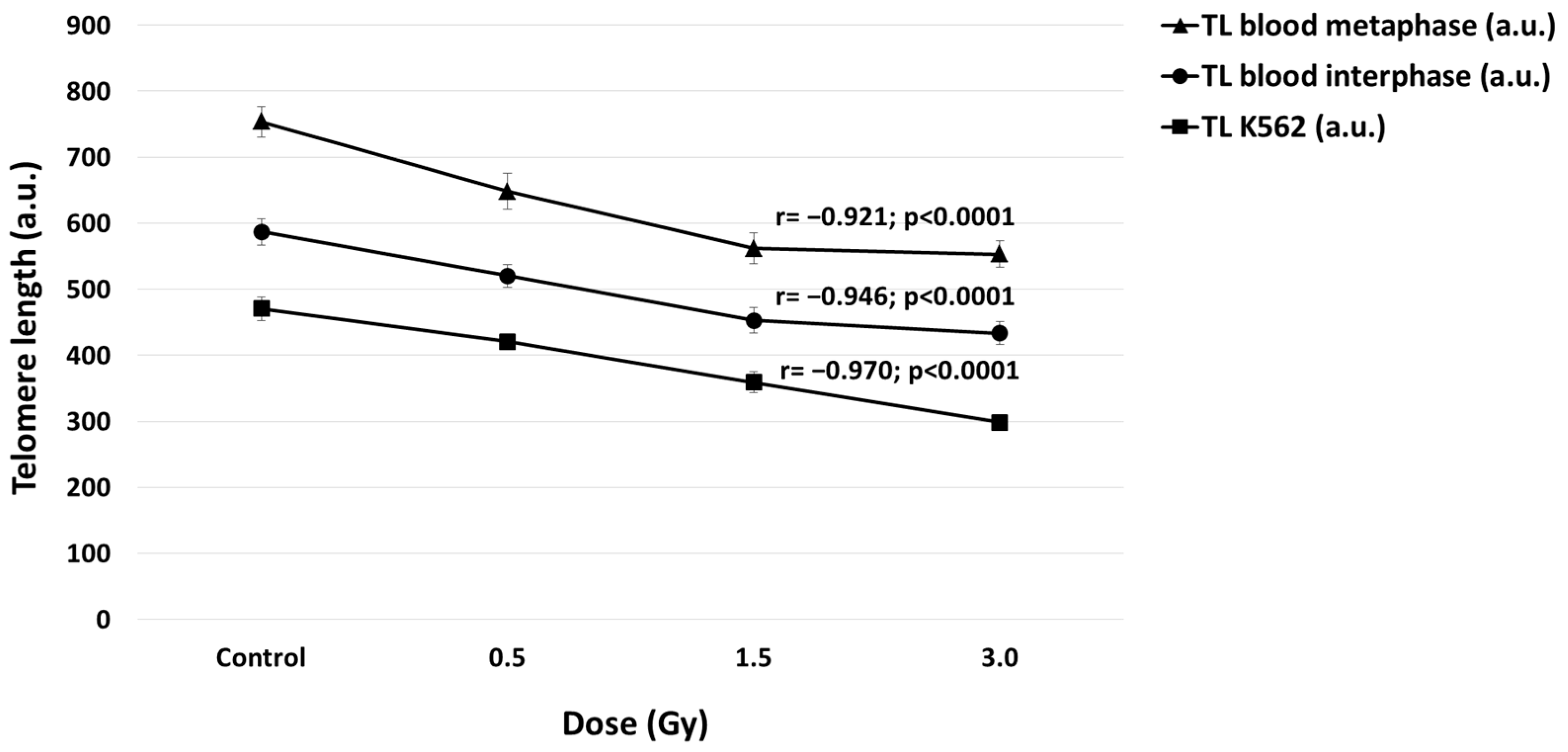
2.3. Correlation between TL and Doses in Blood and K562 Cells
2.4. Distribution of TLs in Blood and K562 Cells
3. Discussion
4. Materials and Methods
4.1. Blood and K562 Cells Cultivation and Irradiation
4.2. Telomere Q-FISH
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofmann, K.M.; Schell, S.; Wilkens, J.J. Laser-Driven Beam Lines for Delivering Intensity Modulated Radiation Therapy with Particle Beams. J. Biophotonics 2012, 5, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Karsch, L.; Beyreuther, E.; Enghardt, W.; Gotz, M.; Masood, U.; Schramm, U.; Zeil, K.; Pawelke, J. Towards Ion Beam Therapy Based on Laser Plasma Accelerators. Acta Oncol. (Madr.) 2017, 56, 1359–1366. [Google Scholar] [CrossRef]
- Rigaud, O.; Fortunel, N.O.; Vaigot, P.; Cadio, E.; Martin, M.T.; Lundh, O.; Faure, J.; Rechatin, C.; Malka, V.; Gauduel, Y.A. Exploring Ultrashort High-Energy Electron-Induced Damage in Human Carcinoma Cells. Cell Death Dis. 2010, 1, e73. [Google Scholar] [CrossRef] [PubMed]
- Faure, J.; Glinec, Y.; Pukhov, A.; Klselev, S.; Gordienko, S.; Lefebvre, E.; Rousseau, J.P.; Burgy, F.; Malka, V. A Laser-Plasma Accelerator Producing Monoenergetic Electron Beams. Nature 2004, 431, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Laschinsky, L.; Baumann, M.; Beyreuther, E.; Enghardt, W.; Kaluza, M.; Karsch, L.; Lessmann, E.; Naumburger, D.; Nicolai, M.; Richter, C.; et al. Radiobiological Effectiveness of Laser Accelerated Electrons in Comparison to Electron Beams from a Conventional Linear Accelerator. J. Radiat. Res. 2012, 53, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Asavei, T.; Bobeica, M.; Nastasa, V.; Manda, G.; Naftanaila, F.; Bratu, O.; Mischianu, D.; Cernaianu, M.O.; Ghenuche, P.; Savu, D.; et al. Laser-Driven Radiation: Biomarkers for Molecular Imaging of High Dose-Rate Effects. Med. Phys. 2019, 46, e726–e734. [Google Scholar] [CrossRef] [PubMed]
- Malka, V.; Faure, J.; Gauduel, Y.A. Ultra-Short Electron Beams Based Spatio-Temporal Radiation Biology and Radiotherapy. Mutat. Res. Rev. Mutat. Res. 2010, 704, 142–151. [Google Scholar] [CrossRef]
- Labate, L.; Palla, D.; Panetta, D.; Avella, F.; Baffigi, F.; Brandi, F.; Di Martino, F.; Fulgentini, L.; Giulietti, A.; Köster, P.; et al. Toward an Effective Use of Laser-Driven Very High Energy Electrons for Radiotherapy: Feasibility Assessment of Multi-Field and Intensity Modulation Irradiation Schemes. Sci. Rep. 2020, 10, 17307. [Google Scholar] [CrossRef] [PubMed]
- Tsakanov, V.M.; Aroutiounian, R.M.; Amatuni, G.A.; Aloyan, L.R.; Aslanyan, L.G.; Avagyan, V.S.; Babayan, N.S.; Buniatyan, V.V.; Dalyan, Y.B.; Davtyan, H.D.; et al. AREAL Low Energy Electron Beam Applications in Life and Materials Sciences. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2016, 829, 248–253. [Google Scholar] [CrossRef][Green Version]
- Labate, L.; Andreassi, M.G.; Baffigi, F.; Basta, G.; Bizzarri, R.; Borghini, A.; Candiano, G.C.; Casarino, C.; Cresci, M.; Di Martino, F.; et al. Small-Scale Laser Based Electron Accelerators for Biology and Medicine: A Comparative Study of the Biological Effectiveness. In Proceedings of the Laser Acceleration of Electrons, Protons, and Ions II; and Medical Applications of Laser-Generated Beams of Particles II; and Harnessing Relativistic Plasma Waves III, Prague, Czech Republic, 15–18 April 2013; Volume 8779, p. 87790O. [Google Scholar] [CrossRef]
- Laschinsky, L.; Karsch, L.; Leßmann, E.; Oppelt, M.; Pawelke, J.; Richter, C.; Schürer, M.; Beyreuther, E. Radiobiological Influence of Megavoltage Electron Pulses of Ultra-High Pulse Dose Rate on Normal Tissue Cells. Radiat. Environ. Biophys. 2016, 55, 381–391. [Google Scholar] [CrossRef]
- Harutyunyan, T.; Hovhannisyan, G.; Sargsyan, A.; Grigoryan, B.; Al-Rikabi, A.H.; Weise, A.; Liehr, T.; Aroutiounian, R. Analysis of Copy Number Variations Induced by Ultrashort Electron Beam Radiation in Human Leukocytes in Vitro. Mol. Cytogenet. 2019, 12, 18. [Google Scholar] [CrossRef]
- Babayan, N.; Hovhannisyan, G.; Grigoryan, B.; Grigoryan, R.; Sarkisyan, N.; Tsakanova, G.; Haroutiunian, S.; Aroutiounian, R. Dose-Rate Effect of Ultrashort Electron Beam Radiation on DNA Damage and Repair in Vitro. J. Radiat. Res. 2017, 58, 894–897. [Google Scholar] [CrossRef]
- Babayan, N.; Grigoryan, B.; Khondkaryan, L.; Tadevosyan, G.; Sarkisyan, N.; Grigoryan, R.; Apresyan, L.; Aroutiounian, R.; Vorobyeva, N.; Pustovalova, M.; et al. Laser-Driven Ultrashort Pulsed Electron Beam Radiation at Doses of 0.5 and 1.0 Gy Induces Apoptosis in Human Fibroblasts. Int. J. Mol. Sci. 2019, 20, 5140. [Google Scholar] [CrossRef]
- Babayan, N.; Vorobyeva, N.; Grigoryan, B.; Grekhova, A.; Pustovalova, M.; Rodneva, S.; Fedotov, Y.; Tsakanova, G.; Aroutiounian, R.; Osipov, A. Low Repair Capacity of Dna Double-Strand Breaks Induced by Laser-Driven Ultrashort Electron Beams in Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9488. [Google Scholar] [CrossRef]
- Tsakanova, G.; Babayan, N.; Karalova, E.; Hakobyan, L.; Abroyan, L.; Avetisyan, A.; Avagyan, H.; Hakobyan, S.; Poghosyan, A.; Baghdasaryan, B.; et al. Low-Energy Laser-Driven Ultrashort Pulsed Electron Beam Irradiation-Induced Immune Response in Rats. Int. J. Mol. Sci. 2021, 22, 11525. [Google Scholar] [CrossRef] [PubMed]
- Andreassi, M.G.; Borghini, A.; Pulignani, S.; Baffigi, F.; Fulgentini, L.; Koester, P.; Cresci, M.; Vecoli, C.; Lamia, D.; Russo, G.; et al. Radiobiological Effectiveness of Ultrashort Laser-Driven Electron Bunches: Micronucleus Frequency, Telomere Shortening and Cell Viability. Radiat. Res. 2016, 186, 245–253. [Google Scholar] [CrossRef]
- Aubert, G.; Hills, M.; Lansdorp, P.M. Telomere Length Measurement-Caveats and a Critical Assessment of the Available Technologies and Tools. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2012, 730, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Vera, E.; Blasco, M.A. Beyond Average: Potential for Measurement of Short Telomeres. Aging 2012, 4, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Montpetit, A.J.; Alhareeri, A.A.; Montpetit, M.; Starkweather, A.R.; Elmore, L.W.; Filler, K.; Mohanraj, L.; Burton, C.W.; Menzies, V.S.; Lyon, D.E.; et al. Telomere Length: A Review of Methods for Measurement. Nurs. Res. 2014, 63, 289–299. [Google Scholar] [CrossRef]
- Slijepcevic, P. Telomere Length Measurement by Q-FISH. Methods Cell Sci. 2001, 23, 17–22. [Google Scholar] [CrossRef]
- Barbaro, V.; Orvieto, A.; Alvisi, G.; Bertolin, M.; Bonelli, F.; Liehr, T.; Harutyunyan, T.; Kankel, S.; Joksic, G.; Ferrari, S.; et al. Analysis and Pharmacological Modulation of Senescence in Human Epithelial Stem Cells. J. Cell. Mol. Med. 2022, 26, 3977–3994. [Google Scholar] [CrossRef] [PubMed]
- Canela, A.; Vera, E.; Klatt, P.; Blasco, M.A. High-Throughput Telomere Length Quantification by FISH and Its Application to Human Population Studies. Proc. Natl. Acad. Sci. USA 2007, 104, 5300–5305. [Google Scholar] [CrossRef] [PubMed]
- M’kacher, R.; Colicchio, B.; Borie, C.; Junker, S.; Marquet, V.; Heidingsfelder, L.; Soehnlen, K.; Najar, W.; Hempel, W.M.; Oudrhiri, N.; et al. Telomere and Centromere Staining Followed by M-Fish Improves Diagnosis of Chromosomal Instability and Its Clinical Utility. Genes 2020, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- Hemann, M.T.; Strong, M.A.; Hao, L.Y.; Greider, C.W. The Shortest Telomere, Not Average Telomere Length, Is Critical for Cell Viability and Chromosome Stability. Cell 2001, 107, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.P.; Zhang, N.; Noh, J.; Mender, I.; Tedone, E.; Huang, E.; Wright, W.E.; Danuser, G.; Shay, J.W. A Method for Measuring the Distribution of the Shortest Telomeres in Cells and Tissues. Nat. Commun. 2017, 8, 1356. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.P.; Wright, W.E.; Shay, J.W. Comparison of Telomere Length Measurement Methods. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160451. [Google Scholar] [CrossRef] [PubMed]
- Tsatsakis, A.; Tsoukalas, D.; Fragkiadaki, P.; Vakonaki, E.; Tzatzarakis, M.; Sarandi, E.; Nikitovic, D.; Tsilimidos, G.; Alegakis, A.K. Developing BioTel: A Semi-Automated Spreadsheet for Estimating Telomere Length and Biological Age. Front. Genet. 2019, 10, 432132. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Pedrero, L.; Ojeda-Rodríguez, A.; Martínez-González, M.A.; Zalba, G.; Bes-Rastrollo, M.; Marti, A. Ultra-Processed Food Consumption and the Risk of Short Telomeres in an Elderly Population of the Seguimiento Universidad de Navarra (SUN) Project. Am. J. Clin. Nutr. 2020, 111, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Pedrero, L.; Ojeda-Rodríguez, A.; Zalba, G.; Razquin, C.; Martínez-González, M.Á.; Bes-Rastrollo, M.; Marti, A. Association between Ideal Cardiovascular Health and Telomere Length in Participants Older than 55 Years Old from the SUN Cohort. Rev. Española Cardiol. (English Ed.) 2022, 75, 308–315. [Google Scholar] [CrossRef]
- Keller, G.; Brassat, U.; Braig, M.; Heim, D.; Wege, H.; Brümmendorf, T.H. Telomeres and Telomerase in Chronic Myeloid Leukaemia: Impact for Pathogenesis, Disease Progression and Targeted Therapy. Hematol. Oncol. 2009, 27, 123–129. [Google Scholar] [CrossRef]
- Samassekou, O. Dynamic Length Changes of Telomeres and Their Nuclear Organization in Chronic Myeloid Leukemia. Cancers 2013, 5, 1086–1102. [Google Scholar] [CrossRef] [PubMed]
- Fehrer, C.; Voglauer, R.; Wieser, M.; Pfister, G.; Brunauer, R.; Cioca, D.; Grubeck-Loebenstein, B.; Lepperdinger, G. Techniques in Gerontology: Cell Lines as Standards for Telomere Length and Telomerase Activity Assessment. Exp. Gerontol. 2006, 41, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Deregowska, A.; Pepek, M.; Pruszczyk, K.; Machnicki, M.M.; Wnuk, M.; Stoklosa, T. Differential Regulation of Telomeric Complex by Bcr-Abl1 Kinase in Human Cellular Models of Chronic Myeloid Leukemia—From Single Cell Analysis to next-Generation Sequencing. Genes 2020, 11, 1145. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, T.C.; Wall, M.; Ververis, K.; Pitsillou, E.; Tortorella, S.M.; Wood, P.A.; Rafehi, H.; Khurana, I.; Maxwell, S.S.; Hung, A.; et al. Characterization of K562 Cells: Uncovering Novel Chromosomes, Assessing Transferrin Receptor Expression, and Probing Pharmacological Therapies. Cell. Mol. Life Sci. 2023, 80, 80. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, E.C.; Velinov, M.T.; Ye, L.; Gu, H.; Li, S.; Jenkins, E.C.; Brooks, S.S.; Pang, D.; Devenny, D.A.; Zigman, W.B.; et al. Telomere Shortening in T Lymphocytes of Older Individuals with Down Syndrome and Dementia. Neurobiol. Aging 2006, 27, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, E.C.; Ye, L.; Gu, H.; Ni, S.A.; Velinov, M.; Pang, D.; Krinsky-McHale, S.J.; Zigman, W.B.; Schupf, N.; Silverman, W.P. Shorter Telomeres May Indicate Dementia Status in Older Individuals with Down Syndrome. Neurobiol. Aging 2010, 31, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Choi, J.; See, C.J.; Kim, J.A.; Park, S.N.; Im, K.; Kim, S.M.; Lee, D.S.; Hwang, S.M. Dysregulation of Telomere Lengths and Telomerase Activity in Myelodysplastic Syndrome. Ann. Lab. Med. 2017, 37, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Harley, C.B. Telomerase and Cancer Therapeutics. Nat. Rev. Cancer 2008, 8, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Barthel, F.P.; Wei, W.; Tang, M.; Martinez-Ledesma, E.; Hu, X.; Amin, S.B.; Akdemir, K.C.; Seth, S.; Song, X.; Wang, Q.; et al. Systematic Analysis of Telomere Length and Somatic Alterations in 31 Cancer Types. Nat. Genet. 2017, 49, 349–357. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Oikonomopoulou, T.; Nikolouzakis, T.K.; Vakonaki, E.; Tzatzarakis, M.; Flamourakis, M.; Renieri, E.; Fragkiadaki, P.; Iliaki, E.; Bachlitzanaki, M.; et al. Role of Telomere Length in Human Carcinogenesis (Review). Int. J. Oncol. 2023, 63, 78. [Google Scholar] [CrossRef]
- Roka, K.; Solomou, E.E.; Kattamis, A. Telomere Biology: From Disorders to Hematological Diseases. Front. Oncol. 2023, 13, 1167848. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing Radiation-Induced Metabolic Oxidative Stress and Prolonged Cell Injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of Ionizing Radiation on Biological Molecules—Mechanisms of Damage and Emerging Methods of Detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, L.; Picano, E.; Andreassi, M.G. Telomere Shortening and Ionizing Radiation: A Possible Role in Vascular Dysfunction? Int. J. Radiat. Biol. 2012, 88, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The Impact of Oxidative DNA Damage and Stress on Telomere Homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Serra, V.; Von Zglinicki, T.; Lorenz, M.; Saretzki, G. Extracellular Superoxide Dismutase Is a Major Antioxidant in Human Fibroblasts and Slows Telomere Shortening. J. Biol. Chem. 2003, 278, 6824–6830. [Google Scholar] [CrossRef] [PubMed]
- Ayouaz, A.; Raynaud, C.; Heride, C.; Revaud, D.; Sabatier, L. Telomeres: Hallmarks of Radiosensitivity. Biochimie 2008, 90, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Shim, G.; Ricoul, M.; Hempel, W.M.; Azzam, E.I.; Sabatier, L. Crosstalk between Telomere Maintenance and Radiation Effects: A Key Player in the Process of Radiation-Induced Carcinogenesis. Mutat. Res. Rev. Mutat. Res. 2014, 760, 1–17. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Johnson, S.A.; Opresko, P.L. Roles for the 8-Oxoguanine DNA Repair System in Protecting Telomeres From Oxidative Stress. Front. Cell Dev. Biol. 2021, 9, 758402. [Google Scholar] [CrossRef]
- Coluzzi, E.; Leone, S.; Sgura, A. Oxidative Stress Induces Telomere Dysfunction and Senescence by Replication Fork Arrest. Cells 2019, 8, 19. [Google Scholar] [CrossRef]
- Miller, D.; Reynolds, G.E.; Mejia, R.; Stark, J.M.; Murnane, J.P. Subtelomeric Regions in Mammalian Cells Are Deficient in DNA Double-Strand Break Repair. In DNA Repair; AMS: Providence, RI, USA, 2011; Volume 10, pp. 536–544. [Google Scholar] [CrossRef]
- Hewitt, G.; Jurk, D.; Marques, F.D.M.; Correia-Melo, C.; Hardy, T.; Gackowska, A.; Anderson, R.; Taschuk, M.; Mann, J.; Passos, J.F. Telomeres Are Favoured Targets of a Persistent DNA Damage Response in Ageing and Stress-Induced Senescence. Nat. Commun. 2012, 3, 708. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Rossiello, F.; Clerici, M.; Barozzi, S.; Cittaro, D.; Kaplunov, J.M.; Bucci, G.; Dobreva, M.; Matti, V.; Beausejour, C.M.; et al. Telomeric DNA Damage Is Irreparable and Causes Persistent DNA-Damage-Response Activation. Nat. Cell Biol. 2012, 14, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.; Boonekamp, J. Does Oxidative Stress Shorten Telomeres in Vivo? A Meta-Analysis. Ageing Res. Rev. 2023, 85, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hou, M.; Lou, F.; Björkholm, M.; Xu, D. Telomere Dysfunction Induced by Chemotherapeutic Agents and Radiation in Normal Human Cells. Int. J. Biochem. Cell Biol. 2012, 44, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Sishc, B.J.; Nelson, C.B.; McKenna, M.J.; Battaglia, C.L.R.; Herndon, A.; Idate, R.; Liber, H.L.; Bailey, S.M. Telomeres and Telomerase in the Radiation Response: Implications for Instability, Reprograming, and Carcinogenesis. Front. Oncol. 2015, 5, 257. [Google Scholar] [CrossRef] [PubMed]
- Sgura, A.; Antoccia, A.; Berardinelli, F.; Cherubini, R.; Gerardi, S.; Zilio, C.; Tanzarella, C. Telomere Length in Mammalian Cells Exposed to Low- and High-LET Radiations. Radiat. Prot. Dosimetry 2006, 122, 176–179. [Google Scholar] [CrossRef]
- Berardinelli, F.; Antoccia, A.; Buonsante, R.; Gerardi, S.; Cherubini, R.; De Nadal, V.; Tanzarella, C.; Sgura, A. The Role of Telomere Length Modulation in Delayed Chromosome Instability Induced by Ionizing Radiation in Human Primary Fibroblasts. Environ. Mol. Mutagen. 2013, 54, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Saini, D.; Jain, V.; Das, B. Evaluation of Natural Chronic Low Dose Radiation Exposure on Telomere Length and Transcriptional Response of Shelterin Complex in Individuals Residing in Kerala Coast, India. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2022, 825, 111797. [Google Scholar] [CrossRef]
- Movahedi, A.; Mostajaboddavati, M.; Rajabibazl, M.; Mirfakhraie, R.; Enferadi, M. Association of Telomere Length with Chronic Exposure to Ionizing Radiation among Inhabitants of Natural High Background Radiation Areas of Ramsar, Iran. Int. J. Radiat. Biol. 2019, 95, 1113–1121. [Google Scholar] [CrossRef]
- Andreassi, M.G.; Piccaluga, E.; Gargani, L.; Sabatino, L.; Borghini, A.; Faita, F.; Bruno, R.M.; Padovani, R.; Guagliumi, G.; Picano, E. Subclinical Carotid Atherosclerosis and Early Vascular Aging from Long-Term Low-Dose Ionizing Radiation Exposure: A Genetic, Telomere, and Vascular Ultrasound Study in Cardiac Catheterization Laboratory Staff. JACC Cardiovasc. Interv. 2015, 8, 616–627. [Google Scholar] [CrossRef]
- Tričković, J.F.; Šobot, A.V.; Joksić, I.; Joksić, G. Lomljivost Telomera u Bolničkih Radnika Profesionalno Izloženih Niskim Dozama Ionizirajućega Zračenja. Arh. Hig. Rada Toksikol. 2022, 73, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Nakamura, K.; Atsumi, K.; Hirakawa, M.; Ueda, Y.; Makino, N. Radiation-Associated Changes in the Length of Telomeres in Peripheral Leukocytes from Inpatients with Cancer. Int. J. Radiat. Biol. 2013, 89, 106–109. [Google Scholar] [CrossRef] [PubMed]
- M’kacher, R.; Girinsky, T.; Colicchio, B.; Ricoul, M.; Dieterlen, A.; Jeandidier, E.; Heidingsfelder, L.; Cuceu, C.; Shim, G.; Frenze, M.; et al. Telomere Shortening: A New Prognostic Factor for Cardiovascular Disease Post-Radiation Exposure. Radiat. Prot. Dosim. 2015, 164, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Ilyenko, I.; Lyaskivska, O.; Bazyka, D. Analysis of Relative Telomere Length and Apoptosis in Humans Exposed to Ionising Radiation. Exp. Oncol. 2011, 33, 235–238. [Google Scholar]
- Fernandes, S.G.; Dsouza, R.; Pandya, G.; Kirtonia, A.; Tergaonkar, V.; Lee, S.Y.; Garg, M.; Khattar, E. Role of Telomeres and Telomeric Proteins in Human Malignancies and Their Therapeutic Potential. Cancers 2020, 12, 1901. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Dubey, D.B.; Agarwal, K.; Dubey, D.B.; Verma, S.; Shabbir, N.; Kushwaha, R.; Reddy, D.H.; Singh, U.S.; Ali, W. Circulating Cell-Free DNA Level in Prediction of COVID-19 Severity and Mortality: Correlation of with Haematology and Serum Biochemical Parameters. Indian J. Clin. Biochem. 2023, 38, 172–181. [Google Scholar] [CrossRef]
- Mavragani, I.V.; Nikitaki, Z.; Souli, M.P.; Aziz, A.; Nowsheen, S.; Aziz, K.; Rogakou, E.; Georgakilas, A.G. Complex DNA Damage: A Route to Radiation-Induced Genomic Instability and Carcinogenesis. Cancers 2017, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Flores, E.R.; Yu, J.; Chang, S. Dysfunctional Telomeres Induce P53-Dependent and Independent Apoptosis to Compromise Cellular Proliferation and Inhibit Tumor Formation. Aging Cell 2016, 15, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.H.; Zhang, Y.; Tan, W.H.; Chng, W.J.; Li, B.; Wang, X. Regulation of HTERT by BCR-ABL at Multiple Levels in K562 Cells. BMC Cancer 2011, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Bangs, C.D.; Donlon, T.A. Metaphase Chromosome Preparation from Cultured Peripheral Blood Cells. Curr. Protoc. Hum. Genet. 2005, 45, 4. [Google Scholar] [CrossRef]
- Cabuy, E.; Newton, C.; Roberts, T.; Newbold, R.; Slijepcevic, P. Identification of Subpopulations of Cells with Differing Telomere Lengths in Mouse and Human Cell Lines by Flow FISH. Cytom. Part A 2004, 62, 150–161. [Google Scholar] [CrossRef] [PubMed]
| Irradiation Variant, Gy | TLs in Metaphases (mean ± SE), a.u. | TLs in Interphases (mean ± SE), a.u. |
|---|---|---|
| Control (D1) | 736.82 ± 18.54 | 587.38 ± 14.01 |
| 0.5 (D1) | 658.76 ± 15.45 | 527.45 ± 18.44 |
| 1.5 (D1) | 553.20 ± 19.67 | 436.78 ± 19.50 |
| 3.0 (D1) | 548.58 ± 12.04 | 421.81 ± 11.42 |
| Control (D2) | 767.77 ± 15.89 | 584.60 ± 16.48 |
| 0.5 (D2) | 652.52 ± 22.09 | 516.52 ± 12.32 |
| 1.5 (D2) | 578.64 ± 20.83 | 461.57 ± 16.88 |
| 3.0 (D2) | 562.05 ± 19.71 | 426.35 ± 13.82 |
| Control (D3) | 776.05 ± 30.06 | 586.98 ± 30.05 |
| 0.5 (D3) | 651.62 ± 28.33 | 513.79 ± 16.97 |
| 1.5 (D3) | 551.78 ± 25.95 | 451.69 ± 16.50 |
| 3.0 (D3) | 546.88 ± 21.08 | 453.44 ± 25.28 |
| Control (D4) | 734.80 ± 28.02 | 587.22 ± 19.11 |
| 0.5 (D4) | 630.18 ± 32.54 | 522.62 ± 19.93 |
| 1.5 (D4) | 563.15 ± 28.15 | 461.60 ± 24.58 |
| 3.0 (D4) | 555.34 ± 26.40 | 432.20 ± 19.81 |
| Cells (20th Percentile of Control) | 0.5 Gy (%) | 1.5 Gy (%) | 3.0 Gy (%) |
|---|---|---|---|
| Blood metaphases (640.40 a.u.) | 47.50 | 79.17 | 81.67 |
| Blood interphases (462.48 a.u.) | 22.70 | 55.97 | 63.41 |
| K562 interphases (363.01 a.u.) | 23.17 | 55.49 | 79.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harutyunyan, T.; Sargsyan, A.; Kalashyan, L.; Igityan, H.; Grigoryan, B.; Davtyan, H.; Aroutiounian, R.; Liehr, T.; Hovhannisyan, G. Changes in Telomere Length in Leukocytes and Leukemic Cells after Ultrashort Electron Beam Radiation. Int. J. Mol. Sci. 2024, 25, 6709. https://doi.org/10.3390/ijms25126709
Harutyunyan T, Sargsyan A, Kalashyan L, Igityan H, Grigoryan B, Davtyan H, Aroutiounian R, Liehr T, Hovhannisyan G. Changes in Telomere Length in Leukocytes and Leukemic Cells after Ultrashort Electron Beam Radiation. International Journal of Molecular Sciences. 2024; 25(12):6709. https://doi.org/10.3390/ijms25126709
Chicago/Turabian StyleHarutyunyan, Tigran, Anzhela Sargsyan, Lily Kalashyan, Hovhannes Igityan, Bagrat Grigoryan, Hakob Davtyan, Rouben Aroutiounian, Thomas Liehr, and Galina Hovhannisyan. 2024. "Changes in Telomere Length in Leukocytes and Leukemic Cells after Ultrashort Electron Beam Radiation" International Journal of Molecular Sciences 25, no. 12: 6709. https://doi.org/10.3390/ijms25126709
APA StyleHarutyunyan, T., Sargsyan, A., Kalashyan, L., Igityan, H., Grigoryan, B., Davtyan, H., Aroutiounian, R., Liehr, T., & Hovhannisyan, G. (2024). Changes in Telomere Length in Leukocytes and Leukemic Cells after Ultrashort Electron Beam Radiation. International Journal of Molecular Sciences, 25(12), 6709. https://doi.org/10.3390/ijms25126709








