Discovery of Di(het)arylmethane and Dibenzoxanthene Derivatives as Potential Anticancer Agents
Abstract
1. Introduction
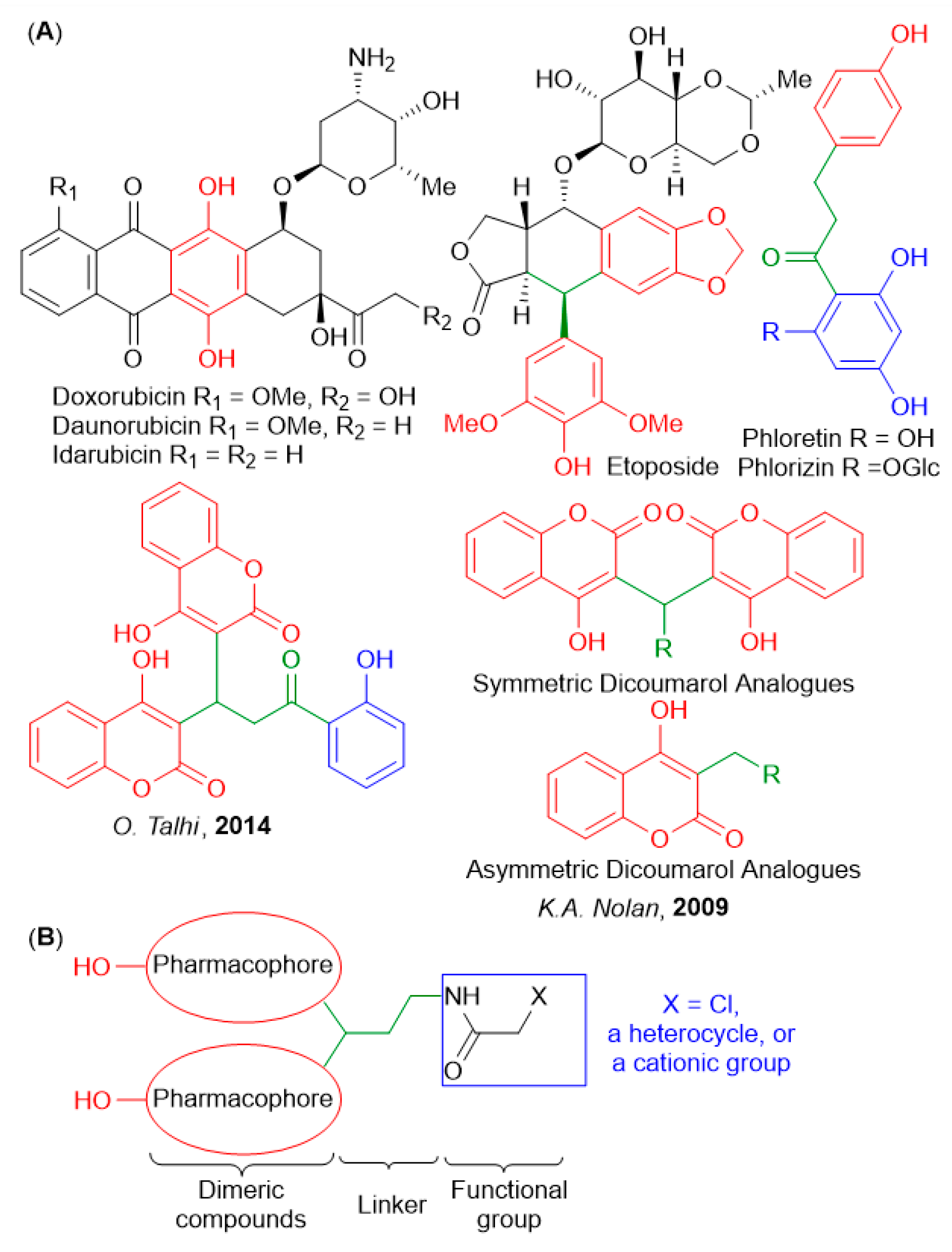
2. Results
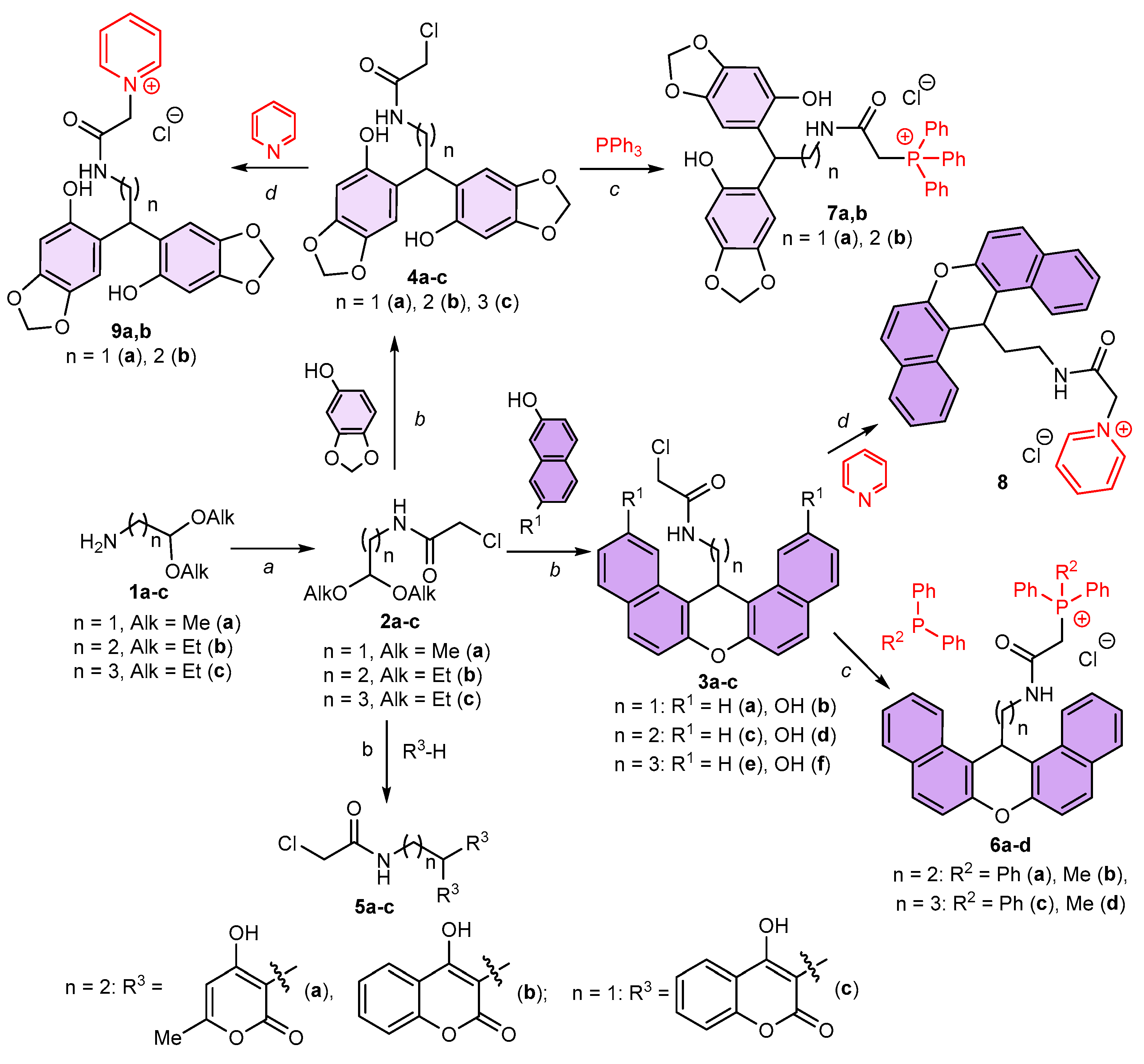
2.1. Synthesis and Characterization of the Compounds
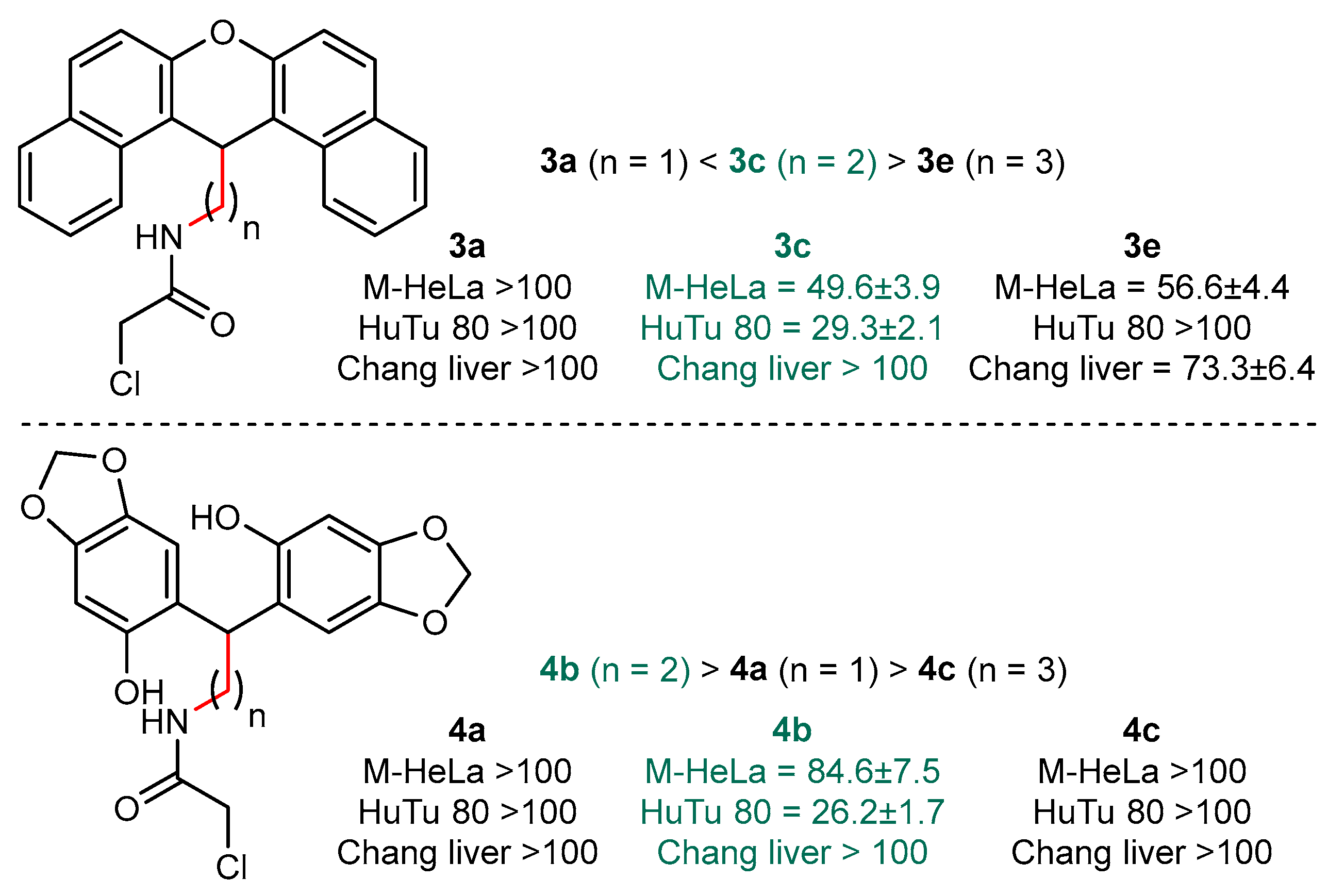
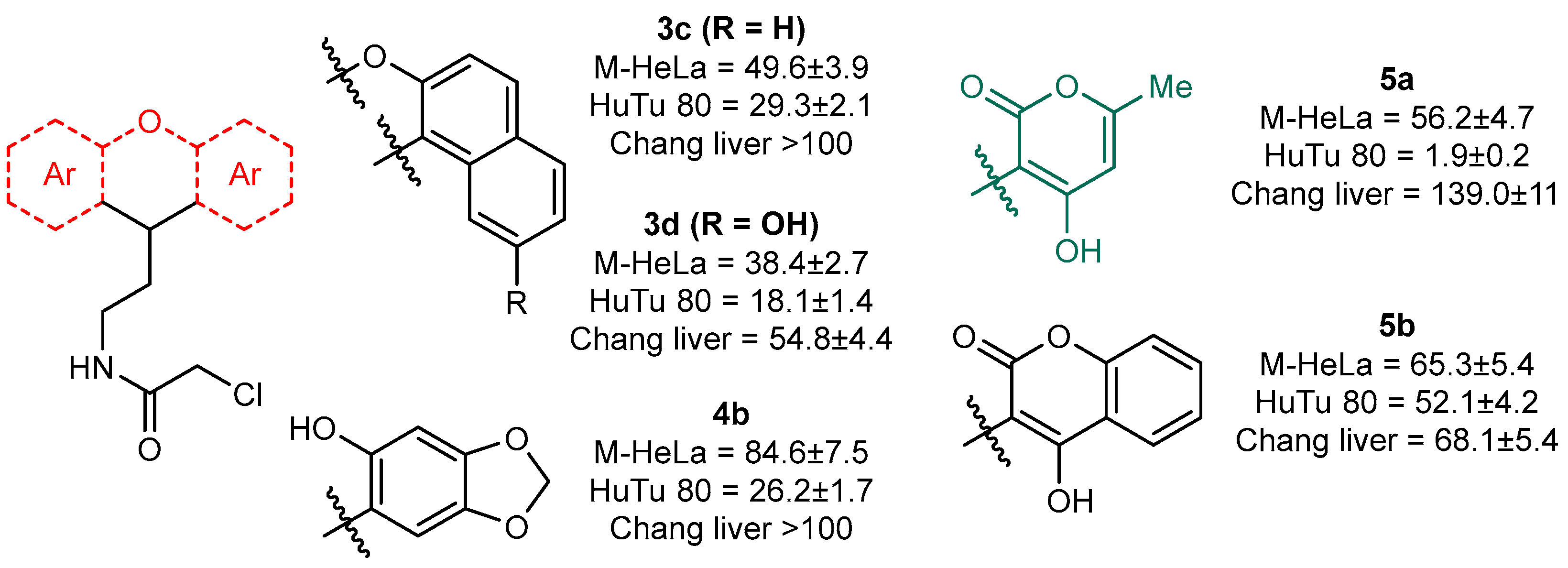
2.2. Structure−Activity Relationships
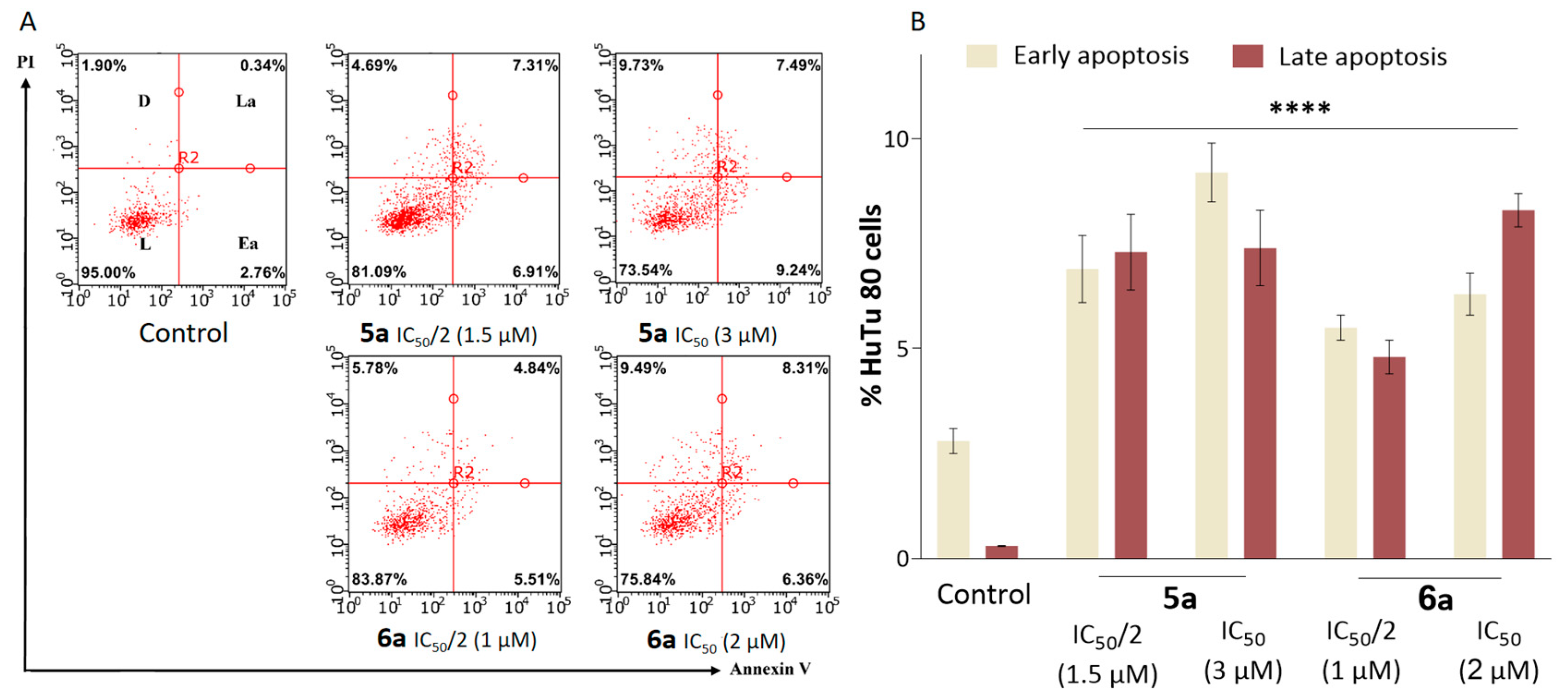
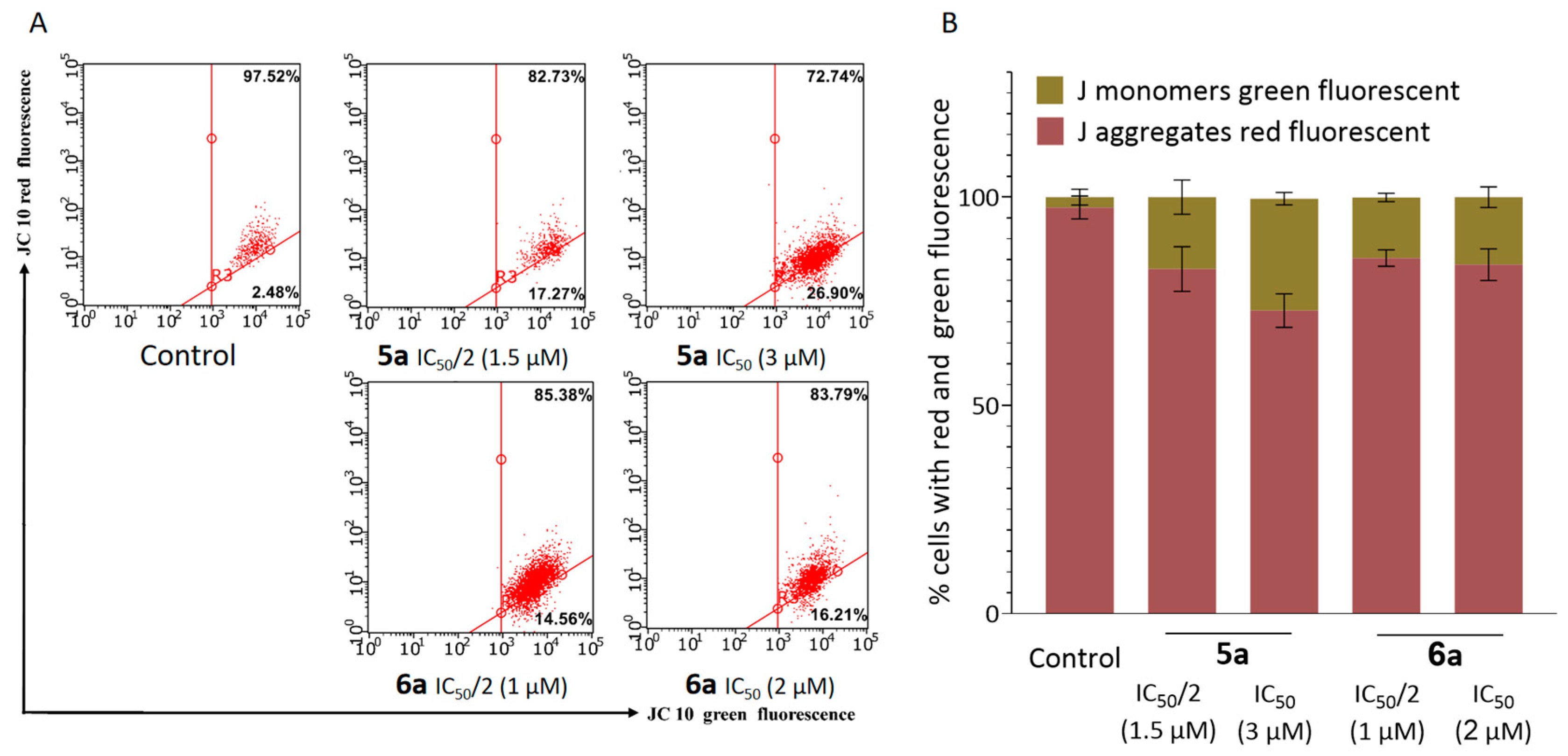
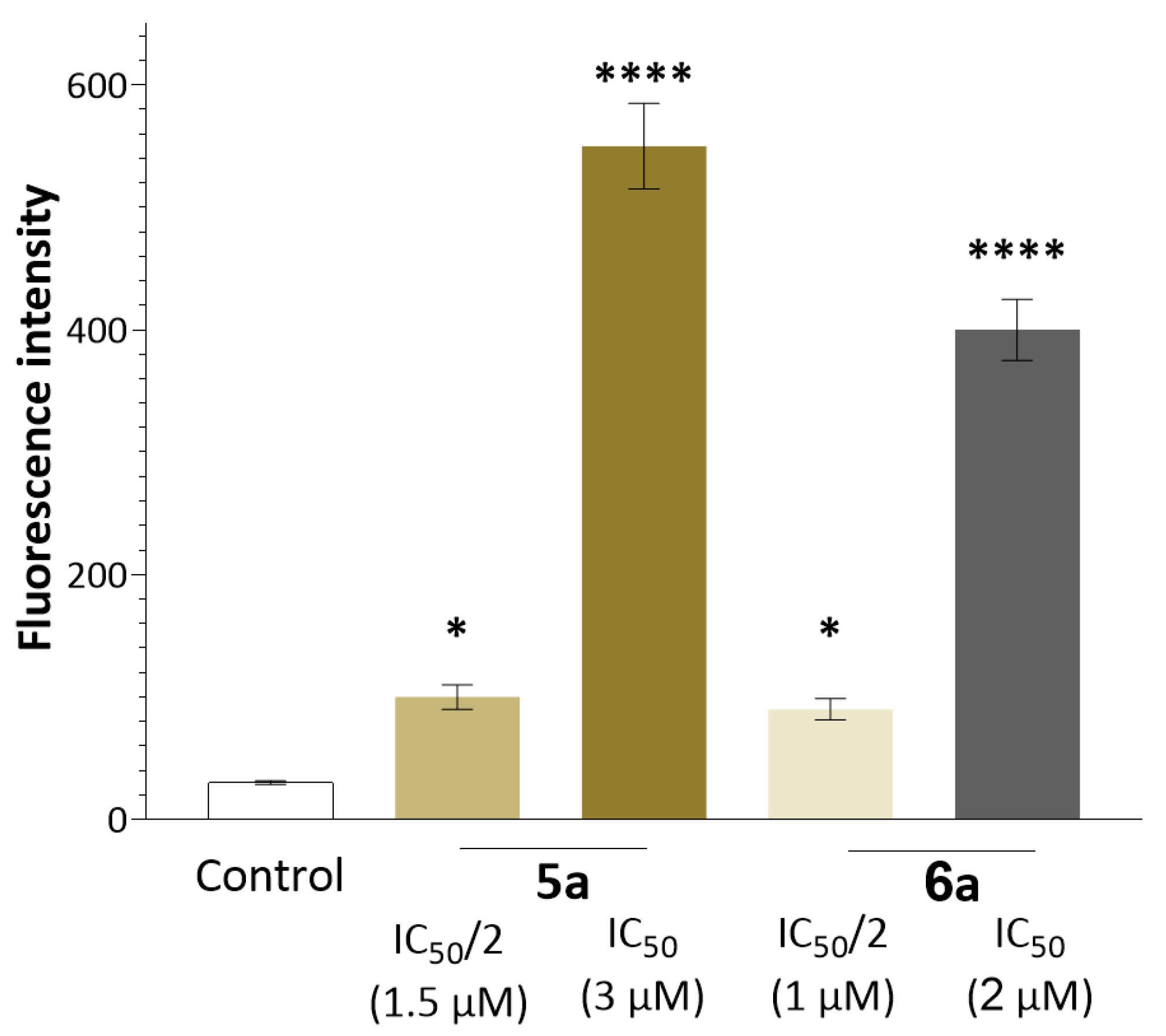
2.3. Antitumor Mechanism
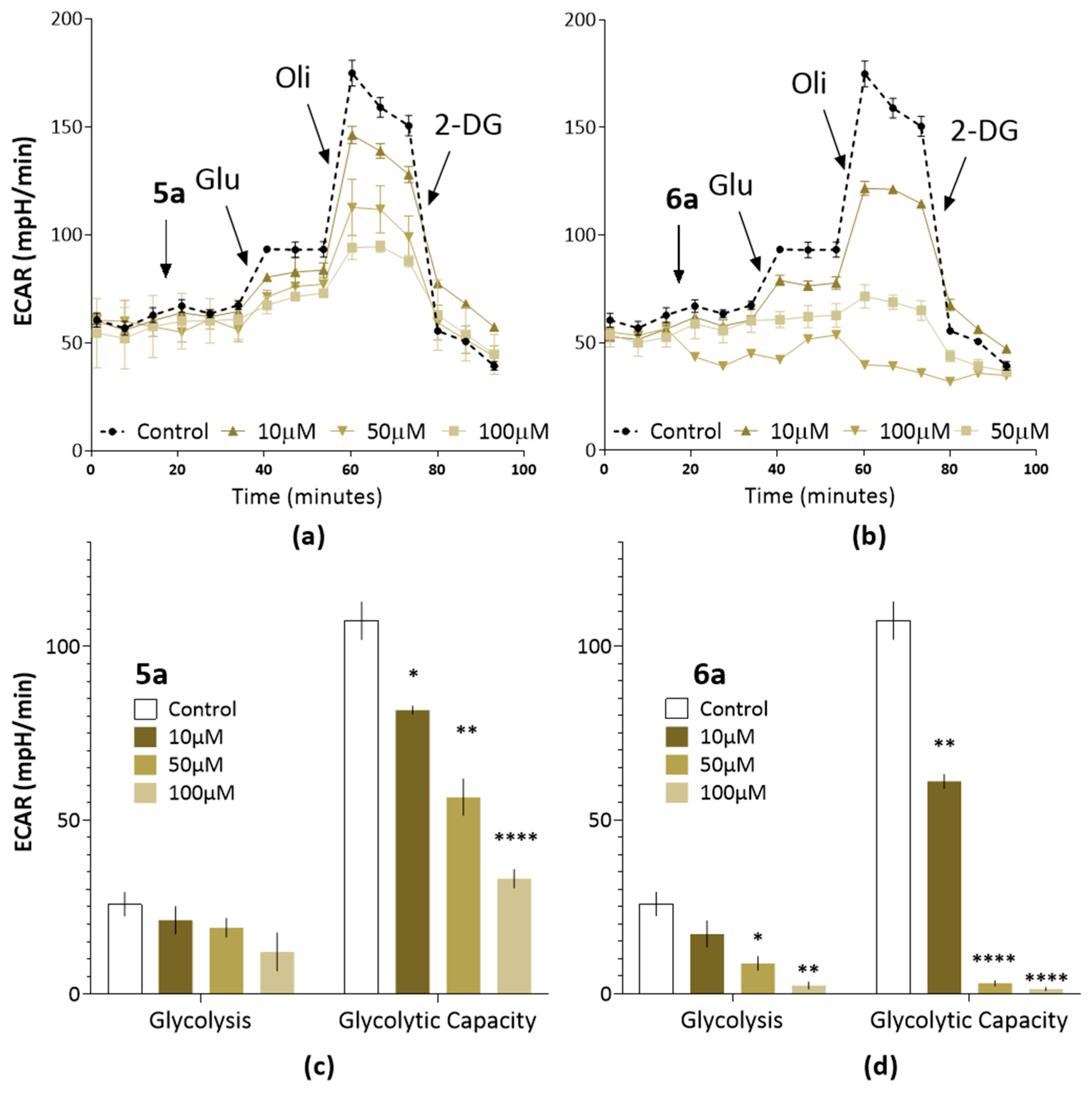
2.4. Suppression of Proliferation and Glycolysis of Human Ovarian Teratocarcinoma Cells PA-1
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hassanpour, S.H.; Dehghani, M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017, 4, 127–129. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Orlikova, B.; Legrand, N.; Panning, J.; Dicato, M.; Diederich, M. Anti-Inflammatory and Anticancer Drugs from Nature. Adv. Nutr. Cancer 2014, 159, 123–143. [Google Scholar] [CrossRef]
- Menezes, J.C.; Diederich, M.F. Natural dimers of coumarin, chalcones, and resveratrol and the link between structure and pharmacology. Eur. J. Med. Chem. 2019, 182, 111637. [Google Scholar] [CrossRef]
- Egan, D.; O’Kennedy, R.; Moran, E.; Cox, D.; Prosser, E.; Thornes, R.D. The Pharmacology, Metabolism, Analysis, and Applications of Coumarin and Coumarin-Related Compounds. Drug Metab. Rev. 1990, 22, 503–529. [Google Scholar] [CrossRef]
- Tatipamula, V.B.; Kukavica, B. Phenolic compounds as antidiabetic, anti-inflammatory, and anticancer agents and improvement of their bioavailability by liposomes. Cell Biochem. Funct. 2021, 39, 926–944. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.; Ferreira, I.C. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Herrmann, L.; Leidenberger, M.; de Morais, A.S.; Mai, C.; Çapci, A.; Silva, M.d.C.B.; Plass, F.; Kahnt, A.; Moreira, D.R.M.; Kappes, B.; et al. Autofluorescent antimalarials by hybridization of artemisinin and coumarin: In vitro/in vivo studies and live-cell imaging. Chem. Sci. 2023, 14, 12941–12952. [Google Scholar] [CrossRef]
- Wu, S.; Huang, Y.; Wang, T.; Li, K.; Lu, J.; Huang, M.; Dong, G.; Sheng, C. Evodiamine-Inspired Topoisomerase-Histone Deacetylase Dual Inhibitors: Novel Orally Active Antitumor Agents for Leukemia Therapy. J. Med. Chem. 2022, 65, 4818–4831. [Google Scholar] [CrossRef]
- Menezes, J.C.J.M.D.S.; Orlikova, B.; Morceau, F.; Diederich, M. Natural and Synthetic Flavonoids: Structure–Activity Relationship and Chemotherapeutic Potential for the Treatment of Leukemia. Crit. Rev. Food Sci. Nutr. 2015, 56, S4–S28. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Aguilar, A.; González-Bakker, A.; Jovanović, M.; Stojanov, S.J.; Puerta, A.; Gargano, A.; Dinić, J.; Vega-Báez, J.L.; Merino-Montiel, P.; Montiel-Smith, S.; et al. Coumarins-lipophilic cations conjugates: Efficient mitocans targeting carbonic anhydrases. Bioorg. Chem. 2024, 145, 107168. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Feng, D.; Lv, J.; Cui, X.; Wang, Y.; Wang, Q.; Zhang, L. Application Prospects of Triphenylphosphine-Based Mitochondria-Targeted Cancer Therapy. Cancers 2023, 15, 666. [Google Scholar] [CrossRef] [PubMed]
- Gibadullina, E.; Neganova, M.; Aleksandrova, Y.; Nguyen, H.B.T.; Voloshina, A.; Khrizanforov, M.; Nguyen, T.T.; Vinyukova, E.; Volcho, K.; Tsypyshev, D.; et al. Hybrids of Sterically Hindered Phenols and Diaryl Ureas: Synthesis, Switch from Antioxidant Activity to ROS Generation and Induction of Apoptosis. Int. J. Mol. Sci. 2023, 24, 12637. [Google Scholar] [CrossRef] [PubMed]
- Chugunova, E.; Gibadullina, E.; Matylitsky, K.; Bazarbayev, B.; Neganova, M.; Volcho, K.; Rogachev, A.; Akylbekov, N.; Nguyen, H.B.T.; Voloshina, A.; et al. Diverse Biological Activity of Benzofuroxan/Sterically Hindered Phenols Hybrids. Pharmaceuticals 2023, 16, 499. [Google Scholar] [CrossRef] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2012, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Angsutararux, P.; Luanpitpong, S.; Issaragrisil, S. Chemotherapy-Induced Cardiotoxicity: Overview of the Roles of Oxidative Stress. Oxidative Med. Cell. Longev. 2015, 2015, 795602. [Google Scholar] [CrossRef] [PubMed]
- Orlikova, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr. 2011, 6, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Orlikova, B.; Schnekenburger, M.; Zloh, M.; Golais, F.; Diederich, M.; Tasdemir, D. Natural chalcones as dual inhibitors of HDACs and NF-κB. Oncol. Rep. 2012, 28, 797–805. [Google Scholar] [CrossRef]
- Talhi, O.; Schnekenburger, M.; Panning, J.; Pinto, D.G.; Fernandes, J.A.; Paz, F.A.A.; Jacob, C.; Diederich, M.; Silva, A.M. Bis(4-hydroxy-2H-chromen-2-one): Synthesis and effects on leukemic cell lines proliferation and NF-κB regulation. Bioorg. Med. Chem. 2014, 22, 3008–3015. [Google Scholar] [CrossRef]
- Nolan, K.A.; Doncaster, J.R.; Dunstan, M.S.; Scott, K.A.; Frenkel, A.D.; Siegel, D.; Ross, D.; Barnes, J.; Levy, C.; Leys, D.; et al. Synthesis and Biological Evaluation of Coumarin-Based Inhibitors of NAD(P)H: Quinone Oxidoreductase-1 (NQO1). J. Med. Chem. 2009, 52, 7142–7156. [Google Scholar] [CrossRef] [PubMed]
- Bérubé, G. An overview of molecular hybrids in drug discovery. Expert Opin. Drug Discov. 2016, 11, 281–305. [Google Scholar] [CrossRef] [PubMed]
- Smolobochkin, A.V.; Gazizov, A.S.; Yakhshilikova, L.J.; Bekrenev, D.D.; Burilov, A.R.; Pudovik, M.A.; Lyubina, A.P.; Amerhanova, S.K.; Voloshina, A.D. Synthesis and Biological Evaluation of Taurine-Derived Diarylmethane and Dibenzoxanthene Derivatives as Possible Cytotoxic and Antimicrobial Agents. Chem. Biodivers. 2022, 19, e202100970. [Google Scholar] [CrossRef]
- Bergeron, K.L.; Murphy, E.L.; Majofodun, O.; Muñoz, L.D.; Williams, J.C.; Almeida, K.H. Arylphosphonium salts interact with DNA to modulate cytotoxicity. Mutat. Res. Toxicol. Environ. Mutagen. 2009, 673, 141–148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, Y.; Wang, J.; Jin, L.; Chang, Y.; Duan, J.; Lu, Y. A new conjugated poly(pyridinium salt) derived from phenanthridine diamine: Its synthesis, optical properties and interaction with calf thymus DNA. Polym. J. 2015, 47, 753–759. [Google Scholar] [CrossRef]
- Kaya, K.; Roy, S.; Nogues, J.C.; Rojas, J.C.; Sokolikj, Z.; Zorio, D.A.R.; Alabugin, I.V. Optimizing Protonation States for Selective Double-Strand DNA Photocleavage in Hypoxic Tumors: pH-Gated Transitions of Lysine Dipeptides. J. Med. Chem. 2016, 59, 8634–8647. [Google Scholar] [CrossRef] [PubMed]
- Breiner, B.; Kaya, K.; Roy, S.; Yang, W.-Y.; Alabugin, I.V. Hybrids of amino acids and acetylenic DNA-photocleavers: Optimising efficiency and selectivity for cancer phototherapy. Org. Biomol. Chem. 2012, 10, 3974–3987. [Google Scholar] [CrossRef] [PubMed]
- Zavarzin, I.V.; Yarovenko, V.N.; Shirokov, A.V.; Smirnova, N.G.; Es’kov, A.A.; Krayushkin, M.M. Synthesis and reactivity of monothio-oxamides. Arkivoc 2003, 2003, 205–223. [Google Scholar] [CrossRef]
- Peña-Morán, O.A.; Villarreal, M.L.; Álvarez-Berber, L.; Meneses-Acosta, A.; Rodríguez-López, V. Cytotoxicity, Post-Treatment Recovery, and Selectivity Analysis of Naturally Occurring Podophyllotoxins from Bursera fagaroides var. fagaroides on Breast Cancer Cell Lines. Molecules 2016, 21, 1013. [Google Scholar] [CrossRef]
- Strobykina, I.Y.; Voloshina, A.D.; Andreeva, O.V.; Sapunova, A.S.; Lyubina, A.P.; Amerhanova, S.K.; Belenok, M.G.; Saifina, L.F.; Semenov, V.E.; Kataev, V.E. Synthesis, antimicrobial activity and cytotoxicity of triphenylphosphonium (TPP) conjugates of 1,2,3-triazolyl nucleoside analogues. Bioorg. Chem. 2021, 116, 105328. [Google Scholar] [CrossRef]
- Voloshina, A.D.; Sapunova, A.S.; Kulik, N.V.; Belenok, M.G.; Strobykina, I.Y.; Lyubina, A.P.; Gumerova, S.K.; Kataev, V.E. Antimicrobial and cytotoxic effects of ammonium derivatives of diterpenoids steviol and isosteviol. Bioorg. Med. Chem. 2021, 32, 115974. [Google Scholar] [CrossRef]
- Shao, M.; Zhang, J.; Zhang, J.; Shi, H.; Zhang, Y.; Ji, R.; Mao, F.; Qian, H.; Xu, W.; Zhang, X. SALL4 promotes gastric cancer progression via hexokinase II mediated glycolysis. Cancer Cell Int. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Amoedo, N.; Obre, E.; Rossignol, R. Drug discovery strategies in the field of tumor energy metabolism: Limitations by metabolic flexibility and metabolic resistance to chemotherapy. Biochim. Biophys. Acta BBA Bioenerg. 2017, 1858, 674–685. [Google Scholar] [CrossRef]
- Romero, N.; Swain, P.; Neilson, A.; Dranka, B.P. Improving Quantification of Cellular Glycolytic Rate Using Agilent Seahorse XF Technology; 5991-7894EN; Agilent Technologies: Santa Clara, CA, USA, 2017. [Google Scholar]
- Mookerjee, S.A.; Goncalves, R.L.S.; Gerencser, A.A.; Nicholls, D.G.; Brand, M.D. The contributions of respiration and glycolysis to extracellular acid production. Biochim. Biophys. Acta 2015, 1847, 171–181. [Google Scholar] [CrossRef]
- Li, L.; Zhu, L.; Hao, B.; Gao, W.; Wang, Q.; Li, K.; Wang, M.; Huang, M.; Liu, Z.; Yang, Q.; et al. iNOS-derived nitric oxide promotes glycolysis by inducing pyruvate kinase M2 nuclear translocation in ovarian cancer. Oncotarget 2017, 8, 33047–33063. [Google Scholar] [CrossRef]
- Wang, Y.; Yun, Y.; Wu, B.; Wen, L.; Wen, M.; Yang, H.; Zhao, L.; Liu, W.; Huang, S.; Wen, N.; et al. FOXM1 promotes reprogramming of glucose metabolism in epithelial ovarian cancer cells via activation of GLUT1 and HK2 transcription. Oncotarget 2016, 7, 47985–47997. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS; Bruker AXS Inc.: Madison, WI, USA, 1997. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Structural Chem. 2014, 71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]



| Test Compounds | IC50 (µM) | ||
|---|---|---|---|
| Cancer Cell Lines | Normal Cell Line | ||
| M-HeLa | HuTu 80 | Chang Liver | |
| 2a | >100 | >100 | >100 |
| 2b | >100 | >100 | >100 |
| 3a | >100 | >100 | >100 |
| 3b | 96.4 ± 8.4 | 53.9 ± 4.3 | 78.2 ± 6.8 |
| 3c | 49.6 ± 3.9 | 29.3 ± 2.1 | >100 |
| 3d | 38.4 ± 2.7 | 18.1 ± 1.4 (SI = 3) | 54.8 ± 4.4 |
| 3e | 56.6 ± 4.4 | >100 | 73.3 ± 6.4 |
| 4a | >100 | >100 | >100 |
| 4b | 84.6 ± 7.5 | 26.2 ± 1.7 | >100 |
| 4c | >100 | >100 | >100 |
| 5a | 56.2 ± 4.7 | 1.9 ± 0.2 (SI = 73) a | 139.0 ± 11 |
| 5b | 65.3 ± 5.4 | 52.1 ± 4.2 | 68.1 ± 5.4 |
| 6a | 11.0 ± 8.7 | 1.7 ± 0.1 (SI = 24) | 41 ± 3.5 |
| 6b | 28.4 ± 1.8 | 64.3 ± 5.2 | >100 |
| 6c | 26.6 ± 1.9 | 7.3 ± 0.6 (SI = 4.6) | 33.4 ± 2.8 |
| 6d | 53.2 ± 4.6 | 18.5 ± 1.5 (SI = 3) | 56.2 ± 4.7 |
| 7a | 73.5 ± 6.2 | 59.7 ± 4.6 | >100 |
| 7b | 65.8 ± 5.8 | 55.4 ± 4.5 | >100 |
| 8 | 85.2 ± 7.4 | 58.4 ± 4.6 | 100 ± 9.2 |
| 9a | >100 | >100 | >100 |
| 9b | 97.8 ± 8.2 | >100 | 95.4 ± 8.7 |
| 10a | >100 | >100 | >100 |
| 11a | >100 | >100 | >100 |
| 11b | 94.3 ± 7.9 | 11.5 ± 0.9 | 53.1 ± 4.3 |
| Sorafenib | 35.6 ± 2.8 | 5.0 ± 0.4 (SI = 7) | 35.0 ± 2.7 |
| Doxorubicin | 3.0 ± 0.2 | 3.0 ± 0.2 (SI = 1) | 3.0 ± 0.1 |
| Test Compound | IC50 (µM) | ||||
|---|---|---|---|---|---|
| Cancer Cell Lines | |||||
| Hep-2 | SH-SY5Y | A549 | PA-1 | MCF-7 | |
| 5a | 65.76 ± 1.18 | 75.78 ± 2.84 | 82.08 ± 3.56 | 80.22 ± 4.05 | 85.63 ± 3.12 |
| 6a | 2.34 ± 0.01 | 10.86 ± 0.23 | 5.47 ± 0.07 | 11.20 ± 1.36 | 7.12 ± 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolobochkin, A.; Niyazova, D.; Gazizov, A.; Syzdykbayev, M.; Voloshina, A.; Amerhanova, S.; Lyubina, A.; Neganova, M.; Aleksandrova, Y.; Babaeva, O.; et al. Discovery of Di(het)arylmethane and Dibenzoxanthene Derivatives as Potential Anticancer Agents. Int. J. Mol. Sci. 2024, 25, 6724. https://doi.org/10.3390/ijms25126724
Smolobochkin A, Niyazova D, Gazizov A, Syzdykbayev M, Voloshina A, Amerhanova S, Lyubina A, Neganova M, Aleksandrova Y, Babaeva O, et al. Discovery of Di(het)arylmethane and Dibenzoxanthene Derivatives as Potential Anticancer Agents. International Journal of Molecular Sciences. 2024; 25(12):6724. https://doi.org/10.3390/ijms25126724
Chicago/Turabian StyleSmolobochkin, Andrey, Dinara Niyazova, Almir Gazizov, Marat Syzdykbayev, Alexandra Voloshina, Syumbelya Amerhanova, Anna Lyubina, Margarita Neganova, Yulia Aleksandrova, Olga Babaeva, and et al. 2024. "Discovery of Di(het)arylmethane and Dibenzoxanthene Derivatives as Potential Anticancer Agents" International Journal of Molecular Sciences 25, no. 12: 6724. https://doi.org/10.3390/ijms25126724
APA StyleSmolobochkin, A., Niyazova, D., Gazizov, A., Syzdykbayev, M., Voloshina, A., Amerhanova, S., Lyubina, A., Neganova, M., Aleksandrova, Y., Babaeva, O., Voronina, J., Appazov, N., Sinyashin, O., Alabugin, I., Burilov, A., & Pudovik, M. (2024). Discovery of Di(het)arylmethane and Dibenzoxanthene Derivatives as Potential Anticancer Agents. International Journal of Molecular Sciences, 25(12), 6724. https://doi.org/10.3390/ijms25126724









