Unique Splicing of Lrp5 in the Brain: A New Player in Neurodevelopment and Brain Maturation
Abstract
1. Introduction
2. Results
2.1. Non-Mendelian Pattern in Lrp5−/− Mouse Births
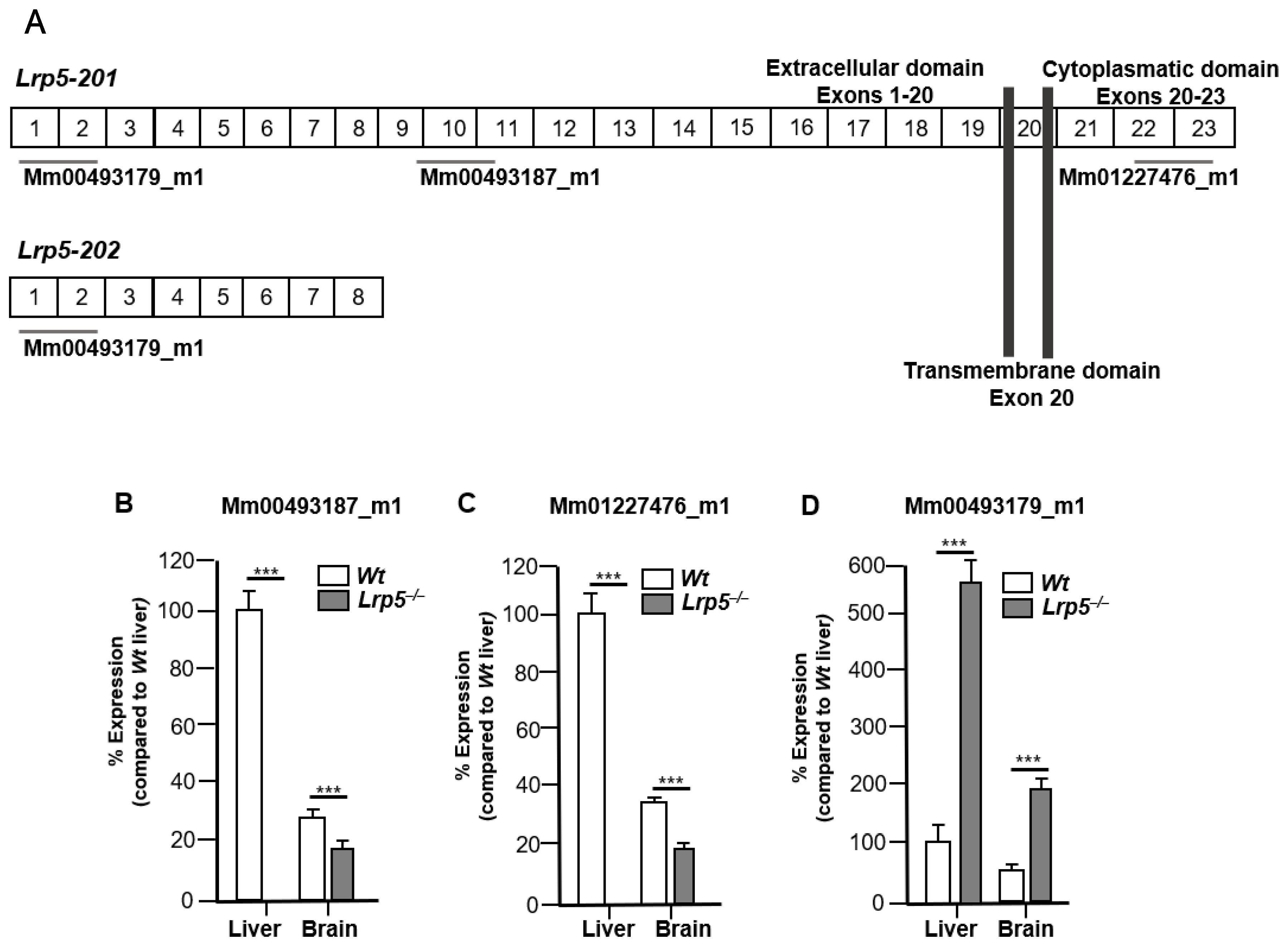
2.2. LRP5 Is Expressed in Brains of Lrp5−/− Mice
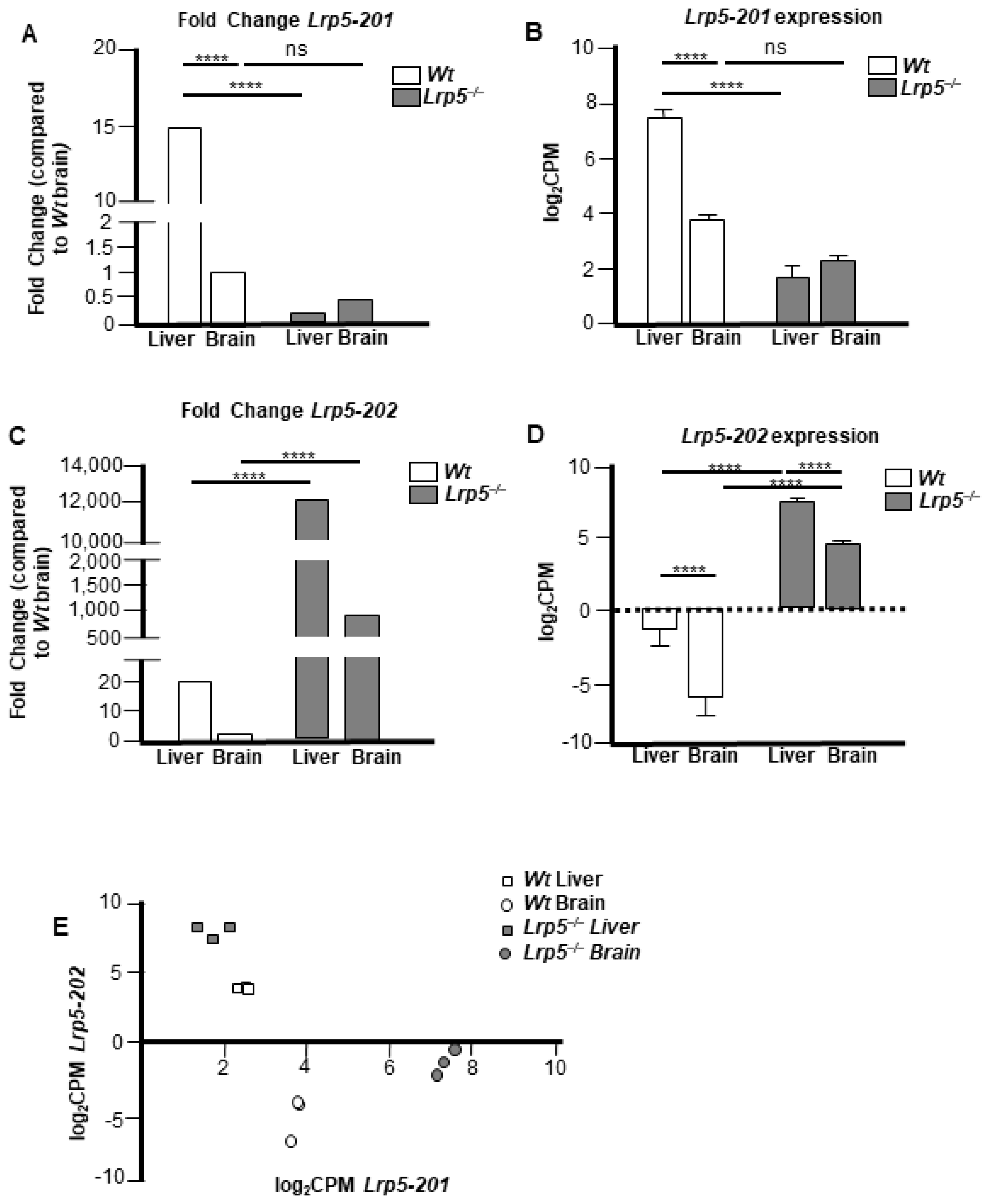
2.3. Lrp5 Transcriptome Is Different in Livers and Brains of Lrp5−/− Mice
2.4. LRP5 Deficiency Leads to Alterations in the Transcriptome of Livers and Brains
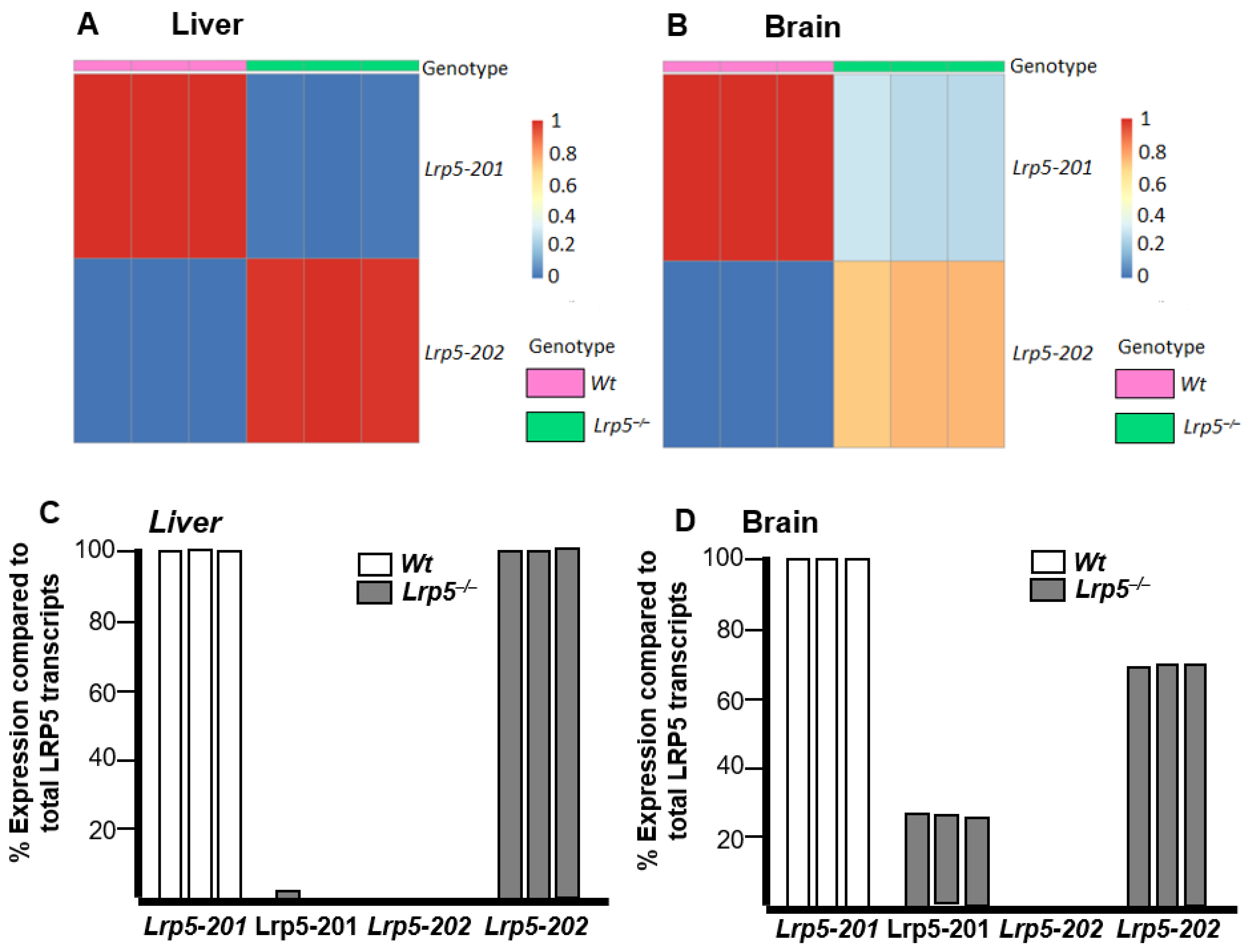
2.5. Lrp5 Quantity Is Different in Livers and Brains of Lrp5−/− Mice
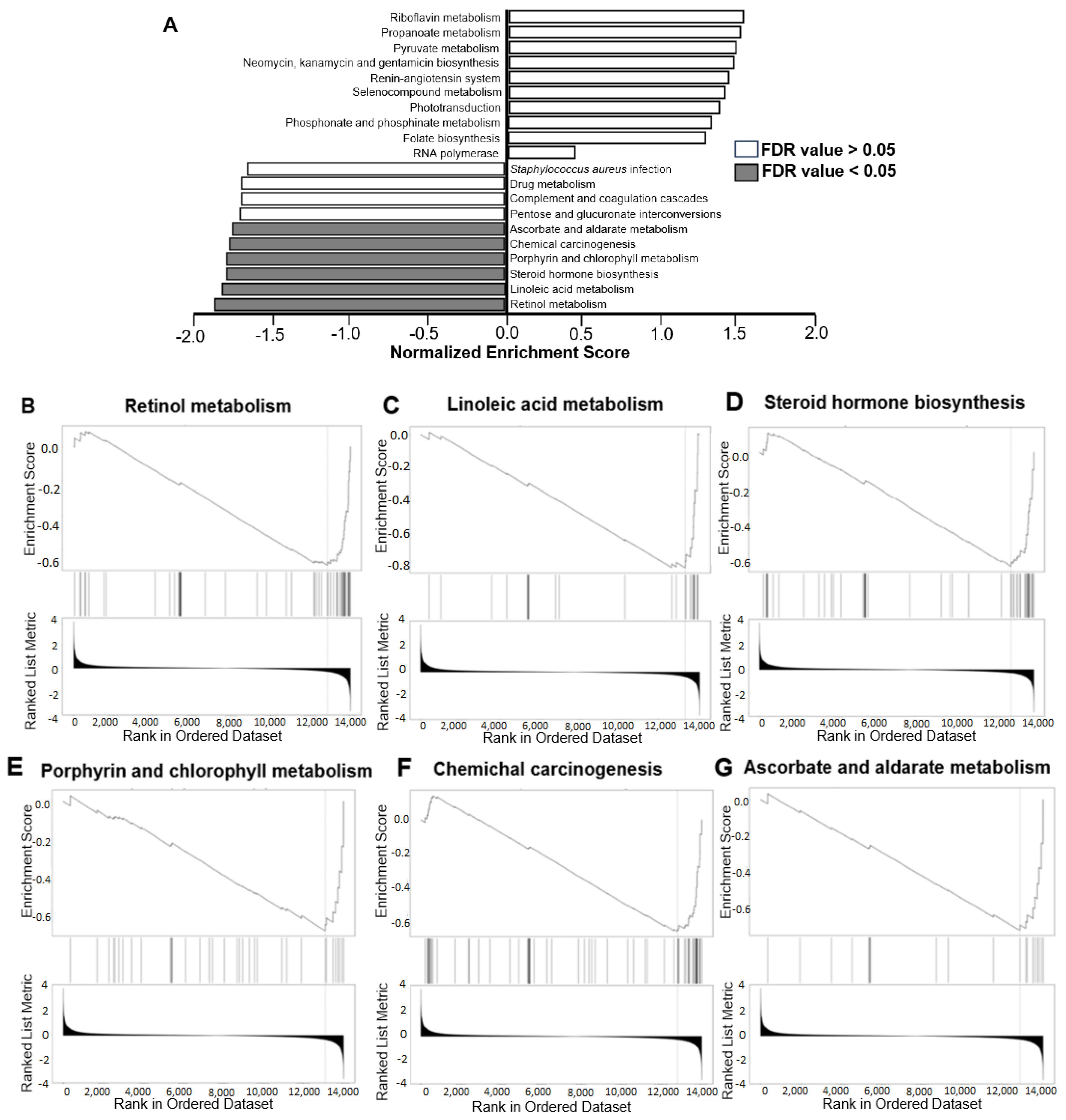
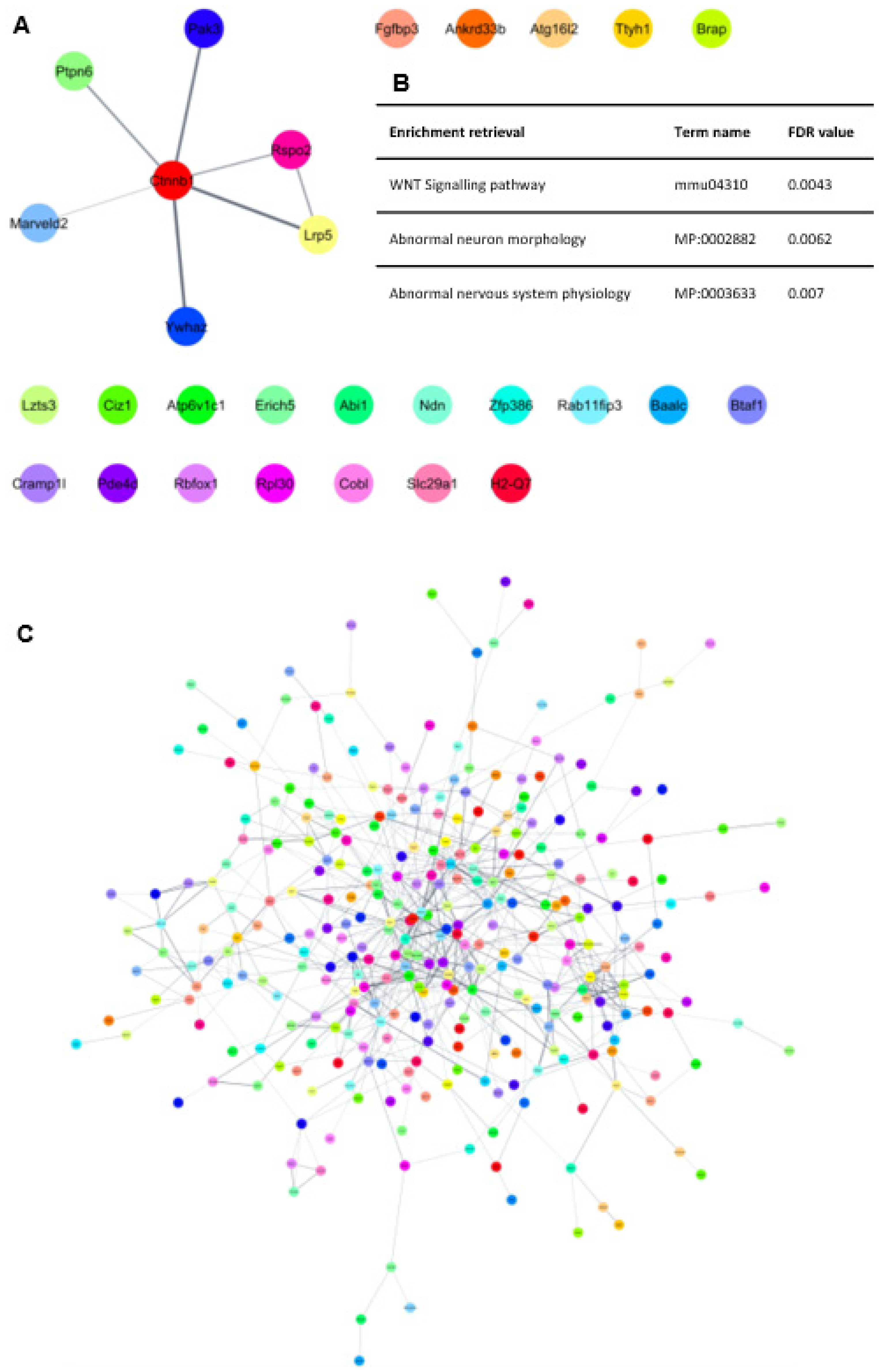
2.6. Functional Studies Show Modified Functions in Brains of Lrp5−/− Mice
2.7. Functional Studies Show Impaired Functions in Livers of Lrp5−/− Mice
3. Discussion
4. Materials and Methods
4.1. Animal Models and Experimental Design
4.2. RNA Isolation and Real-Time PCR
4.3. RNA-Seq Analysis
4.4. In Silico Systems Biology Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Bhanot, P.; Brink, M.; Samos, C.H.; Hsieh, J.C.; Wang, Y.; Macke, J.P.; Andrew, D.; Nathans, J.N.R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 1996, 382, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Yang-Snyder, J.; Miller, J.R.; Brown, J.D.; Lai, C.J.; Moon, R.T. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr. Biol. 1996, 6, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Borrell-Pags, M.; Romero, J.C.; Juan-Babot, O.; Badimon, L. Wnt pathway activation, cell migration, and lipid uptake is regulated by low-density lipoprotein receptor-related protein 5 in human macrophages. Eur. Heart J. 2011, 32, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Wehrli, M.; Dougan, S.T.; Caldwell, K.; O’Keefe, L.; Schwartz, S.; Valzel-Ohayon, D.; Schejter, E.; Tomlinson, A.; DiNardo, S. Arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 2000, 407, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, E.; Chou, T.B.; Perrimon, N. wingless signaling acts through zeste-white 3, the drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell 1992, 71, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Behrens, J.; Jerchow, B.A.; Würtele, M.; Grimm, J.; Asbrand, C.; Wirtz, R.; Kühl, M.; Wedlich, D.; Birchmeier, W. Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science 1998, 280, 596–599. [Google Scholar] [CrossRef]
- Behrens, J.; Von Kries, J.P.; Kühl, M.; Bruhn, L.; Wedlich, D.; Grosschedl, R.; Birchmeier, W. Functional interaction of β-catenin with the transcription factor LEF- 1. Nature 1996, 382, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Billin, A.N.; Thirlwell, H.; Ayer, D.E. β-Catenin–Histone Deacetylase Interactions Regulate the Transition of LEF1 from a Transcriptional Repressor to an Activator. Mol. Cell. Biol. 2000, 20, 6882–6890. [Google Scholar] [CrossRef]
- He, T.C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; Da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of c-MYC as a target of the APC pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef]
- Shtutman, M.; Zhurinsky, J.; Simcha, I.; Albanese, C.; D’Amico, M.; Pestell, R.; Ben-Ze’ev, A. The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 5522–5527. [Google Scholar] [CrossRef]
- Carter, M.; Chen, X.; Slowinska, B.; Minnerath, S.; Glickstein, S.; Shi, L.; Campagne, F.; Weinstein, H.; Ross, M.E. Crooked tail (Cd) model of human folate-responsive neural tube defects is mutated in Wnt coreceptor lipoprotein receptor-related protein 6. Proc. Natl. Acad. Sci. USA 2005, 102, 12843–12848. [Google Scholar] [CrossRef] [PubMed]
- Ciani, L.; Salinas, P.C. WNTs in the vertebrate nervous system: From patterning to neuronal connectivity. Nat. Rev. Neurosci. 2005, 6, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Ahmad-Annuar, A.; Ciani, L.; Simeonidis, I.; Herreros, J.; Fredj, N.B.; Rosso, S.B.; Hall, A.; Brickley, S.; Salinas, P.C. Signaling across the synapse: A role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J. Cell Biol. 2006, 174, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Zechner, D.; Müller, T.; Wende, H.; Walther, I.; Taketo, M.M.; Crenshaw, E.B.; Treier, M.; Birchmeier, W.; Birchmeier, C. Bmp and Wnt/β-catenin signals control expression of the transcription factor Olig3 and the specification of spinal cord neurons. Dev. Biol. 2007, 303, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Varela-Nallar, L.; Alfaro, I.E.; Serrano, F.G.; Parodi, J.; Inestrosa, N.C. Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc. Natl. Acad. Sci. USA 2010, 107, 21164–21169. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.E.J.; Nourse, C.C.; Cooper, H.M. The tangled web of non-canonical wnt signalling in neural migration. NeuroSignals 2012, 20, 202–220. [Google Scholar] [CrossRef] [PubMed]
- Okerlund, N.D.; Cheyette, B.N.R. Synaptic Wnt signaling-a contributor to major psychiatric disorders? J. Neurodev. Disord. 2011, 3, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Valvezan, A.J.; Klein, P.S. GSK-3 and Wnt signaling in neurogenesis and bipolar disorder. Front. Mol. Neurosci. 2012, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Piña-Crespo, J.; Li, Y. Restoring Wnt/β-catenin signaling is a promising therapeutic strategy for Alzheimer’s disease. Mol. Brain 2019, 12, 104. [Google Scholar] [CrossRef]
- Ren, Q.; Chen, J.; Liu, Y. LRP5 and LRP6 in Wnt Signaling: Similarity and Divergence. Front. Cell Dev. Biol. 2021, 9, 670960. [Google Scholar] [CrossRef]
- Grünblatt, E.; Nemoda, Z.; Werling, A.M.; Roth, A.; Angyal, N.; Tarnok, Z.; Thomsen, H.; Peters, T.; Hinney, A.; Hebebrand, J.; et al. The involvement of the canonical Wnt-signaling receptor LRP5 and LRP6 gene variants with ADHD and sexual dimorphism: Association study and meta-analysis. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2019, 180, 365–376. [Google Scholar] [CrossRef]
- Veerapathiran, S.; Teh, C.; Zhu, S.; Kartigayen, I.; Korzh, V.; Matsudaira, P.T.; Wohland, T. Wnt3 distribution in the zebrafish brain is determined by expression, diffusion and multiple molecular interactions. eLife 2020, 9, e59489. [Google Scholar] [CrossRef]
- Borrell-Pages, M.; Vilahur, G.; Romero, J.C.; Casaní, L.; Bejar, M.T.; Badimon, L. LRP5/canonical Wnt signalling and healing of ischemic myocardium. Basic. Res. Cardiol. 2016, 111, 67. [Google Scholar] [CrossRef]
- Borrell-Pagès, M.; Romero, J.C.; Badimon, L. LRP5 deficiency down-regulates Wnt signalling and promotes aortic lipid infiltration in hypercholesterolaemic mice. J. Cell. Mol. Med. 2015, 19, 770–777. [Google Scholar] [CrossRef]
- Borrell-Pages, M.; Carolina Romero, J.; Badimon, L. LRP5 and plasma cholesterol levels modulate the canonical Wnt pathway in peripheral blood leukocytes. Immunol. Cell Biol. 2015, 93, 653–661. [Google Scholar] [CrossRef]
- Badimon, L.; Luquero, A.; Crespo, J.; Peña, E.; Borrell-Pages, M. PCSK9 and LRP5 in macrophage lipid internalization and inflammation. Cardiovasc. Res. 2021, 117, 2054–2068. [Google Scholar] [CrossRef]
- Borrell-Pages, M.; Luquero, A.; Vilahur, G.; Padró, T.; Badimon, L. Canonical Wnt pathway and the LDL receptor superfamily in neuronal cholesterol homeostasis and function. Cardiovasc. Res. 2023, 120, 140–151. [Google Scholar] [CrossRef]
- Kato, M.; Patel, M.S.; Levasseur, R.; Lobov, I.; Chang, B.H.J.; Glass, D.A.; Hartmann, C.; Li, L.; Hwang, T.H.; Brayton, C.F.; et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J. Cell Biol. 2002, 157, 303–314. [Google Scholar] [CrossRef]
- Ensembl. Mouse LRP5 Gene [Internet]. Available online: https://www.ensembl.org/Mus_musculus/Gene/Summary?db=core;g=ENSMUSG00000024913;r=19:3634828-3736564 (accessed on 1 May 2023).
- Badimon, L.; Casaní, L.; Camino-Lopez, S.; Juan-Babot, O.; Borrell-Pages, M. GSK3β inhibition and canonical Wnt signaling in mice hearts after myocardial ischemic damage. PLoS ONE 2019, 14, e0218098. [Google Scholar] [CrossRef]
- Luquero, A.; Vilahur, G.; Casani, L.; Badimon, L.; Borrell-Pages, M. Differential cholesterol uptake in liver cells: A role for PCSK9. FASEB J. 2022, 36, e22291. [Google Scholar] [CrossRef]
- McCaffery, P.; Dräger, U.C. Regulation of retinoic acid signaling in the embryonic nervous system: A master differentiation factor. Cytokine Growth Factor. Rev. 2000, 11, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Maden, M. Retinoid signalling in the development of the central nervous system. Nat. Rev. Neurosci. 2002, 3, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Glaser, T.; Brüstle, O. Retinoic acid induction of ES-cell-derived neurons: The radial glia connection. Trends Neurosci. 2005, 28, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Olson, C.R.; Mello, C.V. Significance of vitamin A to brain function, behavior and learning. Mol. Nutr. Food Res. 2010, 54, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.P.; Da Silva Lima, M.; Barbosa, M.Q.; De Melo, M.F.F.T.; De Menezes Santos Bertozzo, C.C.; De Oliveira, M.E.G.; Branquinho Bessa, R.J.; Almeida Alves, S.P.; Souza, M.I.A.; de Cassia Ramos do Egypto Queiroga, R.; et al. Effect of conjugated linoleic acid on memory and reflex maturation in rats treated during early life. Front. Neurosci. 2019, 13, 370. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.Y. Linoleic acid–good or bad for the brain? NPJ Sci. Food 2020, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Wolffe, A.P.; Cooper, G.M. The Cell: A Molecular Approach. Q. Rev. Biol. 1998, 73, 69–70. [Google Scholar] [CrossRef]
- Guo, Y.; Zi, X.; Koontz, Z.; Kim, A.; Xie, J.; Gorlick, R.; Holcombe, R.F.; Hoang, B.H. Blocking Wnt/LRP5 signaling by a soluble receptor modulates the epithelial to mesenchymal transition and suppresses met and metalloproteinases in osteosarcoma Saos-2 cells. J. Orthop. Res. 2007, 25, 964–971. [Google Scholar] [CrossRef]
- Guo, Y.; Rubin, E.M.; Xie, J.; Zi, X.H.B. Dominant Negative LRP5 Decreases Tumorigenicity and Metastasis of Osteosarcoma in an Animal Model. Clin. Orthop. Relat. Res. 2008, 466, 2039–2045. [Google Scholar] [CrossRef]
- Davisson, M.T. Rules and guidelines for nomenclature of mouse genes. Gene 1994, 147, 157–160. [Google Scholar] [CrossRef]
- Holmen, S.L.; Giambernardi, T.A.; Zylstra, C.R.; Buckner-Berghuis, B.D.; Resau, J.H.; Hess, J.F.; Glatt, V.; Bouxsein, M.L.; Ai, M.; Warman, M.L.; et al. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J. Bone Miner. Res. 2004, 19, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Krueger, F. TrimGalore [Internet]. Available online: https://github.com/FelixKrueger/TrimGalore (accessed on 1 May 2023).
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Tekath, T.; Dugas, M. Differential transcript usage analysis of bulk and single-cell RNA-seq data with DTUrtle. Bioinformatics 2021, 37, 3781–3787. [Google Scholar] [CrossRef] [PubMed]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. G:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Wang, J.; Duncan, D.; Shi, Z.; Zhang, B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): Update 2013. Nucleic Acids Res. 2013, 41, W77–W83. [Google Scholar] [CrossRef]


| Gene Transcripts with Altered Expression in Livers of Lrp5−/− Mice | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mdfic-206 | Myo5a-204 | Lpin2-204 | Wnk2-211 | Rida-201 | Fbxo16-204 | Otud1-201 | Ranbp10-201 | 1500011B03Rik-204 |
| Lrp5-201 | Dpys-201 | Gabrb3-201 | Tlcd4-207 | Xlr3a-201 | Irf6-201 | Aplp2-203 | Atp5pb-203 | 0610030E20Rik-201 |
| Fam222b-203 | Slc13a3-201 | Nat8f2-201 | Wdr77-201 | Eml1-202 | Kif26b-202 | Tbp-211 | Med13l-201 | 1110032F04Rik-201 |
| Tcf7l2-213 | Ppm1k-201 | Papola-202 | Serpinc1-207 | Dph7-201 | Zhx3-202 | Ifnar2-201 | Fech-201 | D5Ertd579e-201 |
| Camsap3-209 | Bend6-201 | Sptan1-202 | Fus-204 | Fzd8-201 | Zfp703-202 | Gmppb-202 | Tmem25-204 | AW209491-202 |
| Dctn1-203 | Fgd6-201 | Hnrnpa1-202 | Gsap-201 | Lipa-201 | Yy1-201 | Mat1a-201 | Btg1-202 | Cdc42bpb-201 |
| Ankrd33b-202 | Clk3-201 | Zmynd8-203 | Xpo4-209 | Ppm1b-201 | Eif5-201 | Lrrc73-204 | Bptf-203 | 2810021J22Rik-201 |
| Lrp5-202 | Hddc3-208 | Dpys-202 | mt-Atp6-201 | St6gal1-205 | Aacs-201 | Ide-201 | Ankrd11-202 | A630089N07Rik-202 |
| Ociad2-205 | Stom-201 | Pxmp2-201 | Tab2-204 | Crebrf-201 | Relch-205 | Tmpo-201 | Mcfd2-204 | 2410002F23Rik-202 |
| Meis3-205 | Tspyl5-201 | Slc8b1-202 | Cyp39a1-203 | Cog8-201 | Map2k3-201 | N4bp2l2-201 | Pwwp2a-203 | Nr1i2-201 |
| Cps1-201 | Zfand5-205 | Irgm1-202 | Bet1l-201 | Rhod-201 | Hes6-202 | Nr1h2-201 | Dcaf12l1-202 | Wbp1l-201 |
| Slc15a2-205 | Inpp5f-208 | Myef2-201 | Rab9-202 | Zfp120-201 | Wnk2-201 | Dst-201 | Elf1-201 | Srp54a-202 |
| Rapgef1-207 | Jmy-201 | Rsph1-201 | Pxmp4-201 | Znfx1-201 | Tfdp1-204 | Gpbp1-202 | Mphosph8-201 | Slc4a2-201 |
| Pnrc1-201 | Traf4-201 | Dclk2-206 | Rnf186-201 | Csnk1d-202 | Scaf11-204 | Rabep1-207 | Chd4-203 | Dtymk-201 |
| Gamt-202 | Brap-201 | Srsf7-201 | Kdm2a-202 | Etv6-202 | Pik3r1-202 | Bhlhe41-201 | Pi4ka-201 | Ip6k2-206 |
| Prxl2c-207 | Gria4-203 | Pdcd11-201 | Esyt2-201 | Stxbp3-201 | Ahctf1-201 | Slc38a2-201 | Pkp4-211 | Gemin5-205 |
| Aktip-204 | Gabarapl1-201 | Slc39a14-202 | Qdpr-201 | Sgsh-201 | Atp6ap2-201 | Gpbp1l1-201 | Per2-201 | Azin1-203 |
| Zfyve1-201 | Irs2-201 | Elfn2-201 | Dnajc13-203 | Rsph4a-201 | Rai1-202 | Cmtm4-201 | Slc43a1-201 | Zrsr2-201 |
| Slc25a33-201 | Spns2-201 | Papss2-201 | Mxd4-201 | Fah-201 | Epm2aip1-201 | Cyp2c70-201 | Grb10-203 | Ttc14-211 |
| Tpm1-215 | Dtx3l-201 | Uox-201 | Hdac5-202 | Csad-211 | Irf2bp2-201 | Pfkfb2-204 | Dusp3-201 | Snrnp48-201 |
| Klf11-201 | Maoa-201 | Inf2-201 | Pspc1-201 | Prpsap1-201 | Tbc1d20-201 | Flcn-203 | Cebpb-201 | Gpcpd1-202 |
| Dctn1-202 | Tor1aip2-205 | Epc2-201 | Rabggtb-201 | Abat-201 | Bdp1-204 | Hbp1-202 | Atrip-201 | Iigp1-202 |
| Evi5l-207 | Pparg-202 | Dennd11-202 | Fn3krp-201 | Serpinf2-202 | Jmjd1c-206 | C9orf72-203 | Riok2-201 | Sstr4-201 |
| Klhl24-201 | Cnppd1-201 | Foxp4-208 | Cdc42bpg-201 | Hnrnpa3-203 | Fnip1-201 | Smc5-202 | Bzw1-201 | Unc13b-201 |
| Cyria-205 | Dach1-202 | Stk24-201 | Aldh3a2-202 | Tro-204 | Tmf1-202 | Atxn2-201 | Slc25a47-201 | Ciart-201 |
| Il13ra1-201 | Erbb3-201 | Serpinb9-201 | Agxt2-204 | Ubiad1-201 | Clic5-203 | Chn2-202 | Zfp955a-201 | Elac1-201 |
| Trim46-201 | Spryd4-201 | Ilrun-203 | Pcdh1-204 | Gorasp1-201 | Upp2-202 | Atpsckmt-201 | Ap4m1-201 | Nfyc-204 |
| Gclc-201 | Sesn2-201 | Mmab-201 | Zfand6-208 | Dyrk3-201 | Csnk1g1-202 | Stat5b-201 | Hsd17b7-201 | Ipmk-203 |
| Dgkb-203 | Dlg4-205 | Thrsp-201 | Fbxl19-201 | Blvrb-201 | Slc25a22-225 | Lats2-201 | Fbxl3-201 | Mef2d-204 |
| Gla-201 | Nr2c2-201 | Klhl42-201 | Ppp1r3b-201 | Pcsk9-201 | Paqr5-201 | Sf3a1-201 | Nars-205 | Rnf125-202 |
| Muc3a-202 | Stard4-201 | Tstd3-201 | Inf2-203 | Cpeb2-202 | Tmc6-201 | Map3k11-201 | Stau2-212 | Dcaf11-202 |
| Aldh1l1-201 | Slc38a3-209 | Bcan-201 | Mtdh-202 | Gnpnat1-201 | Rnd1-201 | Tfe3-201 | Ss18l2-201 | Dhtkd1-202 |
| Smurf1-203 | Ccng2-201 | Atad3a-201 | Mink1-201 | Zfp266-202 | Arhgef3-202 | Elp1-201 | Hmgb1-201 | Ttbk2-202 |
| Wnt7b-201 | Elovl6-201 | Chic1-201 | Pck1-201 | Zswim4-201 | Aqp11-205 | Mthfr-201 | Gpr146-201 | Mapk3-202 |
| Lnx2-201 | Psmc3-210 | Slc38a3-201 | Gtf2ird1-229 | Ccdc39-201 | Slc38a3-202 | Oser1-201 | P2ry1-203 | Heatr1-206 |
| Zfp386-204 | Gprc5b-204 | Serpind1-202 | Fam47e-202 | Arg1-201 | Ankrd13c-202 | Zfp592-201 | Tmem98-201 | Tmub2-202 |
| Creg1-202 | Calcoco1-201 | Nme5-204 | Map1lc3a-201 | Mid1ip1-201 | Ints6-201 | Net1-201 | Zfp322a-201 | Rb1cc1-214 |
| Rbm33-204 | Pou2af2-202 | Laptm4b-201 | Dnajb11-203 | Tbcel-203 | Smad4-201 | Ewsr1-205 | Zkscan8-201 | Kdm3a-201 |
| Fam135a-206 | Rpl30-201 | Fads6-201 | Ppp1r3g-201 | Srsf1-205 | Slc20a2-201 | Slc9a3-203 | Gpx6-201 | Map4k4-209 |
| Rnf38-202 | Pde4b-207 | Lrfn3-201 | Dyrk1b-201 | Ddx42-201 | Map3k5-202 | Hnrnpf-202 | Cstf2t-201 | |
| Ephx1-201 | Sec24c-201 | Gpam-202 | Tacc2-205 | Mbd5-203 | Suds3-202 | Crebbp-205 | Tbc1d14-201 | |
| Rtl5-201 | Stard13-208 | Raf1-201 | Ttc38-203 | Meiob-201 | Plekhm1-201 | Proca1-201 | Acbd5-213 | |
| Uqcc1-204 | Mok-202 | Aox1-201 | Atat1-203 | Fem1a-201 | Net1-202 | Rims2-201 | Mtmr3-203 | |
| Abcb4-201 | Mrtfb-204 | Ube2h-202 | Wac-201 | Cpeb2-204 | Serpina3n-201 | Shroom1-201 | Cyth2-203 | |
| Cpq-201 | Ttll11-202 | Zfp446-203 | Fus-201 | Csad-205 | Lrp6-201 | Ano1-203 | Chrm3-202 | |
| Heca-201 | Septin9-204 | Anks4b-201 | Tomm40-202 | Mul1-201 | Fnbp1-210 | Leng8-203 | Opn3-201 | |
| Nlgn3-201 | Ctdsp2-202 | Kctd7-201 | Tesk1-201 | Efr3a-212 | Taok3-201 | Pnn-201 | H2az1-201 | |
| Map3k13-203 | Nfil3-201 | Gbp7-201 | Hlcs-201 | Evi5-201 | Mpv17l-201 | Pon2-201 | Ppp2r2d-201 | |
| Btg1-201 | Arl4a-201 | Ypel2-201 | Casp7-201 | Ptpn21-203 | Mettl1-201 | Hmgcr-201 | Nup50-201 | |
| Tcp11l2-201 | Zfp740-201 | Plec-218 | Rrp9-201 | Khnyn-203 | Wdr45-204 | Mtss1-201 | Magi1-203 | |
| Tmem64-201 | Pcdh1-203 | Atosa-201 | Hnrnpd-211 | Cyp39a1-204 | Kcna2-202 | Ephb6-201 | Mterf2-201 | |
| Zfp13-201 | Hsdl2-201 | Txndc11-202 | Ankrd46-203 | Zdhhc2-201 | Dtx4-201 | Itgb1-201 | Abhd8-201 | |
| Azin1-201 | Aldh1l1-203 | Nhlrc1-201 | Echdc3-201 | Lrrfip2-205 | Rbbp6-202 | Tmx2-201 | Akap8-206 | |
| Emc2-201 | Gpr17-201 | Znrf3-201 | Dnmbp-206 | Lcorl-212 | Rabl3-201 | Acaca-201 | Csad-201 | |
| Tgoln1-201 | Sppl3-201 | Pomk-201 | Triobp-203 | Rnf11-201 | Phf13-201 | Otud3-201 | Rdx-204 | |
| Pde4dip-201 | Tlcd4-203 | Zfp715-203 | Stat1-206 | Axin1-201 | Tmem44-204 | Ranbp10-203 | Snap25-201 | |
| Gene Transcripts with Altered Expression in Brains of Lrp5−/− Mice | ||||
|---|---|---|---|---|
| Fgfbp3-201 | Pde4d-202 | Cramp1-201 | Ttyh1-201 | Brap-205 |
| Eps8l2-206 | Ankrd33b-202 | H2-Q7-201 | Erich5-201 | Ighg2c-202 |
| Rab11fip3-201 | Cask-210 | Gm17167-201 | Ube2d2a-210 | Abi1-205 |
| Lrp5-202 | Zfp386-204 | Gm8116-201 | Bcat2-205 | Atp6v1c1-202 |
| Gm12191-201 | Rpl30-201 | Aldh1l1-204 | Baalc-202 | Ywhaz-203 |
| Rbfox1-202 | Ciz1-202 | Atp6v1c1-201 | Slc29a1-222 | Lzts3-202 |
| Ndn-201 | Atg16l2-211 | Fn1-204 | Rpl30-ps9-201 | Rspo2-201 |
| Hax1-207 | Gm8276-201 | Cobl-210 | Ankrd46-204 | Pak3-210 |
| Ptpn6-203 | Marveld2-201 | Btaf1-201 | Gm54215-201 | Meg3-201 |
| Ankrd33b-203 | Ywhaz-207 | Eif3s6-ps2-201 | Ywhaz-201 | |
| Altered Function | p-Value | Source | Significantly Altered Transcripts |
|---|---|---|---|
| Cell morphogenesis involved in differentiation | 0.00713631 | GO:BP | Necdin-201; Ptpn6-203; Cask-210; Fn1-204; Cobl-210; Abi-205; Ltzs3-202; Pak3-210 |
| Cell morphogenesis involved in neuron differentiation | 0.03526747 | GO:BP | Necdin-201; Cask-210; Fn1-204; Cobl-210; Abi-205; Ltzs3-202; Pak3-210 |
| Postsynaptic density | 0.00032878 | GO:CC | Cask-210; Rpl30-201; Ywhaz-207; Baalc-202; Abi1-205; Ltzs3-202; Pak3-210 |
| Postsynapse | 0.00037269 | GO:CC | Rab11fip3-201; Slc29a1-222; Cask-210; Rpl30-201; Ywhaz-207; Baalc-202; Abi1-205; Ltzs3-202; Pak3-210 |
| Asymmetric synapse | 0.00043165 | GO:CC | Cask-210; Rpl30-201; Ywhaz-207; Baalc-202; Abi1-205; Ltzs3-202; Pak3-210 |
| Postsynaptic specialization | 0.00060115 | GO:CC | Cask-210; Rpl30-201; Ywhaz-207; Baalc-202; Abi1-205; Ltzs3-202; Pak3-210 |
| Neuron to neuron synapse | 0.00072925 | GO:CC | Cask-210; Rpl30-201; Ywhaz-207; Baalc-202; Abi1-205; Ltzs3-202; Pak3-210 |
| Cell junction | 0.00111901 | GO:CC | Rab11fip3-201; Ptpn6-203; Cask-210; Rpl30-201; Ywhaz-207; Baalc-202; Slc29a1-222; Marveld2-201; Atp6v1c1-201; Ttyh1-201; Abi1-205; Ltzs3-202; Pak3-210 |
| Synapse | 0.00187814 | GO:CC | Rab11fip3-201; Slc29a1-222; Cask-210; Rpl30-201; Ywhaz-207; Baalc-202; Atp6v1c1-201; Abi1-205; Ltzs3-202; Pak3-210 |
| Apical part of cell | 0.01692298 | GO:CC | Hax1-207; Pde4d-202; Marveld2-201; Atp6v1c1-201; Fn1-204; Cobl-210 |
| Plasma membrane region | 0.01918133 | GO:CC | Rab11fip3-201; Eps8l2-206; Hax1-207; Pde4d-202; Cask-210; Marveld2-201; Fn1-204; Ttyh1-201; Slc29a1-222 |
| Protein domain specific binding | 0.00720761 | GO:MF | Hax1-207; Ptpn6-203; Cask-210; Ywhaz-207; Fn1-204; Abi1-205; Lzts3-202; Pak3-210 |
| Protein binding | 0.04096533 | GO:MF | Fgfbp3-201; Eps8l2-206; Rab11fip3-201; Ndn-201; Hax1-207; Ptpn6-203; Pde4d-204; Cask-210; Marveld2-201; Ywhaz-207; Fn1-204; Cobl-210; Ankrd46-204; Abi1-205; Lzts3-202; Pak3-210; Lrp5-202; Ankrd33b-206; Ciz1-202; Atg16l2-211; H2-Q7-201; Aldh1l1-204; Btaf1-201; Ube2d2a-210; Brap-205; Ighg2c-202; Rspo2-201 |
| Altered Function | p-Value | Source | Number of Significantly Altered Transcripts |
|---|---|---|---|
| Regulation of cellular metabolic process | 1.54 × 10−19 | GO:BP | 193 |
| Regulation of cellular process | 2.68 × 10−18 | GO:BP | 325 |
| Regulation of primary metabolic process | 4.22 × 10−18 | GO:BP | 199 |
| Biological regulation | 1.31 × 10−16 | GO:BP | 347 |
| Reguation of metabolic process | 2.41 × 10−16 | GO:BP | 223 |
| Regulation of nitrogen compound metabolic process | 7.82 × 10−16 | GO:BP | 189 |
| Regulation of biological process | 1.66 × 10−15 | GO:BP | 337 |
| Organic substance biosynthetic process | 3.49 × 10−15 | GO:BP | 190 |
| Biosynthetic process | 3.75 × 10−15 | GO:BP | 192 |
| Cellular process | 6.86 × 10−15 | GO:BP | 446 |
| Positive regulation of biological process | 1.45 × 10−14 | GO:BP | 207 |
| Positive regulation of cellular process | 4.05 × 10−14 | GO:BP | 190 |
| Regulation of macromolecule metabolic process | 2.91 × 10−13 | GO:BP | 204 |
| Cellular metabolic process | 2.50 × 10−12 | GO:BP | 299 |
| Cellular biosynthetic process | 3.84 × 10−12 | GO:BP | 173 |
| Regulation of biosynthetic process | 1.43 × 10−11 | GO:BP | 143 |
| Organonitrogen compund metabolic process | 3.46 × 10−11 | GO:BP | 192 |
| Anatomical structural development | 4.95 × 10−11 | GO:BP | 186 |
| Developmental process | 6.58 × 10−11 | GO:BP | 199 |
| Metabolic process | 1.18 × 10−10 | GO:BP | 344 |
| Primary metabolic process | 1.37 × 10−10 | GO:BP | 318 |
| Negative regulation of cellular process | 1.92 × 10−10 | GO:BP | 157 |
| Regulation of macromolecule biosynthetic process | 3.05 × 10−10 | GO:BP | 133 |
| Regulation of cellular biosynthetic process | 3.16 × 10−10 | GO:BP | 136 |
| Multicellular organism development | 3.37 × 10−10 | GO:BP | 154 |
| Positive regulation of cellular metabolic process | 6.09 × 10−10 | GO:BP | 111 |
| System development | 6.42 × 10−10 | GO:BP | 136 |
| Localization | 1.11 × 10−9 | GO:BP | 166 |
| · | |||
| · | |||
| · |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luquero, A.; Pimentel, N.; Vilahur, G.; Badimon, L.; Borrell-Pages, M. Unique Splicing of Lrp5 in the Brain: A New Player in Neurodevelopment and Brain Maturation. Int. J. Mol. Sci. 2024, 25, 6763. https://doi.org/10.3390/ijms25126763
Luquero A, Pimentel N, Vilahur G, Badimon L, Borrell-Pages M. Unique Splicing of Lrp5 in the Brain: A New Player in Neurodevelopment and Brain Maturation. International Journal of Molecular Sciences. 2024; 25(12):6763. https://doi.org/10.3390/ijms25126763
Chicago/Turabian StyleLuquero, Aureli, Noelia Pimentel, Gemma Vilahur, Lina Badimon, and Maria Borrell-Pages. 2024. "Unique Splicing of Lrp5 in the Brain: A New Player in Neurodevelopment and Brain Maturation" International Journal of Molecular Sciences 25, no. 12: 6763. https://doi.org/10.3390/ijms25126763
APA StyleLuquero, A., Pimentel, N., Vilahur, G., Badimon, L., & Borrell-Pages, M. (2024). Unique Splicing of Lrp5 in the Brain: A New Player in Neurodevelopment and Brain Maturation. International Journal of Molecular Sciences, 25(12), 6763. https://doi.org/10.3390/ijms25126763







