“Core/Shell” Nanocomposites as Photocatalysts for the Degradation of the Water Pollutants Malachite Green and Rhodamine B
Abstract
1. Introduction
2. Results
2.1. Core/Shell Structured Composites Based on Sol-Gel (SG)-Produced CoFe2O4
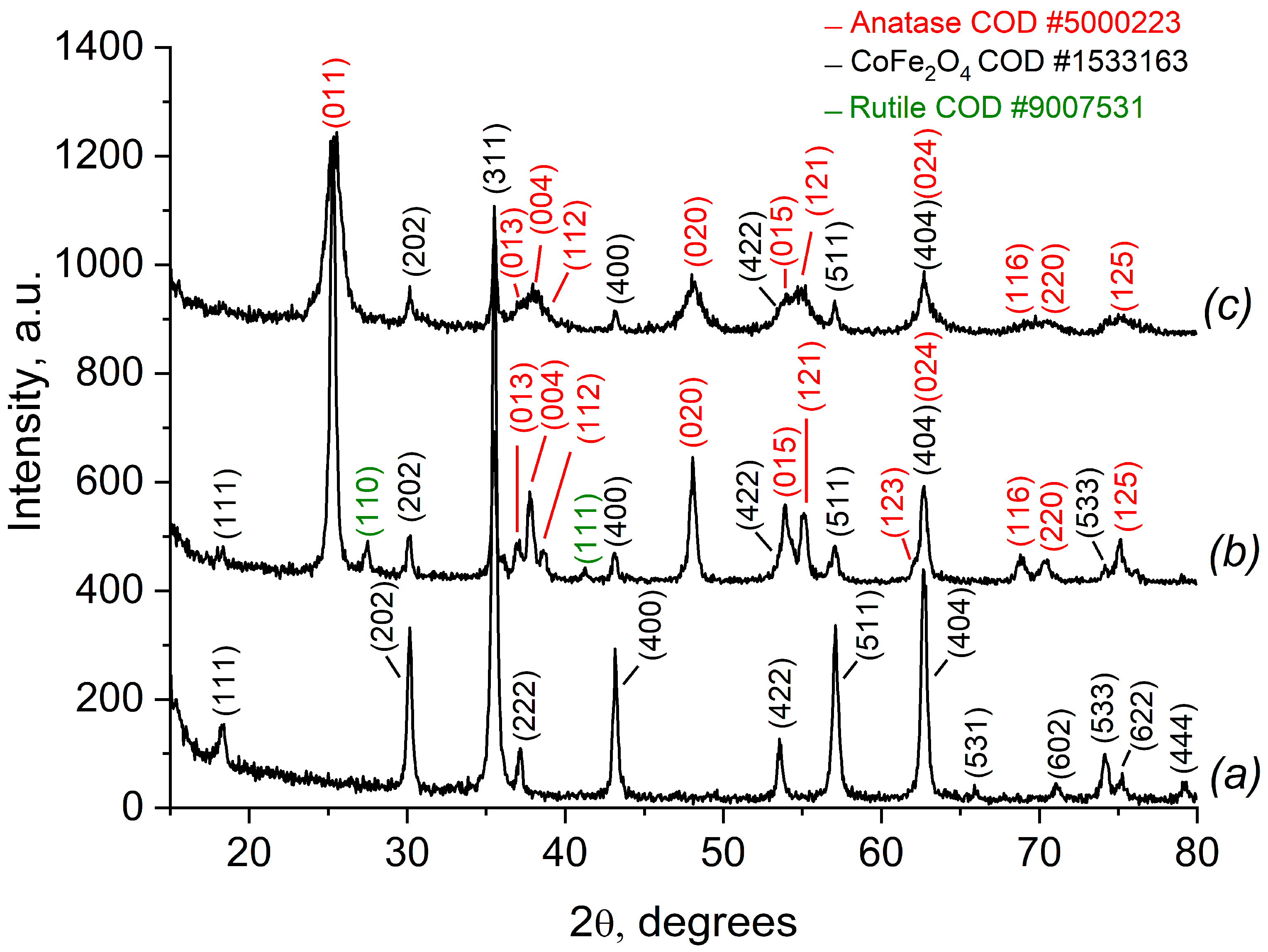
2.1.1. Phase Composition and the Crystal Structure of CoFe2O4, Synthesized by the Sol-Gel Method (SG), and of the Composite CoFe2O4/SiO2/TiO2 (SG) Based on It
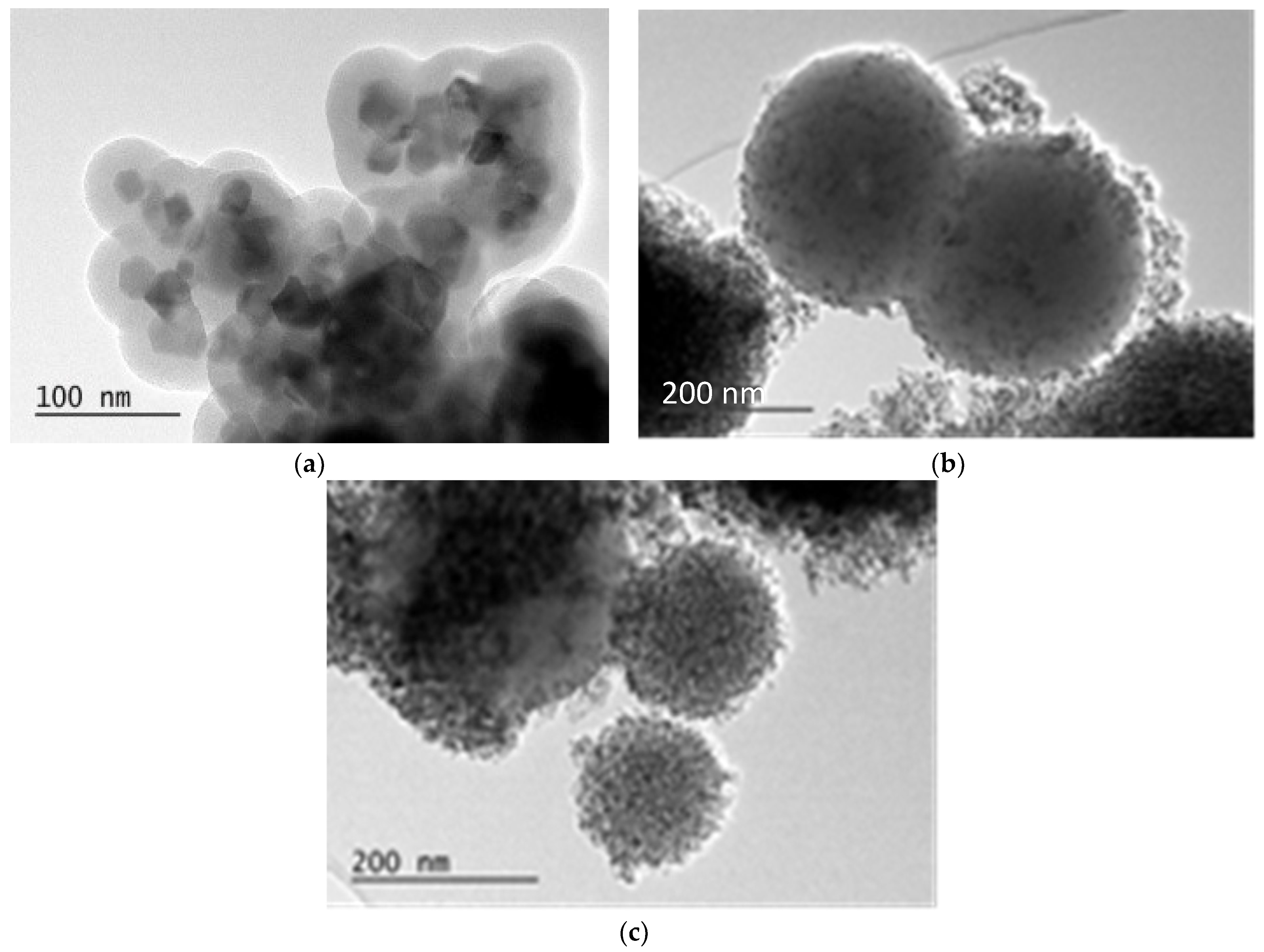
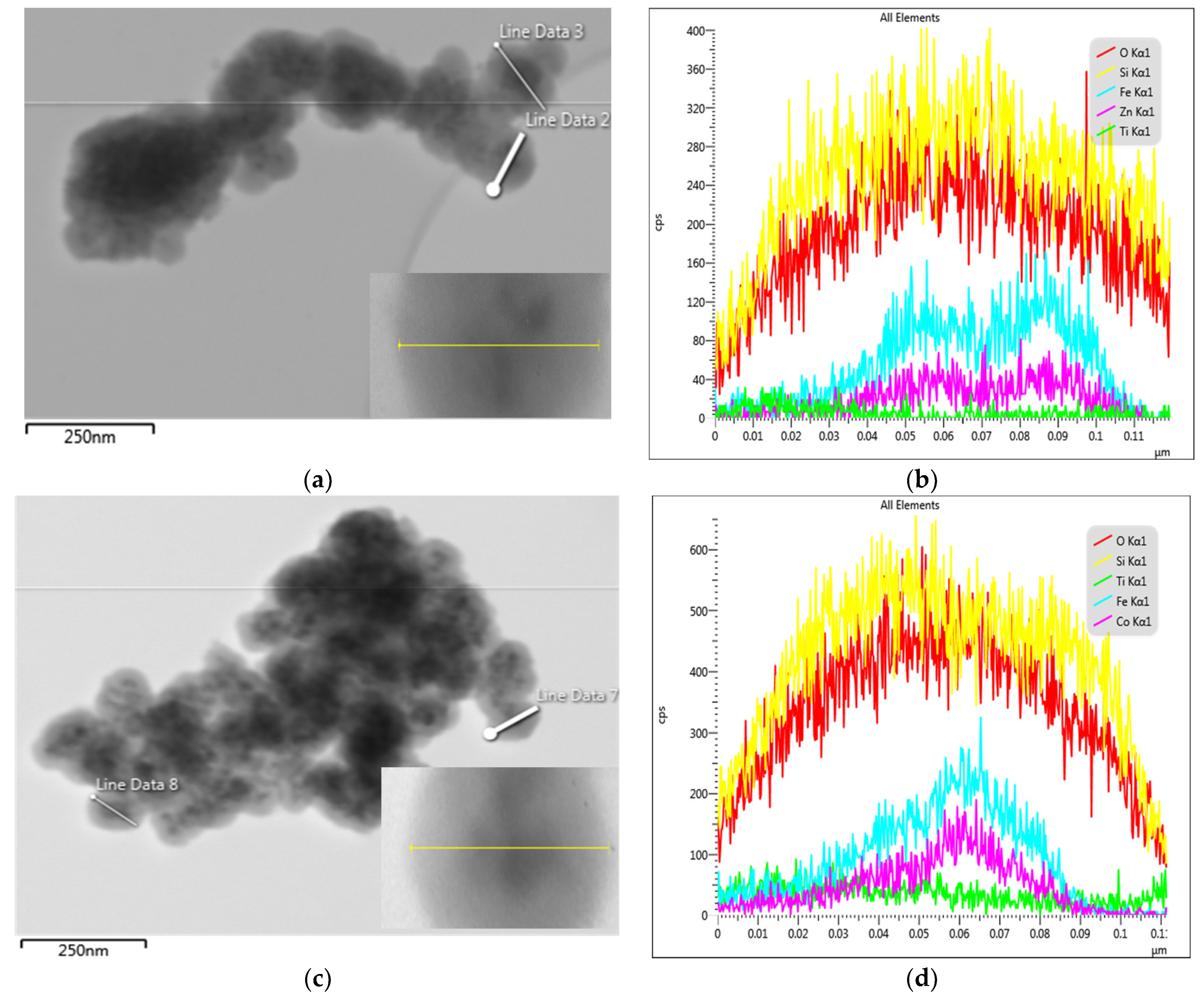
2.1.2. Morphology of the Composites CoFe2O4/SiO2/TiO2 (SG)
2.2. “Core/Shell” Structured Composites MFe2O4/SiO2/TiO2 (M = Zn, Co) Based on Hydrothermally (HT) Produced CoFe2O4 and ZnFe2O4
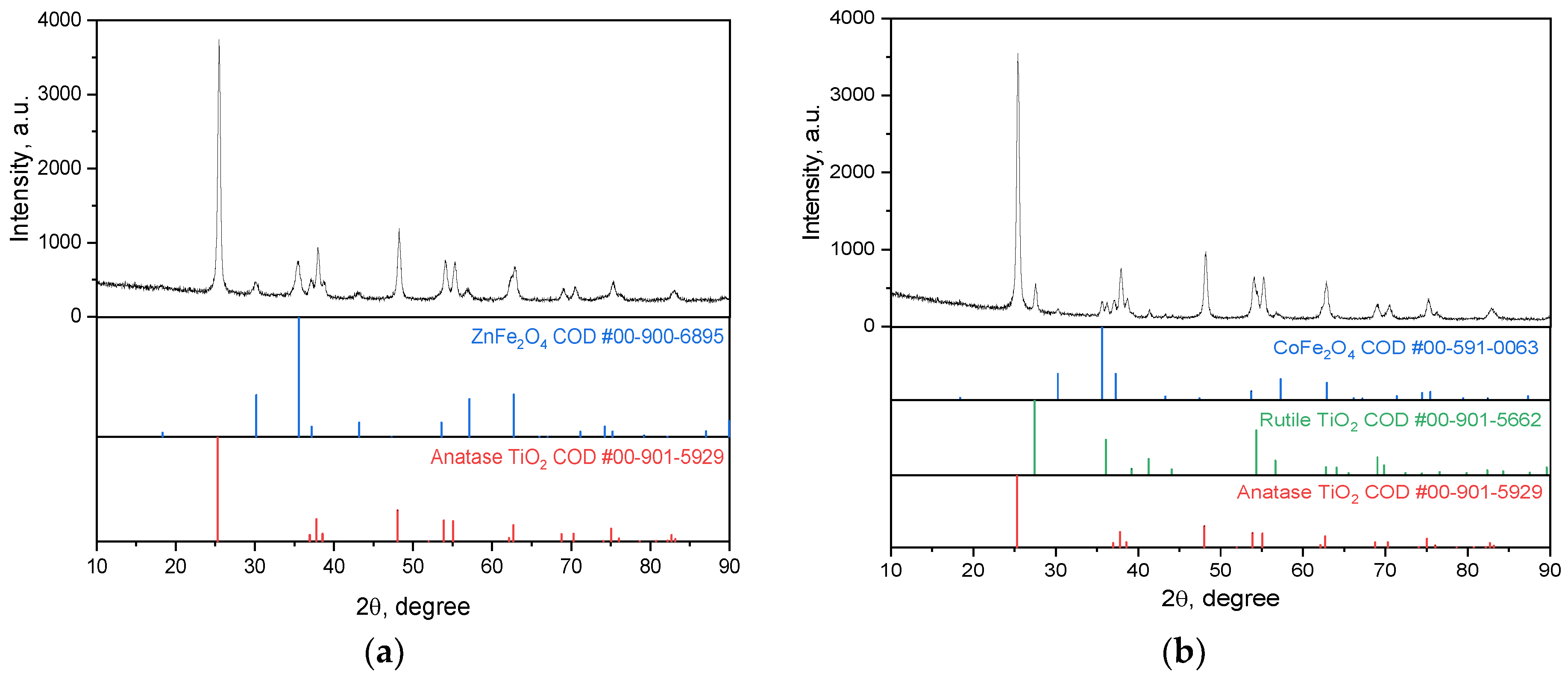
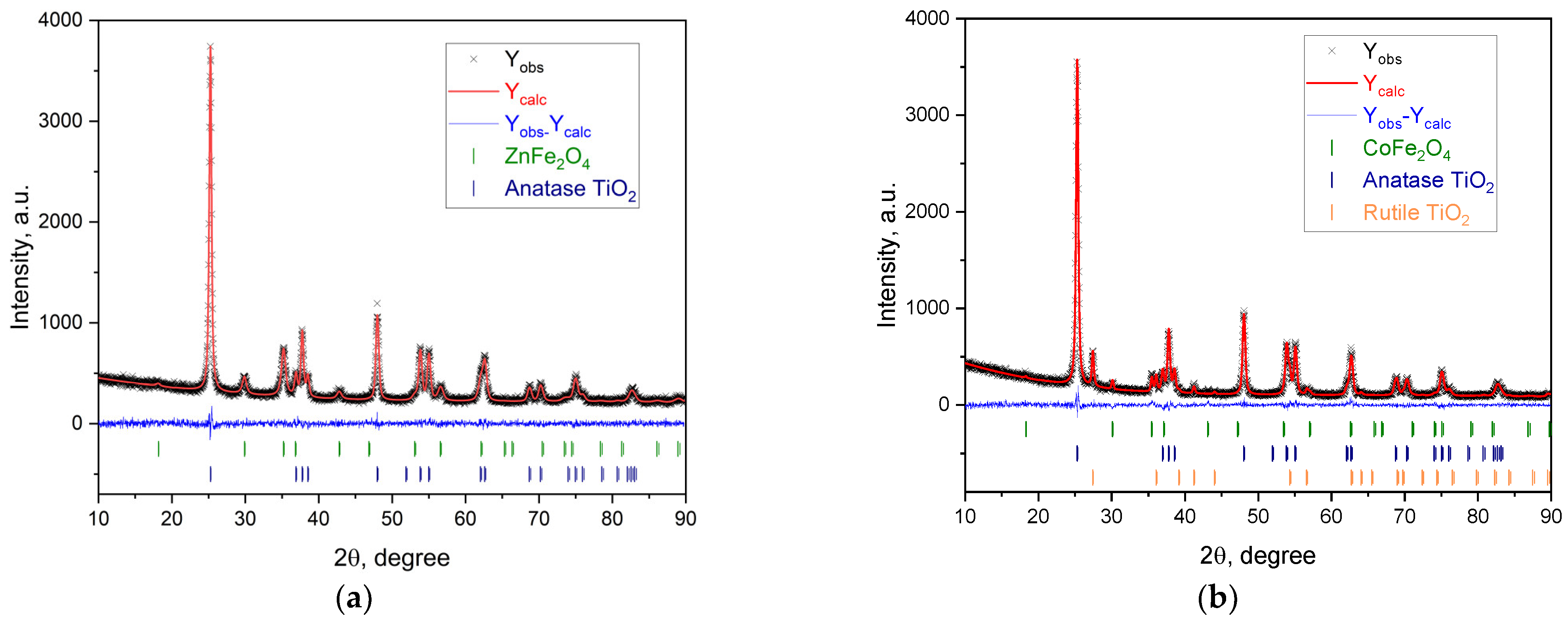
2.2.1. Phase Composition of MFe2O4/SiO2/TiO2 (M = Zn, Co), with CoFe2O4 and ZnFe2O4 Produced via the Hydrothermal (HT) Method
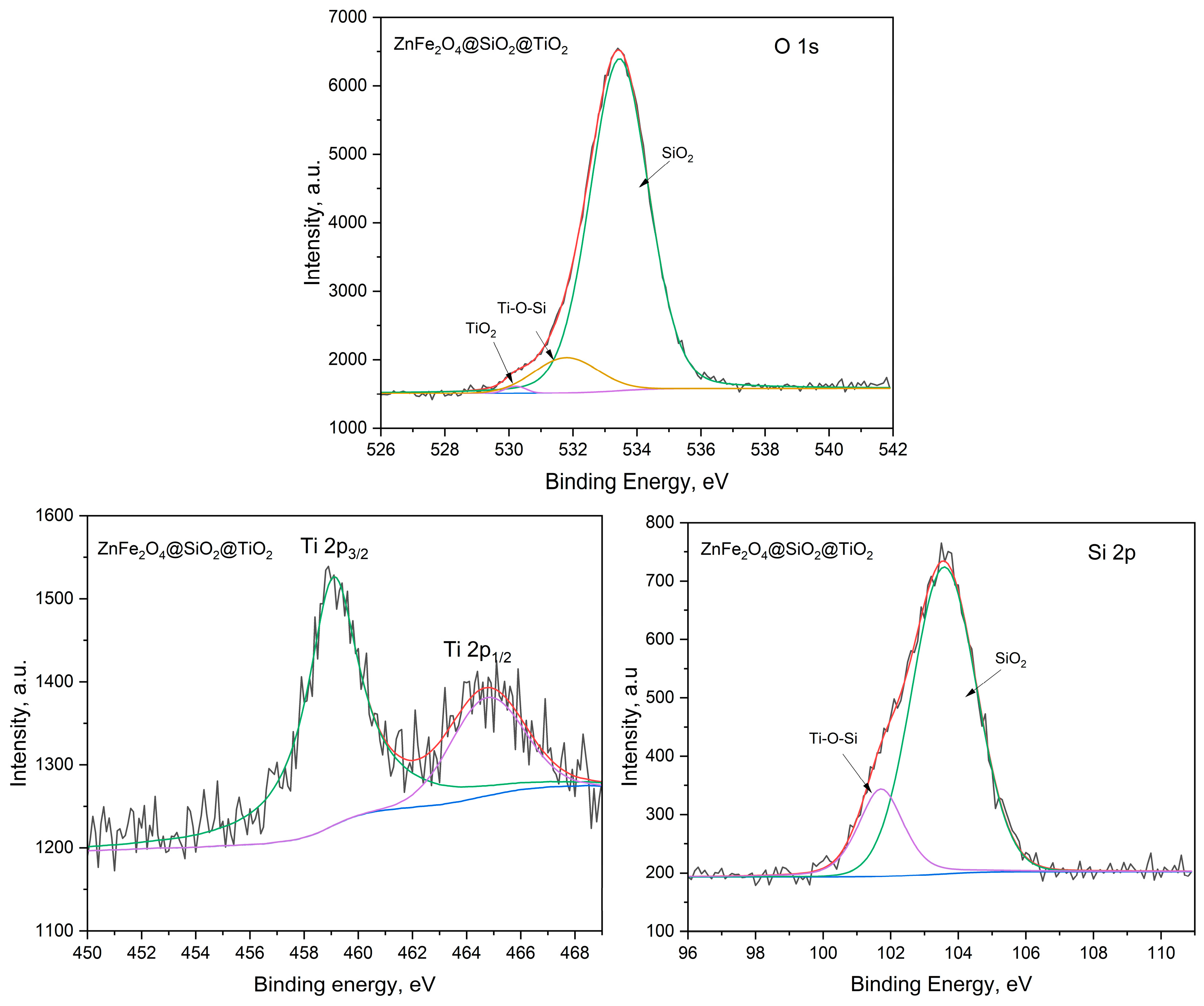
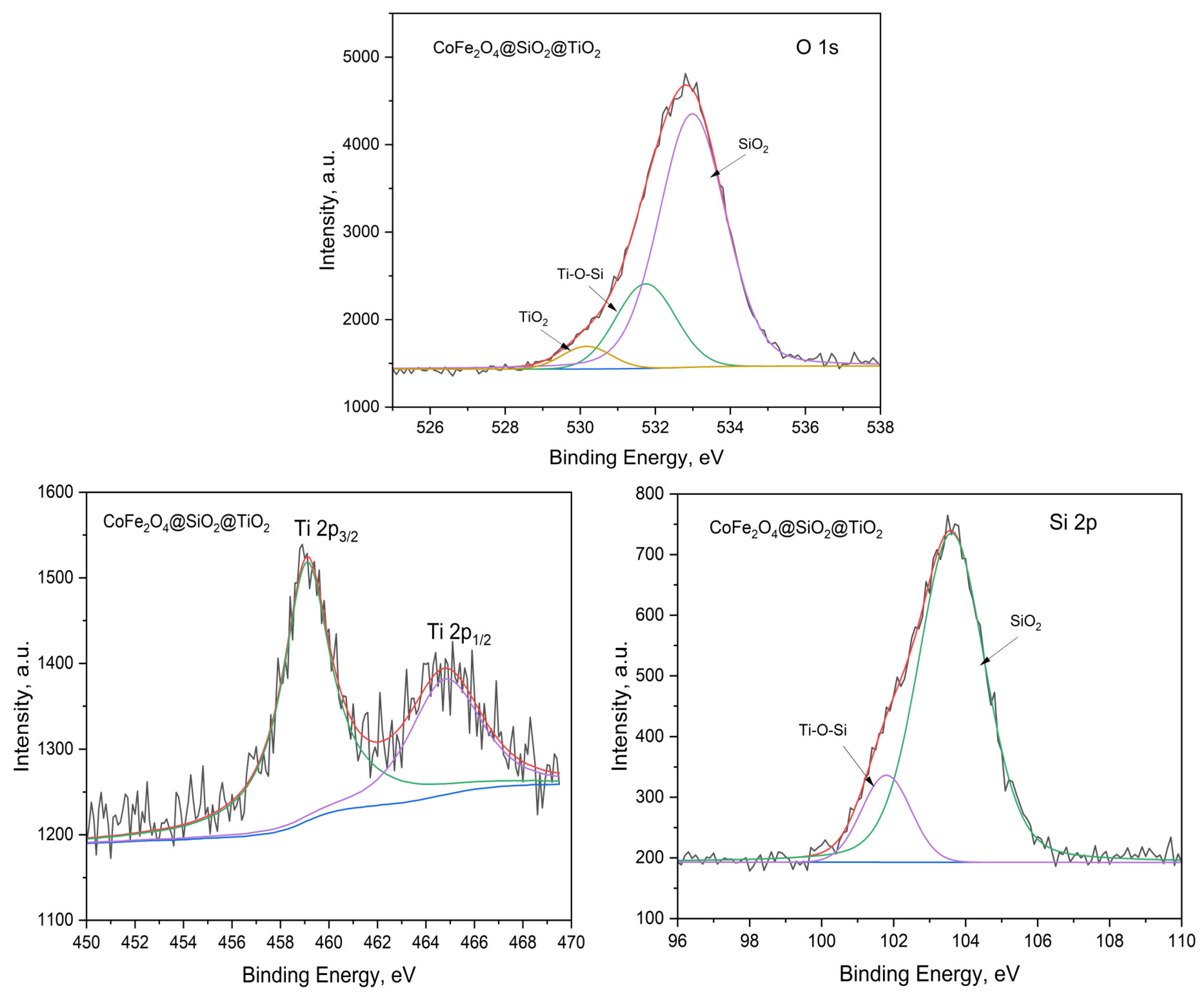
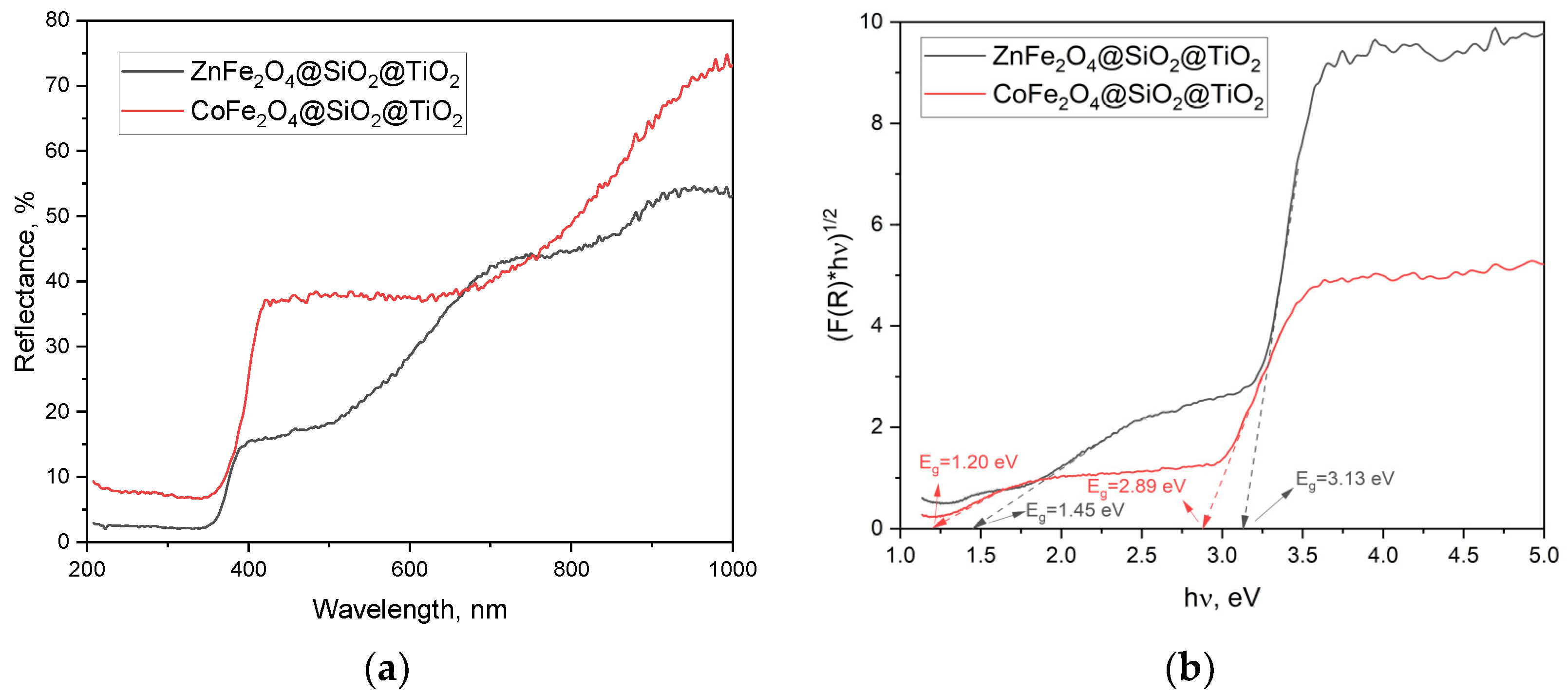
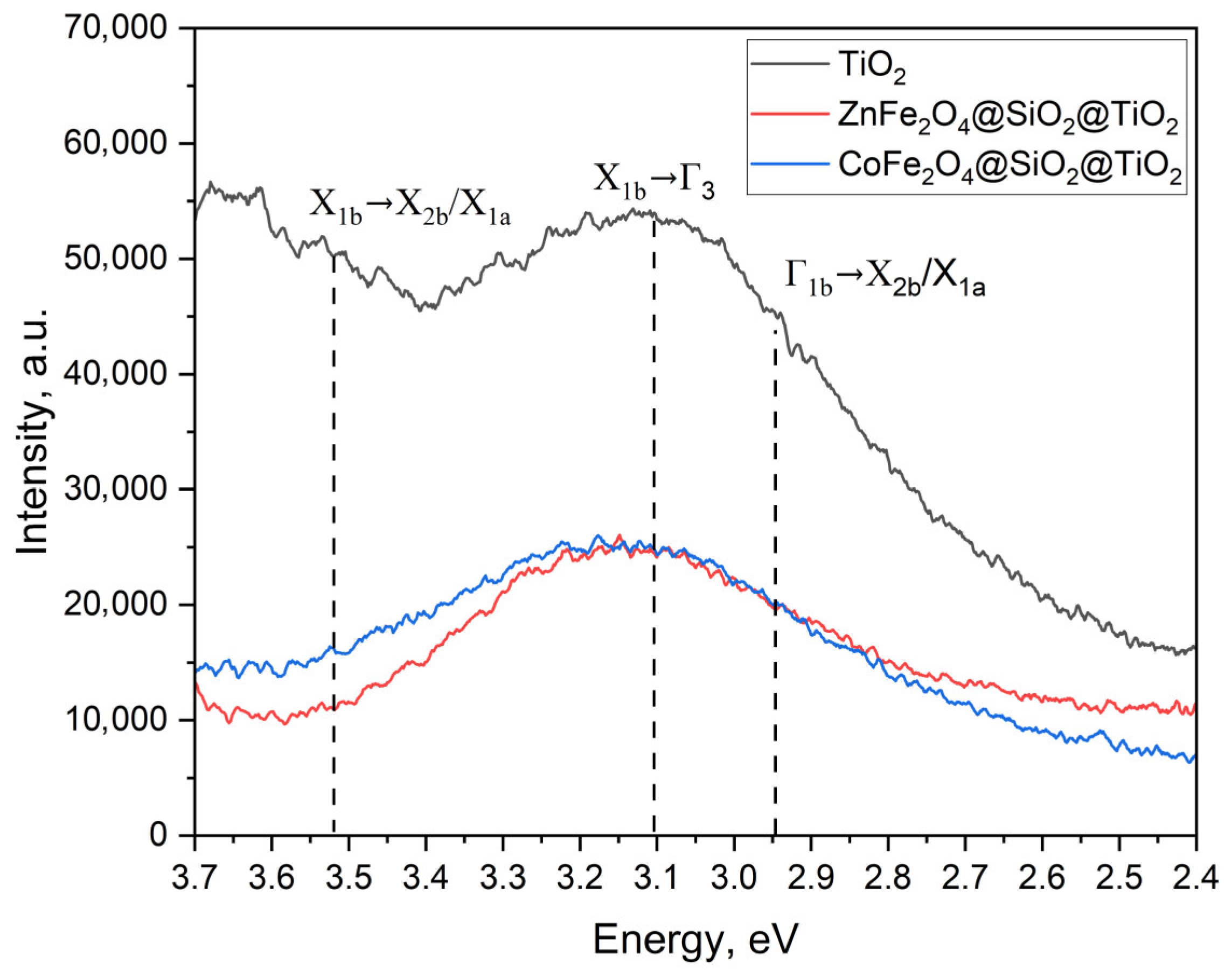
2.2.2. Optical Properties and the Energy Band Gap of MFe2O4/SiO2/TiO2 with MFe2O4 (M = Zn, Co) when HT-Synthesized
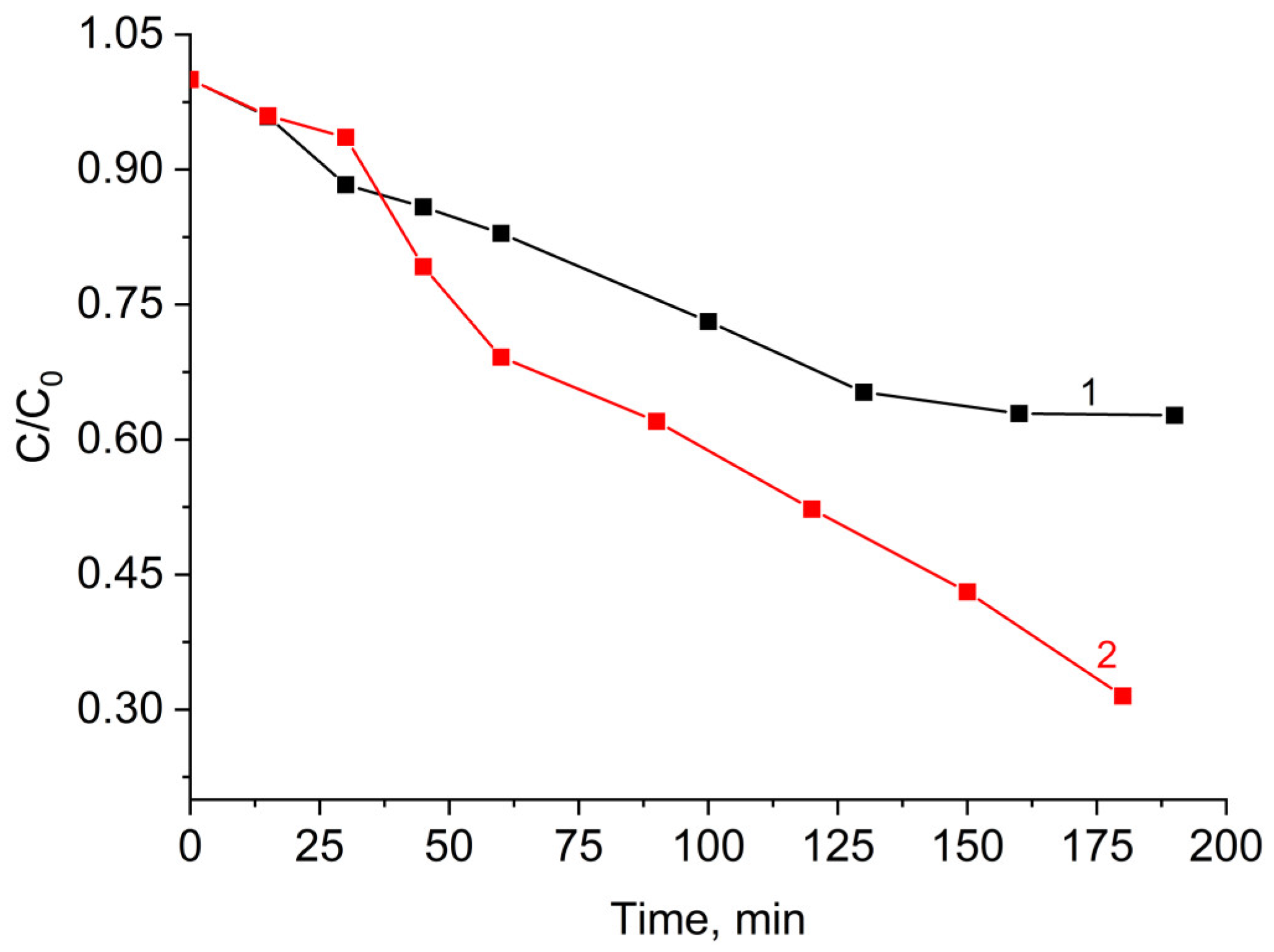
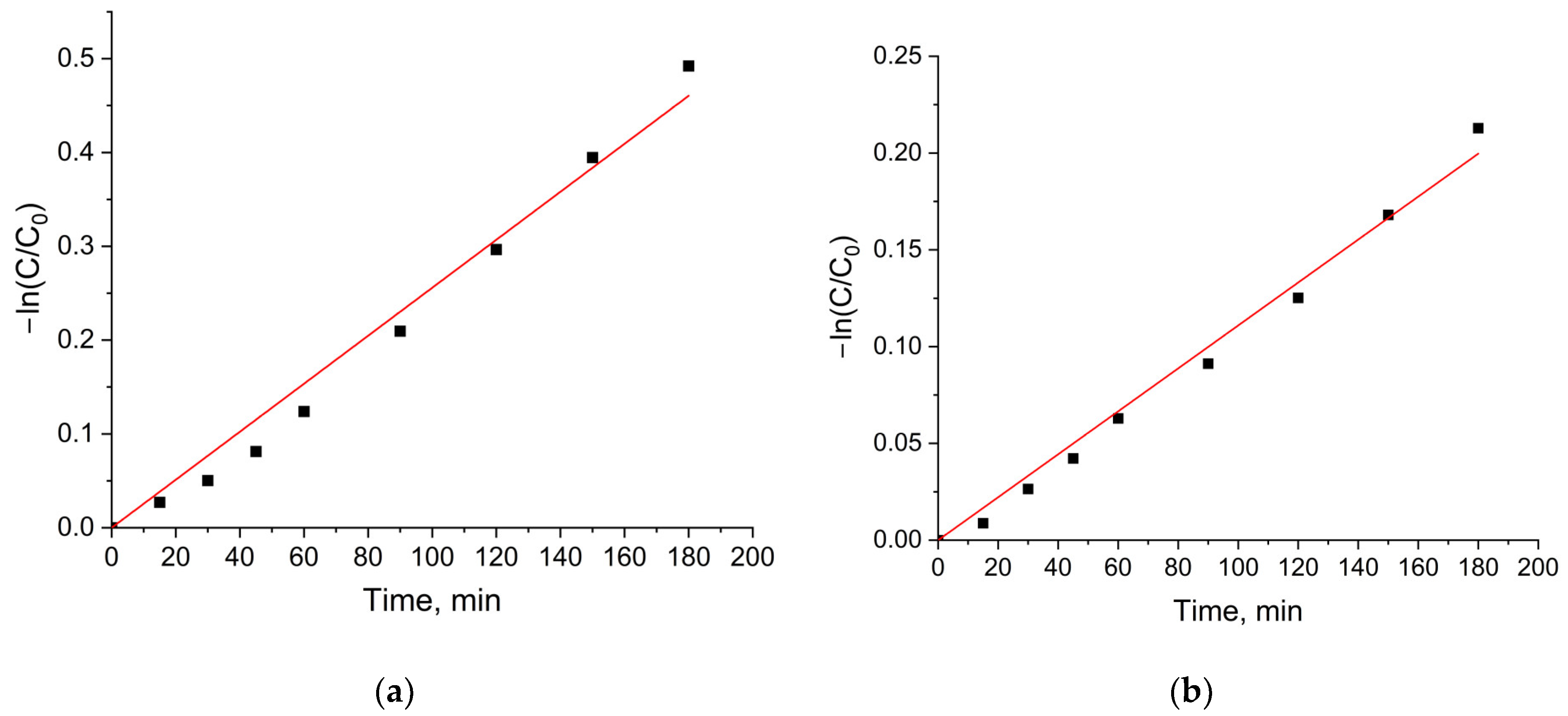
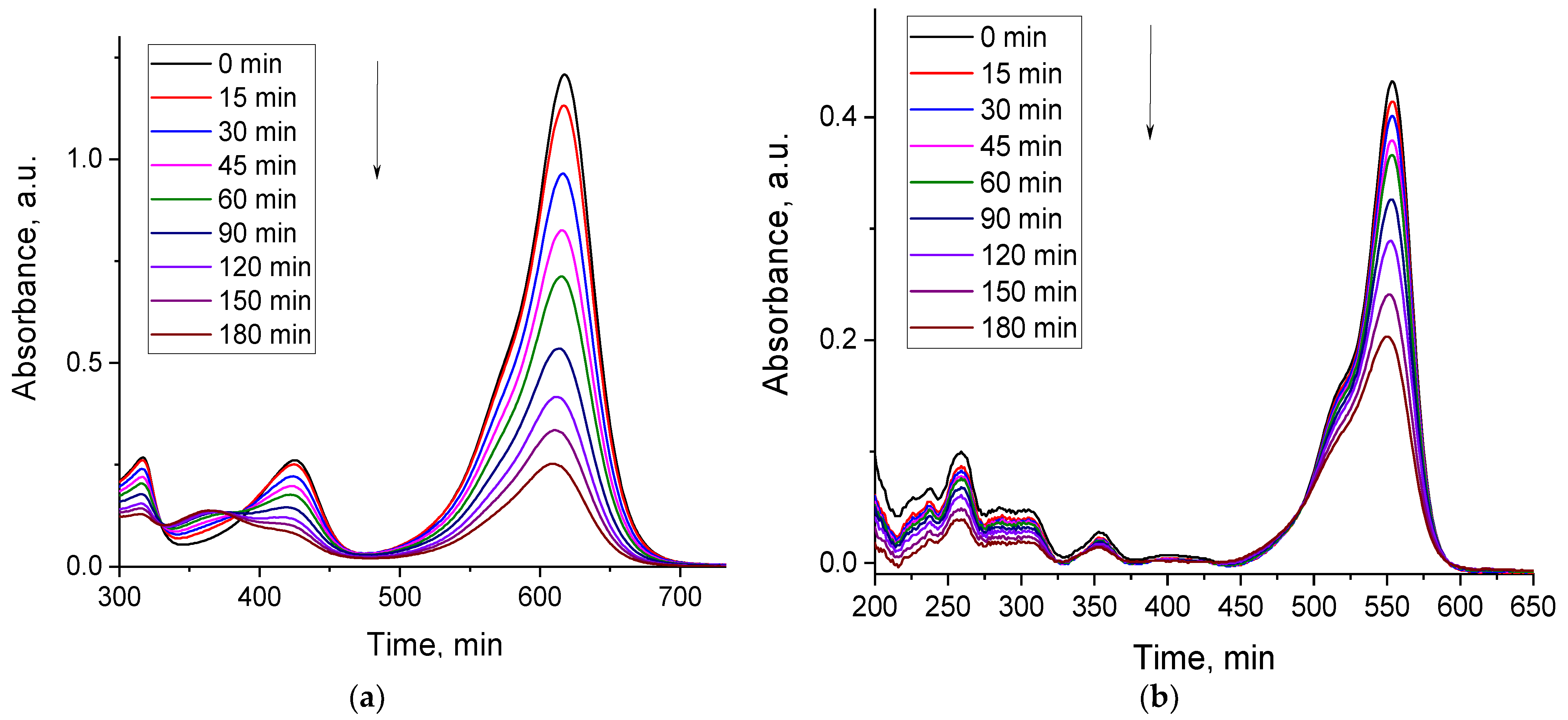
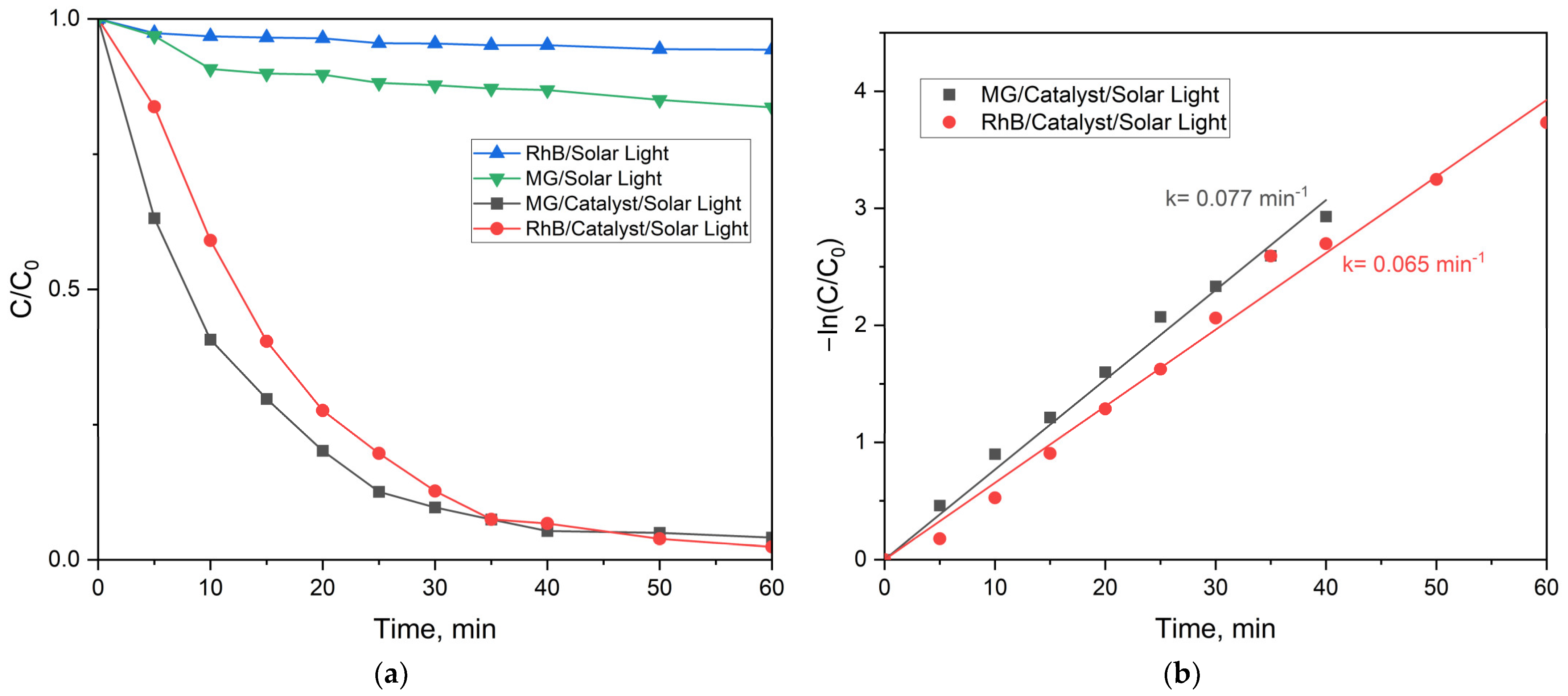
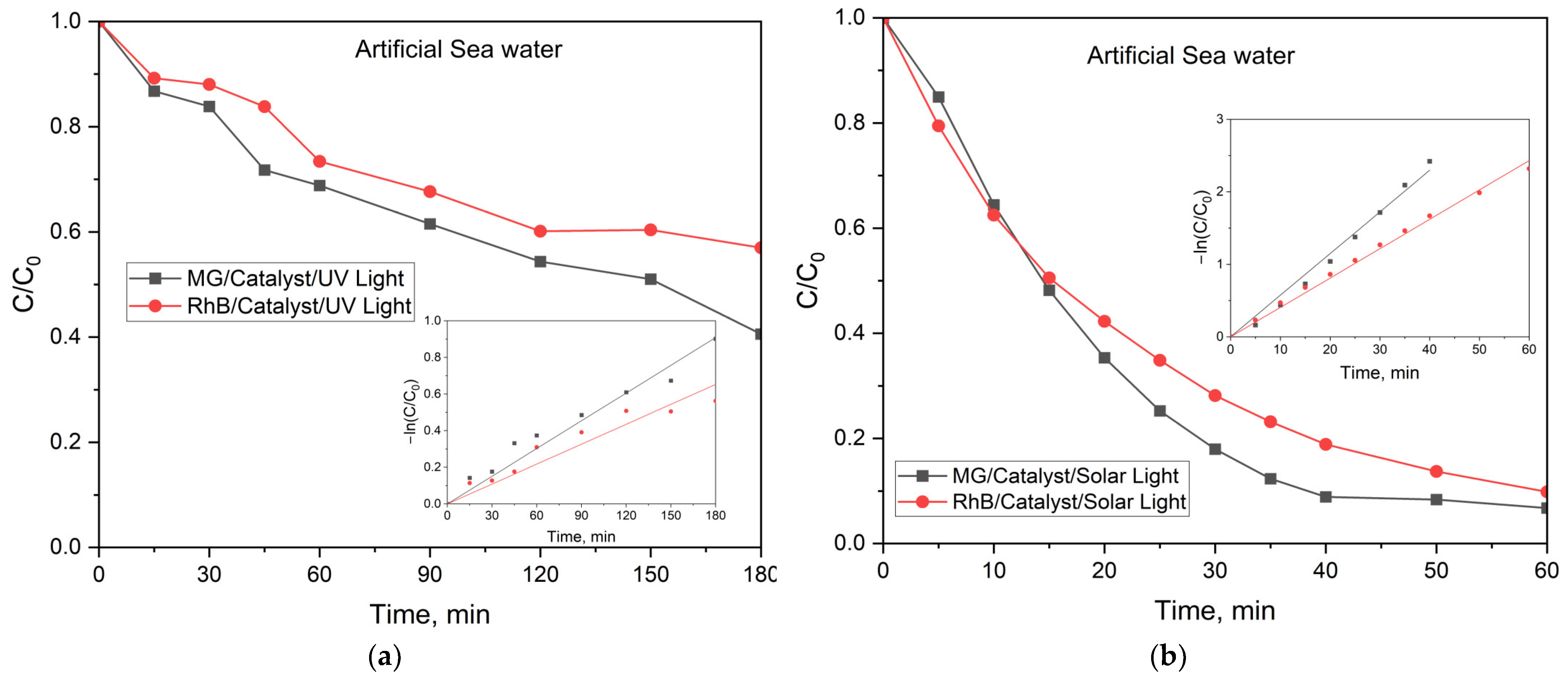
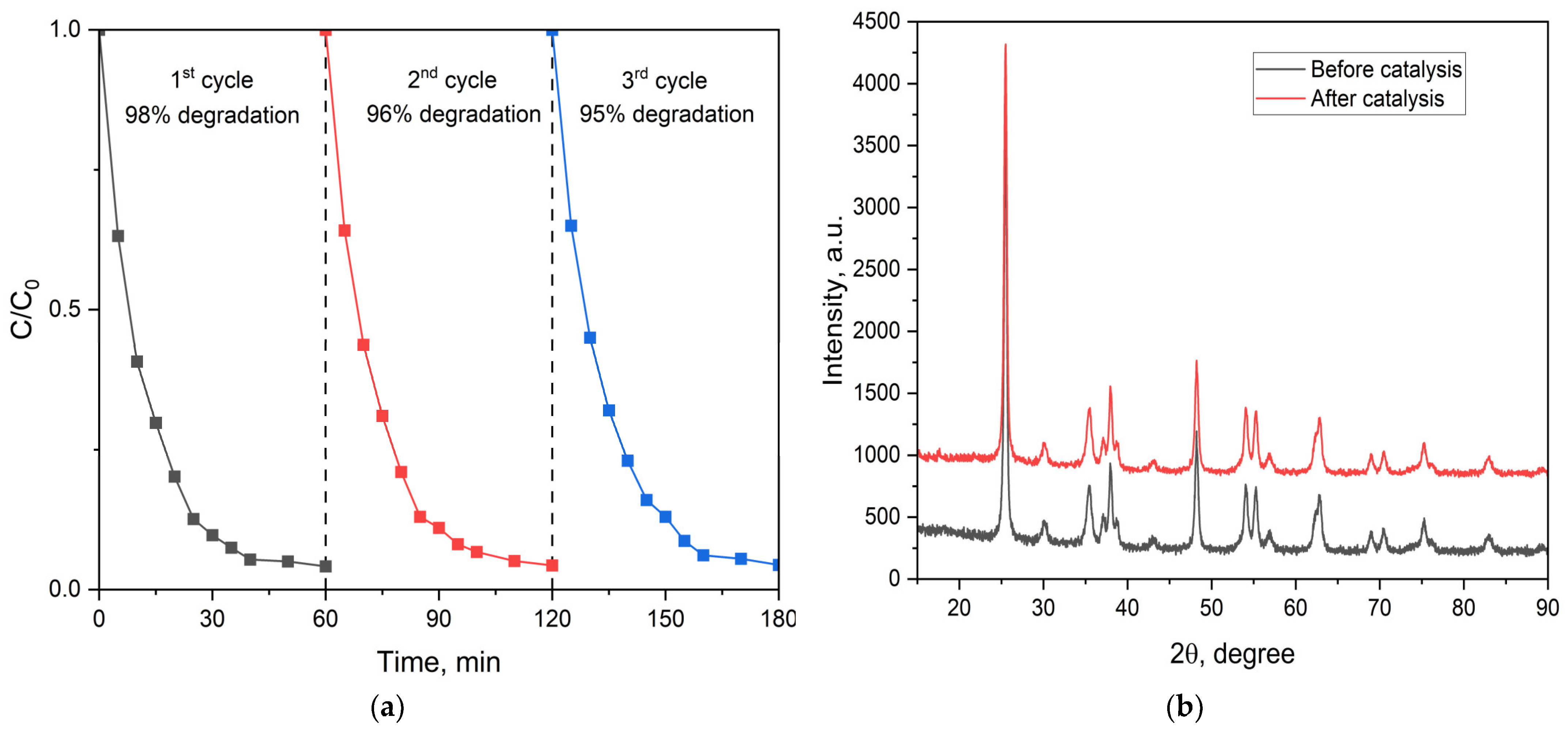
2.2.3. Photocatalytic Activity of the MFe2O4/SiO2/TiO2 (HT) with MFe2O4 (M = Zn, Co) Composites when HT-Synthesized
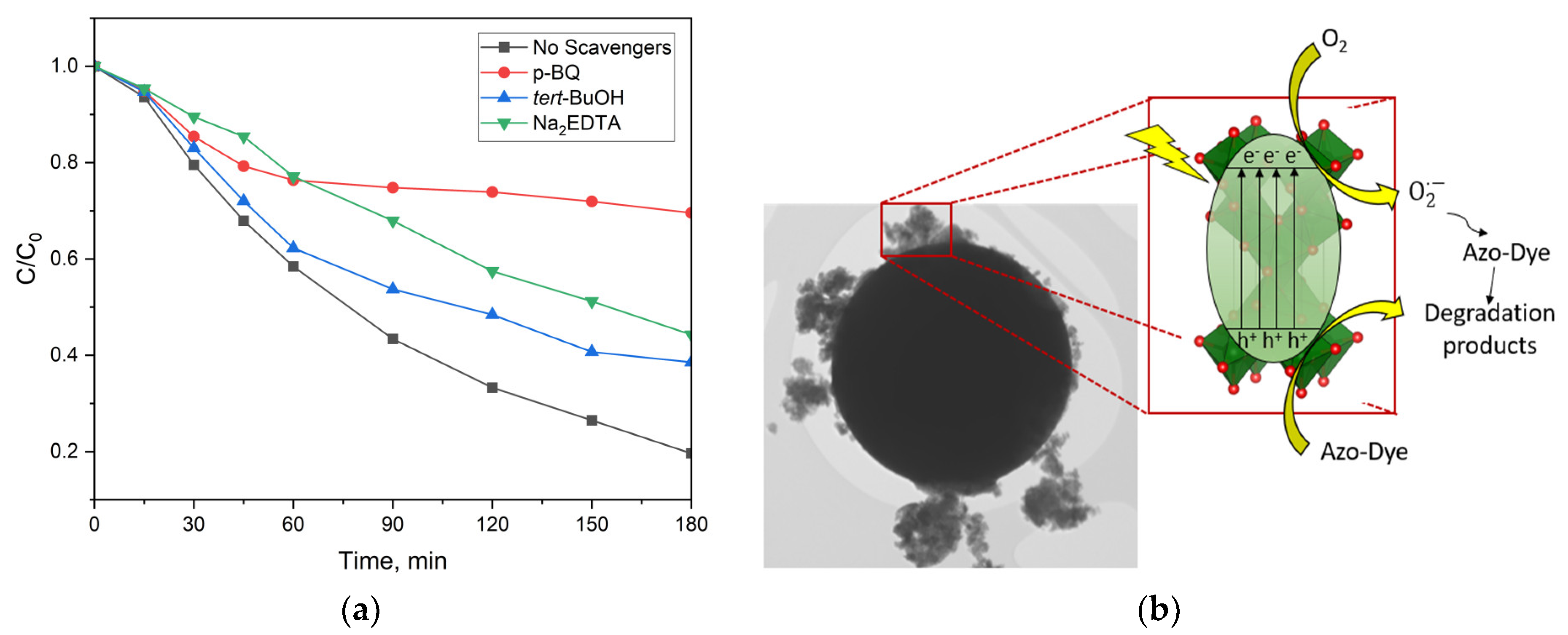
2.2.4. Mechanism of the Photocatalytic Process
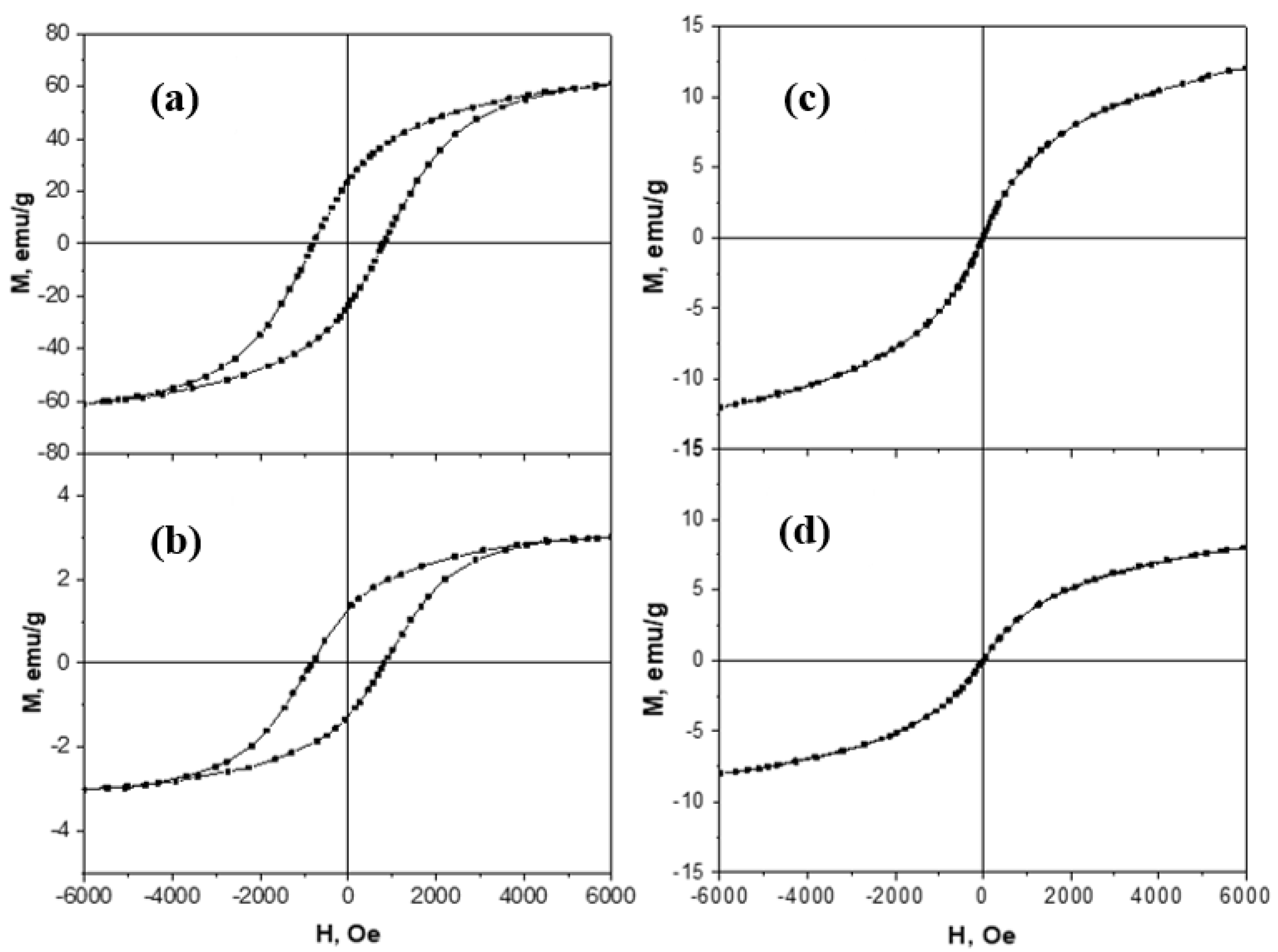
2.2.5. Magnetic Measurements
3. Discussion
- (i)
- Photocatalytic activity of the CoFe2O4/TiO2 (SG) and CoFe2O4/SiO2/TiO2 (SG) composites. The comparison confirms the role of SiO2 as an insulator. The rate constant and the degradation of MG in the process are increasing, i.e., values of 2.9 × 10−3 min−1 (degradation 41%) for CoFe2O4/TiO2 (SG) and (SG) 5.8 × 10−3 min−1 (degradation 48%) for CoFe2O4/SiO2/TiO2 are obtained. A similar system to the kind used for CoFe2O4/SiO2/TiO2 can be experimented with for a different organic dye degradation, Methylene Blue [24], but due to the lack of any rate constant or percentage of degradation, any comparison with our results cannot be made.
- (ii)
- Photocatalytic activity of the CoFe2O4/SiO2/TiO2 (SG) and CoFe2O4/SiO2/TiO2 (HT) composites. Above and beyond the different ferrite “core” MFe2O4 (M = Zn, Co), by XRD, some changes in the phase composition of the nanocomposites, mainly affecting the “shell” TiO2, were detected. Comparing the polymorphs of TiO2 in CoFe2O4/SiO2/TiO2(SG) and CoFe2O4/SiO2/TiO2(HT), it can be seen that the former contains only anatase, but the latter contains both anatase and rutile. The first one, CoFe2O4/SiO2/TiO2(SG), is more active, with a rate constant for MG decomposition under UV light irradiation of 5.8 × 10−3 min−1 (degradation 48%), while the latter, CoFe2O4/SiO2/TiO2(HT), is less active under irradiation of 2.6 × 10−3 min−1 (degradation 39%). The reason for the different activity can be connected with the different polymorphs of TiO2, anatase or anatase/rutile. According to [52,53], anatase demonstrates higher photocatalytic activity in comparison to polymorphs rutile and brookite because of more surface defects and oxygen vacancies in anatase compared to rutile, leading to charge separation and better photocatalytic performance; however, according to [54,55] a combination of A/R displays better photocatalytic activity than each of the polymorphs. A well-known example is the anatase/rutile mixture of TiO2-Degussa P25 with 80% anatase and 20% rutile, being one of the most investigated photocatalysts with good activity under UV light [55]. In CoFe2O4/SiO2/TiO2(HT), the weight fraction (%) of anatase is 87.7 and of rutile is 11.1, apparently differing from that of TiO2-Degussa P25.
- (iii)
- The ZnFe2O4/SiO2/TiO2(HT) and CoFe2O4/SiO2/TiO2(HT) composites. Similarly, ZnFe2O4/SiO2/TiO2(HT) contains anatase, while in CoFe2O4/SiO2/TiO2(HT), both anatase and rutile are detected, with the weight fraction mentioned (%), A/R 87.7/11.1, differing from that of TiO2-Degussa P25. The rate constant for MG decomposition is 9 × 10−3 min−1 (degradation 80%) for ZnFe2O4/SiO2/TiO2(HT), while for the process with CoFe2O4/SiO2/TiO2(HT), 2.6 × 10−3 min−1 (degradation 39%) was obtained.
- (iv)
- Weight ratio of A/R. This happens to be crucial in order to overcome the photocatalytic activity of anatase [56,57]. It is found that an A/R mixture of 90/10 has a weak activity for the decomposition of Methylene Blue, while a mixture of 86/14 is more effective in the same process than commercial TiO2 Degussa P25 [58]. So, our results confirm that not only is the mixture of anatase/rutile essential, but the weight fraction of the polymorphs is also important. For example, the rate constant for the process of RhB degradation by CoFe2O4/SiO2/TiO2 (HT) is close to one obtained [59] for TiO2 containing A/R. However, such a comparison is not correct because of the missing exact value for the A/R ratio in [59]. In addition, differences in the catalyst dose, the pollutant concentration, and the irradiation time should be taken into account. All this is illustrated by the data included in Table S1 [60,61,62,63,64] and Table S2 [59,65].
4. Materials and Methods
4.1. Materials
4.2. Synthetic Procedures
4.2.1. Synthesis of CoFe2O4 by the Sol-Gel Method
4.2.2. Synthesis of CoFe2O4 and ZnFe2O4 by Hydrothermal Method
4.2.3. Coating of MFe2O4 (M = Zn(II), Co(II)) with SiO2 and TiO2
4.3. Methods for Characterization
4.4. Photocatalytic Activity
4.5. Photocatalytic Activity in Real Water Matrix
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 surfaces: Principles, mechanisms and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Xu, D.; Ma, H. Degradation of rhodamine B in water by ultrasound-assisted TiO2 photocatalysis. J. Clean. Prod. 2021, 313, 127758. [Google Scholar] [CrossRef]
- Kralchevska, R.; Milanova, M.; Hristov, D.; Pintar, A.; Todorovsky, D. Synthesis, characterization and photocatalytic activity of neodymium, nitrogen and neodymium-nitrogen doped TiO2. Mater. Res. Bull. 2012, 47, 2165–2177. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, T.J.; Zhou, H.O. Hydrothermal preparation of ZnO-reduced graphene oxide hybrid with high performance in photocatalytic degradation. Appl. Surf. Sci. 2012, 258, 6204–6211. [Google Scholar] [CrossRef]
- Rahman, Q.I.; Ahmad, M.; Misra, S.K.; Lohani, M. Effective photocatalytic degradation of Rhodamine B dye by ZnO nanoparticles. Mater. Lett. 2013, 91, 170–174. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Vasei, H.V.; Masoudpanah, S.M.; Pouya, V.K. Photocatalytic activity of solution combustion synthesized ZnO powders by using a mixture of DTAB and citric acid fuels. J. Phys. Chem. Solids 2021, 151, 109895. [Google Scholar] [CrossRef]
- Qian, L.; Zhu, J.; Du, W.; Qian, X. Solvothermal synthesis, electrochemical and photocatalytic properties of monodispersed CeO2 nanocubes. Mater. Chem. Phys. 2009, 115, 835–840. [Google Scholar] [CrossRef]
- Bellardita, M.; Fiorenza, R.; Palmisano, L.; Scirè, S. Photocatalytic and photothermocatalytic applications of cerium oxide-based materials. In Cerium Oxide (CeO₂): Synthesis, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 109–167. [Google Scholar] [CrossRef]
- Wan, X.; Yuan, M.; Tie, S.-I.; Lan, S. Effects of catalyst characters on the photocatalytic activity and process of NiO nanoparticles in the degradation of methylene blue. Appl. Surf. Sci. 2013, 277, 40–46. [Google Scholar] [CrossRef]
- Chauhan, R.; Kumarb, A.; Chaudhary, R.P. Visible-light photocatalytic degradation of methylene blue with Fe doped CdS nanoparticles. Appl. Surf. Sci. 2013, 270, 655–660. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wei, B.; Zhu, L.; Yua, J.H.; Sun, W.J.; Xu, L.L. Cation exchange synthesis of ZnS-Ag2S microspheric composites with enhanced photocatalytic activity. Appl. Surf. Sci. 2013, 270, 133–138. [Google Scholar] [CrossRef]
- Casbeer, E.; Sharma, V.K.; Li, X.-Z. Synthesis and photocatalytic activity of ferrites under visible light: A review. Sep. Purif. Technol. 2012, 87, 1–14. [Google Scholar] [CrossRef]
- Ch, K.; Dhar, S.S. Rapid catalytic degradation of malachite green by MgFe2O4 nanoparticles in presence of H2O2. J. Alloys Compd. 2020, 828, 154462. [Google Scholar] [CrossRef]
- Krishnamoorthi, A.M.C.; Babu, K.C.; Pavithra, N.C. Dielectric, magnetic hyperthermia, and photocatalytic properties of ZnFe2O4 nanoparticles synthesized by solvothermal reflux method. Appl. Phys. A 2019, 125, 477. [Google Scholar] [CrossRef]
- Tsvetkov, M.; Zaharieva, J.; Milanovа, M. Ferrites, modified with silver nanoparticles, for photocatalytic degradation of malachite green in aqueous solutions. Catal. Today 2020, 357, 453–459. [Google Scholar] [CrossRef]
- Manohar, A.; Chintagumpala, K.; Hyeon, K.K. Mixed Zn–Ni spinel ferrites: Structure, magnetic hyperthermia and photocatalytic properties. Ceram. Intern. 2021, 47, 7052–7061. [Google Scholar] [CrossRef]
- Lassoued, A.; Li, J.F. Magnetic and photocatalytic properties of Ni–Co ferrites. Solid State Sci. 2020, 104, 106199. [Google Scholar] [CrossRef]
- Dojcinovic, M.P.; Vasiljevic, Z.Z.; Pavlovic, V.P.; Barisic, D.; Pajic, D.; Tadic, N.B.; Nikolic, M.V. Mixed Mg–Co spinel ferrites: Structure, morphology, magnetic and photocatalytic properties. J. Alloys Compd. 2021, 855, 157429. [Google Scholar] [CrossRef]
- Kurian, M.; Thankachan, S. Structural diversity and applications of spinel ferrite core—Shell nanostructures—A review. Open Ceram. 2021, 8, 100179. [Google Scholar] [CrossRef]
- Klekotka, U.; Piotrowska, B.; Satuła, D.; Kalska-Szostko, B. Modified ferrite core-shell nanoparticles magneto-structural characterization. Appl. Surf. Sci. 2018, 444, 161–167. [Google Scholar] [CrossRef]
- Masala, O.; Hoffman, D.; Sundaram, N.; Page, K.; Proffen, T.; Lawes, G.; Seshadri, R. Preparation of magnetic spinel ferrite core/shell nanoparticles: Soft ferrites on hard ferrites and vice versa. Solid State Sci. 2006, 8, 1015–1022. [Google Scholar] [CrossRef]
- Greene, D.; Serrano-Garcia, R.; Govan, J.; Gun’ko, Y.K. Synthesis Characterization and Photocatalytic Studies of Cobalt Ferrite-Silica-Titania Nanocomposites. Nanomaterials 2014, 4, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Hamad, H.; Abd El-Latif, M.; Kashyout, A.E.-H.; Sadik, W.; Feteha, M. Synthesis and characterization of core–shell–shell magnetic (CoFe2O4–SiO2–TiO2) nanocomposites and TiO2 nanoparticles for the evaluation of photocatalytic activity under UV and visible irradiation. N. J. Chem. 2015, 39, 3116–3128. [Google Scholar] [CrossRef]
- Harraz, F.A.; Mohamed, R.M.; Rashad, M.M.; Wang, Y.C.; Sigmund, W. Magnetic nanocomposite based on titania–silica/cobalt ferrite for photocatalytic degradation of methylene blue dye. Ceram. Int. 2014, 40, 375–384. [Google Scholar] [CrossRef]
- Ren, X.; Chen, X.; Ding, H.; Yang, H. Luminescent and magnetic properties of CoFe2O4@SiO2@Y2O3:Tb3+ nanocomposites with the core–shell. J. Alloys Compd. 2015, 625, 85–89. [Google Scholar] [CrossRef]
- Dee, G.; Shayoub, H.; McNeill, H.; Sánchez Lozano, I.; Rafferty, A.; Gun’ko, Y.K. MnFe2O4@SiO2@CeO2 core–shell nanostructures for applications in water remediation. RSC Adv. 2023, 13, 10513–10522. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.N.; Reddy, N.L.; Preethi, V.; Karthik, M.; Yu, Y.-T.; Yang, J.M.; Kumari, M.M.; Shankar, M.V. A critical review on core/shell-based nanostructured photocatalysts for improved hydrogen generation. Int. J. Hydrogen Energy 2023, 48, 11754–11774. [Google Scholar] [CrossRef]
- Lee, C.-T.; Lee, H.-Y.; Chen, H.-W. GaN MOS device using SiO2 insulator grown by photoelectrochemical oxidation method. IEEE Electron Device Lett. 2003, 24, 54–56. [Google Scholar] [CrossRef]
- Peng, T.; Zhao, D.; Dai, K.; Shi, W.; Hirao, K. Synthesis of titanium dioxide nanoparticles with mesoporous anatase wall and high photocatalytic activity. J. Phys. Chem. B 2005, 109, 4947–4952. [Google Scholar] [CrossRef]
- Gong, X.-Q.; Selloni, A. Reactivity of anatase TiO2 nanoparticles: The role of the minority (001) surface. J. Phys. Chem. B 2005, 109, 19560–19562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.F.; He, Y.L.; Zhang, M.S.; Yin, Z.; Chen, Q. Raman scattering study on anatase TiO2 nanocrystals. J. Phys. D 2000, 33, 912. [Google Scholar] [CrossRef]
- Yadav, A.A.; Kang, S.-W.; Hunge, Y.M. Photocatalytic degradation of Rhodamine B using graphitic carbon nitride photocatalyst. J. Mater. Sci. Mater. Electron. 2021, 32, 15577–15585. [Google Scholar] [CrossRef]
- Sutili, F.J.; Gressler, L.T. Antimicrobial agents. In Aquaculture Pharmacology; Academic Press: Cambridge, MA, USA, 2021; Chapter 3; pp. 131–168. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Nolan, N.T.; Synnott, D.W.; Seery, M.K.; Hinder, S.J.; Van Wassenhoven, A.; Pillai, S.C. Effect of N-doping on the photocatalytic activity of sol–gel TiO2. J. Hazard. Mater. 2012, 211–212, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Kralchevska, R.; Milanova, M.; Kovacheva, P.; Kolev, J.; Avdeev, G.; Todorovsky, D. In-fluence of ThO2 on the photocatalytic activity of TiO2. Cent. Eur. J. Chem. 2011, 9, 1027–1038. [Google Scholar] [CrossRef]
- Engelhard, M.H.; Droubay, T.C.; Du, Y. X-ray Photoelectron Spectroscopy Applications. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Environmental Molecular Sciences Lab.: Washington, DC, USA, 2017; pp. 716–724. [Google Scholar] [CrossRef]
- Wing, M.; Gong, B.; Yao, X.; Wang, Y.; Lamb, R.N. Preparation and microstructure properties of Al-doped TiO2-SiO2 gel-glass film. Thin Solid Film. 2006, 515, 2055–2058. [Google Scholar] [CrossRef]
- Manole, A.V.; Dobromir, M.; Apetrei, R.; Nica, V.; Lucaet, D. Surface characterization of sputtered N:TiO2 thin films within a wide range of dopant concentration. Ceram. Int. 2014, 40, 9989–9995. [Google Scholar] [CrossRef]
- Guo, N.; Liang, Y.; Lan, S.; Liu, L. Uniform TiO2–SiO2 hollow nanospheres: Synthesis, characterization and enhanced adsorption–photodegradation of azo dyes and phenol. Appl. Surf. Sci. 2014, 305, 562–574. [Google Scholar] [CrossRef]
- Peng, T.; Ray, S.; Veeravalli, S.S.; Lalman, J.A.; Arefi-Khonsari, F. The role of hydrothermal conditions in determining 1D TiO2 nanomaterials bandgap energies and crystal phases. Mater. Res. Bull. 2018, 105, 104–113. [Google Scholar] [CrossRef]
- Ray, S.K.; Dhakal, D.; Lee, S.W. Insight into Malachite Green Degradation, Mechanism and Pathways by Morphology-Tuned α-NiMoO4 Photocatalyst. Photochem. Photobiol. 2018, 94, 552–563. [Google Scholar] [CrossRef]
- Weber, M.; Schulmeister, K. Hazard Assessment of Lamps following the CIE Lamp Safety Standard. 2003. Available online: https://laser-led-lamp-safety.seibersdorf-laboratories.at/fileadmin/uploads/intranet/2003%20cie%20hazard%20assessment%20of%20lamps_%20weber.pdf (accessed on 10 April 2024).
- Stefan, M. More Than Just Light. Solutions in Ultraviolet Light. 100 Years of Innovation Osram. 2016. Available online: https://docs.rs-online.com/45d7/0900766b81506d14.pdf (accessed on 10 April 2024).
- Hu, L.; Wang, P.; Zhang, G.; Liu, G.; Li, Y.; Shen, T.; Crittenden, J.C. Enhanced persulfate oxidation of organic pollutants and removal of total organic carbons using natural magnetite and microwave irradiation. Chem. Eng. J. 2020, 383, 123140. [Google Scholar] [CrossRef]
- Schneider, J.T.; Firak, D.S.; Ribeiro, R.R.; Peralta-Zamora, P. Use of scavenger agents in heterogeneous photocatalysis: Truths, half-truths, and misinterpretations. Phys. Chem. Chem. Phys. 2020, 22, 15723–15733. [Google Scholar] [CrossRef] [PubMed]
- Denisov, N.; Yoo, J.E.; Schmuki, P. Effect of different hole scavengers on the photoelectrochemical properties and photocatalytic hydrogen evolution performance of pristine and Pt-decorated TiO2 nanotubes. Electrochim. Acta 2019, 319, 61–71. [Google Scholar] [CrossRef]
- Slapničar, Š.; Žerjav, G.; Zavašnik, J.; Finšgar, M.; Pintar, A. Synthesis and characterization of plasmonic Au/TiO2 nanorod sol-ids for heterogeneous photocatalysis. J. Environ. Chem. Eng. 2023, 11, 109835. [Google Scholar]
- Serpone, N.; Lawless, D.; Khairutdinov, R. Size effects on the photophysical properties of colloidal anatase TiO2 particles: Size quantization or direct transition in this indirect semiconductor? J. Phys. Chem. 1995, 99, 16646–16654. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]
- Golubović, A.; Scepanovic, M.J.; Kremenovic, A. Raman study of the variation in anatase structure of TiO2 nanopowders due to the changes of sol-gel synthesis conditions. J. Sol-Gel Sci. Technol. 2009, 3, 311–319. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Yu, J.; Yu, H.; Cheng, B.; Zhou, M.; Zhao, X. Enhanced photocatalytic activity of TiO2 powder (P25) by hydrothermal treatment. J. Mol. Catal. A Chem. 2006, 253, 112–118. [Google Scholar] [CrossRef]
- Arbuj, S.S.; Hawaldar, R.R.; Mulik, U.P.; Waniet, B.N.; Amalnerkar, D.P.; Waghmode, S.B. Preparation, characterization and photocatalytic activity of TiO2 towards methylene blue degradation. Mater. Sci. Eng. B 2010, 168, 90–94. [Google Scholar] [CrossRef]
- Yuangpho, N.; Lee, S.T.T.; Treerujiraphapong, T.; Khanitchaidecha, W.; Nakaruk, A. Enhanced photocatalytic performance of TiO2 particles via effect of anatase–rutile ratio. Phys. E Low-Dimens. Syst. Nanostructures 2015, 67, 18–22. [Google Scholar] [CrossRef]
- Nolan, N.T.; Seery, M.K.; Pillai, S.C. Spectroscopic investigation of the anatase-to- rutile transformation of sol–gel-synthesized TiO2 photocatalysts. J. Phys. Chem. C 2009, 113, 16151–16157. [Google Scholar] [CrossRef]
- Mahlambi, M.M.; Mishra, A.K.; Mishra, S.B.; Krause, R.W.; Mamba, B.B.; Raichur, A.M. Comparison of Rhodamine B degradation under UV irradiation by two phases of titania nano-photocatalyst. J. Therm. Anal. Calorim. 2012, 110, 847–855. [Google Scholar] [CrossRef]
- Bachvarova-Nedelcheva, A.; Iordanova, R.; Georgieva, N.; Nemska, V.; Foteva, T.; Stoyanova, A. Influence of Nb2O5 and B2O3 on the photocatalytic and antibacterial activity of sol-gel derived TiO2 nanopowders. J. Chem. Techn. Metall. 2024, 59, 335–342. [Google Scholar] [CrossRef]
- Bachvarova-Nedelcheva, A.; Iordanova, R.; Naydenov, A.; Stoyanova, A.; Georgieva, N.; Nemska, V.; Foteva, T. Sol-Gel Obtaining of TiO2/TeO2 Nanopowders with Biocidal and Environmental Applications. Catalysts 2023, 13, 257. [Google Scholar] [CrossRef]
- Tarigh, G.D.; Shemirani, F.; Mazhari, N.S. Fabrication of a reusable magnetic multi-walled carbon nanotube–TiO2 nanocomposite by electrostatic adsorption: Enhanced photodegradation of malachite green. RSC Adv. 2015, 5, 35070. [Google Scholar] [CrossRef]
- Atalı, Ş.G.; Akbal, F.; Gürbüz, M.; Turan, B.C. Granular titanium dioxide and silicon-doped titanium dioxide as reusable photocatalysts for dye removal. Int. J. Appl. Ceram. Technol. 2023, 21, 2024–2036. [Google Scholar] [CrossRef]
- Ivanova, D.; Mladenova, E.; Kaneva, N. Photo-fixation of Ag onto TiO2 powder films for improved photocatalytic activity and atomic-absorption determination of residual noble metal in dyes solution. C. R. Acad. Bulg. Sci. 2024, 77, 20–29. [Google Scholar] [CrossRef]
- Aarthi, T.; Madras, G. Photocatalytic Degradation of Rhodamine Dyes with Nano-TiO2. Ind. Eng. Chem. Res. 2007, 46, 7–14. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. Match!—Phase Analysis Using Powder Diffraction, Version 3.x, Crystal Impact; GbR, Kreuzherrenstr: Bonn, Germany; Volume 102, p. 53227. Available online: https://www.crystalimpact.de/match (accessed on 5 February 2024).
- Grazulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.T.; Quiros, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An open-access collection of crystal structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Lutterotti, L. Maud: A Rietveld analysis program designed for the internet and experiment integration. Acta Crystallogr. Sect. A Found. Crystallogr. 2000, A56, s54. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Hu, J.; Sun, C.; Wu, L.; Zhao, G.; Liu, H.; Jiao, F. Halogen doped g-C3N4/ZnAl-LDH hybrid as a Z-scheme photocatalyst for efficient degradation for tetracycline in seawater. Sep. Purif. Technol. 2023, 309, 123047. [Google Scholar] [CrossRef]


| Sample | Unit Cell Parameters, Å | Crystallite Size, nm | |
|---|---|---|---|
| CoFe2O4 (SG) | a = 8.3612(2) | 48.1 (2) | |
| CoFe2O4/TiO2 (SG) | Anatase | a = 3.7833 (1) c = 9.4391 (3) | 25.3 (3) |
| Rutile | a = 4.5815 (2) c = 2.9531 (4) | 20.1 (2) | |
| CoFe2O4 | a = 8.3652 (10) | 56.6 (7) | |
| CoFe2O4/SiO2/TiO2 (SG) | Anatase | a = 3.7893 (1) c = 9.4364 (6) | 16.8 (6) |
| CoFe2O4 | a = 8.3631 (9) | 53.1 (4) | |
| ZnFe2O4/SiO2/TiO2 (HT) | CoFe2O4/SiO2/TiO2 (HT) | ||||
|---|---|---|---|---|---|
| ZnFe2O4 | Anatase | CoFe2O4 | Rutile | Anatase | |
| Unit cell parameters, Å | a = b = c = 8.4463 (9) | A = b = 3.7899 (2) c = 9.5230 (7) | a = b = c = 8.3874 (15) | a = b = 4.5953 (6) c = 2.9608 (7) | a = b = 3.7860 (2) c = 9.5185 (7) |
| Crystallite size, nm | 19.3 (5) | 41.2 (1) | 34.2 (3) | 42.8 (9) | 36.8 (3) |
| Microstrains, ×10−3 a.u. | 2.6 (3) | 2.0 (2) | 2.6 (5) | 0.6 (1) | 1.6 (3) |
| Weight fraction,% | 19.5 ± 2.1 | 80.5 ± 2.1 | 1.2 ± 0.3 | 11.1 ± 1.1 | 87.7 ± 5.1 |
| Rwp,% | 5.64 | 7.37 | |||
| χ2 | 1.10 | 1.18 | |||
| Ultrapure H2O | Artificial Sea H2O | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UV Light | Simulated Solar Light | UV Light | Simulated Solar Light | ||||||||||||
| Rate Constant, 10−3 min−1 | Degradation, % | Rate Constant, 10−3 min−1 | Degradation, % | Rate Constant, 10−3 min−1 | Degradation, % | Rate Constant, 10−3 min−1 | Degradation, % | ||||||||
| MG | RhB | MG | RhB | MG | RhB | MG | RhB | MG | RhB | MG | RhB | MG | RhB | MG | RhB |
| 9.0 | - | 80 | 53 | 77 | 65 | 98 | 98 | 5 | 3.6 | 59.5 | 42 | 5.7 | 4.0 | 93 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaharieva, J.; Tsvetkov, M.; Georgieva, M.; Tzankov, D.; Milanova, M. “Core/Shell” Nanocomposites as Photocatalysts for the Degradation of the Water Pollutants Malachite Green and Rhodamine B. Int. J. Mol. Sci. 2024, 25, 6755. https://doi.org/10.3390/ijms25126755
Zaharieva J, Tsvetkov M, Georgieva M, Tzankov D, Milanova M. “Core/Shell” Nanocomposites as Photocatalysts for the Degradation of the Water Pollutants Malachite Green and Rhodamine B. International Journal of Molecular Sciences. 2024; 25(12):6755. https://doi.org/10.3390/ijms25126755
Chicago/Turabian StyleZaharieva, Joana, Martin Tsvetkov, Milena Georgieva, Dimitar Tzankov, and Maria Milanova. 2024. "“Core/Shell” Nanocomposites as Photocatalysts for the Degradation of the Water Pollutants Malachite Green and Rhodamine B" International Journal of Molecular Sciences 25, no. 12: 6755. https://doi.org/10.3390/ijms25126755
APA StyleZaharieva, J., Tsvetkov, M., Georgieva, M., Tzankov, D., & Milanova, M. (2024). “Core/Shell” Nanocomposites as Photocatalysts for the Degradation of the Water Pollutants Malachite Green and Rhodamine B. International Journal of Molecular Sciences, 25(12), 6755. https://doi.org/10.3390/ijms25126755









