Recurrent Glioblastoma—Molecular Underpinnings and Evolving Treatment Paradigms
Abstract
1. Introduction
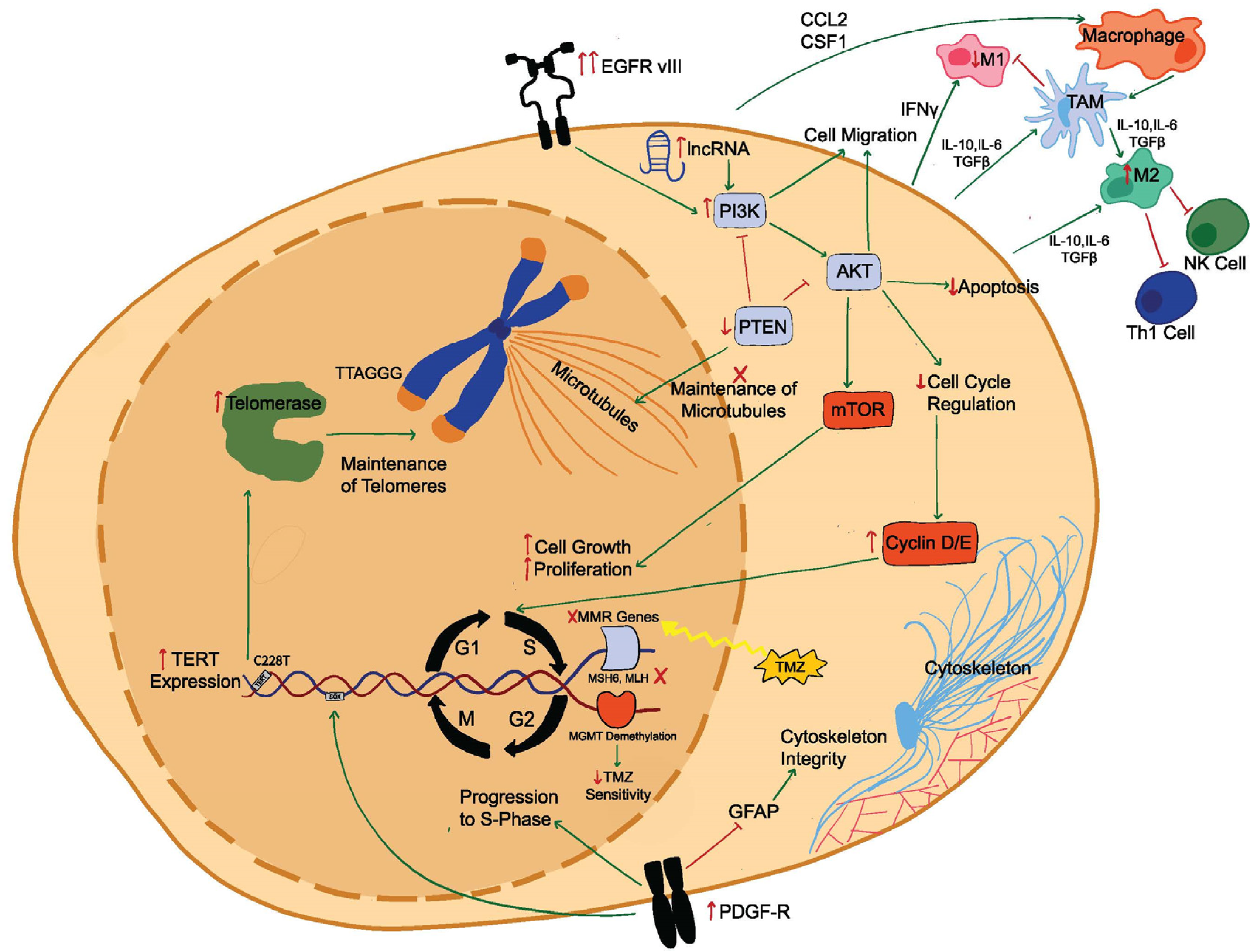
2. Molecular Mechanisms of Recurrence
2.1. Genetic Mutations in Newly Diagnosed Glioblastoma and Recurrent Glioblastoma
2.2. Epigenetic Modifications in Recurrent Glioblastoma
2.3. TME Facilitating Glioblastoma Recurrence
3. The Role of Surgery in Recurrent Glioblastoma
4. Evolving Treatment Paradigms for Recurrent Glioblastoma
4.1. Checkpoint Inhibitors
4.2. CAR-T Cell Therapy
4.3. NK Cell Therapy
4.4. Oncolytic Virotherapy
4.5. Novel Delivery Methods
4.6. Laser Interstitial Thermal Therapy, Electrical Fields, Photodynamic Therapy, and Intratumoral Microdevices
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stupp, R.; Mason, W.P.; Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs. Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef]
- Wick, W.; Platten, M.; Meisner, C.; Felsberg, J.; Tabatabai, G.; Simon, M.; Nikkhah, G.; Papsdorf, K.; Steinbach, J.P.; Sabel, M.; et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: The NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012, 13, 707–715. [Google Scholar] [CrossRef]
- Malmström, A.; Grønberg, B.H.; Marosi, C.; Stupp, R.; Frappaz, D.; Schultz, H.; Abacioglu, U.; Tavelin, B.; Lhermitte, B.; Hegi, M.E.; et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol. 2012, 13, 916–926. [Google Scholar] [CrossRef]
- Perry, J.R.; Laperriere, N.; O’Callaghan, C.J.; Brandes, A.A.; Menten, J.; Phillips, C.; Fay, M.; Nishikawa, R.; Cairncross, J.G.; Roa, W.; et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N. Engl. J. Med. 2017, 376, 1027–1037. [Google Scholar] [CrossRef]
- Marenco-Hillembrand, L.; Wijesekera, O.; Suarez-Meade, P.; Mampre, D.; Jackson, C.; Peterson, J.; Trifiletti, D.; Hammack, J.; Ortiz, K.; Lesser, E.; et al. Trends in glioblastoma: Outcomes over time and type of intervention: A systematic evidence based analysis. J. Neuro-Oncol. 2020, 147, 297–307. [Google Scholar] [CrossRef]
- van Linde, M.E.; Brahm, C.G.; de Witt Hamer, P.C.; Reijneveld, J.C.; Bruynzeel, A.M.E.; Vandertop, W.P.; van de Ven, P.M.; Wagemakers, M.; van der Weide, H.L.; Enting, R.H.; et al. Treatment outcome of patients with recurrent glioblastoma multiforme: A retrospective multicenter analysis. J. Neuro-Oncol. 2017, 135, 183–192. [Google Scholar] [CrossRef]
- Brown, P.D.; Maurer, M.J.; Rummans, T.A.; Pollock, B.E.; Ballman, K.V.; Sloan, J.A.; Boeve, B.F.; Arusell, R.M.; Clark, M.M.; Buckner, J.C. A prospective study of quality of life in adults with newly diagnosed high-grade gliomas: The impact of the extent of resection on quality of life and survival. Neurosurgery 2005, 57, 495–504. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Picart, T.; Pallud, J.; Berthiller, J.; Dumot, C.; Berhouma, M.; Ducray, F.; Armoiry, X.; Margier, J.; Guerre, P.; Varlet, P.; et al. Use of 5-ALA fluorescence-guided surgery versus white-light conventional microsurgery for the resection of newly diagnosed glioblastomas (RESECT study): A French multicenter randomized phase III study. J. Neurosurg. 2024, 140, 987–1000. [Google Scholar] [CrossRef]
- Valdes, P.A.; Juvekar, P.; Agar, N.Y.R.; Gioux, S.; Golby, A.J. Quantitative Wide-Field Imaging Techniques for Fluorescence Guided Neurosurgery. Front. Surg. 2019, 6, 31. [Google Scholar] [CrossRef]
- Valdés, P.A.; Roberts, D.W.; Lu, F.K.; Golby, A. Optical technologies for intraoperative neurosurgical guidance. Neurosurg. Focus. 2016, 40, E8. [Google Scholar] [CrossRef]
- Roberts, D.W.; Valdés, P.A.; Harris, B.T.; Hartov, A.; Fan, X.; Ji, S.; Pogue, B.W.; Leblond, F.; Tosteson, T.D.; Wilson, B.C.; et al. Adjuncts for maximizing resection: 5-aminolevuinic acid. Clin. Neurosurg. 2012, 59, 75–78. [Google Scholar] [CrossRef]
- Adamson, C.; Kanu, O.O.; Mehta, A.I.; Di, C.; Lin, N.; Mattox, A.K.; Bigner, D.D. Glioblastoma multiforme: A review of where we have been and where we are going. Expert. Opin. Investig. Drugs 2009, 18, 1061–1083. [Google Scholar] [CrossRef]
- Andersen, B.M.; Faust Akl, C.; Wheeler, M.A.; Chiocca, E.A.; Reardon, D.A.; Quintana, F.J. Glial and myeloid heterogeneity in the brain tumour microenvironment. Nat. Rev. Cancer 2021, 21, 786–802. [Google Scholar] [CrossRef]
- Birzu, C.; French, P.; Caccese, M.; Cerretti, G.; Idbaih, A.; Zagonel, V.; Lombardi, G. Recurrent glioblastoma: From molecular landscape to new treatment perspectives. Cancers 2021, 13, 47. [Google Scholar] [CrossRef]
- Burger, P.C.; Heinz, E.R.; Shibata, T.; Kleihues, P. Topographic anatomy and CT correlations in the untreated glioblastoma multiforme. J. Neurosurg. 1988, 68, 698–704. [Google Scholar] [CrossRef]
- Campos, B.; Olsen, L.R.; Urup, T.; Poulsen, H.S. A comprehensive profile of recurrent glioblastoma. Oncogene 2016, 35, 5819–5825. [Google Scholar] [CrossRef]
- Montemurro, N.; Pahwa, B.; Tayal, A.; Shukla, A.; De Jesus Encarnacion, M.; Ramirez, I.; Nurmukhametov, R.; Chavda, V.; De Carlo, A. Macrophages in Recurrent Glioblastoma as a Prognostic Factor in the Synergistic System of the Tumor Microenvironment. Neurol. Int. 2023, 15, 595–608. [Google Scholar] [CrossRef]
- Yang, J.; Xu, J.; Wang, W.; Zhang, B.; Yu, X.; Shi, S. Epigenetic regulation in the tumor microenvironment: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 210. [Google Scholar] [CrossRef]
- Wang, J.; Cazzato, E.; Ladewig, E.; Frattini, V.; Rosenbloom, D.I.S.; Zairis, S.; Abate, F.; Liu, Z.; Elliott, O.; Shin, Y.J.; et al. Clonal evolution of glioblastoma under therapy. Nat. Genet. 2016, 48, 768–776. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.W.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef]
- Martinez, R.; Rohde, V.; Schackert, G. Different molecular patterns in glioblastoma multiforme subtypes upon recurrence. J. Neuro-Oncol. 2010, 96, 321–329. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Cavenee, W.K.; Mischel, P.S. Glioblastoma: From molecular pathology to targeted treatment. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 1–25. [Google Scholar] [CrossRef]
- Kim, H.; Zheng, S.; Amini, S.S.; Virk, S.M.; Mikkelsen, T.; Brat, D.J.; Grimsby, J.; Sougnez, C.; Muller, F.; Hu, J.; et al. Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome Res. 2015, 25, 316–327. [Google Scholar] [CrossRef]
- Chen, N.; Peng, C.; Li, D. Epigenetic Underpinnings of Inflammation: A Key to Unlock the Tumor Microenvironment in Glioblastoma. Front. Immunol. 2022, 13, 869307. [Google Scholar] [CrossRef]
- Miki, S.; Satomi, K.; Ohno, M.; Matsushita, Y.; Kitahara, M.; Miyakita, Y.; Takahashi, M.; Matsuda, M.; Ishikawa, E.; Matsumura, A.; et al. Highly sensitive detection of TERT promoter mutations in recurrent glioblastomas using digital PCR. Brain Tumor Pathol. 2020, 37, 154–158. [Google Scholar] [CrossRef]
- Akyerli, C.B.; Yüksel, Ş.; Can, Ö.; Erson-Omay, E.Z.; Oktay, Y.; Coşgun, E.; Ülgen, E.; Erdemgil, Y.; Sav, A.; von Deimling, A.; et al. Use of telomerase promoter mutations to mark specific molecular subsets with reciprocal clinical behavior in IDH mutant and IDH wild-type diffuse gliomas. J. Neurosurg. 2018, 128, 1102–1114. [Google Scholar] [CrossRef]
- He, J.; Zhang, Z.; Ouyang, M.; Yang, F.; Hao, H.; Lamb, K.L.; Yang, J.; Yin, Y.; Shen, W.H. PTEN regulates EG5 to control spindle architecture and chromosome congression during mitosis. Nat. Commun. 2016, 7, 12355. [Google Scholar] [CrossRef]
- Gan, H.K.; Cvrljevic, A.N.; Johns, T.G. The epidermal growth factor receptor variant III (EGFRvIII): Where wild things are altered. FEBS J. 2013, 280, 5350–5370. [Google Scholar] [CrossRef]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.W.; Weiss, W.A. Epidermal growth factor receptor and EGFRvIII in glioblastoma: Signaling pathways and targeted therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef]
- Feng, H.; Hu, B.; Jarzynka, M.J.; Li, Y.; Keezer, S.; Johns, T.G.; Tang, C.K.; Hamilton, R.L.; Vuori, K.; Nishikawa, R.; et al. Phosphorylation of dedicator of cytokinesis 1 (Dock180) at tyrosine residue Y722 by Src family kinases mediates EGFRvIII-driven glioblastoma tumorigenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 3018–3023. [Google Scholar] [CrossRef]
- Cvrljevic, A.N.; Akhavan, D.; Wu, M.; Martinello, P.; Furnari, F.B.; Johnston, A.J.; Guo, D.; Pike, L.; Cavenee, W.K.; Scott, A.M.; et al. Activation of Src induces mitochondrial localisation of de2-7EGFR (EGFRvIII) in glioma cells: Implications for glucose metabolism. J. Cell Sci. 2011, 124, 2938–2950. [Google Scholar] [CrossRef]
- Kim, J.; Lee, I.H.; Cho, H.J.; Park, C.K.; Jung, Y.S.; Kim, Y.; Nam, S.H.; Kim, B.S.; Johnson, M.D.; Kong, D.S.; et al. Spatiotemporal Evolution of the Primary Glioblastoma Genome. Cancer Cell 2015, 28, 318–328. [Google Scholar] [CrossRef]
- Crespo, S.; Kind, M.; Arcaro, A. The role of the PI3K/AKT/mTOR pathway in brain tumor metastasis. J Cancer Metastasis Treat 2016, 2, 80–89. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Liu, H.; Ding, Z.-B.; Xi, H.-P.; Wang, G.-W. lncRNA SNHG16 promotes glioma tumorigenicity through miR-373/EGFR axis by activating PI3K/AKT pathway. Genomics 2020, 112, 1021–1029. [Google Scholar] [CrossRef]
- Pan, J.; Sheng, S.; Ye, L.; Xu, X.; Ma, Y.; Feng, X.; Qiu, L.; Fan, Z.; Wang, Y.; Xia, X.; et al. Extracellular vesicles derived from glioblastoma promote proliferation and migration of neural progenitor cells via PI3K-Akt pathway. Cell Commun. Signal. 2022, 20, 7. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, E.; Wu, Q.; Guryanova, O.; Hitomi, M.; Lathia, J.D.; Serwanski, D.; Sloan, A.E.; Weil, R.J.; Lee, J.; et al. Platelet-derived growth factor receptors differentially inform intertumoral and intratumoral heterogeneity. Genes. Dev. 2012, 26, 1247–1262. [Google Scholar] [CrossRef]
- Lane, R.; Cilibrasi, C.; Chen, J.; Shah, K.; Messuti, E.; Mazarakis, N.K.; Stebbing, J.; Critchley, G.; Song, E.; Simon, T.; et al. PDGF-R inhibition induces glioblastoma cell differentiation via DUSP1/p38(MAPK) signalling. Oncogene 2022, 41, 2749–2763. [Google Scholar] [CrossRef]
- Pixley, F.J.; Stanley, E.R. CSF-1 regulation of the wandering macrophage: Complexity in action. Trends Cell Biol. 2004, 14, 628–638. [Google Scholar] [CrossRef]
- Lamagna, C.; Aurrand-Lions, M.; Imhof, B.A. Dual role of macrophages in tumor growth and angiogenesis. J. Leukoc. Biol. 2006, 80, 705–713. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y. Tumor-associated macrophages: From basic research to clinical application. J. Hematol. Oncol. 2017, 10, 58. [Google Scholar] [CrossRef]
- Zhu, C.; Kros, J.M.; van der Weiden, M.; Zheng, P.; Cheng, C.; Mustafa, D.A.M. Expression site of P2RY12 in residential microglial cells in astrocytomas correlates with M1 and M2 marker expression and tumor grade. Acta Neuropathol. Commun. 2017, 5, 4. [Google Scholar] [CrossRef]
- Pombo Antunes, A.R.; Scheyltjens, I.; Lodi, F.; Messiaen, J.; Antoranz, A.; Duerinck, J.; Kancheva, D.; Martens, L.; De Vlaminck, K.; Van Hove, H.; et al. Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat. Neurosci. 2021, 24, 595–610. [Google Scholar] [CrossRef]
- Vitkovic, L.; Maeda, S.; Sternberg, E. Anti-inflammatory cytokines: Expression and action in the brain. Neuroimmunomodulation 2001, 9, 295–312. [Google Scholar] [CrossRef]
- Gong, D.; Shi, W.; Yi, S.-j.; Chen, H.; Groffen, J.; Heisterkamp, N. TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 2012, 13, 31. [Google Scholar] [CrossRef]
- Takenaka, M.C.; Gabriely, G.; Rothhammer, V.; Mascanfroni, I.D.; Wheeler, M.A.; Chao, C.-C.; Gutiérrez-Vázquez, C.; Kenison, J.; Tjon, E.C.; Barroso, A.; et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci. 2019, 22, 729–740. [Google Scholar] [CrossRef]
- Dong, F.; Qin, X.; Wang, B.; Li, Q.; Hu, J.; Cheng, X.; Guo, D.; Cheng, F.; Fang, C.; Tan, Y.; et al. ALKBH5 Facilitates Hypoxia-Induced Paraspeckle Assembly and IL8 Secretion to Generate an Immunosuppressive Tumor Microenvironment. Cancer Res. 2021, 81, 5876–5888. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, B.S.; Zhou, A.; Lin, K.; Zheng, S.; Lu, Z.; Chen, Y.; Sulman, E.P.; Xie, K.; Bögler, O.; et al. m(6)A Demethylase ALKBH5 Maintains Tumorigenicity of Glioblastoma Stem-like Cells by Sustaining FOXM1 Expression and Cell Proliferation Program. Cancer Cell 2017, 31, 591–606.e596. [Google Scholar] [CrossRef]
- Storey, K.; Leder, K.; Hawkins-Daarud, A.; Swanson, K.; Ahmed, A.U.; Rockne, R.C.; Foo, J. Glioblastoma Recurrence and the Role of O(6)-Methylguanine-DNA Methyltransferase Promoter Methylation. JCO Clin. Cancer Inform. 2019, 3, 1–12. [Google Scholar] [CrossRef]
- Suzuki, T.; Nakada, M.; Yoshida, Y.; Nambu, E.; Furuyama, N.; Kita, D.; Hayashi, Y.; Hayashi, Y.; Hamada, J.-I. The correlation between promoter methylation status and the expression level of O6-methylguanine-DNA methyltransferase in recurrent glioma. Jpn. J. Clin. Oncol. 2011, 41, 190–196. [Google Scholar] [CrossRef][Green Version]
- Christmann, M.; Nagel, G.; Horn, S.; Krahn, U.; Wiewrodt, D.; Sommer, C.; Kaina, B. MGMT activity, promoter methylation and immunohistochemistry of pretreatment and recurrent malignant gliomas: A comparative study on astrocytoma and glioblastoma. Int. J. Cancer 2010, 127, 2106–2118. [Google Scholar] [CrossRef]
- Powter, B.; Jeffreys, S.A.; Sareen, H.; Cooper, A.; Brungs, D.; Po, J.; Roberts, T.; Koh, E.-S.; Scott, K.F.; Sajinovic, M.; et al. Human TERT promoter mutations as a prognostic biomarker in glioma. J. Cancer Res. Clin. Oncol. 2021, 147, 1007–1017. [Google Scholar] [CrossRef]
- Labussière, M.; Boisselier, B.; Mokhtari, K.; Stefano, A.-L.D.; Rahimian, A.; Rossetto, M.; Ciccarino, P.; Saulnier, O.; Paterra, R.; Marie, Y.; et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology 2014, 83, 1200–1206. [Google Scholar] [CrossRef]
- You, H.; Wu, Y.; Chang, K.; Shi, X.; Chen, X.-D.; Yan, W.; Li, R. Paradoxical prognostic impact of TERT promoter mutations in gliomas depends on different histological and genetic backgrounds. CNS Neurosci. Ther. 2017, 23, 790–797. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Li, J.; Huang, T.; Wang, Y.; Chang, L.; Zheng, W.; Ma, Y.; Chen, F.; Gong, X.; et al. Genomic analysis of primary and recurrent gliomas reveals clinical outcome related molecular features. Sci. Rep. 2019, 9, 16058. [Google Scholar] [CrossRef]
- Liaw, D.; Marsh, D.J.; Li, J.; Dahia, P.L.; Wang, S.I.; Zheng, Z.; Bose, S.; Call, K.M.; Tsou, H.C.; Peacocke, M.; et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat. Genet. 1997, 16, 64–67. [Google Scholar] [CrossRef]
- Steck, P.A.; Pershouse, M.A.; Jasser, S.A.; Yung, W.K.; Lin, H.; Ligon, A.H.; Langford, L.A.; Baumgard, M.L.; Hattier, T.; Davis, T.; et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 1997, 15, 356–362. [Google Scholar] [CrossRef]
- Garcia-Cao, I.; Song, M.S.; Hobbs, R.M.; Laurent, G.; Giorgi, C.; de Boer, V.C.J.; Anastasiou, D.; Ito, K.; Sasaki, A.T.; Rameh, L.; et al. Systemic elevation of PTEN induces a tumor-suppressive metabolic state. Cell 2012, 149, 49–62. [Google Scholar] [CrossRef]
- Maehama, T.; Dixon, J.E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998, 273, 13375–13378. [Google Scholar] [CrossRef]
- Song, M.S.; Salmena, L.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor. Nat. Reviews. Mol. Cell Biol. 2012, 13, 283–296. [Google Scholar] [CrossRef]
- Choi, S.W.; Lee, Y.; Shin, K.; Koo, H.; Kim, D.; Sa, J.K.; Cho, H.J.; Shin, H.-m.; Lee, S.J.; Kim, H.; et al. Mutation-specific non-canonical pathway of PTEN as a distinct therapeutic target for glioblastoma. Cell Death Dis. 2021, 12, 374. [Google Scholar] [CrossRef]
- Ohgaki, H.; Kleihues, P. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci. 2009, 100, 2235–2241. [Google Scholar] [CrossRef]
- Akhavan, D.; Cloughesy, T.F.; Mischel, P.S. mTOR signaling in glioblastoma: Lessons learned from bench to bedside. Neuro Oncol. 2010, 12, 882–889. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, Q.; Luo, X.; Lou, K.; Weiss, W.A.; Shokat, K.M. Brain-restricted mTOR inhibition with binary pharmacology. Nature 2022, 609, 822–828. [Google Scholar] [CrossRef]
- Neilsen, B.K.; Sleightholm, R.; McComb, R.; Ramkissoon, S.H.; Ross, J.S.; Corona, R.J.; Miller, V.A.; Cooke, M.; Aizenberg, M.R. Comprehensive genetic alteration profiling in primary and recurrent glioblastoma. J. Neuro-Oncol. 2019, 142, 111–118. [Google Scholar] [CrossRef]
- Koul, D.; Shen, R.; Bergh, S.; Sheng, X.; Shishodia, S.; Lafortune, T.A.; Lu, Y.; de Groot, J.F.; Mills, G.B.; Yung, W.K.A. Inhibition of Akt survival pathway by a small-molecule inhibitor in human glioblastoma. Mol. Cancer Ther. 2006, 5, 637–644. [Google Scholar] [CrossRef]
- Gallia, G.L.; Tyler, B.M.; Hann, C.L.; Siu, I.-M.; Giranda, V.L.; Vescovi, A.L.; Brem, H.; Riggins, G.J. Inhibition of Akt inhibits growth of glioblastoma and glioblastoma stem-like cells. Mol. Cancer Ther. 2009, 8, 386–393. [Google Scholar] [CrossRef]
- Wang, S.-J.; Wang, H.; Zhao, C.-D.; Li, R. Long noncoding RNA LINC01426 promotes glioma progression through PI3K/AKT signaling pathway and serves as a prognostic biomarker. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6358–6368. [Google Scholar] [CrossRef]
- Liu, B.; Cao, W.; Ma, H. Knockdown of lncRNA LSINCT5 suppresses growth and metastasis of human glioma cells via up-regulating miR-451. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2507–2515. [Google Scholar] [CrossRef]
- Jorissen, R.N.; Walker, F.; Pouliot, N.; Garrett, T.P.; Ward, C.W.; Burgess, A.W. Epidermal growth factor receptor: Mechanisms of activation and signalling. Exp. Cell Res. 2003, 284, 31–53. [Google Scholar] [CrossRef]
- Herbst, R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 21–26. [Google Scholar] [CrossRef]
- Dhawan, A.; Manem, V.S.K.; Yeaney, G.; Lathia, J.D.; Ahluwalia, M.S. EGFR Pathway Expression Persists in Recurrent Glioblastoma Independent of Amplification Status. Cancers 2023, 15, 670. [Google Scholar] [CrossRef]
- Ekstrand, A.J.; James, C.D.; Cavenee, W.K.; Seliger, B.; Pettersson, R.F.; Collins, V.P. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991, 51, 2164–2172. [Google Scholar]
- van den Bent, M.J.; Gao, Y.; Kerkhof, M.; Kros, J.M.; Gorlia, T.; van Zwieten, K.; Prince, J.; van Duinen, S.; Sillevis Smitt, P.A.; Taphoorn, M.; et al. Changes in the EGFR amplification and EGFRvIII expression between paired primary and recurrent glioblastomas. Neuro Oncol. 2015, 17, 935–941. [Google Scholar] [CrossRef]
- Nagane, M.; Coufal, F.; Lin, H.; Bögler, O.; Cavenee, W.K.; Huang, H.J. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996, 56, 5079–5086. [Google Scholar]
- Cantanhede, I.G.; de Oliveira, J.R.M. PDGF Family Expression in Glioblastoma Multiforme: Data Compilation from Ivy Glioblastoma Atlas Project Database. Sci. Rep. 2017, 7, 15271. [Google Scholar] [CrossRef]
- Indraccolo, S.; Lombardi, G.; Fassan, M.; Pasqualini, L.; Giunco, S.; Marcato, R.; Gasparini, A.; Candiotto, C.; Nalio, S.; Fiduccia, P.; et al. Genetic, Epigenetic, and Immunologic Profiling of MMR-Deficient Relapsed Glioblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 1828–1837. [Google Scholar] [CrossRef]
- Higuchi, F.; Nagashima, H.; Ning, J.; Koerner, M.V.A.; Wakimoto, H.; Cahill, D.P. Restoration of Temozolomide Sensitivity by PARP Inhibitors in Mismatch Repair Deficient Glioblastoma is Independent of Base Excision Repair. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 1690–1699. [Google Scholar] [CrossRef]
- Kaley, T.; Touat, M.; Subbiah, V.; Hollebecque, A.; Rodon, J.; Lockhart, A.C.; Keedy, V.; Bielle, F.; Hofheinz, R.D.; Joly, F.; et al. BRAF Inhibition in BRAF(V600)-Mutant Gliomas: Results From the VE-BASKET Study. J. Clin. Oncol. 2018, 36, 3477–3484. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Klughammer, J.; Kiesel, B.; Roetzer, T.; Fortelny, N.; Nemc, A.; Nenning, K.H.; Furtner, J.; Sheffield, N.C.; Datlinger, P.; Peter, N.; et al. The DNA methylation landscape of glioblastoma disease progression shows extensive heterogeneity in time and space. Nat. Med. 2018, 24, 1611–1624. [Google Scholar] [CrossRef]
- Eisenbarth, D.; Wang, Y.A. Glioblastoma heterogeneity at single cell resolution. Oncogene 2023, 42, 2155–2165. [Google Scholar] [CrossRef]
- Gangoso, E.; Southgate, B.; Bradley, L.; Rus, S.; Galvez-Cancino, F.; McGivern, N.; Güç, E.; Kapourani, C.-A.; Byron, A.; Ferguson, K.M.; et al. Glioblastomas acquire myeloid-affiliated transcriptional programs via epigenetic immunoediting to elicit immune evasion. Cell 2021, 184, 2454–2470.e2426. [Google Scholar] [CrossRef]
- Cao, M.-F.; Chen, L.; Dang, W.-Q.; Zhang, X.-C.; Zhang, X.; Shi, Y.; Yao, X.-H.; Li, Q.; Zhu, J.; Lin, Y.; et al. Hybrids by tumor-associated macrophages × glioblastoma cells entail nuclear reprogramming and glioblastoma invasion. Cancer Lett. 2019, 442, 445–452. [Google Scholar] [CrossRef]
- Fu, W.; Wang, W.; Li, H.; Jiao, Y.; Huo, R.; Yan, Z.; Wang, J.; Wang, S.; Wang, J.; Chen, D.; et al. Single-Cell Atlas Reveals Complexity of the Immunosuppressive Microenvironment of Initial and Recurrent Glioblastoma. Front. Immunol. 2020, 11, 835. [Google Scholar] [CrossRef]
- Mormino, A.; Cocozza, G.; Fontemaggi, G.; Valente, S.; Esposito, V.; Santoro, A.; Bernardini, G.; Santoni, A.; Fazi, F.; Mai, A.; et al. Histone-deacetylase 8 drives the immune response and the growth of glioma. Glia 2021, 69, 2682–2698. [Google Scholar] [CrossRef]
- Wei, X.; Huo, Y.; Pi, J.; Gao, Y.; Rao, S.; He, M.; Wei, Q.; Song, P.; Chen, Y.; Lu, D.; et al. METTL3 preferentially enhances non-m6A translation of epigenetic factors and promotes tumourigenesis. Nat. Cell Biol. 2022, 24, 1278–1290. [Google Scholar] [CrossRef]
- Gerson, S.L. MGMT: Its role in cancer aetiology and cancer therapeutics. Nat. Rev. Cancer 2004, 4, 296–307. [Google Scholar] [CrossRef]
- Liau, B.B.; Sievers, C.; Donohue, L.K.; Gillespie, S.M.; Flavahan, W.A.; Miller, T.E.; Venteicher, A.S.; Hebert, C.H.; Carey, C.D.; Rodig, S.J.; et al. Adaptive Chromatin Remodeling Drives Glioblastoma Stem Cell Plasticity and Drug Tolerance. Cell Stem Cell 2017, 20, 233–246.e237. [Google Scholar] [CrossRef]
- Murota, Y.; Tabu, K.; Taga, T. Cancer Stem Cell-Associated Immune Microenvironment in Recurrent Glioblastomas. Cells 2022, 11, 2054. [Google Scholar] [CrossRef]
- Phillips, J.P.; Eremin, O.; Anderson, J.R. Lymphoreticular cells in human brain tumours and in normal brain. Br. J. Cancer 1982, 45, 61–69. [Google Scholar] [CrossRef]
- Darmanis, S.; Sloan, S.A.; Croote, D.; Mignardi, M.; Chernikova, S.; Samghababi, P.; Zhang, Y.; Neff, N.; Kowarsky, M.; Caneda, C.; et al. Single-Cell RNA-Seq Analysis of Infiltrating Neoplastic Cells at the Migrating Front of Human Glioblastoma. Cell Rep. 2017, 21, 1399–1410. [Google Scholar] [CrossRef]
- Klemm, F.; Maas, R.R.; Bowman, R.L.; Kornete, M.; Soukup, K.; Nassiri, S.; Brouland, J.-P.; Iacobuzio-Donahue, C.A.; Brennan, C.; Tabar, V.; et al. Interrogation of the Microenvironmental Landscape in Brain Tumors Reveals Disease-Specific Alterations of Immune Cells. Cell 2020, 181, 1643–1660.e1617. [Google Scholar] [CrossRef]
- Wang, L.-J.; Lv, P.; Lou, Y.; Ye, J. Gene Expression-Based Predication of RNA Pseudouridine Modification in Tumor Microenvironment and Prognosis of Glioma Patients. Front. Cell Dev. Biol. 2021, 9, 727595. [Google Scholar] [CrossRef]
- Pyonteck, S.M.; Akkari, L.; Schuhmacher, A.J.; Bowman, R.L.; Sevenich, L.; Quail, D.F.; Olson, O.C.; Quick, M.L.; Huse, J.T.; Teijeiro, V.; et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013, 19, 1264–1272. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. A J. Virtual Libr. 2008, 13, 453–461. [Google Scholar] [CrossRef]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef]
- Zanotto-Filho, A.; Gonçalves, R.M.; Klafke, K.; de Souza, P.O.; Dillenburg, F.C.; Carro, L.; Gelain, D.P.; Moreira, J.C.F. Inflammatory landscape of human brain tumors reveals an NFκB dependent cytokine pathway associated with mesenchymal glioblastoma. Cancer Lett. 2017, 390, 176–187. [Google Scholar] [CrossRef]
- Dumas, A.A.; Pomella, N.; Rosser, G.; Guglielmi, L.; Vinel, C.; Millner, T.O.; Rees, J.; Aley, N.; Sheer, D.; Wei, J.; et al. Microglia promote glioblastoma via mTOR-mediated immunosuppression of the tumour microenvironment. EMBO J. 2020, 39, e103790. [Google Scholar] [CrossRef]
- Wu, L.; Wu, W.; Zhang, J.; Zhao, Z.; Li, L.; Zhu, M.; Wu, M.; Wu, F.; Zhou, F.; Du, Y.; et al. Natural Coevolution of Tumor and Immunoenvironment in Glioblastoma. Cancer Discov. 2022, 12, 2820–2837. [Google Scholar] [CrossRef]
- Tatla, A.S.; Justin, A.W.; Watts, C.; Markaki, A.E. A vascularized tumoroid model for human glioblastoma angiogenesis. Sci. Rep. 2021, 11, 19550. [Google Scholar] [CrossRef]
- Hambardzumyan, D.; Bergers, G. Glioblastoma: Defining Tumor Niches. Trends Cancer 2015, 1, 252–265. [Google Scholar] [CrossRef]
- Gabriely, G.; Wheeler, M.A.; Takenaka, M.C.; Quintana, F.J. Role of AHR and HIF-1alpha in Glioblastoma Metabolism. Trends Endocrinol. Metab. 2017, 28, 428–436. [Google Scholar] [CrossRef]
- Chen, J.; Liu, G.; Wang, X.; Hong, H.; Li, T.; Li, L.; Wang, H.; Xie, J.; Li, B.; Li, T.; et al. Glioblastoma stem cell-specific histamine secretion drives pro-angiogenic tumor microenvironment remodeling. Cell Stem Cell 2022, 29, 1531–1546.e7. [Google Scholar] [CrossRef]
- Barbagallo, G.M.V.; Jenkinson, M.D.; Brodbelt, A.R. ‘Recurrent’ glioblastoma multiforme, when should we reoperate? Br. J. Neurosurg. 2008, 22, 452–455. [Google Scholar] [CrossRef]
- Ringel, F.; Pape, H.; Sabel, M.; Krex, D.; Bock, H.C.; Misch, M.; Weyerbrock, A.; Westermaier, T.; Senft, C.; Schucht, P.; et al. Clinical benefit from resection of recurrent glioblastomas: Results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro-Oncology 2016, 18, 96–104. [Google Scholar] [CrossRef]
- Patrick, H.H.; Sherman, J.H.; Elder, J.B.; Olson, J.J. Congress of neurological surgeons systematic review and evidence-based guidelines update on the role of cytoreductive surgery in the management of progressive glioblastoma in adults. J. Neuro-Oncol. 2022, 158, 167–177. [Google Scholar] [CrossRef]
- Ammirati, M.; Vick, N.; Liao, Y.L.; Ciric, I.; Mikhael, M. Effect of the extent of surgical resection on survival and quality of life in patients with supratentorial glioblastomas and anaplastic astrocytomas. Neurosurgery 1987, 21, 201–206. [Google Scholar] [CrossRef]
- Oppenlander, M.E.; Wolf, A.B.; Snyder, L.A.; Bina, R.; Wilson, J.R.; Coons, S.W.; Ashby, L.S.; Brachman, D.; Nakaji, P.; Porter, R.W.; et al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J. Neurosurg. 2014, 120, 846–853. [Google Scholar] [CrossRef]
- Nava, F.; Tramacere, I.; Fittipaldo, A.; Bruzzone, M.G.; Dimeco, F.; Fariselli, L.; Finocchiaro, G.; Pollo, B.; Salmaggi, A.; Silvani, A.; et al. Survival effect of first- and second-line treatments for patients with primary glioblastoma: A cohort study from a prospective registry, 1997–2010. Neuro-Oncology 2014, 16, 719–727. [Google Scholar] [CrossRef]
- González, V.; Brell, M.; Fuster, J.; Moratinos, L.; Alegre, D.; López, S.; Ibáñez, J. Analyzing the role of reoperation in recurrent glioblastoma: A 15-year retrospective study in a single institution. World J. Surg. Oncol. 2022, 20, 384. [Google Scholar] [CrossRef]
- Dejaegher, J.; Vleeschouwer, S.D. Recurring Glioblastoma: A Case for Reoperation? Exon Publications 2017, 281–296. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Morshed, R.A.; Berger, M.S. FLAIRectomy: Resecting beyond the Contrast Margin for Glioblastoma. Brain Sci. 2022, 12, 544. [Google Scholar] [CrossRef]
- Bloch, O.; Han, S.J.; Cha, S.; Sun, M.Z.; Aghi, M.K.; McDermott, M.W.; Berger, M.S.; Parsa, A.T. Impact of extent of resection for recurrent glioblastoma on overall survival: Clinical article. J. Neurosurg. 2012, 117, 1032–1038. [Google Scholar] [CrossRef]
- Franceschi, E.; Bartolotti, M.; Tosoni, A.; Bartolini, S.; Sturiale, C.; Fioravanti, A.; Pozzati, E.; Galzio, R.; Talacchi, A.; Volpin, L.; et al. The effect of re-operation on survival in patients with recurrent glioblastoma. Anticancer. Res. 2015, 35, 1743–1748. [Google Scholar]
- Woernle, C.M.; Péus, D.; Hofer, S.; Rushing, E.J.; Held, U.; Bozinov, O.; Krayenbühl, N.; Weller, M.; Regli, L. Efficacy of Surgery and Further Treatment of Progressive Glioblastoma. World Neurosurg. 2015, 84, 301–307. [Google Scholar] [CrossRef]
- Voisin, M.R.; Zuccato, J.A.; Wang, J.Z.; Zadeh, G. Surgery for Recurrent Glioblastoma Multiforme: A Retrospective Case Control Study. World Neurosurg. 2022, 166, e624–e631. [Google Scholar] [CrossRef]
- Djamel-Eddine, Y.-C.; De Witte, O.; Mélot, C.; Lefranc, F. Recurrent glioblastomas: Should we operate a second and even a third time? Interdiscip. Neurosurg. 2019, 18, 100551. [Google Scholar] [CrossRef]
- Yong, R.L.; Wu, T.; Mihatov, N.; Shen, M.J.; Brown, M.A.; Zaghloul, K.A.; Park, G.E.; Park, J.K. Residual tumor volume and patient survival following reoperation for recurrent glioblastoma. J. Neurosurg. 2014, 121, 802–809. [Google Scholar] [CrossRef]
- Karschnia, P.; Dono, A.; Young, J.S.; Juenger, S.T.; Teske, N.; Häni, L.; Sciortino, T.; Mau, C.Y.; Bruno, F.; Nunez, L.; et al. Prognostic evaluation of re-resection for recurrent glioblastoma using the novel RANO classification for extent of resection: A report of the RANO resect group. Neuro Oncol. 2023, 25, 1672–1685. [Google Scholar] [CrossRef]
- Stark, A.M.; Nabavi, A.; Mehdorn, H.M.; Blömer, U. Glioblastoma multiforme-report of 267 cases treated at a single institution. Surg. Neurol. 2005, 63, 162–169. [Google Scholar] [CrossRef]
- Helseth, R.; Helseth, E.; Johannesen, T.B.; Langberg, C.W.; Lote, K.; Rønning, P.; Scheie, D.; Vik, A.; Meling, T.R. Overall survival, prognostic factors, and repeated surgery in a consecutive series of 516 patients with glioblastoma multiforme. Acta Neurol. Scand. 2010, 122, 159–167. [Google Scholar] [CrossRef]
- Okita, Y.; Narita, Y.; Miyakita, Y.; Ohno, M.; Fukushima, S.; Kayama, T.; Shibui, S. Pathological findings and prognostic factors in recurrent glioblastomas. Brain Tumor Pathol. 2012, 29, 192–200. [Google Scholar] [CrossRef]
- Park, C.-K.; Kim, J.H.; Nam, D.-H.; Kim, C.-Y.; Chung, S.-B.; Kim, Y.-H.; Seol, H.J.; Kim, T.M.; Choi, S.H.; Lee, S.-H.; et al. A practical scoring system to determine whether to proceed with surgical resection in recurrent glioblastoma. Neuro-Oncology 2013, 15, 1096–1101. [Google Scholar] [CrossRef]
- Suchorska, B.; Weller, M.; Tabatabai, G.; Senft, C.; Hau, P.; Sabel, M.C.; Herrlinger, U.; Ketter, R.; Schlegel, U.; Marosi, C.; et al. Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neuro-Oncology 2016, 18, 549–556. [Google Scholar] [CrossRef]
- Neville, I.S.; Dos Santos, A.G.; Almeida, C.C.; Abaurre, L.B.; Wayhs, S.Y.; Feher, O.; Teixeira, M.J.; Lepski, G. Reoperation for recurrent glioblastomas: What to expect? Surg. Neurol. Int. 2021, 12, 42. [Google Scholar] [CrossRef]
- Li, Y.; Rey-Dios, R.; Roberts, D.W.; Valdés, P.A.; Cohen-Gadol, A.A. Intraoperative fluorescence-guided resection of high-grade gliomas: A comparison of the present techniques and evolution of future strategies. World Neurosurg. 2014, 82, 175–185. [Google Scholar] [CrossRef]
- McCracken, D.J.; Schupper, A.J.; Lakomkin, N.; Malcolm, J.; Painton Bray, D.; Hadjipanayis, C.G. Turning on the light for brain tumor surgery: A 5-aminolevulinic acid story. Neuro Oncol. 2022, 24, S52–S61. [Google Scholar] [CrossRef]
- Roberts, D.W.; Valdés, P.A.; Harris, B.T.; Fontaine, K.M.; Hartov, A.; Fan, X.; Ji, S.; Lollis, S.S.; Pogue, B.W.; Leblond, F.; et al. Coregistered fluorescence-enhanced tumor resection of malignant glioma: Relationships between δ-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article. J. Neurosurg. 2011, 114, 595–603. [Google Scholar] [CrossRef]
- Roberts, D.W.; Valdés, P.A.; Harris, B.T.; Hartov, A.; Fan, X.; Ji, S.; Leblond, F.; Tosteson, T.D.; Wilson, B.C.; Paulsen, K.D. Glioblastoma multiforme treatment with clinical trials for surgical resection (aminolevulinic acid). Neurosurg. Clin. N. Am. 2012, 23, 371–377. [Google Scholar] [CrossRef]
- Valdés, P.A.; Moses, Z.B.; Kim, A.; Belden, C.J.; Wilson, B.C.; Paulsen, K.D.; Roberts, D.W.; Harris, B.T. Gadolinium- and 5-aminolevulinic acid-induced protoporphyrin IX levels in human gliomas: An ex vivo quantitative study to correlate protoporphyrin IX levels and blood-brain barrier breakdown. J. Neuropathol. Exp. Neurol. 2012, 71, 806–813. [Google Scholar] [CrossRef]
- Stummer, W.; Stocker, S.; Wagner, S.; Stepp, H.; Fritsch, C.; Goetz, C.; Goetz, A.E.; Kiefmann, R.; Reulen, H.J. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 1998, 42, 518–525; discussion 525–516. [Google Scholar] [CrossRef]
- Valdés, P.A.; Kim, A.; Brantsch, M.; Niu, C.; Moses, Z.B.; Tosteson, T.D.; Wilson, B.C.; Paulsen, K.D.; Roberts, D.W.; Harris, B.T. δ-aminolevulinic acid-induced protoporphyrin IX concentration correlates with histopathologic markers of malignancy in human gliomas: The need for quantitative fluorescence-guided resection to identify regions of increasing malignancy. Neuro Oncol. 2011, 13, 846–856. [Google Scholar] [CrossRef]
- Valdés, P.A.; Kim, A.; Leblond, F.; Conde, O.M.; Harris, B.T.; Paulsen, K.D.; Wilson, B.C.; Roberts, D.W. Combined fluorescence and reflectance spectroscopy for in vivo quantification of cancer biomarkers in low- and high-grade glioma surgery. J. Biomed. Opt. 2011, 16, 116007. [Google Scholar] [CrossRef]
- Hickmann, A.K.; Nadji-Ohl, M.; Hopf, N.J. Feasibility of fluorescence-guided resection of recurrent gliomas using five-aminolevulinic acid: Retrospective analysis of surgical and neurological outcome in 58 patients. J. Neurooncol 2015, 122, 151–160. [Google Scholar] [CrossRef]
- Kamp, M.A.; Felsberg, J.; Sadat, H.; Kuzibaev, J.; Steiger, H.J.; Rapp, M.; Reifenberger, G.; Dibué, M.; Sabel, M. 5-ALA-induced fluorescence behavior of reactive tissue changes following glioblastoma treatment with radiation and chemotherapy. Acta Neurochir. 2015, 157, 207–213; discussion 213–204. [Google Scholar] [CrossRef]
- Fisher, J.P.; Adamson, D.C. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines 2021, 9, 324. [Google Scholar] [CrossRef]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Weenink, B.; French, P.J.; Sillevis Smitt, P.A.E.; Debets, R.; Geurts, M. Immunotherapy in Glioblastoma: Current Shortcomings and Future Perspectives. Cancers 2020, 12, 751. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Mochizuki, A.Y.; Orpilla, J.R.; Hugo, W.; Lee, A.H.; Davidson, T.B.; Wang, A.C.; Ellingson, B.M.; Rytlewski, J.A.; Sanders, C.M. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 2019, 25, 477–486. [Google Scholar] [CrossRef]
- Schalper, K.A.; Rodriguez-Ruiz, M.E.; Diez-Valle, R.; López-Janeiro, A.; Porciuncula, A.; Idoate, M.A.; Inogés, S.; Andrea, C.; Cerio, A.; Tejada, S. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat. Med. 2019, 25, 470–476. [Google Scholar] [CrossRef]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bähr, O. Effect of Nivolumab vs. Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1003–1010. [Google Scholar] [CrossRef]
- Kurz, S.C.; Cabrera, L.P.; Hastie, D.; Huang, R.; Unadkat, P.; Rinne, M.; Nayak, L.; Lee, E.Q.; Reardon, D.A.; Wen, P.Y. PD-1 inhibition has only limited clinical benefit in patients with recurrent high-grade glioma. Neurology 2018, 91, 1355–1359. [Google Scholar] [CrossRef]
- Chiocca, E.A.; Gelb, A.B.; Chen, C.C.; Rao, G.; Reardon, D.A.; Wen, P.Y.; Bi, W.L.; Peruzzi, P.; Amidei, C.; Triggs, D. Combined immunotherapy with controlled interleukin-12 gene therapy and immune checkpoint blockade in recurrent glioblastoma: An open-label, multi-institutional phase I trial. Neuro-Oncology 2022, 24, 951–963. [Google Scholar] [CrossRef]
- Lee, A.H.; Sun, L.; Mochizuki, A.Y.; Reynoso, J.G.; Orpilla, J.; Chow, F.; Kienzler, J.C.; Everson, R.G.; Nathanson, D.A.; Bensinger, S.J. Neoadjuvant PD-1 blockade induces T cell and cDC1 activation but fails to overcome the immunosuppressive tumor associated macrophages in recurrent glioblastoma. Nat. Commun. 2021, 12, 6938. [Google Scholar] [CrossRef]
- Nassiri, F.; Patil, V.; Yefet, L.S.; Singh, O.; Liu, J.; Dang, R.M.A.; Yamaguchi, T.N.; Daras, M.; Cloughesy, T.F.; Colman, H.; et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: A phase 1/2 trial. Nat. Med. 2023, 29, 1370–1378. [Google Scholar] [CrossRef]
- Nayak, L.; Molinaro, A.M.; Peters, K.; Clarke, J.L.; Jordan, J.T.; de Groot, J.; Nghiemphu, L.; Kaley, T.; Colman, H.; McCluskey, C.; et al. Randomized Phase II and Biomarker Study of Pembrolizumab plus Bevacizumab versus Pembrolizumab Alone for Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2021, 27, 1048–1057. [Google Scholar] [CrossRef]
- Reardon, D.; Kaley, T.; Iwamoto, F.; Baehring, J.; Subramaniam, D.; Rawls, T.; He, Y.; Keler, T.; Yellin, M. ATIM-23. Anti-cd27 agonist antibody varlilumab in combination with nivolumab for recurrent glioblastoma (rgbm): Phase 2 clinical trial results. Neuro-Oncology 2018, 20, vi6. [Google Scholar] [CrossRef]
- Bagley, S.J.; Desai, A.S.; Linette, G.P.; June, C.H.; O’Rourke, D.M.C.A.R. T-cell therapy for glioblastoma: Recent clinical advances and future challenges. Neuro-Oncology 2018, 20, 1429–1438. [Google Scholar] [CrossRef]
- Maggs, L.; Cattaneo, G.; Dal, A.E.; Moghaddam, A.S.; Ferrone, S. CAR T Cell-Based Immunotherapy for the Treatment of Glioblastoma. Front. Neurosci. 2021, 15, 662064. [Google Scholar] [CrossRef]
- Eskilsson, E.; Røsland, G.V.; Solecki, G.; Wang, Q.; Harter, P.N.; Graziani, G.; Verhaak, R.G.W.; Winkler, F.; Bjerkvig, R.; Miletic, H. EGFR heterogeneity and implications for therapeutic intervention in glioblastoma. Neuro-Oncology 2018, 20, 743–752. [Google Scholar] [CrossRef]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette JJ, D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A Single Dose of Peripherally Infused EGFRvIII-Directed CAR T Cells Mediates Antigen Loss and Induces Adaptive Resistance in Patients with Recurrent Glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef]
- Yang, J.; Yan, J.; Liu, B. Targeting EGFRvIII for glioblastoma multiforme. Cancer Lett. 2017, 403, 224–230. [Google Scholar] [CrossRef]
- Chen, M.; Sun, R.; Shi, B.; Wang, Y.; Di, S.; Luo, H.; Sun, Y.; Li, Z.; Zhou, M.; Jiang, H. Antitumor efficacy of chimeric antigen receptor T cells against EGFRvIII-expressing glioblastoma in C57BL/6 mice. Biomed. Pharmacother. = Biomed. Pharmacother. 2019, 113, 108734. [Google Scholar] [CrossRef]
- Durgin, J.S.; Henderson, F.J.; Nasrallah, M.P.; Mohan, S.; Wang, S.; Lacey, S.F.; Melenhorst, J.J.; Desai, A.S.; Lee, J.Y.K.; Maus, M.V. Case Report: Prolonged Survival Following EGFRvIII CAR T Cell Treatment for Recurrent Glioblastoma. Front. Oncol. 2021, 11, 669071. [Google Scholar] [CrossRef]
- Bagley, S.J.; Binder, Z.A.; Lamrani, L.; Marinari, E.; Desai, A.S.; Nasrallah, M.P.; Maloney, E.; Brem, S.; Lustig, R.A.; Kurtz, G.; et al. Repeated Peripheral Infusions of Anti-EGFRvIII CAR T Cells in Combination with Pembrolizumab Show No Efficacy in Glioblastoma: A Phase 1 Trial. Nat. Cancer 2024, 5, 517–531. [Google Scholar] [CrossRef]
- Brown, C.E.; Warden, C.D.; Starr, R.; Deng, X.; Badie, B.; Yuan, Y.C.; Forman, S.J.; Barish, M.E. Glioma IL13Rα2 is associated with mesenchymal signature gene expression and poor patient prognosis. PLoS ONE 2013, 8, 77769. [Google Scholar] [CrossRef]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C. Bioactivity and Safety of IL13Rα2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 4062–4072. [Google Scholar] [CrossRef]
- Tu, M.; Wange, W.; Cai, L.; Zhu, P.; Gao, Z.; Zheng, W. IL-13 receptor α2 stimulates human glioma cell growth and metastasis through the Src/PI3K/Akt/mTOR signaling pathway. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016, 37, 14701–14709. [Google Scholar] [CrossRef]
- Thaci, B.; Brown, C.E.; Binello, E.; Werbaneth, K.; Sampath, P.; Sengupta, S. Significance of interleukin-13 receptor alpha 2-targeted glioblastoma therapy. Neuro-Oncology 2014, 16, 1304–1312. [Google Scholar] [CrossRef]
- Brown, C.E.; Hibbard, J.C.; Alizadeh, D.; Blanchard, M.S.; Natri, H.M.; Wang, D.; Ostberg, J.R.; Aguilar, B.; Wagner, J.R.; Paul, J.A.; et al. Locoregional delivery of IL-13Rα2-targeting CAR-T cells in recurrent high-grade glioma: A phase 1 trial. Nat. Med. 2024, 30, 1001–1012. [Google Scholar] [CrossRef]
- Choi, B.D.; Gerstner, E.R.; Frigault, M.J.; Leick, M.B.; Mount, C.W.; Balaj, L.; Nikiforow, S.; Carter, B.S.; Curry, W.T.; Gallagher, K.; et al. Intraventricular CARv3-TEAM-E T Cells in Recurrent Glioblastoma. N. Engl. J. Med. 2024, 390, 1290–1298. [Google Scholar] [CrossRef]
- Bagley, S.J.; Logun, M.; Fraietta, J.A.; Wang, X.; Desai, A.S.; Bagley, L.J.; Nabavizadeh, A.; Jarocha, D.; Martins, R.; Maloney, E.; et al. Intrathecal bivalent CAR T cells targeting EGFR and IL13Rα2 in recurrent glioblastoma: Phase 1 trial interim results. Nat. Med. 2024, 30, 1320–1329. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Z.; Wang, X.; Zhang, C.; Jiang, X. Natural killer cell awakening: Unleash cancer-immunity cycle against glioblastoma. Cell Death Dis. 2022, 13, 588. [Google Scholar] [CrossRef]
- Natural killer cells for cancer immunotherapy: A new CAR is catching up. EBioMedicine 2019, 39, 1–2. [CrossRef]
- Murakami, T.; Nakazawa, T.; Natsume, A.; Nishimura, F.; Nakamura, M.; Matsuda, R.; Omoto, K.; Tanaka, Y.; Shida, Y.; Park, Y.S. Novel Human NK Cell Line Carrying CAR Targeting EGFRvIII Induces Antitumor Effects in Glioblastoma Cells. Anticancer. Res. 2018, 38, 5049–5056. [Google Scholar] [CrossRef]
- Zhang, C.; Burger, M.C.; Jennewein, L.; Genßler, S.; Schönfeld, K.; Zeiner, P.; Hattingen, E.; Harter, P.N.; Mittelbronn, M.; Tonn, T. ErbB2/HER2-Specific NK Cells for Targeted Therapy of Glioblastoma. J. Natl. Cancer Inst. 2016, 108, djv375. [Google Scholar] [CrossRef]
- Peruzzi, P.P.; Chiocca, E.A. Cancer immunotherapy: A vaccine from plant virus proteins. Nat. Nanotechnol. 2016, 11, 214–215. [Google Scholar] [CrossRef]
- Bernstock, J.D.; Blitz, S.E.; Hoffman, S.E.; Gerstl, J.V.E.; Chiocca, E.A.; Friedman, G.K. Recent oncolytic virotherapy clinical trials outline a roadmap for the treatment of high-grade glioma. Neuro-Oncol. Adv. 2023, 5, vdad081. [Google Scholar] [CrossRef]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: A phase 2 trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef]
- Ballman, K.V.; Buckner, J.C.; Brown, P.D.; Giannini, C.; Flynn, P.J.; LaPlant, B.R.; Jaeckle, K.A. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro-Oncology 2007, 9, 29–38. [Google Scholar] [CrossRef]
- Ling, A.L.; Solomon, I.H.; Landivar, A.M.; Nakashima, H.; Woods, J.K.; Santos, A.; Masud, N.; Fell, G.; Mo, X.; Yilmaz, A.S.; et al. Clinical trial links oncolytic immunoactivation to survival in glioblastoma. Nature 2023, 623, 157–166. [Google Scholar] [CrossRef]
- Markert, J.M.; Medlock, M.D.; Rabkin, S.D.; Gillespie, G.Y.; Todo, T.; Hunter, W.D.; Palmer, C.A.; Feigenbaum, F.; Tornatore, C.; Tufaro, F.; et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: Results of a phase I trial. Gene Ther. 2000, 7, 867–874. [Google Scholar] [CrossRef]
- Markert, J.M.; Liechty, P.G.; Wang, W.; Gaston, S.; Braz, E.; Karrasch, M.; Nabors, L.B.; Markiewicz, M.; Lakeman, A.D.; Palmer, C.A.; et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol. Ther. 2009, 17, 199–207. [Google Scholar] [CrossRef]
- Friedman, G.K.; Johnston, J.M.; Bag, A.K.; Bernstock, J.D.; Li, R.; Aban, I.; Kachurak, K.; Nan, L.; Kang, K.-D.; Totsch, S.; et al. Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N. Engl. J. Med. 2021, 384, 1613–1622. [Google Scholar] [CrossRef]
- Harrow, S.; Papanastassiou, V.; Harland, J.; Mabbs, R.; Petty, R.; Fraser, M.; Hadley, D.; Patterson, J.; Brown, S.M.; Rampling, R. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: Safety data and long-term survival. Gene Ther. 2004, 11, 1648–1658. [Google Scholar] [CrossRef]
- Papanastassiou, V.; Rampling, R.; Fraser, M.; Petty, R.; Hadley, D.; Nicoll, J.; Harland, J.; Mabbs, R.; Brown, M. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: A proof of principle study. Gene Ther. 2002, 9, 398–406. [Google Scholar] [CrossRef]
- Rampling, R.; Cruickshank, G.; Papanastassiou, V.; Nicoll, J.; Hadley, D.; Brennan, D.; Petty, R.; MacLean, A.; Harland, J.; McKie, E.; et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000, 7, 859–866. [Google Scholar] [CrossRef]
- Chiocca, E.A.; Abbed, K.M.; Tatter, S.; Louis, D.N.; Hochberg, F.H.; Barker, F.; Kracher, J.; Grossman, S.A.; Fisher, J.D.; Carson, K.; et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol. Ther. 2004, 10, 958–966. [Google Scholar] [CrossRef]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef]
- Samson, A.; Scott, K.J.; Taggart, D.; West, E.J.; Wilson, E.; Nuovo, G.J.; Thomson, S.; Corns, R.; Mathew, R.K.; Fuller, M.J.; et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci. Transl. Med. 2018, 10, eaam7577. [Google Scholar] [CrossRef]
- Kicielinski, K.P.; Chiocca, E.A.; Yu, J.S.; Gill, G.M.; Coffey, M.; Markert, J.M. Phase 1 clinical trial of intratumoral reovirus infusion for the treatment of recurrent malignant gliomas in adults. Mol. Ther. 2014, 22, 1056–1062. [Google Scholar] [CrossRef]
- Forsyth, P.; Roldán, G.; George, D.; Wallace, C.; Palmer, C.A.; Morris, D.; Cairncross, G.; Matthews, M.V.; Markert, J.; Gillespie, Y.; et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol. Ther. 2008, 16, 627–632. [Google Scholar] [CrossRef]
- Geletneky, K.; Hajda, J.; Angelova, A.L.; Leuchs, B.; Capper, D.; Bartsch, A.J.; Neumann, J.O.; Schöning, T.; Hüsing, J.; Beelte, B.; et al. Oncolytic H-1 Parvovirus Shows Safety and Signs of Immunogenic Activity in a First Phase I/IIa Glioblastoma Trial. Mol. Ther. 2017, 25, 2620–2634. [Google Scholar] [CrossRef]
- Desjardins, A.; Gromeier, M.; Herndon, J.E., 2nd; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef]
- Karandikar, P.V.; Suh, L.; Gerstl, J.V.E.; Blitz, S.E.; Qu, Q.R.; Won, S.Y.; Gessler, F.A.; Arnaout, O.; Smith, T.R.; Peruzzi, P.P. Positioning SUMO as an immunological facilitator of oncolytic viruses for high-grade glioma. Front. Cell Dev. Biol. 2023, 11, 1271575. [Google Scholar] [CrossRef]
- Boockvar, J.A.; Tsiouris, A.J.; Hofstetter, C.P.; Kovanlikaya, I.; Fralin, S.; Kesavabhotla, K.; Seedial, S.M.; Pannullo, S.C.; Schwartz, T.H.; Stieg, P. Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. Clin. Article. J. Neurosurg. 2011, 114, 624–632. [Google Scholar] [CrossRef]
- Sousa, F.; Dhaliwal, H.K.; Gattacceca, F.; Sarmento, B.; Amiji, M.M. Enhanced anti-angiogenic effects of bevacizumab in glioblastoma treatment upon intranasal administration in polymeric nanoparticles. J. Control. Release Off. J. Control. Release Soc. 2019, 309, 37–47. [Google Scholar] [CrossRef]
- Solinge, T.S.; Nieland, L.; Chiocca, E.A.; Broekman, M.L.D. Advances in local therapy for glioblastoma—Taking the fight to the tumour. Nat. Rev. Neurol. 2022, 18, 221–236. [Google Scholar] [CrossRef]
- Bernstock, J.D.; Blitz, S.; Kang, K.D.; Friedman, G.K. Intraventricular immunovirotherapy; a translational step forward. Oncotarget 2023, 14, 40–43. [Google Scholar] [CrossRef]
- Kang, K.D.; Bernstock, J.D.; Totsch, S.K.; Gary, S.E.; Rocco, A.; Nan, L.; Li, R.; Etminan, T.; Han, X.; Beierle, E.A. Safety and Efficacy of Intraventricular Immunovirotherapy with Oncolytic HSV-1 for CNS Cancers. Clin. Cancer Res. 2022, 28, 5419–5430. [Google Scholar] [CrossRef]
- Plaksin, M.; Bercovici, T.; Sat Toltsis, G.G.; Grinfeld, J.; Shapira, B.; Zur, Y.; Picciotto, R.; Zadicario, E.; Siddeeq, M.; Wohl, A. Magnetic resonance imaging analysis predicts nanoparticle concentration delivered to the brain parenchyma. Commun. Biol. 2022, 5, 964. [Google Scholar] [CrossRef]
- Burgess, A.; Shah, K.; Hough, O.; Hynynen, K. Focused ultrasound-mediated drug delivery through the blood-brain barrier. Expert. Rev. Neurother. 2015, 15, 477–491. [Google Scholar] [CrossRef]
- Abrahao, A.; Meng, Y.; Llinas, M.; Huang, Y.; Hamani, C.; Mainprize, T.; Aubert, I.; Heyn, C.; Black, S.E.; Hynynen, K. First-in-human trial of blood-brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat. Commun. 2019, 10, 4373. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Ghobadi, S.N.; Zhou, H.; Huang, A.; Gerosa, M.; Hou, Q.; Keunen, O.; Golebiewska, A.; Habte, F.G. Molecular Identity Changes of Tumor-Associated Macrophages and Microglia After Magnetic Resonance Imaging-Guided Focused Ultrasound-Induced Blood-Brain Barrier Opening in a Mouse Glioblastoma Model. Ultrasound Med. Biol. 2023, 49, 1082–1090. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Huang, Z.; Wang, X.; Jin, Z.; Li, J.; Limsakul, P.; Zhu, L.; Allen, M.; Pan, Y. Control of the activity of CAR-T cells within tumours via focused ultrasound. Nat. Biomed. Eng. 2021, 5, 1336–1347. [Google Scholar] [CrossRef]
- Jain, A.; Garg, N.K.; Tyagi, R.K.; Singh, B.; Katare, O.P.; Webster, T.J.; Soni, V. Surface engineered polymeric nanocarriers mediate the delivery of transferrin-methotrexate conjugates for an improved understanding of brain cancer. Acta Biomater. 2015, 24, 140–151. [Google Scholar] [CrossRef]
- Abdul Razzak, R.; Florence, G.J.; Gunn-Moore, F.J. Approaches to CNS Drug Delivery with a Focus on Transporter-Mediated Transcytosis. Int. J. Mol. Sci. 2019, 20, 3108. [Google Scholar] [CrossRef]
- Hsu, J.F.; Chu, S.M.; Liao, C.C.; Wang, C.J.; Wang, Y.S.; Lai, M.Y.; Wang, H.C.; Huang, H.R.; Tsai, M.H. Nanotechnology and Nanocarrier-Based Drug Delivery as the Potential Therapeutic Strategy for Glioblastoma Multiforme: An Update. Cancers 2021, 13, 195. [Google Scholar] [CrossRef]
- Lu, G.; Wang, X.; Li, F.; Wang, S.; Zhao, J.; Wang, J.; Liu, J.; Lyu, C.; Ye, P.; Tan, H. Engineered biomimetic nanoparticles achieve targeted delivery and efficient metabolism-based synergistic therapy against glioblastoma. Nat. Commun. 2022, 13, 4214. [Google Scholar] [CrossRef]
- Kumthekar, P.; Ko, C.H.; Paunesku, T.; Dixit, K.; Sonabend, A.M.; Bloch, O.; Tate, M.; Schwartz, M.; Zuckerman, L.; Lezon, R. A first-in-human phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Sci. Transl. Med. 2021, 13, eabb3945. [Google Scholar] [CrossRef]
- Hanson, L.R.; Frey, W. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008, 9 (Suppl. S3), 5. [Google Scholar] [CrossRef]
- Mohammadi, A.M.; Hawasli, A.H.; Rodriguez, A.; Schroeder, J.L.; Laxton, A.W.; Elson, P.; Tatter, S.B.; Barnett, G.H.; Leuthardt, E.C. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: A multicenter study. Cancer Med. 2014, 3, 971–979. [Google Scholar] [CrossRef]
- Watanabe, M.; Tanaka, R.; Hondo, H.; Kuroki, M. Effects of antineoplastic agents and hyperthermia on cytotoxicity toward chronically hypoxic glioma cells. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. N. Am. Hyperth. Group. 1992, 8, 131–138. [Google Scholar] [CrossRef]
- Schildkopf, P.; Ott, O.J.; Frey, B.; Wadepohl, M.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Biological rationales and clinical applications of temperature controlled hyperthermia–implications for multimodal cancer treatments. Curr. Med. Chem. 2010, 17, 3045–3057. [Google Scholar] [CrossRef]
- Frey, B.; Weiss, E.M.; Rubner, Y.; Wunderlich, R.; Ott, O.J.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Old and new facts about hyperthermia-induced modulations of the immune system. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. N. Am. Hyperth. Group. 2012, 28, 528–542. [Google Scholar] [CrossRef]
- Man, J.; Shoemake, J.D.; Ma, T.; Rizzo, A.E.; Godley, A.R.; Wu, Q.; Mohammadi, A.M.; Bao, S.; Rich, J.N.; Yu, J.S. Hyperthermia Sensitizes Glioma Stem-like Cells to Radiation by Inhibiting AKT Signaling. Cancer Res. 2015, 75, 1760–1769. [Google Scholar] [CrossRef]
- Hoover, J.M.; Nwojo, M.; Puffer, R.; Mandrekar, J.; Meyer, F.B.; Parney, I.F. Surgical outcomes in recurrent glioma: Clinical article. J. Neurosurg. 2013, 118, 1224–1231. [Google Scholar] [CrossRef]
- Biase, G.; Garcia, D.P.; Bohnen, A.; Quiñones-Hinojosa, A. Perioperative Management of Patients with Glioblastoma. Neurosurg. Clin. N. Am. 2021, 32, 1–8. [Google Scholar] [CrossRef]
- Fabian, D.; Guillermo Prieto Eibl, M.D.P.; Alnahhas, I.; Sebastian, N.; Giglio, P.; Puduvalli, V.; Gonzalez, J.; Palmer, J.D. Treatment of Glioblastoma (GBM) with the Addition of Tumor-Treating Fields (TTF): A Review. Cancers 2019, 11, 174. [Google Scholar] [CrossRef]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004, 64, 3288–3295. [Google Scholar] [CrossRef]
- Dono, A.; Mitra, S.; Shah, M.; Takayasu, T.; Zhu, J.J.; Tandon, N.; Patel, C.B.; Esquenazi, Y.; Ballester, L.Y. PTEN mutations predict benefit from tumor treating fields (TTFields) therapy in patients with recurrent glioblastoma. J. Neurooncol 2021, 153, 153–160. [Google Scholar] [CrossRef]
- Stupp, R.; Wong, E.T.; Kanner, A.A.; Steinberg, D.; Engelhard, H.; Heidecke, V.; Kirson, E.D.; Taillibert, S.; Liebermann, F.; Dbaly, V.; et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur. J. Cancer 2012, 48, 2192–2202. [Google Scholar] [CrossRef]
- Mrugala, M.M.; Engelhard, H.H.; Dinh Tran, D.; Kew, Y.; Cavaliere, R.; Villano, J.L.; Annenelie Bota, D.; Rudnick, J.; Love Sumrall, A.; Zhu, J.J.; et al. Clinical practice experience with NovoTTF-100A system for glioblastoma: The Patient Registry Dataset (PRiDe). Semin. Oncol. 2014, 41 (Suppl. S6), S4–S13. [Google Scholar] [CrossRef]
- Taphoorn, M.J.B.; Dirven, L.; Kanner, A.A.; Lavy-Shahaf, G.; Weinberg, U.; Taillibert, S.; Toms, S.A.; Honnorat, J.; Chen, T.C.; Sroubek, J.; et al. Influence of Treatment With Tumor-Treating Fields on Health-Related Quality of Life of Patients With Newly Diagnosed Glioblastoma: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018, 4, 495–504. [Google Scholar] [CrossRef]
- Rominiyi, O.; Vanderlinden, A.; Clenton, S.J.; Bridgewater, C.; Al-Tamimi, Y.; Collis, S.J. Tumour treating fields therapy for glioblastoma: Current advances and future directions. Br. J. Cancer 2021, 124, 697–709. [Google Scholar] [CrossRef]
- Cramer, S.W.; Chen, C.C. Photodynamic Therapy for the Treatment of Glioblastoma. Front. Surg. 2019, 6, 81. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Vermandel, M.; Dupont, C.; Lecomte, F.; Leroy, H.A.; Tuleasca, C.; Mordon, S.; Hadjipanayis, C.G.; Reyns, N. Standardized intraoperative 5-ALA photodynamic therapy for newly diagnosed glioblastoma patients: A preliminary analysis of the INDYGO clinical trial. J. Neurooncol. 2021, 152, 501–514. [Google Scholar] [CrossRef]
- Akimoto, J.; Haraoka, J.; Aizawa, K. Preliminary clinical report on safety and efficacy of photodynamic therapy using talaporfin sodium for malignant gliomas. Photodiagnosis Photodyn. Ther. 2012, 9, 91–99. [Google Scholar] [CrossRef]
- Beck, T.J.; Kreth, F.W.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Stepp, H.; Stummer, W.; Baumgartner, R. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg. Med. 2007, 39, 386–393. [Google Scholar] [CrossRef]
- Johansson, A.; Faber, F.; Kniebühler, G.; Stepp, H.; Sroka, R.; Egensperger, R.; Beyer, W.; Kreth, F.W. Protoporphyrin IX fluorescence and photobleaching during interstitial photodynamic therapy of malignant gliomas for early treatment prognosis. Lasers Surg. Med. 2013, 45, 225–234. [Google Scholar] [CrossRef]
- Bhanja, D.; Wilding, H.; Baroz, A.; Trifoi, M.; Shenoy, G.; Slagle-Webb, B.; Hayes, D.; Soudagar, Y.; Connor, J.; Mansouri, A. Photodynamic Therapy for Glioblastoma: Illuminating the Path toward Clinical Applicability. Cancers 2023, 15, 3427. [Google Scholar] [CrossRef]
- Peruzzi, P.; Dominas, C.; Fell, G.; Bernstock, J.D.; Blitz, S.; Mazzetti, D.; Zdioruk, M.; Dawood, H.Y.; Triggs, D.V.; Ahn, S.W. Intratumoral drug-releasing microdevices allow in situ high-throughput pharmaco phenotyping in patients with gliomas. Sci. Transl. Med. 2023, 15, eadi0069. [Google Scholar] [CrossRef]
| Gene, Epigenetic Modification, or Paracrine Signaling | Mutational Characteristics in Recurrent Glioblastoma | References |
|---|---|---|
| TERT | Highly conserved mutation with the TERT promoter as the most conserved mutation Correlated with EGFR amplifications, leading to tumor survival and progression | [27] |
| TERT C228T mutation confers poorer prognosis than C250T | [28] | |
| PTEN aberrations lead to microtubule disruptions, resulting in improper cell cycle regulation and mitosis | [29] | |
| EGFR | EGFRvIII results in truncated receptor co-expressed with the wtEGFR, which leads to constitutively activated downstream EGFR signaling EGFRvIII specific signaling includes DOCK1 and SFK pathways | [30,31,32,33] |
| PI3K/Akt | PI3K-activating mutations have been found in 91% of recurrent glioblastomas | [34] |
| Unregulated cell cycle progression | [34,35] | |
| lncRNA upregulates PI3K and leads to stem cell migration | [21,36] | |
| Glioblastoma-derived EVs upregulate PI3K/Akt proliferation and migratory signaling | [37] | |
| PDGF-R α/β | High expression of PDGF-R β maintains GSC renewal and survival | [38] |
| Knockdown of PDGF-R α/β promotes neurites differentiation in GSC and glioblastoma cell lines | [39] | |
| Paracrine signaling via macrophages | CL2 and CSF1 recruit peripheral macrophages | [40,41,42,43] |
| IL-10 and TGF-β signaling polarize TAMs to anti-inflammatory M2 | [44,45,46] | |
| M2 inhibit Th-1 and NK cell responses | [47] | |
| Epigenetic modifications | MSH6, MLH, and LTBP4 lead to resistance to TMZ | [21,26] |
| LTBP4 mutations lead IDH upregulation and increased cell proliferation | [21] | |
| ALKBH5 inhibits CXCL8 in hypoxic TME, leading to GSC proliferation | [48,49] | |
| Demethylation of MGMT conferred to decreased TMZ sensitivity and shorter survival | [50] | |
| MGMT promoter methylation is decreased in recurrent glioblastoma when compared with newly diagnosed glioblastoma | [51,52] |
| Author/Trial Name/Identifier | Agent/Modality | Study Phase | Study Outcome |
|---|---|---|---|
| Reardon et al., 2020 [143], CheckMate-143, NCT02017717 | Nivolumab vs. bevacizumab | Phase 3 | mPFS = 1.5 months (nivolumab) vs. 3.5 months (bevacizumab); mOS = 9.8 months (nivolumab) vs. 10.0 months (bevacizumab). |
| Nayak et al., 2021 [148], NCT02337491 | Pembrolizumab + bevacizumab vs. pembrolizumab monotherapy | Phase 2 | PFS-6 = 26.0% (pembrolizumab + bevacizumab) vs. 6.7% (pembrolizumab monotherapy); mOS = 8.8 months (pembrolizumab + bevacizumab) vs. 10.3 months (pembrolizumab monotherapy); ORR = 20% (pembrolizumab + bevacizumab) vs. 0% (pembrolizumab monotherapy). |
| Cloughesy et al., 2019 [141] | Pembrolizumab (neoadjuvant + adjuvant) vs. pembrolizumab (adjuvant only) | Phase 2 | mPFS = 3.3 months (neoadjuvant + adjuvant) vs. 2.4 months (adjuvant only); mOS = 13.7 months (neoadjuvant + adjuvant) vs. 7.5 months (adjuvant only). |
| Schalper et al., 2019 [142], NCT02550249 | Neoadjuvant nivolumab (single arm) | Phase 2 | mPFS = 4.1 months; mOS = 7.3 months; Higher immune cell infiltration and TCR diversity. |
| Reardon et al., 2018 [149], NCT02335918 | Nivolumab + varlilumab | Phase 2 | mOS = 9.7 months. |
| Author/Trial Name/Identifier | Status | Agent/Modality | Primary Objective | Study Phase |
|---|---|---|---|---|
| NCT04145115 | Recruiting | Ipilimumab + nivolumab | Tumor response by modified RANO in recurrent glioblastoma with high mutational burden. | Phase 2 |
| NCT04323046 | Active, not recruiting | Ipilimumab + nivolumab | TME changes following neoadjuvant nivolumab and placebo, ipilimumab and placebo, and nivolumab and ipilimumab. Safety and tolerability of neoadjuvant nivolumab and placebo, ipilimumab and placebo, and nivolumab and ipilimumab. | Phase 1 |
| NCT03890952 | Active, not recruiting | Nivolumab + bevacizumab (BEV) | Number of indels as determined using mRNA sequencing. | Phase 2 |
| NCT04201873 | Recruiting | Pembrolizumab + ATL-DC vaccine | Influence of pembrolizumab on cell cycle-related genetic signatures within the TME. Influence of ATL-DC vaccination on peripheral T cell response. Safety/tolerability of pembrolizumab and ATL-DC vaccination. | Phase 1 |
| NCT04013672 | Active, not recruiting | SurVaxM + Sargramostim + Montanide ISA 51 | PFS-6 | Phase 2 |
| NCT04013672 | Active, not recruiting | SurVaxM + Sargramostim + Montanide ISA 51 | PFS-6 | Phase 2 |
| NCT05465954 | Recruiting | Pembrolizumab + efineptakin alfa | OS-9 | Phase 2 |
| NCT05465954 | Recruiting | Pembrolizumab + efineptakin alfa | OS-9 | Phase 2 |
| NCT04479241 | Active, not recruiting | Pembrolizumab + lerapolturev | ORR; DOR; DRR | Phase 2 |
| NCT05053880 | Recruiting | Pembrolizumab + ACT001 | Adverse events; PFS-6 | Phase 1b/2 |
| NCT05463848 | Recruiting | Pembrolizumab + Olaparib + Temozolomide | TIL density; PFS-6 | Phase 2 |
| Trial Identifier | Status | Agent/Modality | Study Phase | Treatment Approach |
| NCT01454596 | Completed | EGFRvIII CAR transduced PBL | Phase 3 | Infusion |
| NCT00730613 | Completed | Autologous lymphocytes | Phase 1 | Leukapheresis + infusion |
| NCT01109095 | Completed | HER-2 CAR CMV-specific CTLs | Phase 1 | Infusion |
| NCT02208362 | Active, not recruiting | IL13Ralpha2-specific Hinge-optimized 41BB co-stimulatory CAR truncated CD-19 expressing autologous TN/MEM cells | Phase 1 | Intratumoral catheter |
| NCT05241392 | Recruiting | B7-H3 targeting CAR-T cells | Phase 1 | Ommaya device |
| NCT04077866 | Recruiting | B7-H3 CAR-T + TMZ | Phase 1/2 | ICT/ICV injection (B7-H3 CAR-T) + Oral (TMZ) |
| NCT05366179 | Recruiting | CAR B7-H3T cells | Phase 1 | ICV |
| NCT05474378 | Recruiting | B7-H3CART | Phase 1 | ICV or dual ICV/ICT |
| NCT04214392 | Recruiting | Chlorotoxin (EQ)-CD28-CD3zeta-CD19t-expressing CAR T-lymphocyte | Phase 1 | ICT or dual ICT/ICV |
| NCT03389230 | Active, not recruiting | HER2(EQ)BBZ/CD19 + T cells | Phase 1 | Intratumoral/intracavitary |
| NCT05540873 | Recruiting | IL13alpha2 CAR-T cells | Phase 1 | Infusion |
| NCT04003649 | Recruiting | IL13Ralpha2-specific Hinge-optimized 41BBco-stimulatory CAR/Truncated CD19 expressing autologous TN/MEM+Ipilimumab+nivolumab | Phase 1 | ICV (IL13Ralpha2 CAR T) IV (ipilimumab+nivoluma) |
| NCT05627323 | Recruiting | CHM-1101 CAR-T cells | Phase 1 | ICT/ICV dual delivery |
| NCT05353530 | Recruiting | Ex-vivo expanded autologous IL-8 receptor (CXCR2) modified CD70 CAR (8R70CAR) T cells | Phase 1 | Infusion |
| NCT05577091 | Not yet recruiting | Autologous Tris-CAR-T cells | Phase 1 | Infusion |
| NCT05168423 | Recruiting | CART-EGFR-IL13Rα2 | Phase 1 | ICV |
| NCT05660369 | Recruiting | CARv3-TEAM-E T cells | Phase 1 | ICV |
| Trial Identifier | Status | Agent/Modality | Study Phase | Treatment Approach |
|---|---|---|---|---|
| NCT03383978 | Recruiting | NK-92/5.28z + Ezabenlimab | Phase 1 | ICT (NK-92/5.28z), infusion (Ezabenlimab) |
| NCT04254419 | Not yet recruiting | NK cells | Phase 1 | Infusion |
| NCT02100891 | Active, not recruiting | Donor NK cell | Phase 2 | Infusion |
| Trial | Virus | Study Phase | Treatment Approach | Outcomes |
|---|---|---|---|---|
| Todo et al., 2022 [171], UMIN000015995 | G47∆ | Phase 2 | ICT | mOS 20.02 months; OS-1yr = 84.2% |
| Ling et al., 2023 [173], NCT03152318 | CAN-3110 | Phase 1 | ICT | mOS = 11.6 months |
| Markert et al., 2014 [174], NCT00157703 | G207 | Phase 1 | ICT | mOS = 7.5 months; no DLT |
| Markert et al., 2009 [175] | G207 | Phase 1b | ICT | mOS = 6.6 months; 1 case of transient fever, delirium, hemiparesis |
| Friedman et al., 2021 [176], NCT02457845 | G207 | Phase 1 | ICT | mOS = 12.2 months; radiographic, neuropathologica,l or clinical responses in 11/12 patients |
| Harrow et al., 2004 [177] | HSV1716 | Phase 1 | ICT | No DLT |
| Papanastassiou et al., 2002 [178] | HSV1716 | Phase 1 | ICT | No DLT |
| Rampling et al., 2000 [179] | HSV1716 | Phase 1 | ICT | No DLT |
| Nassiri [147], NCT02798406 | DXN-2401 + Pembrolizumab | Phase 1/2 | ICT (DXN-2401); infusion (Pembrolizumab) | No DLT; ORR = 10.4%; OS-1yr = 52.7% |
| Chiocca et al., 2004 [180] | ONYX-015 | Phase 1 | ICT | No DLT; mOS = 6.2 months; mPFS = 46 days |
| Lang et al., 2018 [181] | DNX-2401 | Phase 1 | Single ICT; ICT followed by resection and injection into resection cavity | mOS (single ICT) = 9.3 months; mOS (ICT + resection) = 13.0 months |
| Samson et al., 2018 [182] | Reolysin | Phase 1b | IV infusion of 1010 TCID5 | Reovirus RNA extensively detected in tumor cells, increased levels of PD-1/PD-L1 in treated patients |
| Kicielinski et al., 2014 [183] | Reolysin | Phase 1 | ICT | mOS = 4.6 months; no severe treatment related adverse events |
| Forsyth et al., 2008 [184] | Reolysin | Phase 1 | ICT | mOS = 4.8 months |
| Geletneky et al., 2017 [185] | Parvoryx01 | Phase 1/2 | (1) ICT followed by resection and injection around resection cavity; (2) Infusions for 5 days prior to resection and injection around resection cavity. | mOS = 15.3 months |
| Desjardins et al., 2018 [186] | PVSRIPO | Phase 1 | ICT | mOS = 12.5 months |
| Trial | Status | Virus | Study Phase | Treatment Approach |
|---|---|---|---|---|
| NCT04482933 | Not yet recruiting | G207 | Phase 2 | ICT |
| NCT02062827 | Active, not recruiting | M032 | Phase 1 | ICT |
| NCT03657576 | Recruiting | C134 | Phase 1 | ICT |
| NCT03896568 | Recruiting | Allogeneic bone marrow-derived human mesenchymal stem cells loaded with DNX-2401 | Phase 1 | Infusion |
| NCT03911388 | Recruiting | G207 | Phase 1 | ICT (cerebellum) |
| NCT03043391 | Active, not recruiting | PVSRIPO | Phase 1 | CED |
| NCT01582516 | Completed | DNX-2401 | Phase 1/2 | CED |
| NCT01956734 | Completed | DNX-2401 | Phase 1 | ICT |
| NCT00390299 | Completed | MV-CEA | Phase 1 | ICT |
| NCT02986178 | Active, not recruiting | PVSRIPO: | Phase 2 | CED |
| NCT02197169 | DNX-2401 | DNX-2401 with IFN-γ | Phase 1 | ICT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.; Chavarro, V.S.; Gerstl, J.V.E.; Blitz, S.E.; Spanehl, L.; Dubinski, D.; Valdes, P.A.; Tran, L.N.; Gupta, S.; Esposito, L.; et al. Recurrent Glioblastoma—Molecular Underpinnings and Evolving Treatment Paradigms. Int. J. Mol. Sci. 2024, 25, 6733. https://doi.org/10.3390/ijms25126733
Chang C, Chavarro VS, Gerstl JVE, Blitz SE, Spanehl L, Dubinski D, Valdes PA, Tran LN, Gupta S, Esposito L, et al. Recurrent Glioblastoma—Molecular Underpinnings and Evolving Treatment Paradigms. International Journal of Molecular Sciences. 2024; 25(12):6733. https://doi.org/10.3390/ijms25126733
Chicago/Turabian StyleChang, Christopher, Velina S. Chavarro, Jakob V. E. Gerstl, Sarah E. Blitz, Lennard Spanehl, Daniel Dubinski, Pablo A. Valdes, Lily N. Tran, Saksham Gupta, Luisa Esposito, and et al. 2024. "Recurrent Glioblastoma—Molecular Underpinnings and Evolving Treatment Paradigms" International Journal of Molecular Sciences 25, no. 12: 6733. https://doi.org/10.3390/ijms25126733
APA StyleChang, C., Chavarro, V. S., Gerstl, J. V. E., Blitz, S. E., Spanehl, L., Dubinski, D., Valdes, P. A., Tran, L. N., Gupta, S., Esposito, L., Mazzetti, D., Gessler, F. A., Arnaout, O., Smith, T. R., Friedman, G. K., Peruzzi, P., & Bernstock, J. D. (2024). Recurrent Glioblastoma—Molecular Underpinnings and Evolving Treatment Paradigms. International Journal of Molecular Sciences, 25(12), 6733. https://doi.org/10.3390/ijms25126733





