Upscaling and Risk Evaluation of the Synthesis of the 3,5-Diamino-1H-Pyrazole, Disperazol
Abstract
:1. Introduction
1.1. Safety Assessment of the Active Compound
1.2. Synthetic Route
2. Results and Discussion
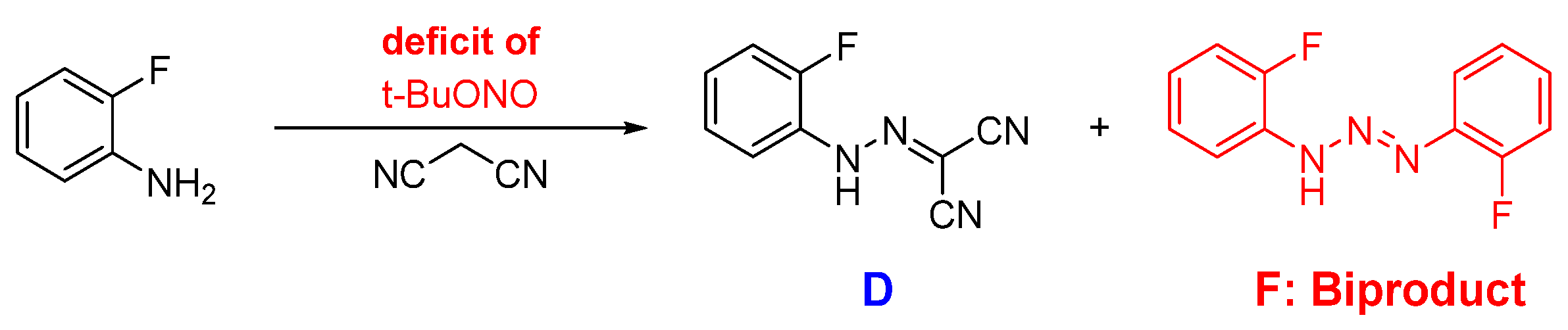
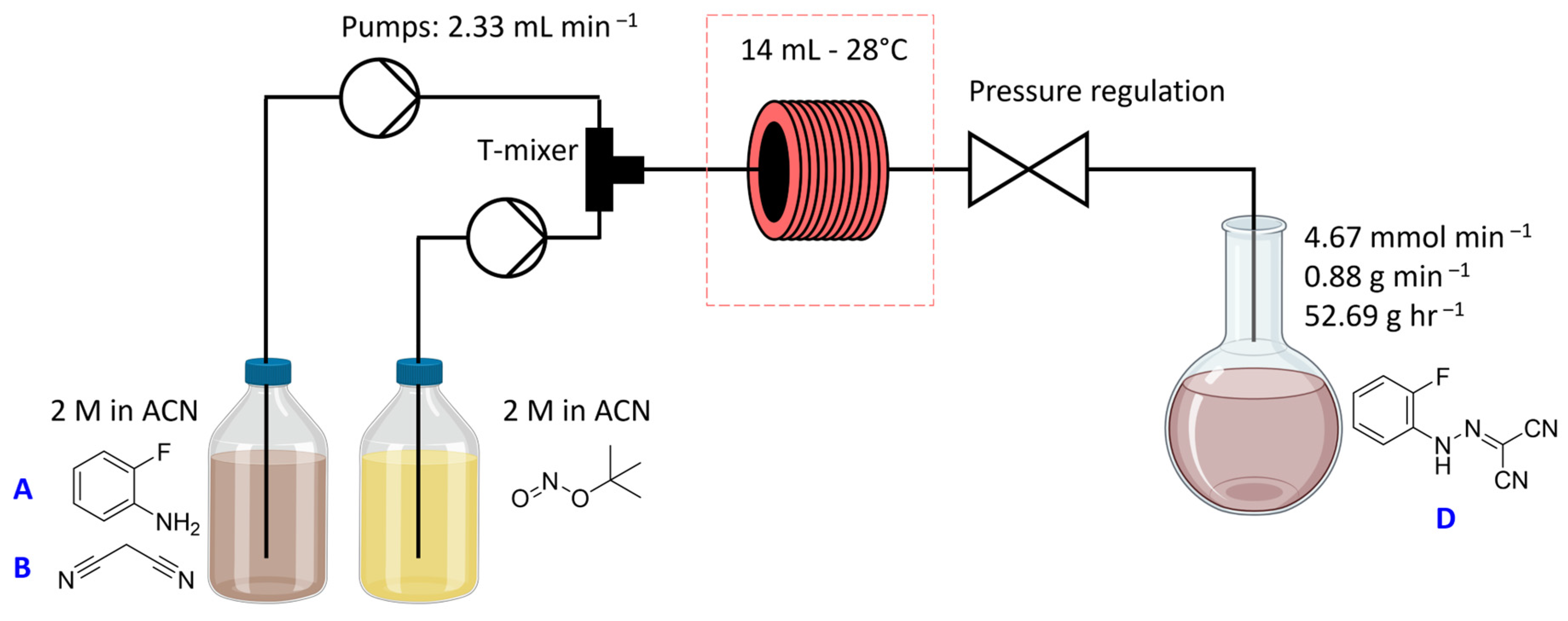
2.1. Step 1—Diazotisation
2.2. Step 2—Ring Closure of Pyrazole
2.3. Step 3—Formulation as a Salt
2.4. Summary
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersen, J.B.; Hultqvist, L.D.; Jansen, C.U.; Jakobsen, T.H.; Nilsson, M.; Rybtke, M.; Uhd, J.; Fritz, B.G.; Seifert, R.; Berthelsen, J.; et al. Identification of small molecules that interfere with c-di-GMP signaling and induce dispersal of Pseudomonas aeruginosa biofilms. Npj Biofilms Microbiomes 2021, 7, 59. [Google Scholar] [CrossRef]
- Hultqvist, L.D.; Andersen, J.B.; Nilsson, C.M.; Jansen, C.U.; Rybtke, M.; Jakobsen, T.H.; Nielsen, T.E.; Qvortrup, K.; Moser, C.; Graz, M.; et al. High efficacy treatment of murine Pseudomonas aeruginosa catheter-associated urinary tract infections using the c-di-GMP modulating anti-biofilm compound Disperazol in combination with ciprofloxacin. Antimicrob. Agents Chemother. 2024, 68, e01481-23. [Google Scholar] [CrossRef]
- Jansen, C.U.; Uhd, J.; Andersen, J.B.; Hultqvist, L.D.; Jakobsen, T.H.; Nilsson, M.; Nielsen, T.E.; Givskov, M.; Tolker-Nielsen, T.; Qvortrup, K.M. SAR study of 4-arylazo-3,5-diamino-1 H-pyrazoles: Identification of small molecules that induce dispersal of Pseudomonas aeruginosa biofilms. RSC Med. Chem. 2021, 12, 1868–1878. [Google Scholar] [CrossRef]
- Manner, C.; Dias Teixeira, R.; Saha, D.; Kaczmarczyk, A.; Zemp, R.; Wyss, F.; Jaeger, T.; Laventie, B.-J.; Boyer, S.; Malone, J.G.; et al. A genetic switch controls Pseudomonas aeruginosa surface colonization. Nat. Microbiol. 2023, 8, 1520–1533. [Google Scholar] [CrossRef]
- Tarselli, M.A. Life and death with nitrogen. Nat. Chem. 2012, 4, 686. [Google Scholar] [CrossRef]
- Warawdekar, M.G. Challenges in Scale-Up of Specialty Chemicals—A Development Chemist’s Perspective. In Industrial Catalytic Processes for Fine and Specialty Chemicals; Elsevier: Amsterdam, The Netherlands, 2016; pp. 721–736. [Google Scholar] [CrossRef]
- Partington, S.; Waldram, S.P. Runaway Reaction during Production of an Azo Dye Intermediate. Process Saf. Environ. Prot. 2002, 80, 33–39. [Google Scholar] [CrossRef]
- Sheng, M.; Frurip, D.; Gorman, D. Reactive chemical hazards of diazonium salts. J. Loss Prev. Process Ind. 2015, 38, 114–118. [Google Scholar] [CrossRef]
- Filimonov, V.D.; Krasnokutskaya, E.A.; Bondarev, A.A. Structures, Stability, and Safety of Diazonium Salts. In Aryl Diazonium Salts and Related Compounds; Chehimi, M.M., Pinson, J., Mousli, F., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 35–57. [Google Scholar] [CrossRef]
- Bretherick, L. Bretherick’s Handbook of Reactive Chemical Hazards: An Indexed Guide to Published Data, 8th ed.; Urben, P.G., Ed.; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2017. [Google Scholar]
- Ullrich, R.; Grewer, T. Decomposition of aromatic diazonium compounds. Thermochim. Acta 1993, 225, 201–211. [Google Scholar] [CrossRef]
- Kittsley, S. Need for minimum standards in evaluating doctoral programs. J. Chem. Educ. 1971, 48, 419. [Google Scholar] [CrossRef]
- Firth, J.D.; Fairlamb, I.J.S. A Need for Caution in the Preparation and Application of Synthetically Versatile Aryl Diazonium Tetrafluoroborate Salts. Org. Lett. 2020, 22, 7057–7059. [Google Scholar] [CrossRef]
- Bondarev, A.A.; Naumov, E.V.; Kassanova, A.Z.; Krasnokutskaya, E.A.; Stankevich, K.S.; Filimonov, V.D. First Study of the Thermal and Storage Stability of Arenediazonium Triflates Comparing to 4-Nitrobenzenediazonium Tosylate and Tetrafluoroborate by Calorimetric Methods. Org. Process Res. Dev. 2019, 23, 2405–2415. [Google Scholar] [CrossRef]
- Deadman, B.J.; Collins, S.G.; Maguire, A.R. Taming Hazardous Chemistry in Flow: The Continuous Processing of Diazo and Diazonium Compounds. Chem.-Eur. J. 2015, 21, 2298–2308. [Google Scholar] [CrossRef]
- Jacq, J.; Pasau, P. Multistep Flow Synthesis of 5-Amino-2-aryl-2 H-[1,2,3]-triazole-4-carbonitriles. Chem.-Eur. J. 2014, 20, 12223–12233. [Google Scholar] [CrossRef]
- Crisóstomo, F.P.; Martín, T.; Carrillo, R. Ascorbic Acid as an Initiator for the Direct C-H Arylation of (Hetero)arenes with Anilines Nitrosated In Situ. Angew. Chem. Int. Ed. 2014, 53, 2181–2185. [Google Scholar] [CrossRef]
- Qiu, D.; Meng, H.; Jin, L.; Wang, S.; Tang, S.; Wang, X.; Mo, F.; Zhang, Y.; Wang, J. Synthesis of Aryl Trimethylstannanes from Aryl Amines: A Sandmeyer-Type Stannylation Reaction. Angew. Chem. Int. Ed. 2013, 52, 11581–11584. [Google Scholar] [CrossRef]
- Chakraborty, A.; Jana, S.; Kibriya, G.; Dey, A.; Hajra, A. tert-Butyl nitrite mediated azo coupling between anilines and imidazoheterocycles. RSC Adv. 2016, 6, 34146–34152. [Google Scholar] [CrossRef]
- He, L.; Qiu, G.; Gao, Y.; Wu, J. Removal of amino groups from anilines through diazonium salt-based reactions. Org. Biomol. Chem. 2014, 12, 6965. [Google Scholar] [CrossRef]
- Hu, T.; Baxendale, I.; Baumann, M. Exploring Flow Procedures for Diazonium Formation. Molecules 2016, 21, 918. [Google Scholar] [CrossRef]
- Mihelač, M.; Siljanovska, A.; Košmrlj, J. A convenient approach to arenediazonium tosylates. Dyes Pigment. 2021, 184, 108726. [Google Scholar] [CrossRef]
- Oger, N.; d’Halluin, M.; Le Grognec, E.; Felpin, F.-X. Using Aryl Diazonium Salts in Palladium-Catalyzed Reactions under Safer Conditions. Org. Process Res. Dev. 2014, 18, 1786–1801. [Google Scholar] [CrossRef]
- Mo, F.; Jiang, Y.; Qiu, D.; Zhang, Y.; Wang, J. Direct Conversion of Arylamines to Pinacol Boronates: A Metal-Free Borylation Process. Angew. Chem. Int. Ed. 2010, 49, 1846–1849. [Google Scholar] [CrossRef]
- Oger, N.; Le Grognec, E.; Felpin, F.-X. Handling diazonium salts in flow for organic and material chemistry. Org. Chem. Front. 2015, 2, 590–614. [Google Scholar] [CrossRef]
- Callonnec, F.L.; Fouquet, E.; Felpin, F.-X. Unprecedented Substoichiometric Use of Hazardous Aryl Diazonium Salts in the Heck-Matsuda Reaction via a Double Catalytic Cycle. Org. Lett. 2011, 13, 2646–2649. [Google Scholar] [CrossRef]
- Susperregui, N.; Miqueu, K.; Sotiropoulos, J.-M.; Le Callonnec, F.; Fouquet, E.; Felpin, F.-X. Sustainable Heck-Matsuda Reaction with Catalytic Amounts of Diazonium Salts: An Experimental and Theoretical Study. Chem.-Eur. J. 2012, 18, 7210–7218. [Google Scholar] [CrossRef]
- Ahmed-Omer, B.; Barrow, D.A.; Wirth, T. Heck reactions using segmented flow conditions. Tetrahedron Lett. 2009, 50, 3352–3355. [Google Scholar] [CrossRef]
- Movsisyan, M.; Delbeke, E.I.P.; Berton, J.K.E.T.; Battilocchio, C.; Ley, S.V.; Stevens, C.V. Taming hazardous chemistry by continuous flow technology. Chem. Soc. Rev. 2016, 45, 4892–4928. [Google Scholar] [CrossRef]
- Smith, C.J.; Smith, C.D.; Nikbin, N.; Ley, S.V.; Baxendale, I.R. Flow synthesis of organic azides and the multistep synthesis of imines and amines using a new monolithic triphenylphosphine reagent. Org. Biomol. Chem. 2011, 9, 1927. [Google Scholar] [CrossRef]
- Nielsen, M.A.; Nielsen, M.K.; Pittelkow, T. Scale-Up and Safety Evaluation of a Sandmeyer Reaction. Org. Process Res. Dev. 2004, 8, 1059–1064. [Google Scholar] [CrossRef]
- Li, B.; Widlicka, D.; Boucher, S.; Hayward, C.; Lucas, J.; Murray, J.C.; O’Neil, B.T.; Pfisterer, D.; Samp, L.; VanAlsten, J.; et al. Telescoped Flow Process for the Syntheses of N-Aryl Pyrazoles. Org. Process Res. Dev. 2012, 16, 2031–2035. [Google Scholar] [CrossRef]

| TEST | Compound E |
|---|---|
| Drop-weight impact/Fallhammer (bulk sample) | >100 J |
| Friction sensitivity (bulk sample) | >360 N |
| Explosion severity, ES; Ignition sensitivity, IS (dust cloud) | Pmax = 9.6 bar (dP/dt)max = 1128 bar/s Kmax = 306 bar·m/s ES = 2.9 IS = 13.1 |
| Minimum explosible concentration, MEC (dust cloud) | 50–60 g/m3 Estimate: 56 g/m3 |
| Minimum ignition energy, MIE (dust cloud) | 3–10 mJ Estimate: 8 mJ |
| Minimum autoignition temperature, MIT (dust cloud) | >600 °C |
| Total combustible content (bulk sample) | 100 wt% > 700 °C |
| Parameters | Range Tested |
|---|---|
| Concentration of A | 0.3 M, 0.4 M, 0.6 M, 0.8 M, 1.0 M, 2.0 M |
| Temperature | Room temperature (ranging from 23–26 °C), 28 °C, 30 °C |
| Coil resident time | 2.5 min, 3 min, 4 min, 5 min, 6.5 min, 7.5 min, 8 min, 10 min, 12 min, 15 min |
| Reagents ratio (A:B:TBN) | 1:1:1, 1:1:1.3, 1:1:1.7, 1:1:2, 1:1:2.3, 1:1.1:1, 1:1.2:1, 1:1.3:2, 1:1.3:1.3, 1:1.5:1.3, 1:2:1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen, C.U.; Grier, K.E.; Andersen, J.B.; Hultqvist, L.D.; Nilsson, M.; Moser, C.; Graz, M.; Tolker-Nielsen, T.; Givskov, M.; Qvortrup, K. Upscaling and Risk Evaluation of the Synthesis of the 3,5-Diamino-1H-Pyrazole, Disperazol. Int. J. Mol. Sci. 2024, 25, 6737. https://doi.org/10.3390/ijms25126737
Jansen CU, Grier KE, Andersen JB, Hultqvist LD, Nilsson M, Moser C, Graz M, Tolker-Nielsen T, Givskov M, Qvortrup K. Upscaling and Risk Evaluation of the Synthesis of the 3,5-Diamino-1H-Pyrazole, Disperazol. International Journal of Molecular Sciences. 2024; 25(12):6737. https://doi.org/10.3390/ijms25126737
Chicago/Turabian StyleJansen, Charlotte Uldahl, Katja Egeskov Grier, Jens Bo Andersen, Louise Dahl Hultqvist, Martin Nilsson, Claus Moser, Michael Graz, Tim Tolker-Nielsen, Michael Givskov, and Katrine Qvortrup. 2024. "Upscaling and Risk Evaluation of the Synthesis of the 3,5-Diamino-1H-Pyrazole, Disperazol" International Journal of Molecular Sciences 25, no. 12: 6737. https://doi.org/10.3390/ijms25126737








