Adaptation of the Invasive Plant Sphagneticola trilobata to Flooding Stress by Hybridization with Native Relatives
Abstract
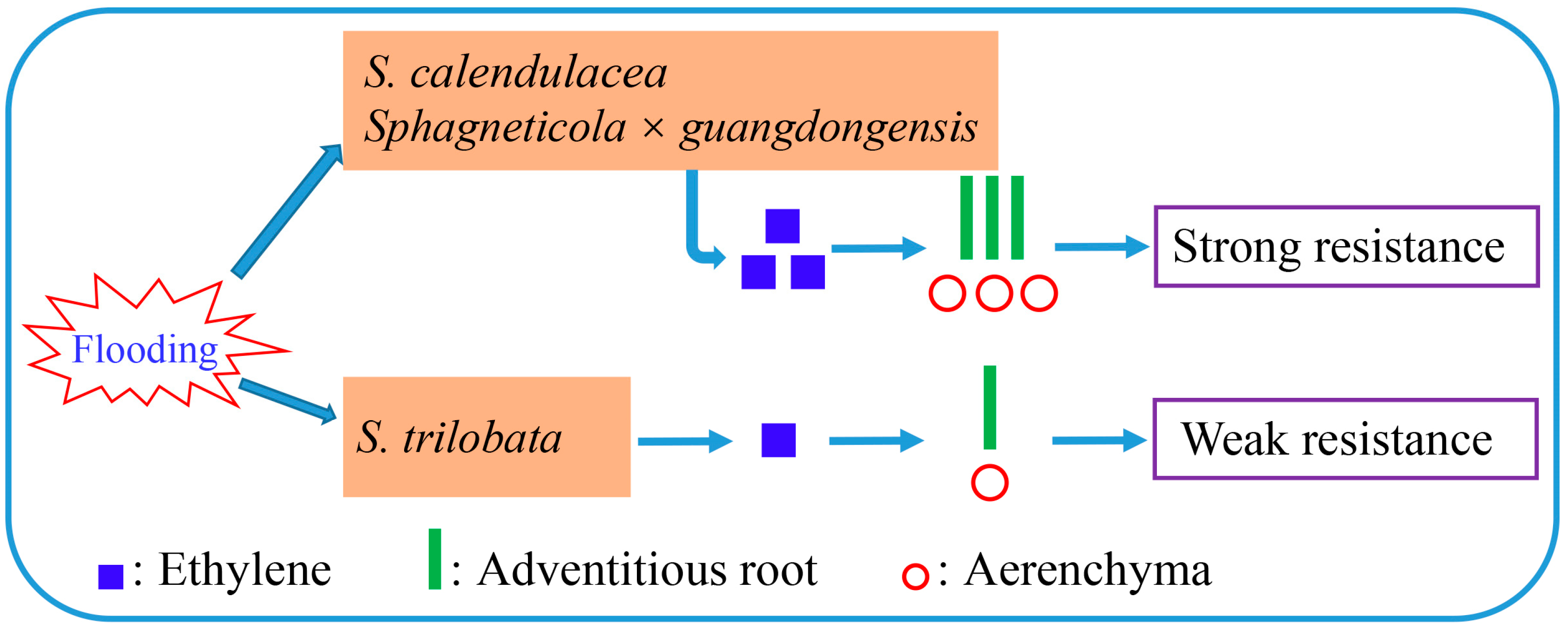
:1. Introduction
2. Results
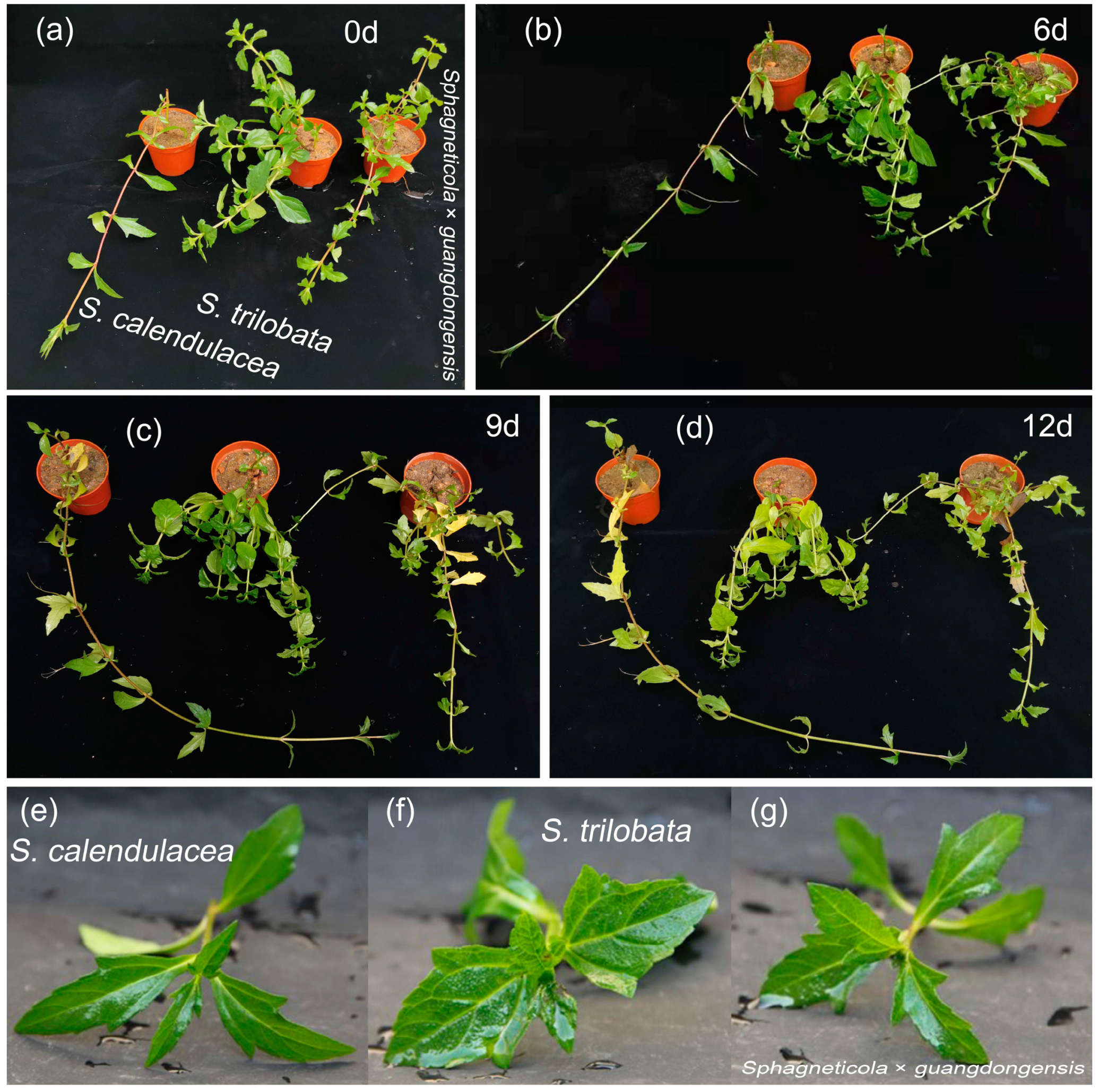
2.1. Phenotypic Characteristics
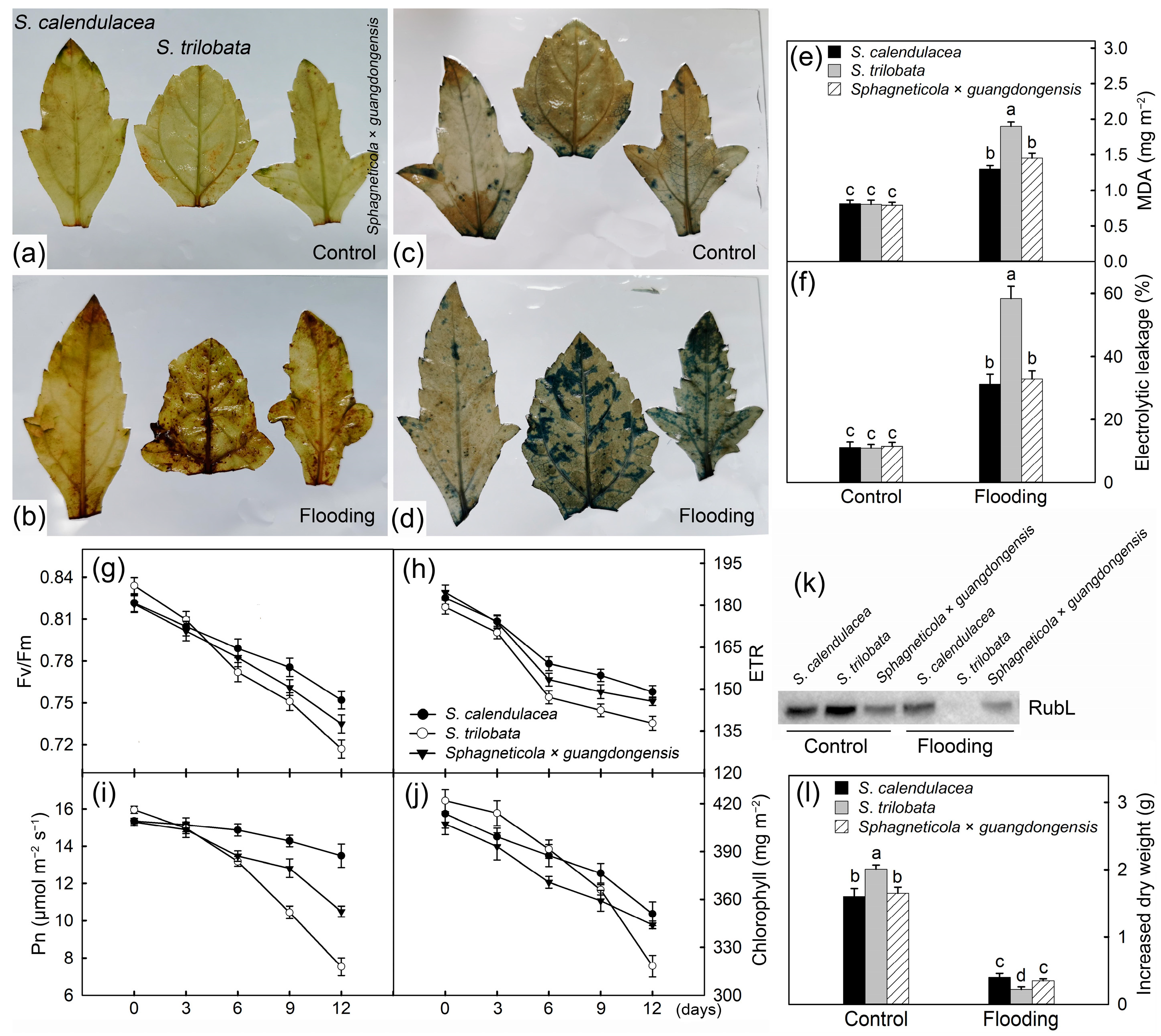
2.2. Accumulation of ROS and Changes in Photosynthetic Capacity
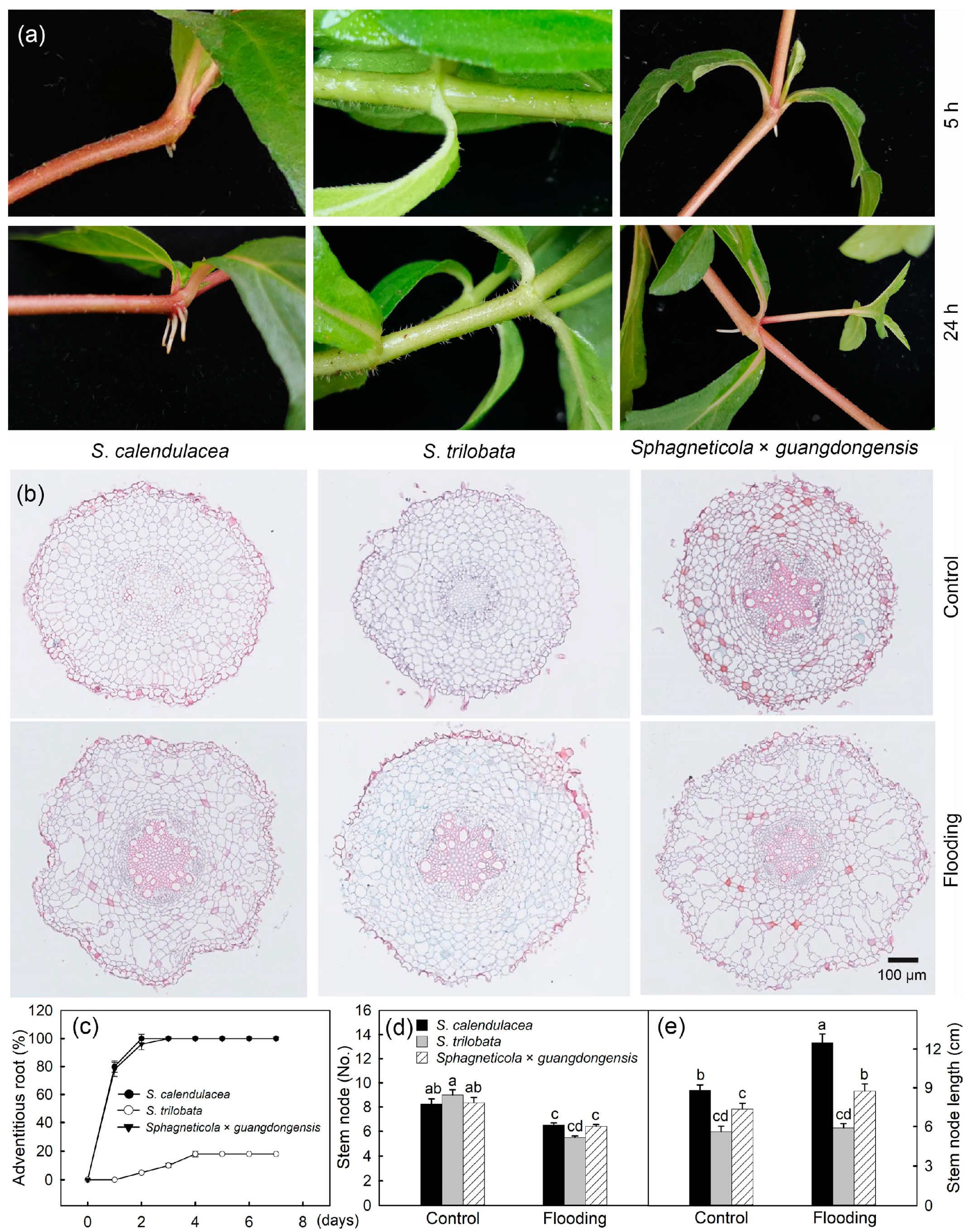
2.3. Adventitious Roots and Aerenchyma
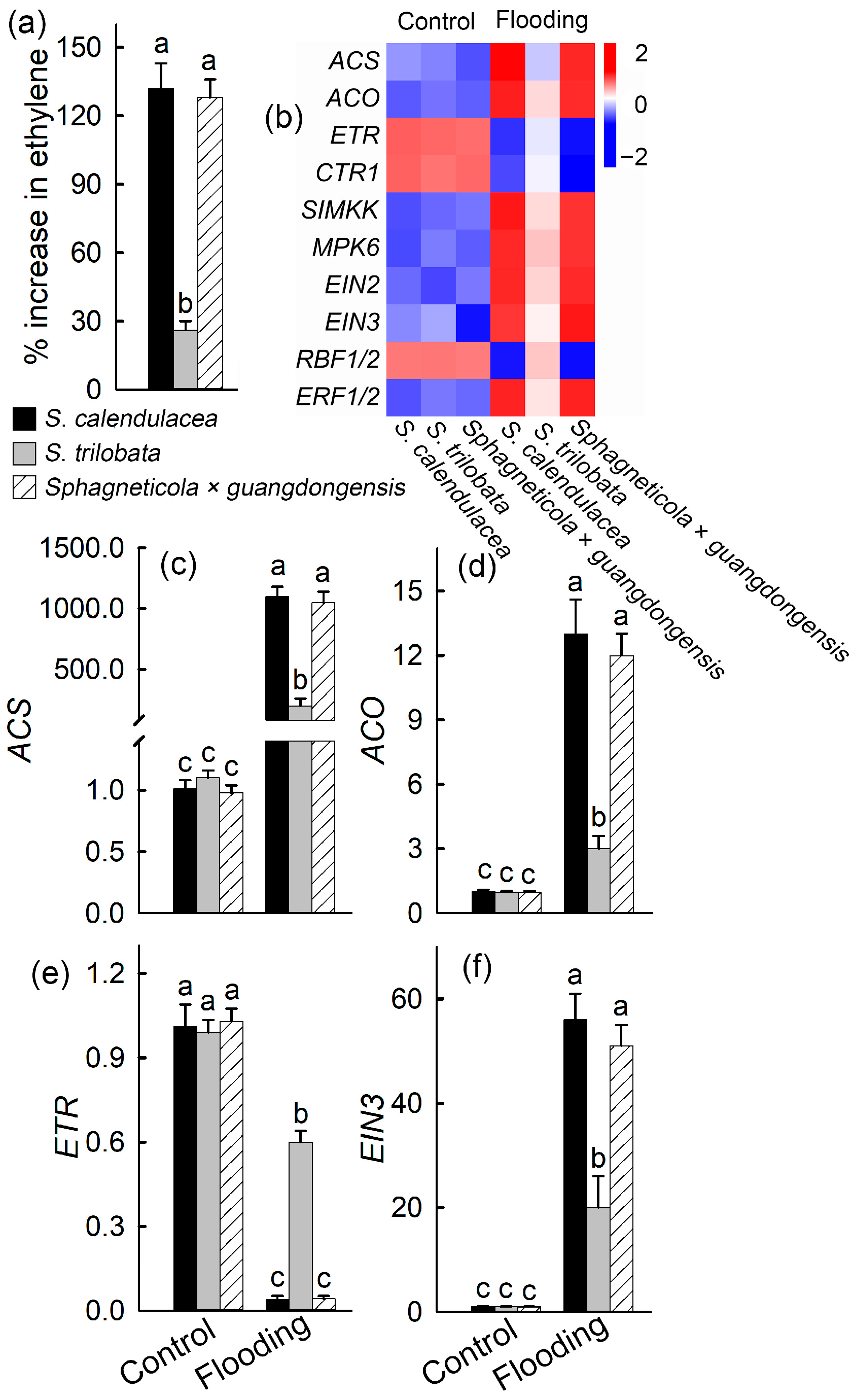
2.4. Expression Levels of Ethylene Synthesis and Transduction-Related Genes
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Net Photosynthetic Rate and Chlorophyll Fluorescence Parameters
4.3. Rubisco Protein Western Blotting
4.4. Measurement of Electrolyte Leakage Rates and Malondialdehyde
4.5. Histochemical Staining
4.6. Anatomical Analysis
4.7. Ethylene Measurement
4.8. Transcriptome Sequencing and Gene Expression Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahood, A.L.; Jones, R.O.; Board, D.I.; Balch, J.K.; Chambers, J.C. Interannual climate variability mediates changes in carbon and nitrogen pools caused by annual grass invasion in a semiarid shrubland. Glob. Chang. Biol. 2022, 28, 267–284. [Google Scholar] [CrossRef]
- Bock, D.G.; Baeckens, S.; Pita-Aquino, J.N.; Chejanovski, Z.A.; Michaelides, S.N.; Muralidhar, P.; Lapiedra, O.; Park, S.; Menke, D.B.; Geneva, A.J.; et al. Changes in selection pressure can facilitate hybridization during biological invasion in a Cuban lizard. Proc. Natl. Acad. Sci. USA 2021, 118, e2108638118. [Google Scholar] [CrossRef]
- Qiao, H.; Liu, W.; Zhang, Y.; Zhang, Y.; Li, Q.Q. Genetic admixture accelerates invasion via provisioning rapid adaptive evolution. Mol. Ecol. 2019, 28, 4012–4027. [Google Scholar] [CrossRef]
- Xia, L.; Zhao, H.; Yang, W.; An, S.Q. Genetic diversity, ecotype hybrid, and mixture of invasive Spartina alterniflora loisel in Coastal China. Clean-Soil Air Water 2015, 43, 1672–1681. [Google Scholar] [CrossRef]
- Abbott, R.J.; Brennan, A.C.; James, J.K.; Forbes, D.G.; Hegarty, M.J.; Hiscock, S.J. Recent hybrid origin and invasion of the British Isles by a self-incompatible species, Oxford ragwort (Senecio squalidus L., Asteraceae). Biol. Invasions 2009, 11, 1145–1158. [Google Scholar] [CrossRef]
- Lexer, C.; Welch, M.E.; Durphy, J.L.; Rieseberg, L.H. Natural selection for salt tolerance quantitative trait loci (QTLs) in wild sunflower hybrids: Implications for the origin of Helianthus paradoxus, a diploid hybrid species. Mol. Ecol. 2003, 12, 1225–1235. [Google Scholar] [CrossRef]
- Gallego-Tévar, B.; Grewell, B.J.; Drenovsky, R.E.; Castillo, J.M. Transgressivity in key functional traits rather than phenotypic plasticity promotes stress tolerance in a hybrid cordgrass. Plants 2019, 8, 594. [Google Scholar] [CrossRef]
- Hamilton, J.A.; Royauté, R.; Wright, J.W.; Hodgskiss, P.; Ledig, F.T. Genetic conservation and management of the California endemic, Torrey pine (Pinus torreyana Parry): Implications of genetic rescue in a genetically depauperate species. Ecol. Evol. 2017, 7, 7370–7381. [Google Scholar] [CrossRef]
- Sprunger, C.D.; Lindsey, A.; Lightcap, A. Above- and belowground linkages during extreme moisture excess: Leveraging knowledge from natural ecosystems to better understand implications for row-crop agroecosystems. J. Exp. Bot. 2023, 74, 2845–2859. [Google Scholar] [CrossRef]
- Tanoue, M.; Hirabayashi, Y.; Ikeuchi, H. Global-scale river flood vulnerability in the last 50 years. Sci. Rep. 2016, 6, 36021. [Google Scholar] [CrossRef]
- Campbell, D.; Keddy, P. The roles of competition and facilitation in producing zonation along an experimental flooding gradient: A tale of two tails with ten freshwater marsh plants. Wetlands 2022, 42, 1–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Ge, W.; Wang, J.; Guo, X.; Wang, T.; Li, W. Assessment of the impact of floods on terrestrial plant biodiversity. J. Clean. Prod. 2022, 339, 130722. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, F.; Meng, Y.; Chandrasekaran, U.; Luo, X.; Yang, W.; Shu, K. Plant waterlogging/flooding stress responses: From seed germination to maturation. Plant Physiol. Biochem. 2020, 148, 228–236. [Google Scholar] [CrossRef]
- Langan, P.; Bernád, V.; Walsh, J.; Henchy, J.; Khodaeiaminjan, M.; Mangina, E.; Negrão, S. Phenotyping for waterlogging tolerance in crops: Current trends and future prospects. J. Exp. Bot. 2022, 73, 5149–5169. [Google Scholar] [CrossRef]
- Leeggangers, H.A.; Rodriguez-Granados, N.Y.; Macias-Honti, M.G.; Sasidharan, R. A helping hand when drowning: The versatile role of ethylene in root flooding resilience. Environ. Exp. Bot. 2023, 213, 105422. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Q.; Lu, L.; Gao, H.; Liu, Q.; Liu, Y.; Yang, C.; Peng, C. The role of ascorbic acid in rice leaf senescence and photo-carbon imbalance. Funct. Plant Biol. 2020, 47, 263–278. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, J.; Dong, S.; Liu, P.; Zhao, B. Root and shoot responses of summer maize to waterlogging at different stages. Agron. J. 2016, 108, 1060–1069. [Google Scholar] [CrossRef]
- Martínez-Arias, C.; Witzell, J.; Solla, A.; Martin, J.A.; Rodríguez-Calcerrada, J. Beneficial and pathogenic plant-microbe interactions during flooding stress. Plant, Cell Environ. 2022, 45, 2875–2897. [Google Scholar] [CrossRef]
- Zhong, Y.; Guo, Z.; Wei, M.; Wang, J.; Song, S.; Chi, B.; Zhang, Y.; Liu, J.; Li, J.; Zhu, X.; et al. Hydrogen sulfide upregulates the alternative respiratory pathway in mangrove plant Avicennia marina to attenuate waterlogging-induced oxidative stress and mitochondrial damage in a calcium-dependent manner. Plant Cell Environ. 2023, 46, 1521–1539. [Google Scholar] [CrossRef]
- Pan, R.; Buitrago, S.; Feng, X.; Hu, A.; Zhou, M.; Zhang, W. Ethylene regulates aerenchyma formation in cotton under hypoxia stress by inducing the accumulation of reactive oxygen species. Environ. Exp. Bot. 2022, 197, 104826. [Google Scholar] [CrossRef]
- Tian, L.-X.; Zhang, Y.-C.; Chen, P.-L.; Zhang, F.-F.; Li, J.; Yan, F.; Dong, Y.; Feng, B.-L. How does the waterlogging regime affect crop yield? A global meta-analysis. Front. Plant Sci. 2021, 12, 634898. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Loreti, E.; van Veen, H.; Perata, P. Plant responses to flooding stress. Curr. Opin. Plant Biol. 2016, 33, 64–71. [Google Scholar] [CrossRef]
- Jia, W.; Ma, M.; Chen, J.; Wu, S. Plant morphological, physiological and anatomical adaption to flooding stress and the underlying molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 1088. [Google Scholar] [CrossRef]
- Yin, C.; Huang, Y.; Zhang, X.; Zhou, Y.; Chen, S.; Zhang, J. Ethylene-mediated regulation of coleoptile elongation in rice seedlings. Plant Cell Environ. 2023, 46, 1060–1074. [Google Scholar] [CrossRef]
- Daniel, K.; Hartman, S. How plant roots respond to waterlogging. J. Exp. Bot. 2024, 75, 511–525. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Trivellini, A.; Chhillar, H.; Chopra, P.; Ferrante, A.; Khan, N.A.; Ismail, A.M. The significance and functions of ethylene in flooding stress tolerance in plants. Environ. Exp. Bot. 2020, 179, 104188. [Google Scholar] [CrossRef]
- McGee, T.; Shahid, M.A.; Beckman, T.G.; Chaparro, J.X.; Schaffer, B.; Sarkhosh, A. Physiological and biochemical characterization of six Prunus rootstocks in response to flooding. Environ. Exp. Bot. 2021, 183, 104368. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, R.-C.; Ni, G.-Y.; Shen, H.; Ge, X.-J. Is a new invasive herb emerging? Molecular confirmation and preliminary evaluation of natural hybridization between the invasive Sphagneticola trilobata (Asteraceae) and its native congener S. calendulacea in South China. Biol. Invasions 2013, 15, 75–88. [Google Scholar] [CrossRef]
- Li, H.M.; Ren, C.; Yang, Q.-E.; Yuan, Q. A new natural hybrid of Sphagneticola (Asteraceae, Heliantheae) from Guangdong, China. Phytotaxa 2015, 221, 71. [Google Scholar]
- Ni, G.Y.; Zhao, P.; Wu, W.; Lu, X.K.; Zhao, X.H.; Zhu, L.W.; Niu, J.F. A hybrid of the invasive plant has similar competitive ability but different response to nitrogen deposition compared to parent. Ecol. Res. 2014, 29, 331–339. [Google Scholar] [CrossRef]
- Gao, L.; Cai, M.; Zeng, L.; Zhang, Q.; Zhu, H.; Gu, X.; Peng, C. Adaptation of the invasive plant (Sphagneticola trilobata L. Pruski) to a high cadmium environment by hybridizing with native relatives. Front. Plant Sci. 2022, 13, 905577. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, G.; Shao, L.; Gu, X.; Huang, J.; Peng, C. The hybridization between Sphagneticola trilobata (L.) Pruski and Sphagneticola calendulacea (L.) Pruski improved the tolerance of hybrid to cadmium stress. Chemosphere 2020, 249, 126540. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Chen, G.X.; Huang, J.D.; Peng, C.L. Comparison of the ability to control water loss in the detached leaves of Wedelia trilobata, Wedelia chinensis, and their hybrid. Plants 2020, 9, 1227. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Huang, J.D.; Ke, W.Q.; Cai, M.L.; Chen, G.X.; Peng, C.L. Responses of Sphagneticola trilobata, Sphagneticola calendulacea and their hybrid to drought stress. Int. J. Mol. Sci. 2021, 22, 11288. [Google Scholar] [CrossRef]
- Culley, T.M.; Hardiman, N.A. The role of intraspecific hybridization in the evolution of invasiveness: A case study of the ornamental pear tree Pyrus calleryana. Biol. Invasions 2009, 11, 1107–1119. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, Y.; Schaefer, V.; Liang, H.; Li, W.; Huang, S.; Peng, C. Responses of the hybrid between Sphagneticola trilobata and Sphagneticola calendulacea to low temperature and weak light characteristic in South China. Sci. Rep. 2015, 5, 16906. [Google Scholar] [CrossRef]
- Ding, Z.; Sun, Q. Effects of flooding depth on metal(loid) absorption and physiological characteristics of Phragmites australis in acid mine drainage phytoremediation. Environ. Technol. Innov. 2021, 22, 101512. [Google Scholar] [CrossRef]
- Mozo, I.; Rodríguez, M.E.; Monteoliva, S.; Luquez, V.M.C. Floodwater depth causes different physiological responses during post-flooding in Willows. Front. Plant Sci. 2021, 12, 575090. [Google Scholar] [CrossRef]
- Haque, A.; Rafii, M.Y.Y.; Yusoff, M.M.; Ali, N.S.; Yusuff, O.; Arolu, F.; Anisuzzaman, M. Flooding tolerance in Rice: Adaptive mechanism and marker-assisted selection breeding approaches. Mol. Biol. Rep. 2023, 50, 2795–2812. [Google Scholar] [CrossRef]
- Muhammad, T.; Zhang, J.; Ma, Y.; Li, Y.; Zhang, F.; Zhang, Y.; Liang, Y. Overexpression of a mitogen-activated protein kinase SlMAPK3 positively regulates tomato tolerance to cadmium and drought stress. Molecules 2019, 24, 556. [Google Scholar] [CrossRef]
- Mushtaq, N.; Wang, Y.; Fan, J.M.; Li, Y.; Ding, J. Down-regulation of Cytokinin receptor gene SlHK2 improves plant tolerance to drought, heat, and combined stresses in tomato. Plants 2022, 11, 154. [Google Scholar] [CrossRef]
- Mao, C.; Lu, S.; Lv, B.; Zhang, B.; Shen, J.; He, J.; Luo, L.; Xi, D.; Chen, X.; Ming, F. A rice NAC transcription factor promotes leaf senescence via ABA biosynthesis. Plant Physiol. 2017, 174, 1747–1763. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Van Ha, C.; Nishiyama, R.; Watanabe, Y.; Leyva-González, M.A.; Fujita, Y.; Tran, U.T.; Li, W.Q.; Tanaka, M.; Seki, M.; et al. Arabidopsis type B cytokinin response regulators ARR1, ARR10, and ARR12 negatively regulate plant responses to drought. Proc. Natl. Acad. Sci. USA 2016, 113, 3090–3095. [Google Scholar] [CrossRef]
- Yamauchi, T.; Nakazono, M. Mechanisms of lysigenous aerenchyma formation under abiotic stress. Trends Plant Sci. 2022, 27, 13–15. [Google Scholar] [CrossRef]
- Yeung, E.; van Veen, H.; Vashisht, D.; Paiva, A.L.S.; Hummel, M.; Rankenberg, T.; Steffens, B.; Steffen-Heins, A.; Sauter, M.; de Vries, M.; et al. A stress recovery signaling network for enhanced flooding tolerance inArabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2018, 115, E6085–E6094. [Google Scholar] [CrossRef]
- Xu, H.; Wang, S.; Larkin, R.M.; Zhang, F. The transcription factors DcHB30 and DcWRKY75 antagonistically regulate ethylene-induced petal senescence in carnation (Dianthus caryophyllus). J. Exp. Bot. 2022, 73, 7326–7343. [Google Scholar] [CrossRef]
- Zhang, H.M.; Li, S.; Yang, L.; Cai, G.H.; Chen, H.M.; Gao, D.L.; Lin, T.; Cui, Q.Z.; Wang, D.H.; Li, Z.; et al. Gain-of-function of the 1-aminocyclopropane-1-carboxylate synthase gene induces female flower development in cucumber gynoecy. Plant Cell 2021, 33, 306–321. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhai, J.; Shao, L.; Lin, W.; Peng, C. Accumulation of anthocyanins: An adaptation strategy of Mikania micrantha to low temperature in winter. Front. Plant Sci. 2019, 10, 1049. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Krall, J.P.; Edwards, G.E. Relationship between photosystem II activity and CO2 fixation in leaves. Physiol. Plantarum 1992, 86, 180–187. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components—Calculation of qP and Fv’/Fm’; without measuring Fo’. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Lee, S.-H.; Sakuraba, Y.; Lee, T.; Kim, K.-W.; An, G.; Lee, H.Y.; Paek, N.-C. Mutation of Oryza sativa CORONATINE INSENSITIVE 1b (OsCOI1b) delays leaf senescence. J. Integr. Plant Biol. 2015, 57, 562–576. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, D.; Pike, S.; Pallardy, S.; Gassmann, W.; Zhang, S. Chloroplast-generated reactive oxygen species are involved in hypersensitive response-like cell death mediated by a mitogen-activated protein kinase cascade. Plant J. 2007, 51, 941–954. [Google Scholar] [CrossRef]
- Lim, T.S.; Chitra, T.R.; Han, P.; Pua, E.C.; Yu, H. Cloning and characterization of and flavin-containing amine oxidases. J. Exp. Bot. 2006, 57, 4155–4169. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gallego-Tévar, B.; Grewell, B.J.; Futrell, C.J.; Drenovsky, R.E.; Castillo, J.M. Interactive effects of salinity and inundation on native Spartina foliosa, invasive S. densiflora and their hybrid from San Francisco Estuary, California. Ann. Bot. 2020, 125, 377–389. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Chen, G.; Ke, W.; Peng, C. Adaptation of the Invasive Plant Sphagneticola trilobata to Flooding Stress by Hybridization with Native Relatives. Int. J. Mol. Sci. 2024, 25, 6738. https://doi.org/10.3390/ijms25126738
Zhang Q, Chen G, Ke W, Peng C. Adaptation of the Invasive Plant Sphagneticola trilobata to Flooding Stress by Hybridization with Native Relatives. International Journal of Molecular Sciences. 2024; 25(12):6738. https://doi.org/10.3390/ijms25126738
Chicago/Turabian StyleZhang, Qilei, Guangxin Chen, Weiqian Ke, and Changlian Peng. 2024. "Adaptation of the Invasive Plant Sphagneticola trilobata to Flooding Stress by Hybridization with Native Relatives" International Journal of Molecular Sciences 25, no. 12: 6738. https://doi.org/10.3390/ijms25126738





