Hydrogen-Rich Alkaline Water Supplementation Restores a Healthy State and Redox Balance in H2O2-Treated Mice
Abstract
:1. Introduction
2. Results
2.1. Systemic Response to the HAW Treatment
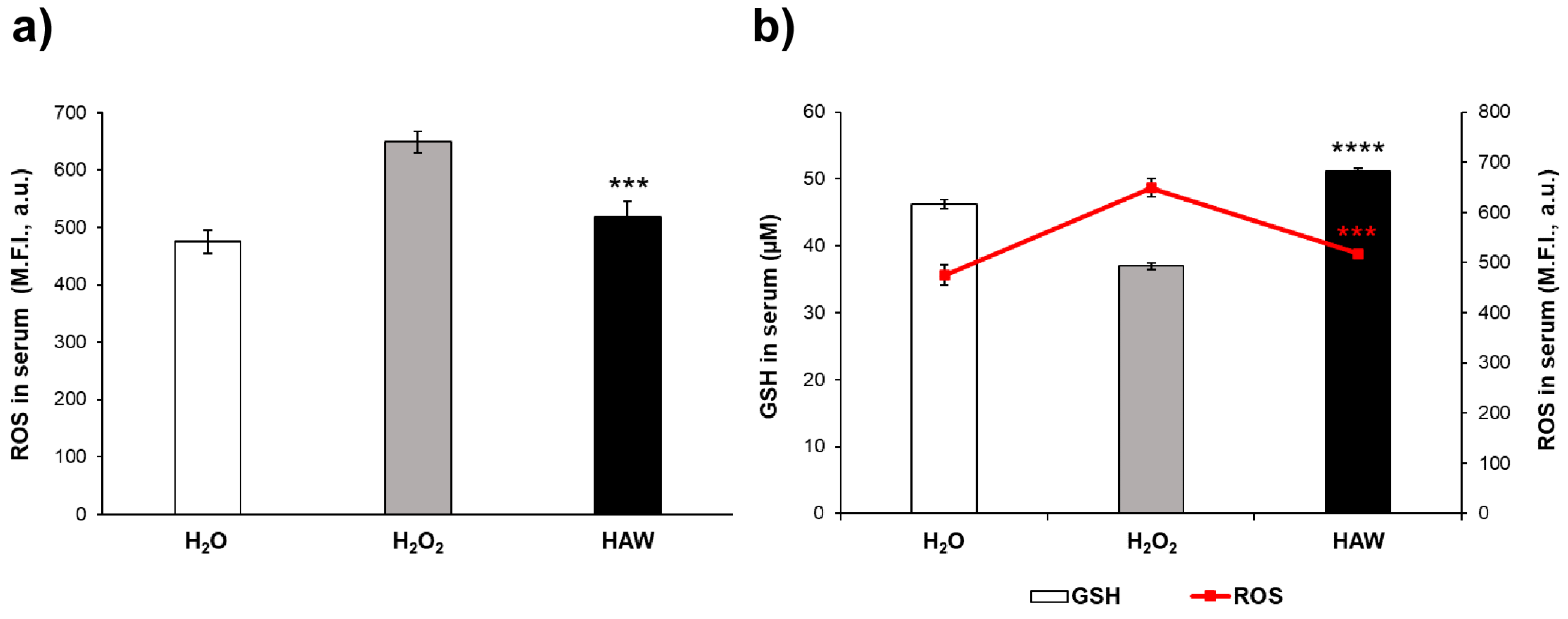
2.1.1. The Antioxidant Effect of HAW
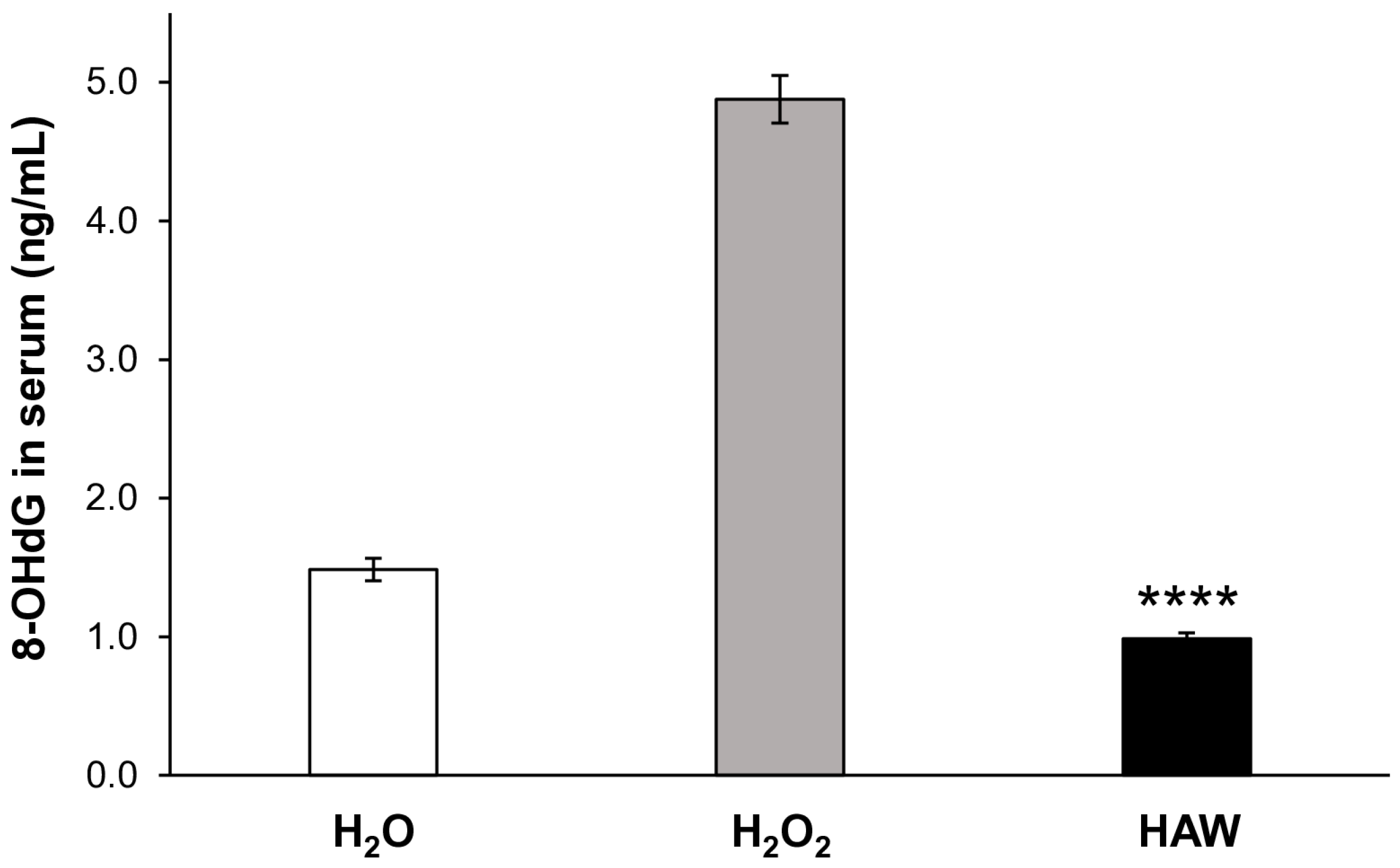
2.1.2. DNA Damage
2.2. The Response at the Organ Level
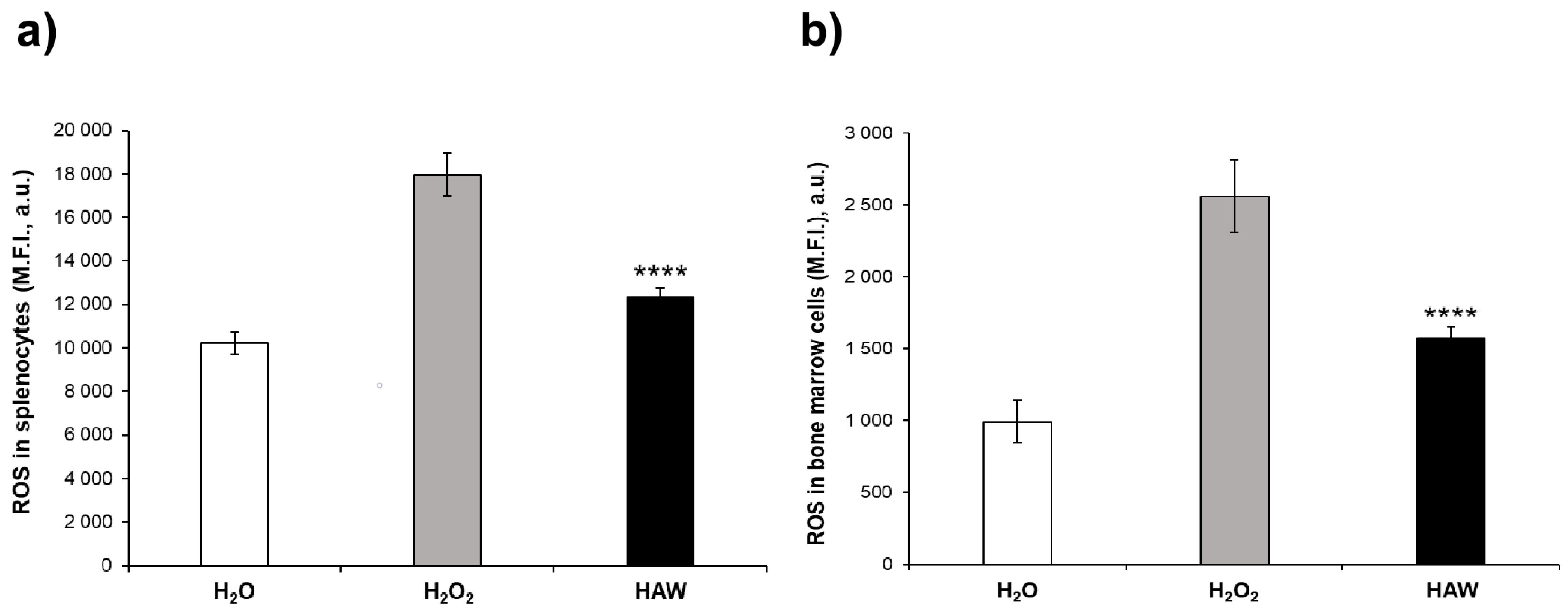
2.2.1. Oxidative Stress
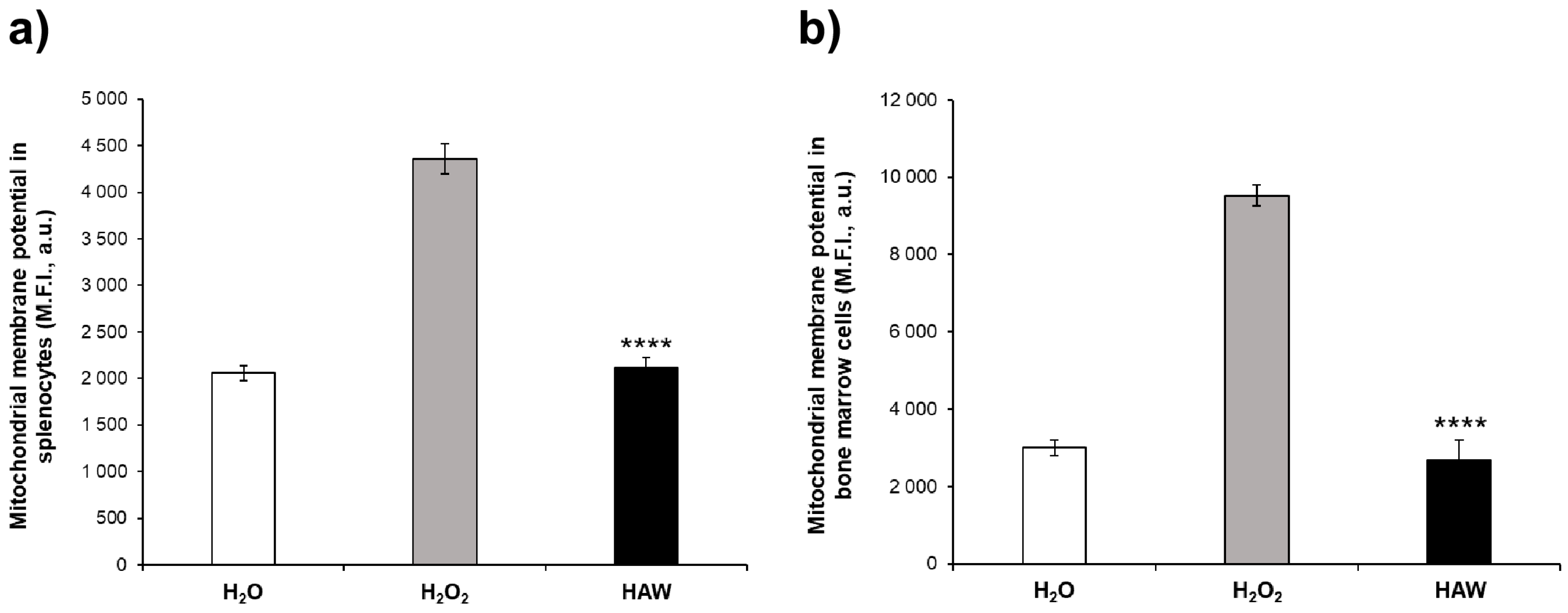
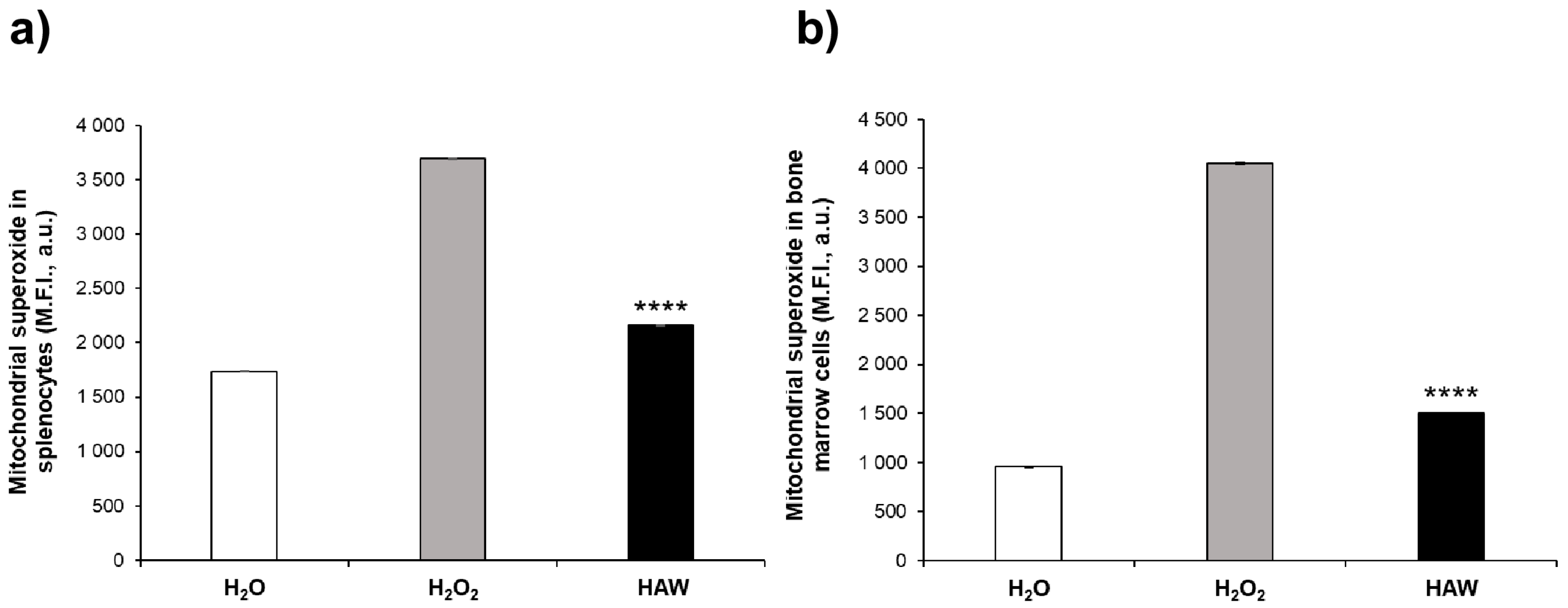
2.2.2. Oxidative Stress in Mitochondria of Bone Marrow Cells and Splenocytes
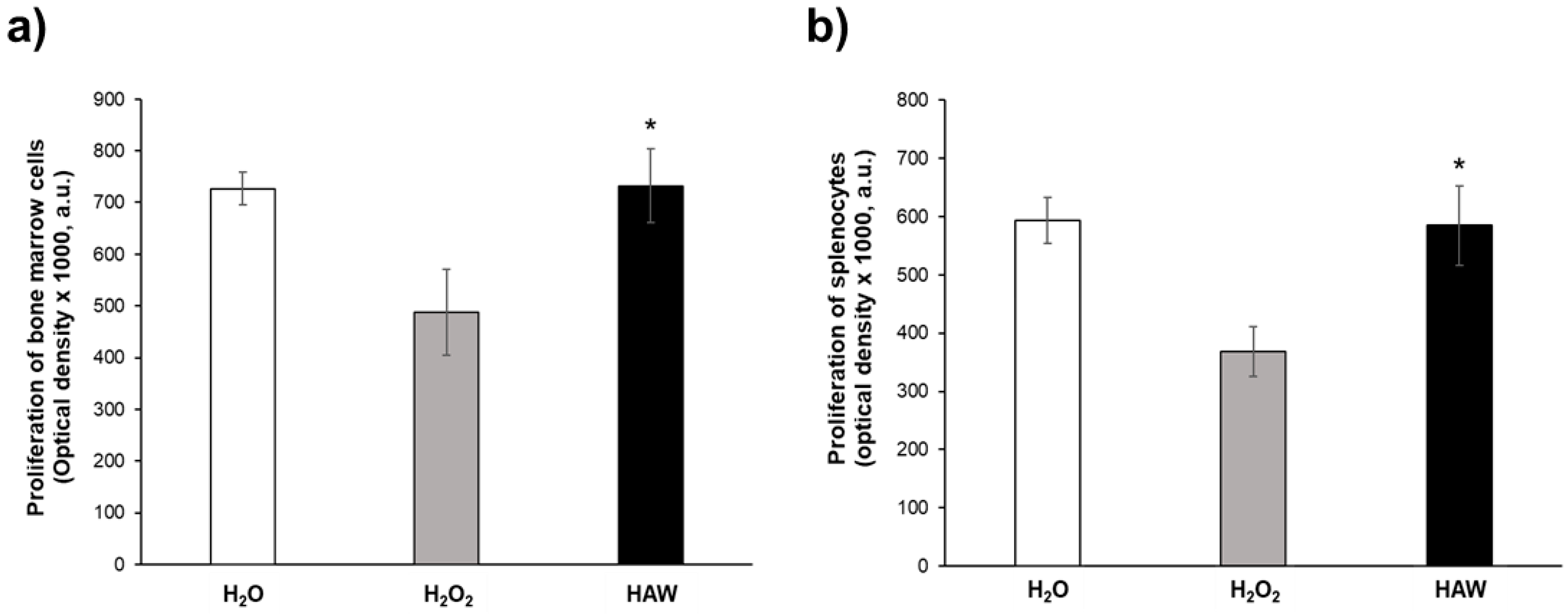
2.2.3. Ex Vivo Proliferative Response of Single-Cell Suspensions from Spleen and Bone Marrow
3. Discussion
4. Materials and Methods
4.1. In Vivo Studies
4.2. Collection and Processing of Murine Plasma from Blood Samples
4.3. Bone Marrow and Splenocytes Cells Recovery from C57BL/6J Mice
4.4. Hydrogen-Rich Alkaline Water Supplementation (HAW)
4.5. Total ROS Assay
4.6. GSH Detection and Quantification Assay
4.7. Mitochondrial Membrane Potential Measurement
4.8. Mitochondrial Superoxide Detection
4.9. Cell Proliferation Assay
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galloway, A. The Evolutionary Biology of Aging. By Michael, R. Rose. New York: Oxford University Press. 1991. Ix + 221 Pp. ISBN 0-19-506133-0. $35.00 (Cloth). Am. J. Phys. Anthropol. 1993, 91, 260–262. [Google Scholar] [CrossRef]
- Partridge, L.; Barton, N.H. Optimally, Mutation and the Evolution of Ageing. Nature 1993, 362, 305–311. [Google Scholar] [CrossRef]
- Jin, K. Modern Biological Theories of Aging. Aging Dis. 2010, 1, 72–74. [Google Scholar]
- Logozzi, M.; Mizzoni, D.; Di Raimo, R.; Andreotti, M.; Macchia, D.; Spada, M.; Fais, S. In Vivo Antiaging Effects of Alkaline Water Supplementation. J. Enzym. Inhib. Med. Chem. 2020, 35, 657–664. [Google Scholar] [CrossRef]
- Abrabam Guy, E.; Flebas Jorge, D. The Effect of Daily Consumption of 2 Liters of Electrolyzed Water for 2 Months on Body Composition and Several Physiological Parameters in Four Obese Subjects: A Preliminary Report; The Free Library: Philadelphia, PA, USA, 2011. [Google Scholar]
- Magro, M.; Corain, L.; Ferro, S.; Baratella, D.; Bonaiuto, E.; Terzo, M.; Corraducci, V.; Salmaso, L.; Vianello, F. Alkaline Water and Longevity: A Murine Study. Evid.-Based Complement. Altern. Med. 2016, 2016, 3084126. [Google Scholar] [CrossRef]
- Astigiano, S.; Puglisi, A.; Mastracci, L.; Fais, S.; Barbieri, O. Systemic Alkalinisation Delays Prostate Cancer Cell Progression in TRAMP Mice. J. Enzym. Inhib. Med. Chem. 2017, 32, 363–368. [Google Scholar] [CrossRef]
- Spugnini, E.P.; Buglioni, S.; Carocci, F.; Francesco, M.; Vincenzi, B.; Fanciulli, M.; Fais, S. High Dose Lansoprazole Combined with Metronomic Chemotherapy: A Phase I/II Study in Companion Animals with Spontaneously Occurring Tumors. J. Transl. Med. 2014, 12, 225. [Google Scholar] [CrossRef]
- Albina, P.; Durban, N.; Bertron, A.; Albrecht, A.; Robinet, J.-C.; Erable, B. Influence of Hydrogen Electron Donor, Alkaline pH, and High Nitrate Concentrations on Microbial Denitrification: A Review. Int. J. Mol. Sci. 2019, 20, 5163. [Google Scholar] [CrossRef]
- Barzel, U.S. Acid-Induced Osteoporosis: An Experimental Model of Human Osteoporosis. Calcif. Tissue Res. 1976, 21, 417–422. [Google Scholar] [CrossRef]
- Sebastian, A.; Harris, S.T.; Ottaway, J.H.; Todd, K.M.; Morris, R.C. Improved Mineral Balance and Skeletal Metabolism in Postmenopausal Women Treated with Potassium Bicarbonate. N. Engl. J. Med. 1994, 330, 1776–1781. [Google Scholar] [CrossRef]
- Ostojic, S.M. Serum Alkalinization and Hydrogen-Rich Water in Healthy Men. Mayo Clin. Proc. 2012, 87, 501–502. [Google Scholar] [CrossRef]
- Sim, M.; Kim, C.-S.; Shon, W.-J.; Lee, Y.-K.; Choi, E.Y.; Shin, D.-M. Hydrogen-Rich Water Reduces Inflammatory Responses and Prevents Apoptosis of Peripheral Blood Cells in Healthy Adults: A Randomized, Double-Blind, Controlled Trial. Sci. Rep. 2020, 10, 12130. [Google Scholar] [CrossRef]
- Botek, M.; Krejčí, J.; McKune, A.; Valenta, M.; Sládečková, B. Hydrogen Rich Water Consumption Positively Affects Muscle Performance, Lactate Response, and Alleviates Delayed Onset of Muscle Soreness After Resistance Training. J. Strength Cond. Res. 2022, 36, 2792–2799. [Google Scholar] [CrossRef]
- Dobashi, S.; Takeuchi, K.; Koyama, K. Hydrogen-Rich Water Suppresses the Reduction in Blood Total Antioxidant Capacity Induced by 3 Consecutive Days of Severe Exercise in Physically Active Males. Med. Gas Res. 2020, 10, 21. [Google Scholar] [CrossRef]
- Li, S.-W.; Takahara, T.; Que, W.; Fujino, M.; Guo, W.-Z.; Hirano, S.-I.; Ye, L.-P.; Li, X.-K. Hydrogen-Rich Water Protects against Liver Injury in Nonalcoholic Steatohepatitis through HO-1 Enhancement via IL-10 and Sirt 1 Signaling. Am. J. Physiol.-Gastrointest. Liver Physiol. 2021, 320, G450–G463. [Google Scholar] [CrossRef]
- Kawakami, K.; Matsuo, H.; Yamada, T.; Matsumoto, K.; Sasaki, D.; Nomura, M. Effects of Hydrogen-Rich Water and Ascorbic Acid Treatment on Spontaneously Hypertensive Rats. Exp. Anim. 2022, 71, 347–355. [Google Scholar] [CrossRef]
- Song, L.; Zhang, Y.; Zhu, C.; Ding, X.; Yang, L.; Yan, H. Hydrogen-Rich Water Partially Alleviate Inflammation, Oxidative Stress and Intestinal Flora Dysbiosis in DSS-Induced Chronic Ulcerative Colitis Mice. Adv. Med. Sci. 2022, 67, 29–38. [Google Scholar] [CrossRef]
- Zheng, M.; Yu, H.; Xue, Y.; Yang, T.; Tu, Q.; Xiong, K.; Deng, D.; Lu, L.; Huang, N. The Protective Effect of Hydrogen-Rich Water on Rats with Type 2 Diabetes Mellitus. Mol. Cell. Biochem. 2021, 476, 3089–3097. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Liu, L.; Zhang, Z.; Li, S.; Zhang, Z.; Zhou, Y.; Liu, F. Metabolomics Analysis of the Effect of Hydrogen-Rich Water on Myocardial Ischemia-Reperfusion Injury in Rats. J. Bioenerg. Biomembr. 2020, 52, 257–268. [Google Scholar] [CrossRef]
- Qian, L.; Liu, J.; Ma, W.; Liu, Y.; Wang, X.; Liu, D. Hydrogen-Rich Water Ameliorates Murine Chronic Graft-versus-Host Disease through Antioxidation. Oxid. Med. Cell. Longev. 2021, 2021, 1165928. [Google Scholar] [CrossRef]
- Mizutani, A.; Endo, A.; Saito, M.; Hara, T.; Nakagawa, M.; Sakuraya, K.; Murano, Y.; Nishizaki, N.; Hirano, D.; Fujinaga, S.; et al. Hydrogen-Rich Water Reduced Oxidative Stress and Renal Fibrosis in Rats with Unilateral Ureteral Obstruction. Pediatr. Res. 2022, 91, 1695–1702. [Google Scholar] [CrossRef]
- Li, H.; Yin, Y.; Liu, J.; Lu, B.; Wan, H.; Yang, L.; Wang, W.; Li, R. Hydrogen-Rich Water Attenuates the Radiotoxicity Induced by Tritium Exposure in Vitro and in Vivo. J. Radiat. Res. 2021, 62, 34–45. [Google Scholar] [CrossRef]
- Köktürk, M.; Atalar, M.N.; Odunkıran, A.; Bulut, M.; Alwazeer, D. Evaluation of the Hydrogen-Rich Water Alleviation Potential on Mercury Toxicity in Earthworms Using ATR-FTIR and LC–ESI–MS/MS Spectroscopy. Environ. Sci. Pollut. Res. 2022, 29, 19642–19656. [Google Scholar] [CrossRef]
- Saitoh, Y.; Harata, Y.; Mizuhashi, F.; Nakajima, M.; Miwa, N. Biological Safety of Neutral-pH Hydrogen-Enriched Electrolyzed Water upon Mutagenicity, Genotoxicity and Subchronic Oral Toxicity. Toxicol. Ind. Health 2010, 26, 203–216. [Google Scholar] [CrossRef]
- Li, J.; Hong, Z.; Liu, H.; Zhou, J.; Cui, L.; Yuan, S.; Chu, X.; Yu, P. Hydrogen-Rich Saline Promotes the Recovery of Renal Function after Ischemia/Reperfusion Injury in Rats via Anti-Apoptosis and Anti-Inflammation. Front. Pharmacol. 2016, 7, 106. [Google Scholar] [CrossRef]
- Meng, X.; Chen, H.; Wang, G.; Yu, Y.; Xie, K. Hydrogen-Rich Saline Attenuates Chemotherapy-Induced Ovarian Injury via Regulation of Oxidative Stress. Exp. Ther. Med. 2015, 10, 2277–2282. [Google Scholar] [CrossRef]
- Bai, X.; Liu, S.; Yuan, L.; Xie, Y.; Li, T.; Wang, L.; Wang, X.; Zhang, T.; Qin, S.; Song, G.; et al. Hydrogen-Rich Saline Mediates Neuroprotection through the Regulation of Endoplasmic Reticulum Stress and Autophagy under Hypoxia-Ischemia Neonatal Brain Injury in Mice. Brain Res. 2016, 1646, 410–417. [Google Scholar] [CrossRef]
- Chu, X.; Cao, L.; Yu, Z.; Xin, D.; Li, T.; Ma, W.; Zhou, X.; Chen, W.; Liu, D.; Wang, Z. Hydrogen-Rich Saline Promotes Microglia M2 Polarization and Complement-Mediated Synapse Loss to Restore Behavioral Deficits Following Hypoxia-Ischemic in Neonatal Mice via AMPK Activation. J. Neuroinflamm. 2019, 16, 104. [Google Scholar] [CrossRef]
- Ke, H.; Liu, D.; Li, T.; Chu, X.; Xin, D.; Han, M.; Wang, S.; Wang, Z. Hydrogen-Rich Saline Regulates Microglial Phagocytosis and Restores Behavioral Deficits Following Hypoxia-Ischemia Injury in Neonatal Mice via the Akt Pathway. DDDT 2020, 14, 3827–3839. [Google Scholar] [CrossRef]
- Schulpis, K.; Papassotiriou, I.; Tsakiris, S. 8-Hydroxy-2-Desoxyguanosine Serum Concentrations as a Marker of DNA Damage in Patients with Classical Galactosaemia. Acta Paediatr. 2006, 95, 164–169. [Google Scholar] [CrossRef]
- Dai, L.; Watanabe, M.; Qureshi, A.R.; Mukai, H.; Machowska, A.; Heimbürger, O.; Barany, P.; Lindholm, B.; Stenvinkel, P. Serum 8-Hydroxydeoxyguanosine, a Marker of Oxidative DNA Damage, Is Associated with Mortality Independent of Inflammation in Chronic Kidney Disease. Eur. J. Intern. Med. 2019, 68, 60–65. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′ -Deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Environ. Sci. Health Part C 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Chuma, M.; Hige, S.; Nakanishi, M.; Ogawa, K.; Natsuizaka, M.; Yamamoto, Y.; Asaka, M. 8-Hydroxy-2′-deoxy-guanosine Is a Risk Factor for Development of Hepatocellular Carcinoma in Patients with Chronic Hepatitis C Virus Infection. J. Gastrol. Hepatol. 2008, 23, 1431–1436. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. [Malondialdehyde (MDA) as a lipid peroxidation marker]. Wiad. Lek. 2004, 57, 453–455. [Google Scholar]
- Finkel, T. Signal Transduction by Reactive Oxygen Species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Arnold, R.S.; Shi, J.; Murad, E.; Whalen, A.M.; Sun, C.Q.; Polavarapu, R.; Parthasarathy, S.; Petros, J.A.; Lambeth, J.D. Hydrogen Peroxide Mediates the Cell Growth and Transformation Caused by the Mitogenic Oxidase Nox1. Proc. Natl. Acad. Sci. USA 2001, 98, 5550–5555. [Google Scholar] [CrossRef]
- Brand, M.D.; Affourtit, C.; Esteves, T.C.; Green, K.; Lambert, A.J.; Miwa, S.; Pakay, J.L.; Parker, N. Mitochondrial Superoxide: Production, Biological Effects, and Activation of Uncoupling Proteins. Free. Radic. Biol. Med. 2004, 37, 755–767. [Google Scholar] [CrossRef]
- Velarde, M.C.; Flynn, J.M.; Day, N.U.; Melov, S.; Campisi, J. Mitochondrial Oxidative Stress Caused by Sod2 Deficiency Promotes Cellular Senescence and Aging Phenotypes in the Skin. Aging 2012, 4, 3–12. [Google Scholar] [CrossRef]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial Membrane Potential Probes and the Proton Gradient: A Practical Usage Guide. BioTechniques 2011, 50, 98–115. [Google Scholar] [CrossRef]
- Izyumov, D.S.; Avetisyan, A.V.; Pletjushkina, O.Y.; Sakharov, D.V.; Wirtz, K.W.; Chernyak, B.V.; Skulachev, V.P. “Wages of Fear”: Transient Threefold Decrease in Intracellular ATP Level Imposes Apoptosis. Biochim. Biophys. Acta (BBA)-Bioenerg. 2004, 1658, 141–147. [Google Scholar] [CrossRef]
- McMahon, B.H.; Fabian, M.; Tomson, F.; Causgrove, T.P.; Bailey, J.A.; Rein, F.N.; Dyer, R.B.; Palmer, G.; Gennis, R.B.; Woodruff, W.H. FTIR Studies of Internal Proton Transfer Reactions Linked to Inter-Heme Electron Transfer in Bovine Cytochrome c Oxidase. Biochim. Biophys. Acta (BBA)-Bioenerg. 2004, 1655, 321–331. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial Membrane Potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Packer, L.; Traber, M.G. Proceedings of the International Symposium on Natural Antioxidants; AOCS Publishing: Champaign, IL, USA, 1996; ISBN 978-1-4398-3205-9. [Google Scholar]
- Starkov, A.A.; Fiskum, G. Regulation of Brain Mitochondrial H2O2 Production by Membrane Potential and NAD(P)H Redox State. J. Neurochem. 2003, 86, 1101–1107. [Google Scholar] [CrossRef]
- Skulachev, V.P. Role of Uncoupled and Non-Coupled Oxidations in Maintenance of Safely Low Levels of Oxygen and Its One-Electron Reductants. Quart. Rev. Biophys. 1996, 29, 169–202. [Google Scholar] [CrossRef]
- Ježek, P.; Holendová, B.; Garlid, K.D.; Jabůrek, M. Mitochondrial Uncoupling Proteins: Subtle Regulators of Cellular Redox Signaling. Antioxid. Redox Signal. 2018, 29, 667–714. [Google Scholar] [CrossRef]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Andreotti, M.; Spada, M.; Macchia, D.; Fais, S. Beneficial Effects of Fermented Papaya Preparation (FPP®) Supplementation on Redox Balance and Aging in a Mouse Model. Antioxidants 2020, 9, 144. [Google Scholar] [CrossRef]

| SUBSTANCE | DOSE FOR 1 CPS | MAX DAILY DOSE | %Nutrient Reference Values (NRV) |
|---|---|---|---|
| Potassium | 250.3 mg | 751.1 mg | 37.56% |
| Sodium | 79.34 mg | 238.02 mg | - |
| Vitamin C | 333.33 mg | 1000 mg | 1250% |
| Chloride | 60.9 mg | 182.72 mg | 22.84% |
| Chromium | 6.66 mcg | 20 mcg | 50% |
| Selenium | 9.16 mcg | 27.5 mcg | 50% |
| Salt | Source | Powder % |
|---|---|---|
| Sodium Chloride (NaCl) | Bolivian RoseTM—Andes Mountain Salt from Andes Mountain Range (Bolivia) (Woodinville, WA, USA) | 95.5 |
| Natural Alps Mountains Salt, coarse from Bergkern (Austria) | 94.5 | |
| HALITE SALT from rock salt mines (Pakistan) | 99.35 | |
| Sodium (Na) | Bolivian RoseTM—Andes Mountain Salt from Andes Mountain Range (Bolivia) (Woodinville, WA, USA) | / |
| Natural Alps Mountains Salt, coarse from Bergkern (Austria) | 35.4 | |
| HALITE SALT from rock salt mines (Pakistan) | / | |
| Calcium (Ca) | Bolivian RoseTM—Andes Mountain Salt from Andes Mountain Range (Bolivia) (Woodinville, WA, USA) | 0.7 |
| Natural Alps Mountains Salt, coarse from Bergkern (Austria) | 0.25 | |
| HALITE SALT from rock salt mines (Pakistan) | 0.19 | |
| Magnesium (Ma) | Bolivian RoseTM—Andes Mountain Salt from Andes Mountain Range (Bolivia) (Woodinville, WA, USA) | 0.208 |
| Natural Alps Mountains Salt, coarse from Bergkern (Austria) | 0.1 | |
| HALITE SALT from rock salt mines (Pakistan) | 0.16 | |
| Potassium (K) | Bolivian RoseTM—Andes Mountain Salt from Andes Mountain Range (Bolivia) (Woodinville, WA, USA) | 0.646 |
| Natural Alps Mountains Salt, coarse from Bergkern (Austria) | 0.3 | |
| HALITE SALT from rock salt mines (Pakistan) | / | |
| Iron (Fe) | Bolivian RoseTM—Andes Mountain Salt from Andes Mountain Range (Bolivia) (Woodinville, WA, USA) | 0.303 |
| Natural Alps Mountains Salt, coarse from Bergkern (Austria) | / | |
| HALITE SALT from rock salt mines (Pakistan) | / | |
| Sulphate (SO4) | Bolivian RoseTM—Andes Mountain Salt from Andes Mountain Range (Bolivia) (Woodinville, WA, USA) | / |
| Natural Alps Mountains Salt, coarse from Bergkern (Austria) | 2.0 | |
| HALITE SALT from rock salt mines (Pakistan) | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizzoni, D.; Logozzi, M.; Di Raimo, R.; Spada, M.; Fais, S. Hydrogen-Rich Alkaline Water Supplementation Restores a Healthy State and Redox Balance in H2O2-Treated Mice. Int. J. Mol. Sci. 2024, 25, 6736. https://doi.org/10.3390/ijms25126736
Mizzoni D, Logozzi M, Di Raimo R, Spada M, Fais S. Hydrogen-Rich Alkaline Water Supplementation Restores a Healthy State and Redox Balance in H2O2-Treated Mice. International Journal of Molecular Sciences. 2024; 25(12):6736. https://doi.org/10.3390/ijms25126736
Chicago/Turabian StyleMizzoni, Davide, Mariantonia Logozzi, Rossella Di Raimo, Massimo Spada, and Stefano Fais. 2024. "Hydrogen-Rich Alkaline Water Supplementation Restores a Healthy State and Redox Balance in H2O2-Treated Mice" International Journal of Molecular Sciences 25, no. 12: 6736. https://doi.org/10.3390/ijms25126736
APA StyleMizzoni, D., Logozzi, M., Di Raimo, R., Spada, M., & Fais, S. (2024). Hydrogen-Rich Alkaline Water Supplementation Restores a Healthy State and Redox Balance in H2O2-Treated Mice. International Journal of Molecular Sciences, 25(12), 6736. https://doi.org/10.3390/ijms25126736







