Pregnant Women with Multiple Sclerosis: An Overview of Gene Expression and Molecular Interaction Using Bioinformatics Analysis
Abstract
:1. Introduction
2. Treatment during Pregnancy
3. Expression of Genes Related to MS in Pregnancy
4. Materials and Methods
Bioinformatics Data Tools: Protein–Protein Interactions among Associated Genes
5. Results
Molecular Interaction of Genes
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Primers 2018, 4, 43. [Google Scholar] [CrossRef]
- Walz, L.; Brooks, J.C.; Shavelle, R.M.; Robertson, N.; Harding, K.E. Life expectancy in multiple sclerosis by EDSS score. Mult. Scler. Relat. Disord. 2022, 68, 104219. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Axisa, P.P.; Hafler, D.A. Multiple sclerosis: Genetics, biomarkers, treatments. Curr. Opin. Neurol. 2016, 29, 345–353. [Google Scholar] [CrossRef]
- Confavreux, C.; Hutchinson, M.; Hours, M.M.; Cortinovis-Tourniaire, P.; Moreau, T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N. Engl. J. Med. 1998, 339, 285–291. [Google Scholar] [CrossRef]
- Vukusic, S.; Hutchinson, M.; Hours, M.; Moreau, T.; Cortinovis-Tourniaire, P.; Adeleine, P.; Confavreux, C.; Pregnancy In Multiple Sclerosis Group. Pregnancy and multiple sclerosis (the PRIMS study): Clinical predictors of post-partum relapse. Brain 2004, 127, 1353–1360. [Google Scholar] [CrossRef]
- Vukusic, S.; Confavreux, C. Pregnancy and multiple sclerosis: The children of PRIMS. Clin. Neurol. Neurosurg. 2006, 108, 266–270. [Google Scholar] [CrossRef]
- Finkelsztejn, A.; Brooks, J.B.; Paschoal, F.M., Jr.; Fragoso, Y.D. What can we really tell women with multiple sclerosis regarding pregnancy? A systematic review and meta-analysis of the literature. BJOG 2011, 118, 790–797. [Google Scholar] [CrossRef]
- Portaccio, E.; Ghezzi, A.; Hakiki, B.; Martinelli, V.; Moiola, L.; Patti, F.; La Mantia, L.; Mancardi, G.L.; Solaro, C.; Tola, M.R.; et al. Breastfeeding is not related to postpartum relapses in multiple sclerosis. Neurology 2011, 77, 145–150. [Google Scholar] [CrossRef]
- Houtchens, M.K.; Edwards, N.C.; Phillips, A.L. Relapses and disease-modifying drug treatment in pregnancy and live birth in US women with MS. Neurology 2018, 91, e1570–e1578. [Google Scholar] [CrossRef]
- Dobson, R.; Jokubaitis, V.G.; Giovannoni, G. Change in pregnancy-associated multiple sclerosis relapse rates over time: A meta-analysis. Mult. Scler. Relat. Disord. 2020, 44, 102241. [Google Scholar] [CrossRef]
- Modrego, P.J.; Urrea, M.A.; de Cerio, L.D. The effects of pregnancy on relapse rates, disability and peripartum outcomes in women with multiple sclerosis: A systematic review and meta-analysis. J. Comp. Eff. Res. 2021, 10, 175–186. [Google Scholar] [CrossRef]
- Rodrigues, R.; Rocha, R.; Bonifacio, G.; Ferro, D.; Sabenca, F.; Goncalves, A.I.; Correia, F.; Pinheiro, J.; Loureiro, J.L.; Guerreiro, R.P.; et al. Therapeutic inertia in relapsing-remitting multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 55, 103176. [Google Scholar] [CrossRef]
- Hellwig, K.; Verdun di Cantogno, E.; Sabido, M. A systematic review of relapse rates during pregnancy and postpartum in patients with relapsing multiple sclerosis. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211051012. [Google Scholar] [CrossRef]
- Nguyen, A.L.; Eastaugh, A.; van der Walt, A.; Jokubaitis, V.G. Pregnancy and multiple sclerosis: Clinical effects across the lifespan. Autoimmun. Rev. 2019, 18, 102360. [Google Scholar] [CrossRef]
- Krysko, K.M.; Bove, R.; Dobson, R.; Jokubaitis, V.; Hellwig, K. Treatment of Women with Multiple Sclerosis Planning Pregnancy. Curr. Treat. Options Neurol. 2021, 23, 11. [Google Scholar] [CrossRef]
- Lopez, C.; Comabella, M.; Tintore, M.; Sastre-Garriga, J.; Montalban, X. Variations in chemokine receptor and cytokine expression during pregnancy in multiple sclerosis patients. Mult. Scler. 2006, 12, 421–427. [Google Scholar] [CrossRef]
- Zenere, A.; Hellberg, S.; Papapavlou Lingehed, G.; Svenvik, M.; Mellergard, J.; Dahle, C.; Vrethem, M.; Raffetseder, J.; Khademi, M.; Olsson, T.; et al. Prominent epigenetic and transcriptomic changes in CD4(+) and CD8(+) T cells during and after pregnancy in women with multiple sclerosis and controls. J. Neuroinflamm. 2023, 20, 98. [Google Scholar] [CrossRef]
- Gilli, F.; Lindberg, R.L.; Valentino, P.; Marnetto, F.; Malucchi, S.; Sala, A.; Capobianco, M.; di Sapio, A.; Sperli, F.; Kappos, L.; et al. Learning from nature: Pregnancy changes the expression of inflammation-related genes in patients with multiple sclerosis. PLoS ONE 2010, 5, e8962. [Google Scholar] [CrossRef]
- Neuteboom, R.F.; Verbraak, E.; Wierenga-Wolf, A.F.; Voerman, J.S.; Meurs, M.; Swagemakers, S.M.; van de Spek, P.J.; Steegers, E.A.; de Groot, C.J.; Laman, J.D.; et al. The monocyte transcriptome during pregnancy in multiple sclerosis: Prominent expression of the Fc-receptor CD64. Mult. Scler. 2011, 17, 389–396. [Google Scholar] [CrossRef]
- Mimpen, M.; Smolders, J.; Hupperts, R.; Damoiseaux, J. Natural killer cells in multiple sclerosis: A review. Immunol. Lett. 2020, 222, 1–11. [Google Scholar] [CrossRef]
- Badam, T.V.; Hellberg, S.; Mehta, R.B.; Lechner-Scott, J.; Lea, R.A.; Tost, J.; Mariette, X.; Svensson-Arvelund, J.; Nestor, C.E.; Benson, M.; et al. CD4(+) T-cell DNA methylation changes during pregnancy significantly correlate with disease-associated methylation changes in autoimmune diseases. Epigenetics 2022, 17, 1040–1055. [Google Scholar] [CrossRef]
- Mehta, D.; Wani, S.; Wallace, L.; Henders, A.K.; Wray, N.R.; McCombe, P.A. Cumulative influence of parity-related genomic changes in multiple sclerosis. J. Neuroimmunol. 2019, 328, 38–49. [Google Scholar] [CrossRef]
- Campagna, M.P.; Xavier, A.; Stankovich, J.; Maltby, V.E.; Slee, M.; Yeh, W.Z.; Kilpatrick, T.; Scott, R.J.; Butzkueven, H.; Lechner-Scott, J.; et al. Parity is associated with long-term differences in DNA methylation at genes related to neural plasticity in multiple sclerosis. Clin. Epigenetics 2023, 15, 20. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Ogata, H.; Goto, S.; Fujibuchi, W.; Kanehisa, M. Computation with the KEGG pathway database. Biosystems 1998, 47, 119–128. [Google Scholar] [CrossRef]
- Wickham, H.; Navarro, D.; Pedersen, T.L. ggplot2: Elegant Graphics for Data Analysis (3e); Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Lumley, T. Analysis of complex survey samples. J. Stat. Softw. 2004, 9, 1–19. [Google Scholar] [CrossRef]
- Lumley, T. Survey: Analysis of Complex Survey Samples, R package version 4.4.1; United States Environmental Protection Agency: Washington, DC, USA, 2023. [Google Scholar]
- Lorenzo, P.M.; Izquierdo, A.G.; Diaz-Lagares, A.; Carreira, M.C.; Macias-Gonzalez, M.; Sandoval, J.; Cueva, J.; Lopez-Lopez, R.; Casanueva, F.F.; Crujeiras, A.B. ZNF577 Methylation Levels in Leukocytes From Women With Breast Cancer Is Modulated by Adiposity, Menopausal State, and the Mediterranean Diet. Front. Endocrinol. 2020, 11, 245. [Google Scholar] [CrossRef]
- Levine, K.S.; Leonard, H.L.; Blauwendraat, C.; Iwaki, H.; Johnson, N.; Bandres-Ciga, S.; Ferrucci, L.; Faghri, F.; Singleton, A.B.; Nalls, M.A. Virus exposure and neurodegenerative disease risk across national biobanks. Neuron 2023, 111, 1086–1093.e2. [Google Scholar] [CrossRef]
- Moraes, A.; da Luz, R.; Fernandes, A.L.M.; Barbosa, M.X.S.; de Andrade, L.V.; Armstrong, A.D.C.; de Souza, C.D.F.; do Carmo, R.F. Association of PTX3 gene polymorphisms and PTX3 plasma levels with leprosy susceptibility. BMC Infect. Dis. 2023, 23, 853. [Google Scholar] [CrossRef]
- Wirestam, L.; Pihl, S.; Saleh, M.; Wettero, J.; Sjowall, C. Plasma C-Reactive Protein and Pentraxin-3 Reference Intervals During Normal Pregnancy. Front. Immunol. 2021, 12, 722118. [Google Scholar] [CrossRef]
- Piedra-Quintero, Z.L.; Wilson, Z.; Nava, P.; Guerau-de-Arellano, M. CD38: An Immunomodulatory Molecule in Inflammation and Autoimmunity. Front. Immunol. 2020, 11, 597959. [Google Scholar] [CrossRef]
- Dogan, S.; Deshpande, D.A.; Kannan, M.S.; Walseth, T.F. Changes in CD38 expression and ADP-ribosyl cyclase activity in rat myometrium during pregnancy: Influence of sex steroid hormones. Biol. Reprod. 2004, 71, 97–103. [Google Scholar] [CrossRef]
- Gilli, F.; Navone, N.D.; Perga, S.; Marnetto, F.; Caldano, M.; Capobianco, M.; Pulizzi, A.; Malucchi, S.; Bertolotto, A. Loss of braking signals during inflammation: A factor affecting the development and disease course of multiple sclerosis. Arch. Neurol. 2011, 68, 879–888. [Google Scholar] [CrossRef]
- Perga, S.; Montarolo, F.; Martire, S.; Berchialla, P.; Malucchi, S.; Bertolotto, A. Anti-inflammatory genes associated with multiple sclerosis: A gene expression study. J. Neuroimmunol. 2015, 279, 75–78. [Google Scholar] [CrossRef]
- Stumpo, D.J.; Byrd, N.A.; Phillips, R.S.; Ghosh, S.; Maronpot, R.R.; Castranio, T.; Meyers, E.N.; Mishina, Y.; Blackshear, P.J. Chorioallantoic fusion defects and embryonic lethality resulting from disruption of Zfp36L1, a gene encoding a CCCH tandem zinc finger protein of the Tristetraprolin family. Mol. Cell Biol. 2004, 24, 6445–6455. [Google Scholar] [CrossRef]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef]
- Conti, L.; De Palma, R.; Rolla, S.; Boselli, D.; Rodolico, G.; Kaur, S.; Silvennoinen, O.; Niccolai, E.; Amedei, A.; Ivaldi, F.; et al. Th17 cells in multiple sclerosis express higher levels of JAK2, which increases their surface expression of IFN-gammaR2. J. Immunol. 2012, 188, 1011–1018. [Google Scholar] [CrossRef]
- Morris, R.; Kershaw, N.J.; Babon, J.J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018, 27, 1984–2009. [Google Scholar] [CrossRef]
- Lashgari, N.A.; Roudsari, N.M.; Momtaz, S.; Sathyapalan, T.; Abdolghaffari, A.H.; Sahebkar, A. The involvement of JAK/STAT signaling pathway in the treatment of Parkinson’s disease. J. Neuroimmunol. 2021, 361, 577758. [Google Scholar] [CrossRef]
- Russo, R.C.; Garcia, C.C.; Teixeira, M.M.; Amaral, F.A. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert. Rev. Clin. Immunol. 2014, 10, 593–619. [Google Scholar] [CrossRef]
- Gibbons, D.; Fleming, P.; Virasami, A.; Michel, M.L.; Sebire, N.J.; Costeloe, K.; Carr, R.; Klein, N.; Hayday, A. Interleukin-8 (CXCL8) production is a signatory T cell effector function of human newborn infants. Nat. Med. 2014, 20, 1206–1210. [Google Scholar] [CrossRef]
- Li, P.; Furusawa, Y.; Wei, Z.L.; Sakurai, H.; Tabuchi, Y.; Zhao, Q.L.; Saiki, I.; Kondo, T. TAK1 promotes cell survival by TNFAIP3 and IL-8 dependent and NF-kappaB independent pathway in HeLa cells exposed to heat stress. Int. J. Hyperth. 2013, 29, 688–695. [Google Scholar] [CrossRef]
- Platnich, J.M.; Muruve, D.A. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [Google Scholar] [CrossRef]
- Boro, M.; Balaji, K.N. CXCL1 and CXCL2 Regulate NLRP3 Inflammasome Activation via G-Protein-Coupled Receptor CXCR2. J. Immunol. 2017, 199, 1660–1671. [Google Scholar] [CrossRef]
- Angeletti, R.H.; D’Amico, T.; Ashok, S.; Russell, J. The chemokine interleukin-8 regulates parathyroid secretion. J. Bone Miner. Res. 1998, 13, 1232–1237. [Google Scholar] [CrossRef]
- Li, H.; Zheng, C.; Han, J.; Zhu, J.; Liu, S.; Jin, T. PD-1/PD-L1 Axis as a Potential Therapeutic Target for Multiple Sclerosis: A T Cell Perspective. Front. Cell Neurosci. 2021, 15, 716747. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Tian, M.; Tang, M.X.; Liu, Z.Z.; Liao, A.H. Recent Insight into the Role of the PD-1/PD-L1 Pathway in Feto-Maternal Tolerance and Pregnancy. Am. J. Reprod. Immunol. 2015, 74, 201–208. [Google Scholar] [CrossRef]
- Harjunpaa, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Zheng, J.; Wang, S.; Liu, C.; Yao, X.; Ren, Y.; Wang, X. Integrative study reveals the prognostic and immunotherapeutic value of CD274 and PDCD1LG2 in pan-cancer. Front. Genet. 2022, 13, 990301. [Google Scholar] [CrossRef]
- Wu, S.; Gessner, R.; von Stackelberg, A.; Kirchner, R.; Henze, G.; Seeger, K. Cytokine/cytokine receptor gene expression in childhood acute lymphoblastic leukemia: Correlation of expression and clinical outcome at first disease recurrence. Cancer 2005, 103, 1054–1063. [Google Scholar] [CrossRef]
- Bugbee, E.; Wang, A.A.; Gommerman, J.L. Under the influence: Environmental factors as modulators of neuroinflammation through the IL-10/IL-10R axis. Front. Immunol. 2023, 14, 1188750. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.L.; Zhao, S.J.; Lin, X.X.; Liao, A.H. IL-10: A bridge between immune cells and metabolism during pregnancy. J. Reprod. Immunol. 2022, 154, 103750. [Google Scholar] [CrossRef]
- Hernandez-Preciado, M.R.; Torres-Mendoza, B.M.; Mireles-Ramirez, M.A.; Kobayashi-Gutierrez, A.; Sanchez-Rosales, N.A.; Vazquez-Valls, E.; Marquez-Pedroza, J. Gene expression in multiple sclerosis during pregnancy based on integrated bioinformatics analysis. Mult. Scler. Relat. Disord. 2024, 82, 105373. [Google Scholar] [CrossRef]
- Charvet, C.; Canonigo, A.J.; Billadeau, D.D.; Altman, A. Membrane localization and function of Vav3 in T cells depend on its association with the adapter SLP-76. J. Biol. Chem. 2005, 280, 15289–15299. [Google Scholar] [CrossRef]
- Pollard, S.M.; Wallbank, R.; Tomlinson, S.; Grotewold, L.; Smith, A. Fibroblast growth factor induces a neural stem cell phenotype in foetal forebrain progenitors and during embryonic stem cell differentiation. Mol. Cell Neurosci. 2008, 38, 393–403. [Google Scholar] [CrossRef]
- Karachrysafi, S.; Georgiou, P.; Kavvadas, D.; Papafotiou, F.; Isaakidou, S.; Grammatikakis, I.E.; Papamitsou, T. Immunohistochemical study of MMP-2, MMP-9, EGFR and IL-8 in decidual and trophoblastic specimens of recurrent pregnancy loss cases. J. Matern. Fetal Neonatal Med. 2023, 36, 2218523. [Google Scholar] [CrossRef]

| Author | Year | Type | Sample Size | Disability and Relapses |
|---|---|---|---|---|
| [8] | 2006 | Original | 254 women with multiple sclerosis. | Decrease in the relapse/year rate during the third trimester of pregnancy (0.2) versus a year before pregnancy (0.7) and an increase in the first three months after delivery (1.2). Disability progression steadily increased mean disability levels during the entire study period. |
| [9] | 2011 | Literature review and meta- analysis | 1221 pregnancies reported in 13 papers. | During the year preceding pregnancy, women presented 0.435 relapses/year. During pregnancy, the relapse rate decreased to 0.26 relapses/year. After delivery, the relapse rate increased to 0.758 relapses/year. |
| [10] | 2011 | Original | 302 pregnancies in 298 women. | The relapse rate significantly decreased during pregnancy, particularly in the third trimester, and increased in the postpartum, particularly in the first trimester (6.162, p = 0.001). The breastfeeding group (BG) had fewer relapses during pregnancy (0.06 vs. 0.14; p = 0.041) and 12 months after delivery (0.35 vs. 0.66; p = 0.001). Patients in the BG group had significantly lower EDSS scores at conception (1.3 vs. 1.6; p = 0.004) compared with the no BG group. |
| [11] | 2018 | Retrospective database research | 2158 eligible patients. | Odds of relapse (OR) declined significantly (p < 0.001) during pregnancy (OR 0.623), increased during puerperium (OR 1.710), and remained higher than pre-pregnancy levels for 6 months postpartum (OR 1.216). The odds of relapse requiring hospitalization increased significantly during the third trimester (OR 1.849; p = 0.0011) and the puerperium. |
| [12] | 2020 | Meta-analysis | 6430 women were included. | The annual relapse rate fell from 0.57 pre-pregnancy to 0.36 in the first trimester, 0.29 in the second trimester, and 0.16 during thirst trimesters, with a postpartum rebound (0.85). Relapses/year increased in the 3-month puerperium relative to the pre-pregnancy year period; the increase was 0.22 relapses/year p < 0.001. The range of the median EDSS was 0.7–2.4. |
| [13] | 2021 | Systematic review and meta-analysis | 1469 abstracts were screened; 1293 articles. | The relapses/year rate increased in the 3-month puerperium relative to the pre-pregnancy year period; the mean increase was 0.22 (p < 0.001). Disability does not change significantly after pregnancy. |
| Author | Patients | Gene | Result |
|---|---|---|---|
| [18] | 6 MS patients during pregnancy. | IL-10/IFN-g. | The IL-10/IFN-g ratio was increased during pregnancy, especially in the third trimester, and decreased during the postpartum period compared with the third trimester (p = 0.043). |
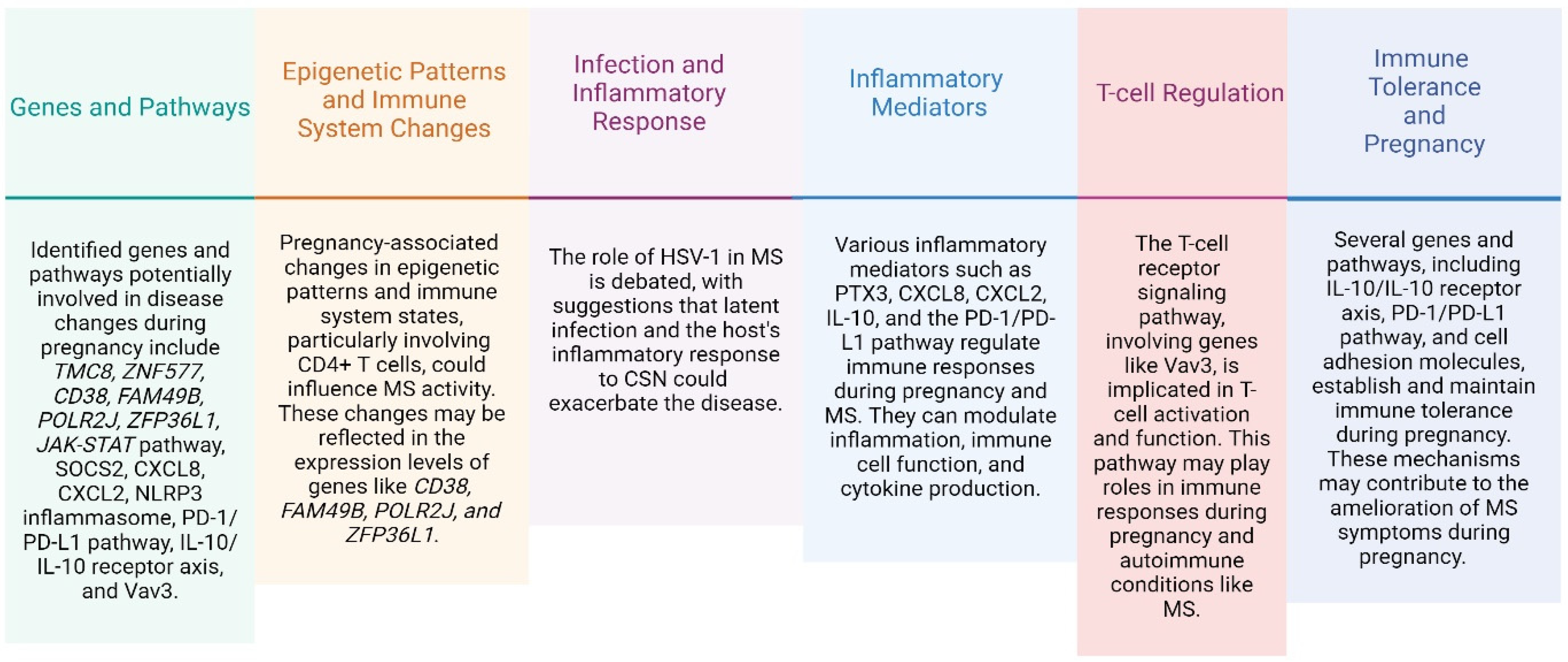
| [20] | 7 MS patients and 5 healthy controls followed during the 1st, 2nd, and 3rd trimesters and after pregnancy. | SOCS2, TNFAIP3, NR4A2, CXCR4, ZFP36L1, POLR2J, FAM49B, and STAG3L1. | Altered expression was observed in 347 transcripts in non-pregnant MS patients compared to healthy non-pregnant controls. Expression changes that occurred during pregnancy reversed the previous imbalance, particularly for seven inflammation-related transcripts (SOCS2, TNFAIP3, NR4A2, CXCR4, POLR2J, FAM49B, and STAG3L1). The deregulation of gene expression returned to “normal” within the third month of gestation. |
| [21] | 10 women with MS and 9 healthy women. | CD64, IL8, CXCL2, CD38, PTX3, JAK2, and STAT1. | Increased CD64 expression during pregnancy is indicative of enhanced innate immune functions. A significant increase was observed in MS patients directly after delivery in the expression of JAK2 and STAT1 (p < 0.05) compared to before pregnancy or until the third trimester. No statistically significant differences were found in the expression of the other genes when comparing the initial sample with the third trimester sample in patients with MS. |
| [24] | 47 women with MS. | 574 genes associated with parity. | The majority of differentially expressed genes (85%) were upregulated among women with MS who had fathered children. A total of 16 of the 574 genes had been reported to be clinically actionable genes. The results were not conclusive due to the sample size. |
| [22] | 5 pregnant RRMS patients, 5 healthy controls, 12 MS third trimester patients, 12 MS postpartum patients, and 12 MS patient with no treatment. | 754 miRNAs. | 21 miRNAs were differentially expressed in the 3rd trimester compared to the postpartum period in MS patients. miR-1 in samples from the 3rd trimester of pregnancy in the non-pregnant patients showed a suggestive downregulation of miR-1 (p = 0.023). Only miR-18a was differentially expressed and was 1.3-fold higher in the 3rd trimester than in the postpartum period of pregnancy. |
| [23] | 12 non-pregnant healthy women, 23 pregnant women, 11 first trimester women, and 12 second trimester women. | 22 genes. | Twenty genes were hypermethylated (e.g., CD28, CD86, PRKCB, TP73, PLK1, and VAV3) and two were hypomethylated (SPTBN4, MAML3) when comparing second trimester pregnant and healthy non-pregnant women. These changes could be an important immune regulatory mechanism during pregnancy. |
| [19] | 11 pregnant women with MS during the 1st, 2nd, and 3rd trimesters and after pregnancy. | CD4+ (n = 13,440 genes); CD8+ (n = 13,448 genes) and CpGs (N = 740,552 for both cell types). | DNA methylation and RNA sequencing in CD4+ and CD8+ T cells revealed a prominent regulation, mostly peaking in the third semester and reversing postpartum, thus minoring the clinical course with improvement followed by a worsening in disease activity. |
| [25] | 192 women with relapse-onset MS (nulligravida = 96, parous = 96). | 2965 differentially methylated positions in the whole blood. | It found 22 differentially methylated positions and 366 differentially methylated genes in epigenetic changes associated with parity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marquez-Pedroza, J.; Hernández-Preciado, M.R.; Valdivia-Tangarife, E.R.; Alvarez-Padilla, F.J.; Mireles-Ramírez, M.A.; Torres-Mendoza, B.M. Pregnant Women with Multiple Sclerosis: An Overview of Gene Expression and Molecular Interaction Using Bioinformatics Analysis. Int. J. Mol. Sci. 2024, 25, 6741. https://doi.org/10.3390/ijms25126741
Marquez-Pedroza J, Hernández-Preciado MR, Valdivia-Tangarife ER, Alvarez-Padilla FJ, Mireles-Ramírez MA, Torres-Mendoza BM. Pregnant Women with Multiple Sclerosis: An Overview of Gene Expression and Molecular Interaction Using Bioinformatics Analysis. International Journal of Molecular Sciences. 2024; 25(12):6741. https://doi.org/10.3390/ijms25126741
Chicago/Turabian StyleMarquez-Pedroza, Jazmin, Martha Rocio Hernández-Preciado, Edgar Ricardo Valdivia-Tangarife, Francisco J. Alvarez-Padilla, Mario Alberto Mireles-Ramírez, and Blanca Miriam Torres-Mendoza. 2024. "Pregnant Women with Multiple Sclerosis: An Overview of Gene Expression and Molecular Interaction Using Bioinformatics Analysis" International Journal of Molecular Sciences 25, no. 12: 6741. https://doi.org/10.3390/ijms25126741






