Modulation of Photosystem II Function in Celery via Foliar-Applied Salicylic Acid during Gradual Water Deficit Stress
Abstract
1. Introduction
2. Results
2.1. Soil and Leaf Water Content under Treatments
2.2. Chlorophyll Content in Water-Sprayed and Salicylic Acid-Sprayed Plants
2.3. Light Energy Partitioning in Water-Sprayed and Salicylic Acid-Sprayed Plants
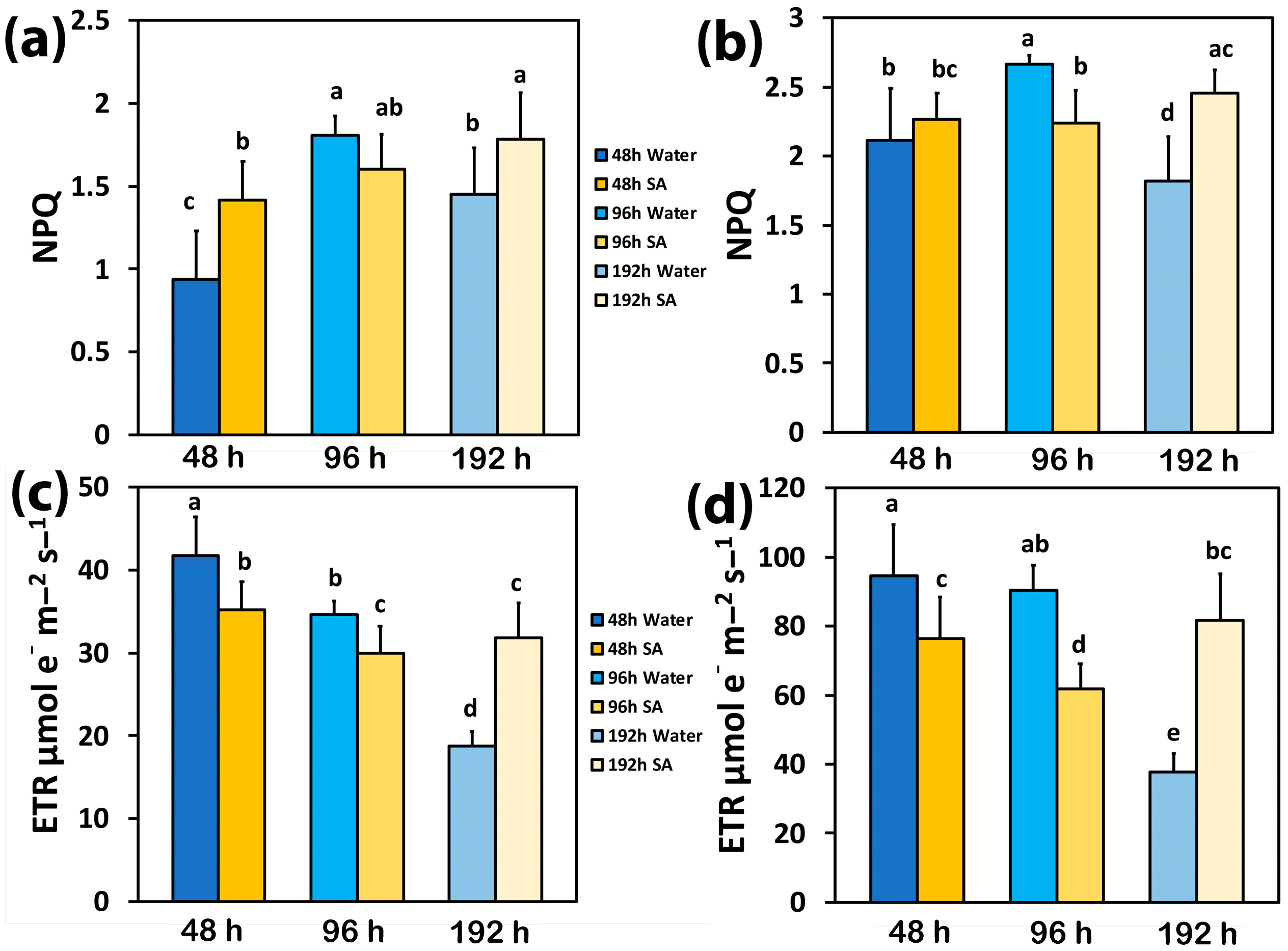
2.4. The Photoprotective Heat Dissipation and the Electron Transport Rate in Water-Sprayed and Salicylic Acid-Sprayed Plants
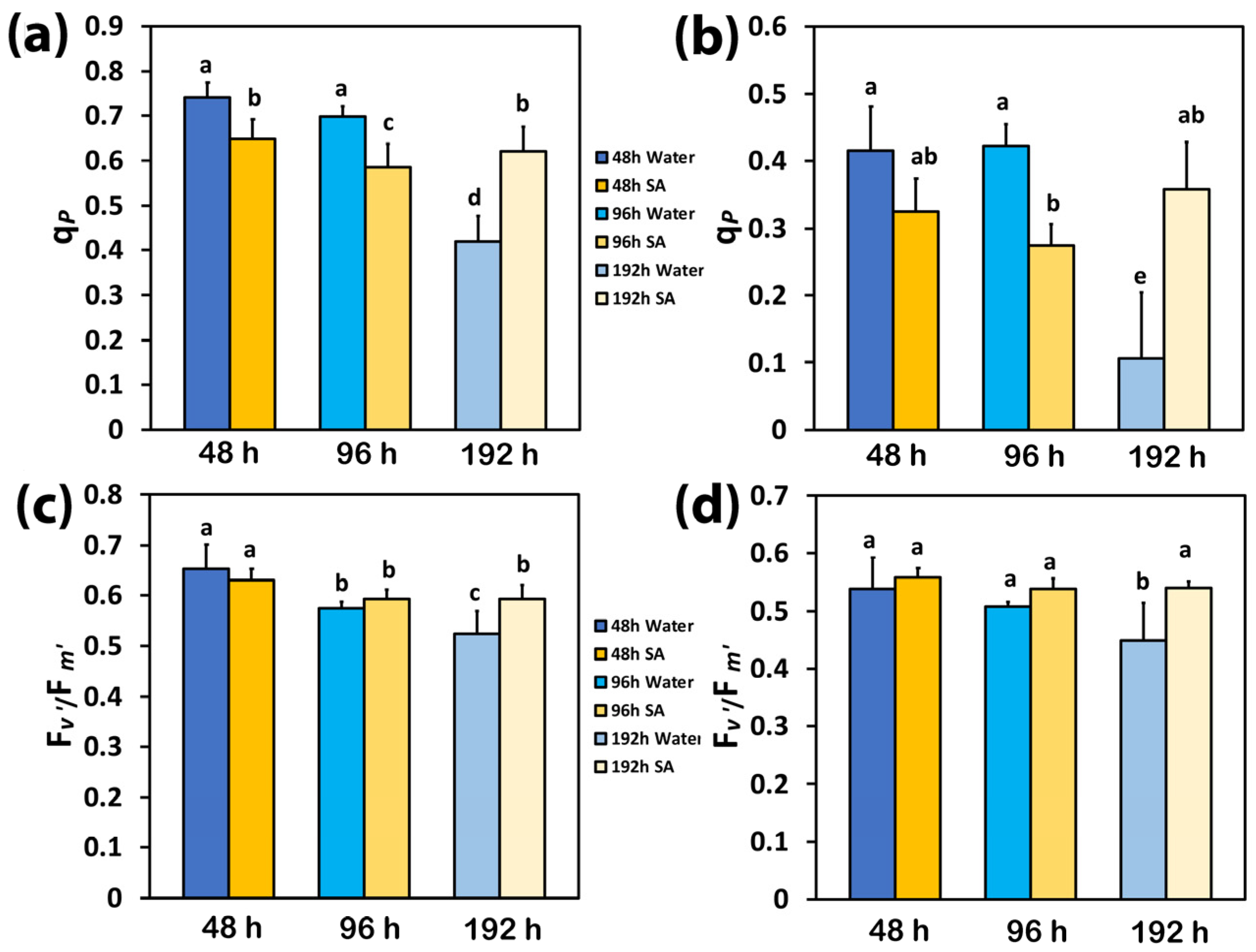
2.5. The Fraction of Open PSII Reaction Centers and Their Efficiency in Water-Sprayed and Salicylic Acid-Sprayed Plants
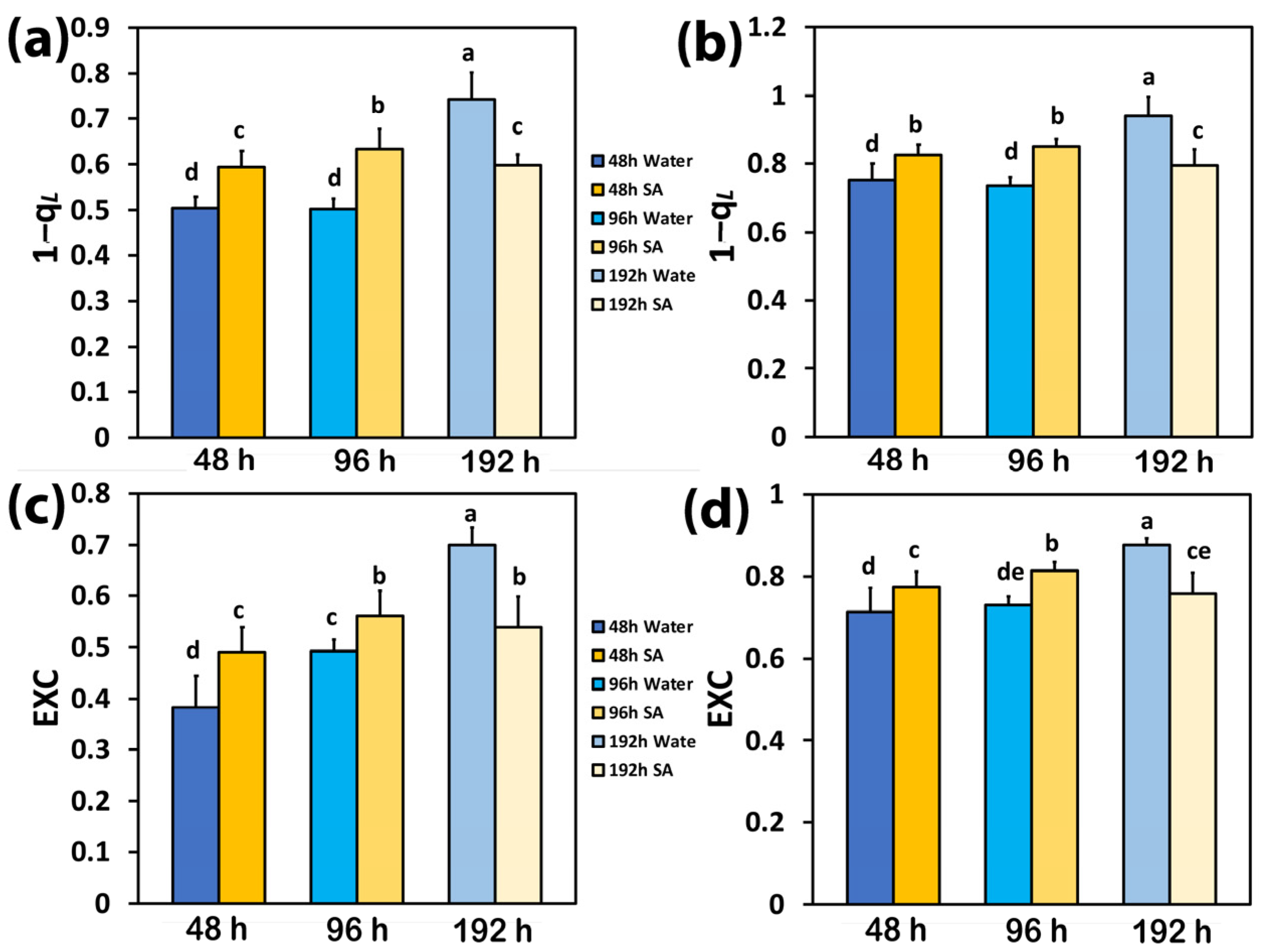
2.6. Excess Excitation Energy and PSII Excitation Pressure in Water-Sprayed and Salicylic Acid-Sprayed Plants
2.7. The Spatiotemporal Heterogeneity of PSII Function in Water-Sprayed and Salicylic Acid-Sprayed Plants
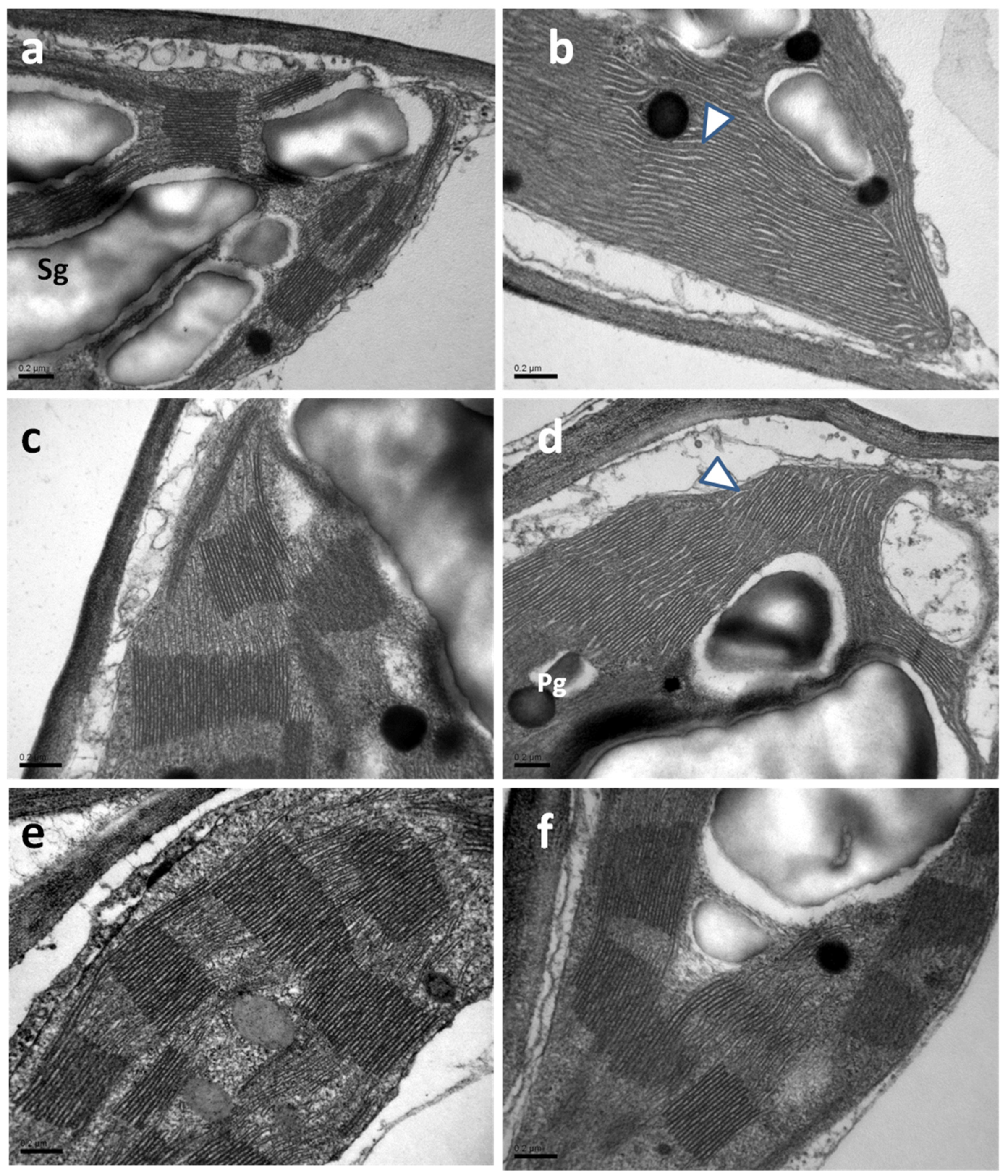
2.8. Chloroplast Ultrastructure in Water-Sprayed and Salicylic Acid-Sprayed Plants
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Salicylic Acid Treatments
4.3. Soil Volumetric Water Content
4.4. Leaf Water Content
4.5. Chlorophyll Content
4.6. Chlorophyll Fluorescence Imaging Analysis
4.7. Transmission Electron Microscopy
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fregonezi, B.F.; Pereira, A.E.S.; Ferreira, J.M.; Fraceto, L.F.; Gomes, D.G.; Oliveira, H.C. Seed priming with nanoencapsulated gibberellic acid triggers beneficial morphophysiological and biochemical responses of tomato plants under different water conditions. Agronomy 2024, 14, 588. [Google Scholar] [CrossRef]
- Wing, I.S.; De Cian, E.; Mistry, M.N. Global vulnerability of crop yields to climate change. J. Environ. Econ. Manag. 2021, 109, 102462. [Google Scholar] [CrossRef]
- Placide, R.; Hirut, G.B.; Stephan, N.; Fekadu, B. Assessment of drought stress tolerance in root and tuber crops. Afr. J. Plant Sci. 2014, 8, 214–224. [Google Scholar] [CrossRef][Green Version]
- Sperdouli, I.; Mellidou, I.; Moustakas, M. Harnessing chlorophyll fluorescence for phenotyping analysis of wild and cultivated tomato for high photochemical efficiency under water deficit for climate change resilience. Climate 2021, 9, 154. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Moustaka, J. Early drought stress warning: Color pictures of photosystem II photochemistry. Climate 2022, 10, 179. [Google Scholar] [CrossRef]
- Moustaka, J.; Moustakas, M. Early-stage detection of biotic and abiotic stress on plants by chlorophyll fluorescence imaging analysis. Biosensors 2023, 13, 796. [Google Scholar] [CrossRef]
- Akin, S.; Kaya, C. Impact of salicylic acid and sodium hydrosulfide applied singly or in combination on drought tolerance and grain yield in wheat plants. Food Energy Secur. 2024, 13, e532. [Google Scholar] [CrossRef]
- Zhang, J.; Corpas, F.J.; Li, J.; Xie, Y. Hydrogen sulfide and reactive oxygen species, antioxidant defense, abiotic stress tolerance mechanisms in plants. Int. J. Mol. Sci. 2022, 23, 9463. [Google Scholar] [CrossRef]
- Li, J.; Lardon, R.; Mangelinckx, S.; Geelen, D. Practical guide toward discovery of biomolecules with biostimulant activity. J. Exp. Bot. 2024; in press. [Google Scholar] [CrossRef]
- Martinez, C.; Pons, E.; Prats, G.; Leon, J. Salicylic acid regulates flowering time and links defence responses and reproductive development. Plant J. 2004, 37, 209–217. [Google Scholar] [CrossRef]
- Stevens, J.; Senaratna, T.; Sivasithamparam, K. Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): Associated changes in gas exchange, water relations and membrane stabilisation. Plant Growth Regul. 2006, 49, 77–83. [Google Scholar]
- Ramirez, A.; Rodriguez, D.; Reyes, D.; Angel Jimenez, J.; Nicolas, G.; Lopez Climent, M.; Gómez-Cadenas, A.; Nicolás, C. Evidence for a role of gibberellins in salicylic acid-modulated early plant responses to abiotic stress in Arabidopsis seeds. Plant Physiol. 2009, 150, 1335–1344. [Google Scholar] [CrossRef]
- Ding, P.; Ding, Y. Stories of salicylic acid: A plant defense hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic acid as a safe plant protector and growth regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Li, A.; Sun, X.; Liu, L. Action of salicylic acid on plant growth. Front. Plant Sci. 2022, 13, 878076. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, Z.; Chu, Z. Emerging roles of salicylic acid in plant saline stress tolerance. Int. J. Mol. Sci. 2023, 24, 3388. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.; Song, L.Y.; Zeng, L.L.; Guo, Z.J.; Ma, D.N.; Wei, M.Y.; Zhang, L.D.; Wang, X.X.; Zheng, H.L. NADPH oxidase-dependent H2O2 production mediates salicylic acid-induced salt tolerance in mangrove plant Kandelia obovata by regulating Na+/K+ and redox homeostasis. Plant J. 2024, 118, 1119–1135. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Zhong, Q.; Hu, H.; Fan, B.; Zhu, C.; Chen, Z. Biosynthesis and roles of salicylic acid in balancing stress response and growth in plants. Int. J. Mol. Sci. 2021, 22, 11672. [Google Scholar] [CrossRef]
- Hanif, S.; Mahmood, A.; Javed, T.; Bibi, S.; Zia, M.A.; Asghar, S.; Naeem, Z.; Ercisli, S.; Rahimi, M.; Ali, B. Exogenous application of salicylic acid ameliorates salinity stress in barley (Hordeum vulgare L.). BMC Plant Biol. 2024, 24, 270. [Google Scholar] [CrossRef]
- Jia, X.; Wang, L.; Zhao, H.; Zhang, Y.; Chen, Z.; Xu, L.; Yi, K. The origin and evolution of salicylic acid signaling and biosynthesis in plants. Mol. Plant 2023, 16, 245–259. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, W.; Zhao, Q. Salicylic acid biosynthesis is not from phenylalanine in Arabidopsis. J. Integr. Plant Biol. 2023, 65, 881–887. [Google Scholar] [CrossRef]
- Raskin, I. Role of salicylic acid in plants. Ann. Rev. Plant Biol. 1992, 43, 439–463. [Google Scholar] [CrossRef]
- Mimouni, H.; Wasti, S.; Manaa, A.; Gharbi, E.; Chalh, A.; Vandoorne, B.; Lutts, S.; Ben Ahmed, H. Does salicylic acid (SA) improve tolerance to salt stress in plants? A study of SA effects on tomato plant growth, water dynamics, photosynthesis, and biochemical parameters. OMICS 2016, 20, 180–910. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, R.; Gheith, E.M.S.; Hussain, H.A.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Role of exogenous-applied salicylic acid, zinc and glycine betaine to improve drought-tolerance in wheat during reproductive growth stages. BMC Plant Biol. 2021, 21, 574. [Google Scholar] [CrossRef]
- Janda, T.; Gondor, O.K.; Yordanova, R.; Szalai, G.; Pál, M. Salicylic acid and photosynthesis: Signalling and effects. Acta Physiol. Plant. 2014, 36, 2537–2546. [Google Scholar] [CrossRef]
- Khan, W.; Prithiviraj, B.; Smith, D.L. Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant Physiol. 2003, 160, 485–492. [Google Scholar] [CrossRef]
- Janda, T.; Szalai, G.; Tari, I.; Páldi, E. Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta 1999, 208, 175–180. [Google Scholar] [CrossRef]
- Ananieva, E.A.; Alexieva, V.S.; Popova, L.P. Treatment with salicylic acid decreases the effects of paraquat on photosynthesis. J. Plant Physiol. 2002, 159, 685–693. [Google Scholar] [CrossRef]
- Henschel, J.M.; Dantas, E.F.O.; Soares, V.A.; Santos, S.K.D.; Santos, L.W.O.D.; Dias, T.J.; Batista, D.S. Salicylic acid mitigates the effects of mild drought stress on radish (Raphanus sativus) growth. Funct. Plant Biol. 2022, 49, 822–831. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustaka, J.; Ouzounidou, G.; Moustakas, M. Leaf age-dependent photosystem II photochemistry and oxidative stress responses to drought stress in Arabidopsis thaliana are modulated by flavonoid accumulation. Molecules 2021, 26, 4157. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Ouzounidou, G.; Moustakas, M. Hormesis responses of photosystem II in Arabidopsis thaliana under water deficit stress. Int. J. Mol. Sci. 2023, 24, 9573. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Moustakas, M. Plant Photochemistry, Reactive Oxygen Species, and Photoprotection. Photochem 2022, 2, 5–8. [Google Scholar] [CrossRef]
- Zhu, X.G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef]
- Wu, A.; Hammer, G.L.; Doherty, A.; von Caemmerer, S.; Farquhar, G.D. Quantifying impacts of enhancing photosynthesis on crop yield. Nat. Plants 2019, 5, 380388. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.J.; Masclaux-Daubresse, C.; Strittmatter, G.; Weber, A.P.M.; Taylor, S.H.; Harbinson, J.; Yin, X.; Long, S.; Paul, M.J.; Westhoff, P.; et al. Improving crop yield potential: Underlying biological processes and future prospects. Food Energy Secur. 2023, 12, e435. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Gaju, O.; Bowerman, A.F.; Buck, S.A.; Evans, J.R.; Furbank, R.T.; Gilliham, M.; Millar, A.H.; Pogson, B.J.; Reynolds, M.P.; et al. Enhancing crop yields through improvements in the efficiency of photosynthesis and respiration. New Phytol. 2023, 237, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Ruban, A.V. Dynamic non-photochemical quenching in plants: From molecular mechanism to productivity. Plant J. 2020, 101, 885–896. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.P.; Burgess, S.J.; Doran, L.; Hansen, J.; Manukyan, L.; Maryn, N.; Gotarkar, D.; Leonelli, L.; Niyogi, K.K.; Long, S.P. Soybean photosynthesis and crop yield are improved by accelerating recovery from photoprotection. Science 2022, 377, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Leister, D. Enhancing the light reactions of photosynthesis: Strategies, controversies, and perspectives. Mol. Plant. 2023, 16, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D. Perspectives of improving rice photosynthesis for higher grain yield. Crop Environ. 2024, 3, 123–137. [Google Scholar] [CrossRef]
- Poór, P.; Tari, I. Regulation of stomatal movement and photosynthetic activity in guard cells of tomato abaxial epidermal peels by salicylic acid. Funct. Plant Biol. 2012, 39, 1028–1037. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.-D.S.; Moustaka, J.; İşgören, S.; Şaş, B. Harnessing the role of foliar applied salicylic acid in decreasing chlorophyll content to reassess photosystem II photoprotection in crop plants. Int. J. Mol. Sci. 2022, 23, 7038. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Moustaka, J.; Şaş, B.; İşgören, S.; Morales, F. Mechanistic insights on salicylic acid mediated enhancement of photosystem II function in oregano seedlings subjected to moderate drought stress. Plants 2023, 12, 518. [Google Scholar] [CrossRef] [PubMed]
- Janda, K.; Hideg, E.; Szalai, G.; Kovács, L.; Janda, T. Salicylic acid may indirectly influence the photosynthetic electron transport. J. Plant Physiol. 2012, 169, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Ullah, C.; Chen, Y.H.; Ortega, M.A.; Tsai, C.J. The diversity of salicylic acid biosynthesis and defense signaling in plants: Knowledge gaps and future opportunities. Curr. Opin. Plant Biol. 2023, 72, 102349. [Google Scholar] [CrossRef] [PubMed]
- Fragkiska, M. Wild and cultivated vegetables, herbs and spices in Greek antiquity (900 B.C. to 400 B.C.). Environ. Archaeol. 2013, 10, 73–82. [Google Scholar]
- Liu, J.X.; Wang, H.; Feng, K.; Li, T.; Liu, Y.H.; Duan, A.Q.; Shu, S.; Liu, H.; Xiong, A.S. AgDHAR2, a chloroplast-located dehydroascorbate reductase, modulates the ascorbate accumulation and drought stress response in celery. Environ. Exp. Bot. 2022, 202, 105006. [Google Scholar] [CrossRef]
- Li, M.Y.; Hou, X.L.; Wang, F.; Tan, G.F.; Xu, Z.S.; Xiong, A.S. Advances in the research of celery, an important Apiaceae vegetable crop. Crit. Rev. Biotechnol. 2018, 38, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, N.; Bemani, N.M.; Mohammadinejad, A.; Mohajeri, S.A. Beneficial effects of celery (Apium graveolens) on metabolic syndrome: A review of the existing evidences. Phytother. Res. 2019, 33, 3040–3053. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, J.; Wang, C.; Han, K.; Hu, L.; Niu, T.; Yang, Y.; Chang, Y.; Xie, J. Exogenous proline enhances systemic defense against salt stress in celery by regulating photosystem, phenolic compounds, and antioxidant system. Plants 2023, 12, 928. [Google Scholar] [CrossRef]
- Ma, J.Z.; Zhang, M.; Liu, Z.G.; Wang, M.; Sun, Y.; Zheng, W.K.; Lu, H. Copper-based-zinc-boron foliar fertilizer improved yield, quality, physiological characteristics, and microelement concentration of celery (Apium graveolens L.). Environ. Pollut. Bioavailab. 2019, 31, 261–271. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. Interaction of proline, sugars, and anthocyanins during photosynthetic acclimation of Arabidopsis thaliana to drought stress. J. Plant Physiol. 2012, 169, 577–585. [Google Scholar] [CrossRef]
- Smeekens, S. Drought resistance: Spraying for yield. Nat. Plants 2017, 3, 17023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Rosental, L.; Ji, B.; Brotman, Y.; Dai, M. Metabolite-mediated adaptation of crops to drought and the acquisition of tolerance. Plant J. 2024, 118, 626–644. [Google Scholar] [CrossRef] [PubMed]
- Loutfy, N.; El-Tayeb, M.A.; Hassanen, A.M.; Moustafa, M.F.; Sakuma, Y.; Inouhe, M. Changes in the water status and osmotic solute contents in response to drought and salicylic acid treatments in four different cultivars of wheat (Triticum aestivum). J. Plant Res. 2012, 125, 173–184. [Google Scholar] [PubMed]
- Shi, Q.; Bao, Z.; Zhu, Z.; Ying, Q.; Qian, Q. Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul. 2006, 48, 127–135. [Google Scholar] [CrossRef]
- Abdelhameed, R.E.; Abdel Latef, A.A.H.; Shehata, R.S. Physiological responses of salinized fenugreek (Trigonella foenum-graecum L.) plants to foliar application of salicylic acid. Plants 2021, 10, 657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, S.; Yang, S.; Chen, Y. Salicylic acid alleviates cadmium-induced inhibition of growth and photosynthesis through upregulating antioxidant defense system in two melon cultivars (Cucumis melo L.). Protoplasma 2015, 252, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, H.; Huang, X.; Xing, J.; Yao, J.; Yin, T.; Jiang, J.; Wang, P.; Xu, B. STAYGREEN-mediated chlorophyll a catabolism is critical for photosystem stability during heat-induced leaf senescence in perennial ryegrass. Plant Cell Environ. 2022, 45, 1412–1427. [Google Scholar] [CrossRef]
- Pancheva, T.V.; Popova, L.P.; Uzunova, A.M. Effect of salicylic acid on growth and photosynthesis in barley plants. J. Plant Physiol. 1996, 149, 57–63. [Google Scholar] [CrossRef]
- Anandhi, S.; Ramanujam, M.P. Effect of salicylic acid on black gram (Vigna mungo) cultivars. Ind. J. Plant Physiol. 1997, 2, 138–141. [Google Scholar]
- Chandra, A.; Bhatt, R.K. Biochemical and physiological response to salicylic acid in relation to the systemic acquired resistance. Photosynthetica 1998, 45, 255–258. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Hayat, S.; Ahmad, A. Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity and seed yield in Brassica juncea. Photosynthetica 2003, 41, 281–284. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Arfan, M.; Athar, H.R.; Ashraf, M. Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress? J. Plant Physiol. 2007, 164, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Torun, H.; Novák, O.; Mikulík, J.; Strnad, M.; Ayaz, F.A. The effects of exogenous salicylic acid on endogenous phytohormone status in Hordeum vulgare L. under salt stress. Plants 2022, 11, 618. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A. Singlet oxygen production in photosynthesis. J. Exp. Bot. 2005, 56, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.E.; Ivanov, A.G.; Öquist, G.; Grodzinski, B.; Sarhan, F.; Huner, N.P.A. Energy balance, organellar redox status, and acclimation to environmental stress. Can. J. Bot. 2006, 84, 1355–1370. [Google Scholar] [CrossRef]
- Gawroński, P.; Witoń, D.; Vashutina, K.; Bederska, M.; Betliński, B.; Rusaczonek, A.; Karpiński, S. Mitogen-activated protein kinase 4 is a salicylic acid-independent regulator of growth but not of photosynthesis in Arabidopsis. Mol. Plant 2014, 7, 1151–1166. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PSII quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Kasajima, I.; Takahara, K.; Kawai-Yamada, M.; Uchimiya, H. Estimation of the relative sizes of rate constants for chlorophyll de-excitation processes through comparison of inverse fluorescence intensities. Plant Cell Physiol. 2009, 50, 1600–1616. [Google Scholar] [CrossRef]
- Moustakas, M.; Dobrikova, A.; Sperdouli, I.; Hanć, A.; Moustaka, J.; Adamakis, I.-D.S.; Apostolova, E. Photosystem II tolerance to excess zinc exposure and high light stress in Salvia sclarea L. Agronomy 2024, 14, 589. [Google Scholar] [CrossRef]
- Mattila, H.; Sotoudehnia, P.; Kuuslampi, T.; Stracke, R.; Mishra, K.B.; Tyystjärvi, E. Singlet oxygen, flavonols and photoinhibition in green and senescing silver birch leaves. Trees 2021, 35, 1267–1282. [Google Scholar] [CrossRef]
- Adir, N.; Zer, H.; Shochat, S.; Ohad, I. Photoinhibition–a historical perspective. Photosynth. Res. 2003, 76, 343–370. [Google Scholar] [CrossRef]
- Li, L.; Aro, E.M.; Millar, A.H. Mechanisms of photodamage and protein turnover in photoinhibition. Trends Plant Sci. 2018, 23, 667–676. [Google Scholar] [CrossRef]
- Horton, P.; Ruban, A.V.; Walters, R.G. Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 655–684. [Google Scholar] [CrossRef] [PubMed]
- Melis, A. Photosystem-II damage and repair cycle in chloroplasts: What modulates the rate of photodamage in vivo? Trends Plant Sci. 1999, 4, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V.; Johnson, M.P.; Duffy, C.D. The photoprotective molecular switch in the photosystem II antenna. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 167–181. [Google Scholar] [CrossRef]
- Zavafer, A.; Mancilla, C. Concepts of photochemical damage of photosystem II and the role of excessive excitation. J. Photochem. Photobiol. C Photochem. Rev. 2021, 47, 100421. [Google Scholar] [CrossRef]
- Yotsova, E.K.; Dobrikova, A.G.; Stefanov, M.A.; Kouzmanova, M.; Apostolova, E.L. Improvement of the rice photosynthetic apparatus defence under cadmium stress modulated by salicylic acid supply to roots. Theor. Exp. Plant Physiol. 2018, 30, 57–70. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Aziz, U.; Alsahli, A.A.; Alyemeni, M.N.; Ahmad, P. Influence of exogenous salicylic acid and nitric oxide on growth, photosynthesis, and ascorbate-glutathione cycle in salt stressed Vigna angularis. Biomolecules 2020, 10, 42. [Google Scholar] [CrossRef]
- Maslenkova, L.; Peeva, V.; Stojnova, Z.; Popova, L. Salicylic acid-induced changes in photosystem II reactions in barley plants. Biotechnol. Biotechnol. Equip. 2009, 23, 297–300. [Google Scholar] [CrossRef]
- Goggin, F.L.; Fischer, H.D. Singlet oxygen signalling and its potential roles in plant biotic interactions. Plant Cell Environ. 2024, 47, 1957–1970. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Tanou, G.; Adamakis, I.D.; Eleftheriou, E.P.; Moustakas, M. Leaf age dependent photoprotective and antioxidative mechanisms to paraquat-induced oxidative stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 13989–14006. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.-D.S.; Şaş, B.; İşgören, S.; Moustaka, J.; Morales, F. Mechanistic approach on melatonin-induced hormesis of photosystem II function in the medicinal plant Mentha spicata. Plants 2023, 12, 4025. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.D.S. Editorial: Reactive oxygen species in chloroplasts and chloroplast antioxidants under abiotic stress. Front. Plant Sci. 2023, 14, 1208247. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signaling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Lambrev, P.H.; Miloslavina, Y.; Jahns, P.; Holzwarth, A.R. On the relationship between non-photochemical quenching and photoprotection of photosystem II. Biochim. Biophys. Acta 2012, 1817, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Hanć, A.; Dobrikova, A.; Sperdouli, I.; Adamakis, I.D.S.; Apostolova, E. Spatial heterogeneity of cadmium effects on Salvia sclarea leaves revealed by chlorophyll fluorescence imaging analysis and laser ablation inductively coupled plasma mass spectrometry. Materials 2019, 12, 2953. [Google Scholar] [CrossRef]
- Pfalz, J.; Liebers, M.; Hirth, M.; Grübler, B.; Holtzegel, U.; Schröter, Y.; Dietzel, L.; Pfannschmidt, T. Environmental control of plant nuclear gene expression by chloroplast redox signals. Front. Plant Sci. 2012, 3, 257. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J.; Turkan, I.; Krieger-Liszkay, A. Redox- and reactive oxygen species-dependent signaling into and out of the photosynthesizing chloroplast. Plant Physiol. 2016, 171, 1541–1550. [Google Scholar] [CrossRef]
- Leverne, L.; Roach, T.; Perreau, F.; Maignan, F.; Krieger-Liszkay, A. Increased drought resistance in state transition mutants is linked to modified plastoquinone pool redox state. Plant Cell Environ. 2023, 46, 3737–3747. [Google Scholar] [CrossRef] [PubMed]
- Bräutigam, K.; Dietzel, L.; Kleine, T.; Ströher, E.; Wormuth, D.; Dietz, K.J.; Radke, D.; Wirtz, M.; Hell, R.; Dörmann, P.; et al. Dynamic plastid redox signals integrate gene expression and metabolism to induce distinct metabolic states in photosynthetic acclimation in Arabidopsis. Plant Cell 2009, 21, 2715–2732. [Google Scholar] [CrossRef]
- Dietz, K.J.; Pfannschmidt, T. Novel regulators in photosynthetic redox control of plant metabolism and gene expression. Plant Physiol. 2011, 155, 1477–1485. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustaka, J.; Antonoglou, O.; Adamakis, I.D.S.; Dendrinou-Samara, C.; Moustakas, M. Leaf age dependent effects of foliar-sprayed CuZn nanoparticles on photosynthetic efficiency and ROS generation in Arabidopsis thaliana. Materials 2019, 12, 2498. [Google Scholar] [CrossRef] [PubMed]
- Borisova-Mubarakshina, M.M.; Vetoshkina, D.V.; Ivanov, B.N. Antioxidant and signaling functions of the plastoquinone pool in higher plants. Physiol. Plant. 2019, 166, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Messant, M.; Krieger-Liszkay, A.; Shimakawa, G. Dynamic changes in protein-membrane association for regulating photosynthetic electron transport. Cells 2021, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Mao, H.T.; Wu, N.; Mohi Ud Din, A.; Khan, A.; Zhang, H.Y.; Yuan, S. Salicylic acid protects photosystem II by alleviating photoinhibition in Arabidopsis thaliana under high light. Int. J. Mol. Sci. 2020, 21, 1229. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Panteris, E.; Moustaka, J.; Aydın, T.; Bayçu, G.; Moustakas, M. Mechanistic insights on salicylic acid-induced enhancement of photosystem II function in basil plants under non-stress or mild drought stress. Int. J. Mol. Sci. 2024, 25, 5728. [Google Scholar] [CrossRef]
- Ali, A.; Kant, K.; Kaur, N.; Gupta, S.; Jindal, P.; Gill, S.S.; Naeem, M. Salicylic acid: Homeostasis, signalling and phytohormone crosstalk in plants under environmental challenges. S. Afr. J. Bot. 2024, 169, 314–335. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. Spatio-temporal heterogeneity in Arabidopsis thaliana leaves under drought stress. Plant Biol. 2012, 14, 118–128. [Google Scholar] [CrossRef]
- Borek, M.; Bączek-Kwinta, R.; Rapacz, M. Photosynthetic activity of variegated leaves of Coleus × hybridus hort. cultivars characterised by chlorophyll fluorescence techniques. Photosynthetica 2016, 54, 331–339. [Google Scholar] [CrossRef]
- Moustaka, J.; Panteris, E.; Adamakis, I.D.S.; Tanou, G.; Giannakoula, A.; Eleftheriou, E.P.; Moustakas, M. High anthocyanin accumulation in poinsettia leaves is accompanied by thylakoid membrane unstacking, acting as a photoprotective mechanism, to prevent ROS formation. Environ. Exp. Bot. 2018, 154, 44–55. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Hendrickson, L.; Furbank, R.T.; Chow, W.S. A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth. Res. 2004, 82, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll fluorescence as a non-intrusive indicator for rapid assessment of in vivo photosynthesis. In Ecophysiology of Photosynthesis; Schulze, E.D., Caldwell, M.M., Eds.; Series Ecological Studies; Springer: Berlin/Heidelberg, Germany, 1994; Volume 100, pp. 49–70. [Google Scholar]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Bilger, W.; Schreiber, U.; Bock, M. Determination of the quantum efficiency of photosystem II and of non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia 1995, 102, 425–432. [Google Scholar] [CrossRef]



| Treatments | Soil Water Content (%) * | Relative Leaf Water Content (%) | Chlorophyll Content ** |
|---|---|---|---|
| Water-sprayed plants after 48 h | 70 ± 3% | 81 ± 0.4% | 6.14 ± 0.52 |
| Water-sprayed plants after 96 h | 27 ± 2% | 79 ± 0.2% | 7.11 ± 2.33 |
| Water-sprayed plants after 192 h | 5 ± 1% | 73 ± 0.1% | 2.82 ± 0.97 |
| Salicylic acid-sprayed plants after 48 h | 82 ± 4% | 85 ± 0.3% | 4.04 ± 1.12 |
| Salicylic acid-sprayed plants after 96 h | 38 ± 3% | 83 ± 0.3% | 12.80 ± 1.99 |
| Salicylic acid-sprayed plants after 192 h | 9 ± 2% | 80 ± 0.2% | 20.23 ± 4.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moustakas, M.; Panteris, E.; Moustaka, J.; Aydın, T.; Bayçu, G.; Sperdouli, I. Modulation of Photosystem II Function in Celery via Foliar-Applied Salicylic Acid during Gradual Water Deficit Stress. Int. J. Mol. Sci. 2024, 25, 6721. https://doi.org/10.3390/ijms25126721
Moustakas M, Panteris E, Moustaka J, Aydın T, Bayçu G, Sperdouli I. Modulation of Photosystem II Function in Celery via Foliar-Applied Salicylic Acid during Gradual Water Deficit Stress. International Journal of Molecular Sciences. 2024; 25(12):6721. https://doi.org/10.3390/ijms25126721
Chicago/Turabian StyleMoustakas, Michael, Emmanuel Panteris, Julietta Moustaka, Tuğba Aydın, Gülriz Bayçu, and Ilektra Sperdouli. 2024. "Modulation of Photosystem II Function in Celery via Foliar-Applied Salicylic Acid during Gradual Water Deficit Stress" International Journal of Molecular Sciences 25, no. 12: 6721. https://doi.org/10.3390/ijms25126721
APA StyleMoustakas, M., Panteris, E., Moustaka, J., Aydın, T., Bayçu, G., & Sperdouli, I. (2024). Modulation of Photosystem II Function in Celery via Foliar-Applied Salicylic Acid during Gradual Water Deficit Stress. International Journal of Molecular Sciences, 25(12), 6721. https://doi.org/10.3390/ijms25126721










