Abstract
The incorporation of histone variants has structural ramifications on nucleosome dynamics and stability. Due to their unique sequences, histone variants can alter histone–histone or histone–DNA interactions, impacting the folding of DNA around the histone octamer and the overall higher-order structure of chromatin fibers. These structural modifications alter chromatin compaction and accessibility of DNA by transcription factors and other regulatory proteins to influence gene regulatory processes such as DNA damage and repair, as well as transcriptional activation or repression. Histone variants can also generate a unique interactome composed of histone chaperones and chromatin remodeling complexes. Any of these perturbations can contribute to cellular plasticity and the progression of human diseases. Here, we focus on a frequently overlooked group of histone variants lying within the four human histone gene clusters and their contribution to breast cancer.
1. Introduction
The regulated expression of histone genes and tissue-specific utilization of histone variants are intriguing aspects of chromatin structure that differ from the well-studied histone post-translational modifications (PTMs) [1,2,3]. Histone variants contribute to nucleosome structural diversity and chromatin architecture and regulate processes like DNA repair, centromere function, and gene activation or silencing [4,5,6,7,8,9]. However, our understanding of how histone variants are regulated, incorporated into chromatin, and interact with other regulatory proteins remains limited, especially in human diseases such as breast cancer.
Decades-long consensus has held that the development and progression of breast cancer is mediated by driver mutations in commonly known genes. These genetic alterations make individuals more prone to disease development and confer a competitive edge to the tumor in terms of growth. While genetic mutations have long been recognized as the key drivers of cancer development, emerging research has also highlighted the crucial role of epigenetic changes in this process. These modifications drive changes in gene expression that do not involve alterations in the underlying DNA sequence. The epigenetic aspect of the cell is defined by the status of DNA methylation, covalent modifications of histones, chromatin structure, and the network of chromatin modifiers. The integrity of this regulatory system is highly important to maintain normal gene expression regulation. In addition, like genetic mutations, aberrant changes to this epigenetic system can have profound effects on cellular behavior. Growing interest in this field has shown that dysregulation of these epigenetic events plays key roles in carcinogenesis and tumor progression. Several research studies have focused on the ‘histone code’ governed by the covalent modification of histones through methylation, acetylation, phosphorylation, ubiquitination, ADP-ribosylation, or SUMOylating and how this language translates into gene expression changes [10]. Beyond this, the incorporation of histone variants and onco-histones into the nucleosome is another mechanism used by the cell to regulate the structure of chromatin, histone modification, chromatin network, and ultimately the accessibility of regulatory elements to target gene promoters. In this paper, we review human histone isoforms and their epigenetic role(s) in breast cancer.
2. Canonical and Non-Canonical Histones
Eukaryotic cells wrap their nuclear DNA around histone proteins to form chromatin. The basic repeating unit of chromatin is the nucleosome, which consists of a histone octamer of four core histone proteins (two copies each of histone: H2A, H2B, H3, and H4). Nucleosome assembly involves the sequential deposition of (H3–H4)2 tetramer and H2A–H2B dimers onto DNA [11,12]. The nucleosome is stabilized via electrostatic interactions between the positively charged histones and the negatively charged DNA phosphate backbone [13]. Linker histone H1 then binds to the entry–exit nucleosomal DNA to form a chromatosome [14], to efficiently store and protect DNA from damage.
Histone deposition is tightly regulated by specific chaperones [15], especially during the S-phase of cell division, where timely and coordinated assembly of newly synthesized DNA into chromatin preserves genome integrity and regulates gene expression [16,17,18,19,20,21]. Cell division significantly increases the histone pool within a specialized subnuclear compartment called the histone locus body (HLB) [18,22], which facilitates spatial assembly of transcription factors and mRNA processing components around histone loci to promote rapid expression and processing of canonical histone mRNAs [23,24]. In eukaryotes, canonical histones lack introns and polyadenylated tails, and their mRNAs feature a unique 3′ end structure that is recognized by stem loop binding protein (SLBP) [20,25]. SLBP expression increases as cells enter the S-phase, where it helps stabilize interactions between U7 snRNP and histone downstream elements (HDEs), leading to endo-nucleolytic cleavage of canonical histone pre-mRNAs [26,27,28]. SLBP degradation at the end of the S-phase [21] results in decreased canonical histone mRNAs and proteins [21,29].
Although histones are among the longest-lived proteins, non-dividing cells outlive those histones that were initially deposited during cell division [9,30,31,32]. Thus, cells have evolved replication-independent mechanisms to incorporate non-canonical histones (i.e., histone variants) into chromatin outside of the S-phase. Numerous histone variants have been discovered in humans [33], differing in their primary sequence, introns, poly-A tails, and other mRNA characteristics [34,35,36]. These variants diverge from canonical histones by single amino acid changes or inclusion/deletion of entire protein domains, thus influencing chromatin architecture and gene regulation [2,37,38,39]. Histone variants and their specific chaperones recruit effector proteins and chromatin remodelers that also influence gene expression [15,40,41,42]. For example, H3.3 is deposited by HIRA and DAXX chaperones in actively transcribed regions during normal cell growth and development [43,44], changing nucleosome stability [45] and altering histone PTMs [46,47,48]. Nucleosomes containing H2A.Z enhance the accessibility of transcriptional machinery [49] to the promoters of estrogen receptor (ERα)-dependent genes in breast cancer [50]. Thus, histone variants shape chromatin structure and function in normal development [51,52] but are also involved in diseases like breast cancer [53,54].
3. Histone Gene Organization and Expression
Human histone proteins (H1, H2A, H2B, H3, and H4) are encoded by a large family of genes, which can be clustered or dispersed across the genome (Figure 1 and Table A1). The major clusters include HIST1 on chromosome 6, HIST2 and HIST3 on chromosome 1, and HIST4 on chromosome 12. These clusters comprise canonical histones, histone variants, and pseudogenes, where some pseudogenes can also produce functional proteins [55,56]. In evolutionarily distant species like Saccharomyces cerevisiae, genes of partner histones (i.e., H2A and H2B that form dimers, and H3 and H4 that form tetramers) are arranged pairwise and are coordinately expressed from the same promoter region [18,57]. Initially, it was thought that clustered histone genes in eukaryotes are functionally identical and expressed at similar levels [58]. However, unlike in yeast, human histone genes and their interacting partners are not arranged in the same tandem repeat organization [4,59,60,61,62] (Figure 1). In fact, human histone genes are regulated by their own promoters and can be differentially expressed to carry out distinct spatial–temporal functions [59].
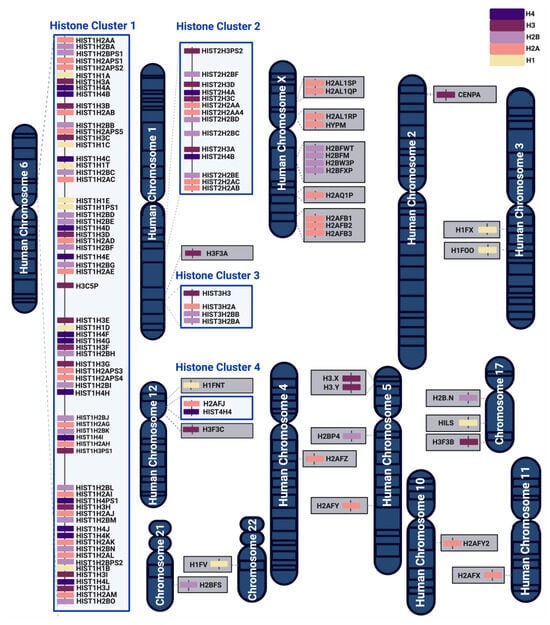
Figure 1.
Chromosomal organization of human histone genes belonging to histone H4 family (in indigo), H3 family (in purple), H2B family (in lilac), H2A family (in salmon), and H1 family (in cream yellow). Most of the histone isoforms are organized in 4 histone clusters, whereas other histone genes are dispersed over various chromosomal regions. The arrangement and orientation of histone genes within the genome allow for their controlled expression by distinct promoters.
Histones within the major histone gene clusters are commonly believed to be expressed solely during cell division. Several recent studies, however, highlight findings that many of these histones are expressed outside of the S-phase and are independent of replication, for instance: 1. Certain histones within these clusters are expressed in non-dividing cells and are independent of SLBP [33,56,63]. As an example, recent studies show that several H2B variants within the human histone clusters are produced in non-dividing cells. These variants differ from canonical histone transcripts in that they possess a poly-adenylated tail and lack the stem loop structures [32,64,65,66,67,68,69,70]. 2. Genes involved in the expression and mRNA processing of histones were found to be expressed in terminally differentiated cells [65]. 3. Depletion of SLBP during the S-phase only modestly reduced the levels of histone mRNAs [71]. These studies therefore show that certain histones are expressed outside of the replication phase, hence the term ‘replication-independent’ histones [70]. Indeed, studies now show that histone genes within histone gene clusters are differentially expressed and are not only ‘replication-independent’, but their expression is cell-type- and tissue-type-specific [7,8,59,72,73,74]. For example, HIST1H2AE shows high expression in breast tissue, indicating its significance in breast tissue specificity [75]. The differential expression of histone variants across cell types suggests their diverse physiological roles [8,59], some of which are outlined below.
4. Histone Isoforms and Nucleosome Structure
4.1. Histone H2A Isoforms
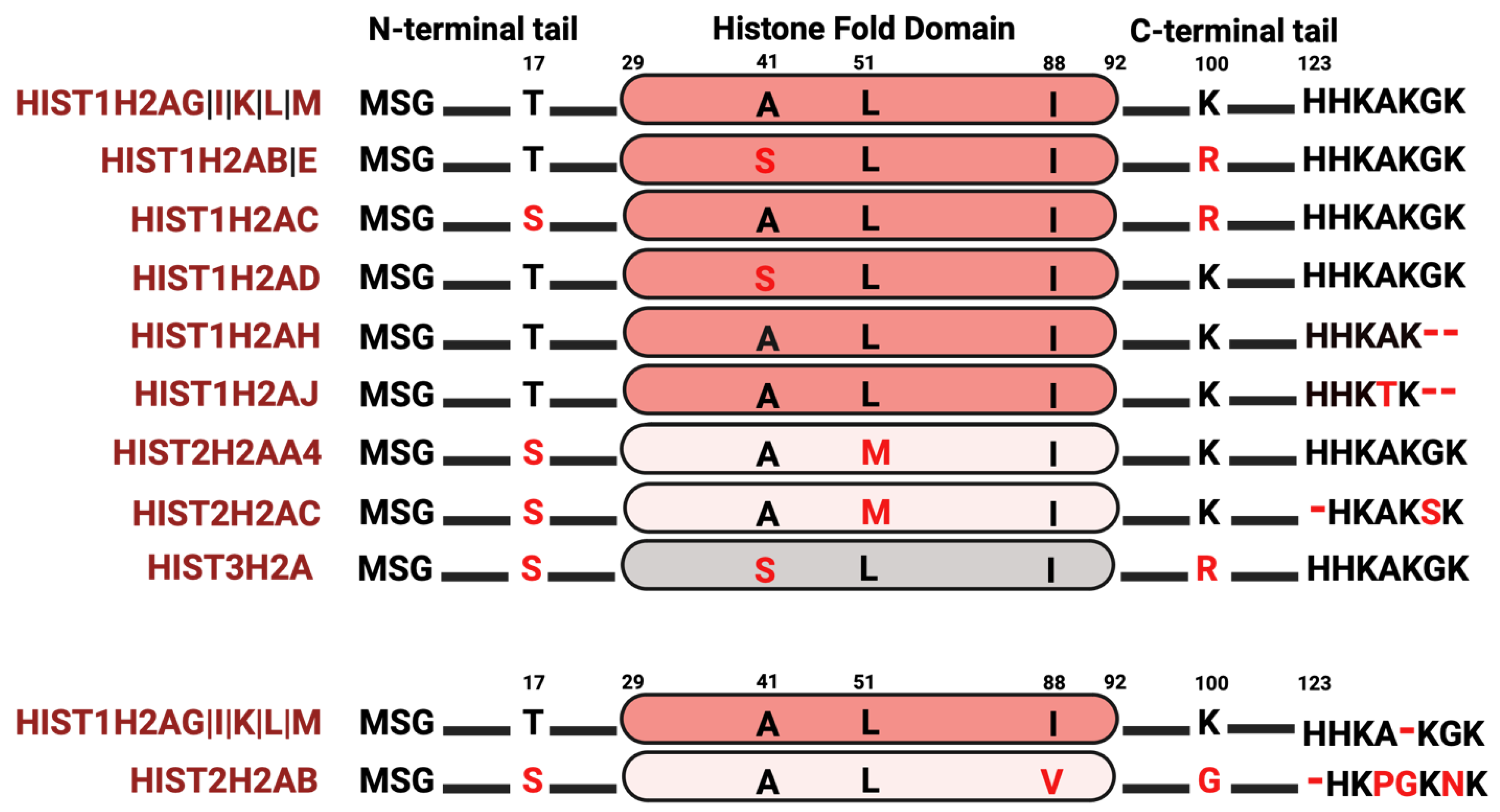
Among the core histone proteins, histone H2A exhibits the highest number of variations. Histone H2A contains a C-terminal docking domain critical in linking the H3–H4 tetramer with the H2A–H2B dimer, with consequences in octamer stability. Additionally, the interaction between two H2A molecules in a nucleosome stabilizes the DNA helix at the back face of the nucleosome. H2A also makes a substantial contribution to the nucleosome surface, facilitating intra-nucleosomal interactions necessary for chromatin compaction; thus, changes in amino acid composition may have a profound effect on nucleosome stability, chromatin structure, and regulation of gene expression. Although several H2A variants, such as macroH2A, H2A.X, and H2A.Z, have been extensively studied [49,76,77,78], the extent of research into the histone H2A variants that are found within the clusters is still limited. Within the histone clusters, the most prevalent H2A protein, known as the “canonical” H2A protein, is encoded by five distinct genes (Figure 2). In analyzing the histone variants, a few of the H2A variants have a serine at position 17 instead of a threonine, compared to the “canonical” histone H2A protein. Phosphorylation at S17 is crucial for responding to DNA damage and hydrogen-peroxide-induced stress [79,80,81], hindering p53 binding protein 1 (53BP1) activity at damaged DNA sites and reducing neuronal apoptosis [82]. Given that several serine–threonine kinases have preferences either for serine or threonine as a phosphate acceptor, the S17T substitution can either add or delete a potential phosphorylation site [83,84], consequently impacting the nucleosome interactome. In addition, some of these H2A variants have amino acid changes in the histone fold domain, including A41S, L51M, and I88V. While some of these are typically considered as “conservative” substitutions in proteins, they can alter histone–histone interactions, PTMs, and ultimately gene expression patterns [85]. The H2A C-terminus exhibits the most sequence diversity, not only in amino acid substitutions but also in sequence length. Since the H2A C-terminus is positioned near the DNA entry and exit site, amino acid changes in this region could influence nucleosome breathing (the transient opening of ~10 bp of DNA from the nucleosome) and DNA accessibility by regulatory factors. Indeed, several studies have shown that the C-terminal tail of H2A is critical in the wrapping and unwrapping of nucleosomal DNA [49,86,87,88,89]. Apart from direct nucleosomal effects, some of these changes could impact PTMs on histones. Arginine residues can be methylated; therefore, the L100R substitution seen in several H2A variants may also be subjected to methylation with consequences in gene expression [8,90,91]. Thus, even subtle amino acid substitutions can alter the structure of a single nucleosome or introduce modifications (such as histone methylation) that influence gene expression.
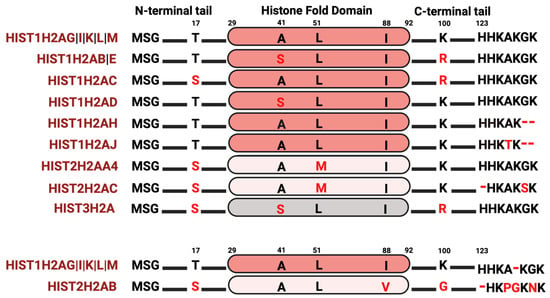
Figure 2.
H2A isoform sequence alignments. The most abundant histone variant is indicated at the top. Red letters indicate divergent amino acids relative to the most abundant isoform.
4.2. Histone H2B Isoforms
Most of the sequence diversity in H2B variants is concentrated in the lysine-enriched N-terminal tails (Figure 3), potentially impacting nucleosome structure. For instance, the P4L substitution in HIST1H2BL changes an amino acid with a cyclic side chain to a residue with a free α-amino group, which should destabilize the structure. The A5S/T substitution in several H2B isoforms introduces a polar (-OH) side chain that could also impact nucleosome structure. Because S and T can be phosphorylated, a change from A to S/T at position 5 might also change the phosphorylation status at this site. Additionally, the A5S/T substitution neighbors a K6 that is frequently methylated and acetylated during the epithelial-to-mesenchymal transition in breast cancer, so changes to S/T might impact potential PTMs of this neighboring site [92]. For example, the residue H2B E35 is one instance of a neighboring effect, where H2B S36 phosphorylation is impaired by E35 ADP-ribosylation [93]. S33 is normally phosphorylated by the signaling kinases adenosine monophosphate (AMP)-activated kinase (AMPK) and ribosomal protein S6 kinase 1 (S6K1) to activate stress response genes and regulate early adipogenesis pathways [94,95], but S33 is replaced by glycine in the HIST3H2BB isoform. Finally, histone HIST1H2BJ and HIST1H2BK variants harbor an alanine instead of serine at position 120, which could alter hydrogen bonds and nucleosome integrity or delete a phosphorylation site.
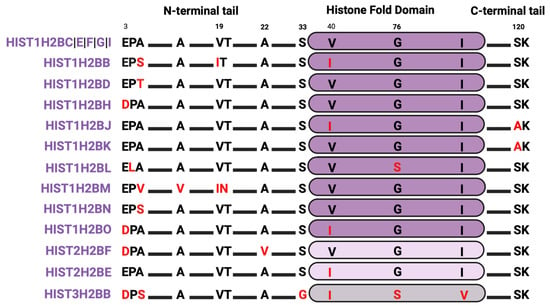
Figure 3.
H2B isoform sequence alignments. Alignments are relative to the most abundant isoform. Red letters indicate divergent amino acids relative to the most abundant isoform.
4.3. Histone H3 Isoforms
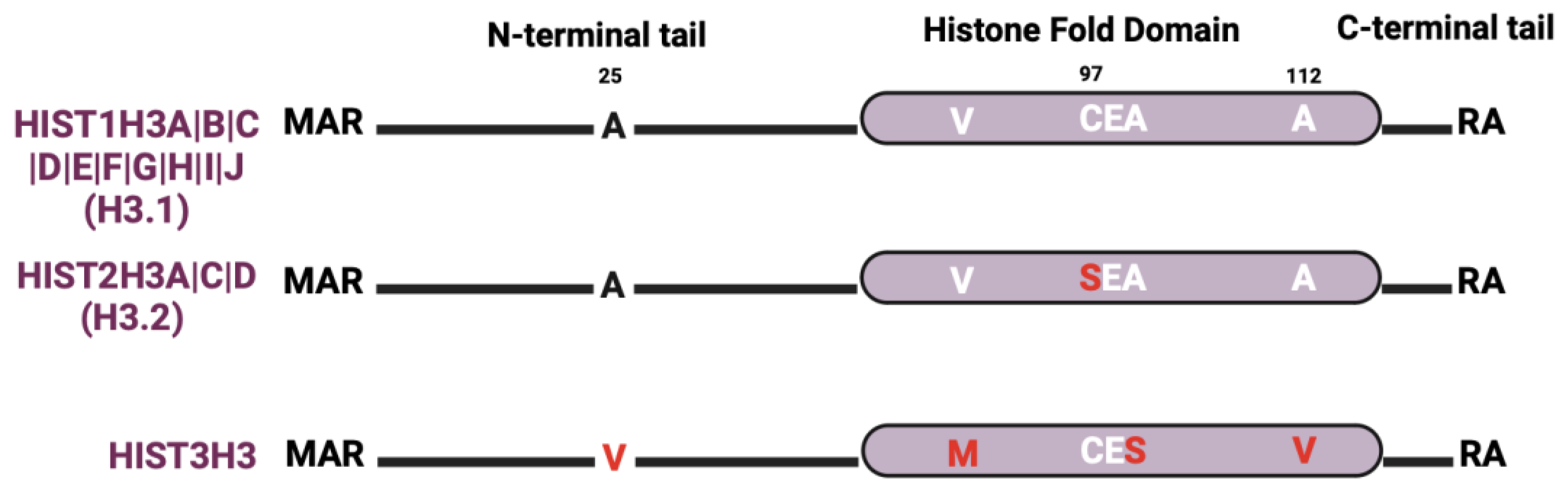
The most studied histone H3 variant is H3.3, which is encoded by three genes (H3F3A, H3F3B, and H3F3C) that are distributed outside of the histone gene clusters (Figure 1). There are relatively few H3 protein isoforms within the histone gene clusters even though there are many H3 genes (Figure 4). The most abundant clustered histone H3 protein is HIST1H3A|B|C|D|E|F|G|H|I|J (also known as histone H3.1). HIST2H3A|C|D (also known as histone H3.2) differs from histone H3.1 by a single amino acid at position 97 (serine-to-cysteine substitution). While crystallography studies suggest that histone H3.1 and H3.2 have no discernable effect on nucleosome structure, S97 in the H3.2 variant is on the H3–H4 tetramer accessible surface, which makes it a possible interaction site for H3.2-specific chaperones [96]. Recent studies also show that H3.1 and H3.2 differ in their PTMs. For instance, H3.1 is enriched in K14 acetylation (a mark associated with gene activation), while H3.2 is enriched in K27 di- and tri-methylation (marks linked to gene silencing) [97,98]. Lastly, HIST3H3 contains four amino acid substitutions (A25V, V72M, V99A, and A112V) that make nucleosomes much more unstable than nucleosomes containing H3.1 or H3.2 by weakening the association of the HIST3H3–H4 tetramer with H2A–H2B dimers [34,99].
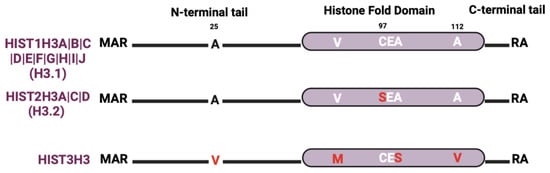
Figure 4.
H3 isoform sequence alignments. Alignments are relative to the most abundant isoform. Red letters indicate divergent amino acids relative to the most abundant isoform.
4.4. Histone H4 Isoforms
As of now, all identified histone H4 genes are distributed within the histone gene clusters (Figure 1), and all encode for the same amino acid sequence except for one isoform, HIST1H4G. The protein sequence of the HIST1H4G variant has substantial differences from the most abundant H4 protein throughout all domains (Figure 5). HIST1H4G differs from canonical H4 by 20 amino acid residues. These substitutions have significant implications for nucleosome stability and the regulation of histone PTMs [100]. In the N-terminal domain, the conformationally flexible glycine is replaced by the hydrophobic valine and alanine at positions 3 and 7, respectively. These substitutions in HIST1H4G increase its N-terminal tail hydrophobicity, which could impact histone tail bridging and the positioning of nucleosomes [101,102]. This is because H4 histone tails play a significant role in the attractive interaction between nucleosomes [103,104]. R18 (with its positively charged side chain) is replaced with cysteine, an alteration that modifies protein thermal stability [105]. In addition, several substitutions in the HIST1H4G histone fold domain enhance the hydrophobic nature of this variant and disrupt several interactions that are essential for nucleosome assembly. For instance, R46H substitution in the histone fold domain was shown to create a less compact nucleosome structure [106]. The C-terminus of HIST1H4G is truncated by five residues that would otherwise stabilize H4 and H2A interactions [107]. In summary, the substitutions in HIST1H4G have been shown to form nucleosomes with an open structure, resulting in less compact chromatin fibers [108,109].
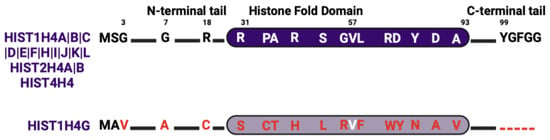
Figure 5.
H4 isoform sequence alignment. Alignments are relative to the most abundant isoform. Red letters indicate divergent amino acids.
5. Dysregulated Histone Genes in Breast Cancer
5.1. Histone H2A Variants
Differential gene expression studies reveal that HIST1H2AC is upregulated in ER-positive clinical breast cancer tissues and ER-positive cell lines and is associated with poor patient prognosis [110]. This same study showed that HIST1H2AC upregulates estrogen receptor target genes BCL2 and c-MYC by recruiting the ERα transcription factor to their respective promoter regions. Depleting HIST1H2AC impaired the estrogen signaling pathway and reduced cell proliferation [110]. Additionally, breast cancer patients with low HIST1H2AC expression benefitted from anthracycline adjuvant chemotherapy, whereas patients with high HIST1H2AC expression were resistant to treatment, suggesting that HIST1H2AC is linked to anthracycline sensitivity [110].
Other studies have shown that HIST1H2AH, HIST1H2AK, HIST1H2AG, and HIST1H2AM are upregulated in breast cancer specimens [111,112,113,114]. Furthermore, in women of Indian descent, the expression levels of HIST3H2A relative to normal tissue were higher compared to those of Western patients [115], indicating ethnic diversity in H2A gene expression in breast cancer. Interestingly, HIST3H2A expression is considerably higher in the most aggressive breast cancer subtypes, particularly in triple-negative breast cancer and brain metastases of the primary breast cancer [116,117]. In investigating the mechanistic role of elevated histone H2A levels, another study discovered that HIST2H2AC was upregulated downstream of the EGFR signaling pathway. This upregulation stimulated oncogenic gene expression in proliferating mammary epithelial (EpH4 and HC11) and breast cancer cells (MC4-L2 and T47-D) [72]. In the same study, silencing of HIST2H2AC expression suppressed EGF-induced Zeb-1 expression and downregulated E-cadherin. Thus, aberrant expression of H2A variants can lead to dysregulated expression patterns that promote human diseases such as breast cancer.
5.2. Histone H2B Variants
Histone H2B variants are also dysregulated in breast cancer. For instance, HIST1H2BE was shown to be upregulated in estrogen-positive breast cancer tumors that exhibited resistance to aromatase inhibitors, indicating a potential association between HIST1H2BE and resistance to aromatase inhibitors [118,119]. In separate studies, upregulation of HIST2H2BC correlated with paclitaxel resistance in triple-negative breast cancer cells [120], while overexpression of HIST1H2BK correlated with increased expression of VEGF165, a vascular endothelial growth factor isoform associated with breast tumor cell invasion of the lungs and bones [121]. Moreover, genome-wide profiling in triple-negative breast cancer patient samples and cell lines revealed overexpression of HIST1H2BO in this highly aggressive breast cancer subtype, suggesting its potential role in tumor initiation, maintenance, or progression [113,122]. Bioinformatic studies also associate HIST1H2BO overexpression with breast cancer brain and spine metastases, as well as poor overall survival [123,124,125]. HIST1H2BO is upregulated in other hormone-dependent cancers and primary brain tumors [126,127,128,129,130,131,132]. In BT-474, MCF7, and ZR-75-1 breast cancer cell lines, histone variants HIST1H2BF and HIST1H2BO were co-expressed with the phosphatase magnesium-dependent 1 delta protein (PPM1D), a breast cancer protein that correlates with poor prognosis [133]. The HIST1H2BJ promoter is hypomethylated in brain metastases of breast cancer, leading to HIST1H2BJ over-expression [134]. Differential transcriptome analysis of primary and metastatic tumors revealed HIST1H2BB, HIST1H2BF, and HIST1H2BC as markers associated with breast cancer metastases to the brain and lymph nodes [135,136]. Furthermore, HIST1H2BB expression in primary breast cancer tumors was linked with recurrence-free survival [118]. Finally, HIST1H2BL was shown to drive histone modification crosstalk to upregulate c-MYC, thus promoting tumor cell proliferation [137]. These findings suggest that the expression of several H2B histone variants influences histone PTMs and activates genes and pathways associated with breast cancer.
5.3. Histone H3 Variants
In recent years, there has been notable interest surrounding histone H3 variants. Specifically, several studies have shown that H3 variants provide an extra level of control in gene expression by replacing their canonical counterpart, and their dysregulation is highly associated with the acquisition of malignant traits [46,54,138].
Although 10 different H3 genes encode the same H3.1 amino acid sequence, a recent study reported that only the HIST1H3H and HIST1H3D genes were differentially regulated in metastatic breast cancer [139]. This suggests that at the transcript level, these histone genes may not be functionally redundant [140,141]. HIST3H3 is also upregulated in breast cancer and is positively associated with activated polymorphonuclear neutrophil (PMN)-induced breast cancer metastasis [142]. Other studies also suggest that several histone variants regulate inflammatory response genes, thus affecting the innate immune response to cancer [143,144]. Indeed, the chronic expression of inflammatory genes can create an environment that is conducive to breast cancer initiation, progression, and invasion [145].
5.4. Histone H4 Variants
According to a study of patient samples from The Human Protein Atlas database, breast cancers exhibit considerably higher HIST1H4G expression than non-cancerous breast tissue [109]. HIST1H4G upregulation was also observed in breast cancer cell lines (MCF7, LCC1, and LCC2) relative to non-cancerous epithelial cells (MCF10A). Relative to other cancers, HIST1H4G exhibited the highest expression in breast cancer, suggesting that it is cancer-type-specific. Notably, HIST1H4G expression progressively increased as tumors progressed to more advanced stages, even though the expression of canonical H4 did not show significant variation. Furthermore, knocking out HIST1H4G reduced tumor formation in a tumor xenograft model [109]. This same study also demonstrated that the nucleolar histone chaperone NPM1 (nucleophosmin/B23) interacts uniquely with HIST1H4G but not with other histone H4 proteins. This suggests that NPM1 is a distinct chaperone for HIST1H4G [109]. The incorporation of HIST1H4G into nucleosome also renders chromatin more accessible and activates global rRNA synthesis [146,147], which is essential for breast cancer cell proliferation.
6. Onco-Histones and Breast Cancer
As described above, dysregulated histone variant gene expression is strongly associated with cancers such as breast cancer. Histone genes can also be mutated, with the mutated histones themselves driving oncogenesis, hence the term “onco-histones” [148]. These mutations on histones mechanistically can act by modifying the nucleosome structure, stability, DNA accessibility, and/or histone–protein interactions to cause changes in gene expression and cancer [148]. One of the most common H2A mutations is R29Q [149]. This residue can be di-methylated by the enzyme protein arginine methyltransferase 6 (PRMT6), and di-methylation on the arginine at position 29 (H2A R29me2) has been linked to transcriptional repression as it localizes to transcriptionally inactive chromatin fibers [150]. The adjacent residues K74 and K75 are known to participate in H2A–H3 and H2A–DNA interactions; however, these residues are commonly mutated to K74N and K75N [151,152]. These cancer-associated histone mutations on H2A have been shown to affect nucleosome sliding and enhance remodeling rates [149,153]. Other prevalent onco-histones implicated in breast cancer are H2A E121Q and H2A E121K [154]. E121 is a conserved residue in the H2A C-terminal tail and is involved in the interactions of the H2A–H2B dimer with the H3–H4 tetramer, and with linker DNA [150]. Recently, it was shown that E121 residue forms a salt bridge with H3K14 [155]. Thus, E121 may play a crucial role in maintaining nucleosome stability or mediating histone–protein interactions that regulate chromatin structure and dynamics [156,157]. Mutation of E121 might disrupt key interactions necessary for nucleosome integrity.
H2B isoforms also have relatively high mutation frequencies. One such mutation is E71K, which was shown to inhibit the differentiation of mesenchymal progenitor cells [149] and is most often associated with breast cancer [154,158], where it activates ADAM19 and genes critical for cancer invasion [159]. In vitro, the H2BE71K onco-histone can combine with H2A to form a dimer. However, the process of nucleosome assembly was hindered as the H2A–H2B E71K dimer was unable to create stable histone octamers with H3 and H4 [149,154]. Other hotspot mutations on H2B include E76K and E76Q, which mechanistically weaken H2B–H4 interactions and are also observed in breast cancer [154]. These E76K/Q mutations occur most frequently in HIST1H2BC, HIST1H2BD, and HIST1H2BH variants and not so frequent in HIST1H2BB, HIST1H2BJ, HIST1H2BK, and HIST1H2BO [158]. The expression of the E76K onco-histone in a non-cancerous breast epithelial cell line (MCF10A) altered chromatin accessibility at gene regulatory elements, leading to enhanced colony formation (an oncogenic phenotype) [154]. Other studies show that H2B E2 is a site of ADP-ribosylation, and that this post-translational modification is associated with increased chromatin relaxation with potential implication in DNA repair and gene regulation [160,161]. Interestingly, E2 is frequently mutated in cancers [149,154]; nevertheless, the malignant potential of this oncogenic mutation is yet to be investigated [154,162,163]. Additionally, the E113K/Q mutation occurs frequently in breast cancer [154,162,163], though its mechanistic role is still unclear. One might hypothesize that because this mutation occurs in the acidic patch of H2B, a region in histones known as the ‘landing dock’ for chromatin remodelers [164], it might affect their binding and thereby affect the balance between active and repressive chromatin states. Another recent study shows that the expression of the onco-histone H2B D51A/N significantly enhanced cell proliferation in breast cancer [165]. H2B D51 is an ADP-ribosylation site that is essential for p300-mediated acetylation inhibition of several lysine residues on H2B. Changes to acetylation patterns of histone H2B and histone acetyltransferases activity is common in breast cancer [166,167]. Therefore, the loss of ADP-ribosylation on H2B D51A/N mutations might disrupt the acetylation pattern on H2B, leading to significant changes in chromatin accessibility at enhancers and promoters, along with alterations in gene expression patterns. Notably, mutation of D51 to A was associated with accelerated breast tumor formation in mouse xenografts [165]. These discoveries imply that both the presence and the specific mutations of these variants play crucial roles in organizing chromatin structure, which in turn influences patterns of gene expression.
Mutations on H3 were among the first mutations discovered in histones and are associated with cancers. H3 mutations such as K27M, K36M, and G34V/R have been discovered by many groups [33,168,169,170,171]. H3 K27M is linked to many cancers including pediatric glioblastoma (pGBM) [172], adult diffuse midline glioma [170], head and neck squamous cell carcinoma [173], melanoma [174], and acute myeloid leukemia [175]. H3 K27M can directly modulate nucleosome assembly but also indirectly modulate the methylation status of histone lysine residues [169,176,177]. In detail, the K27M mutants alter normal H3 methylation patterns, disrupting PRC2-mediated repressive function and the enhancer landscape, leading to widespread epigenetic dysregulation and tumorigenic gene expression profiles [168,178,179,180]. In general, while the N-terminal tail mutations such as K27M, K36M, and G34 have been extensively examined across numerous malignancies, their frequencies and implications, if any, in breast cancer are yet to be investigated. Although the functional implications of mutations linked with the replication-independent histone variant H3.3 have been widely researched in several tumor types and have attracted significant interest, research on mutations in other H3 variants such as H3.2 and HIST3H3 is still in its early stages [171].
Histone H4 genes on the other hand are rarely mutated in cancers. The most common H4 onco-histone carries an R4C mutation, with equal prevalence across all H4 genes [33,149]. R4C is also a site of symmetric demethylation and citrullination, which serve as marks for transcriptional repression and DNA damage. Disrupting these PTMs is associated with oncogenesis [181,182,183]. H4 D67H/N and R93T mutations are also found in cancer patients [148]. These residues form a hydrogen bond with H2B to stabilize the nucleosome structure [184], so altering these residues may result in nucleosome instability.
Overall, onco-histone mutations not only alter chromatin structure but also influence potential post-translational modifications (PTMs) and interactomes, leading to changes in gene expression. Recent studies have begun to elucidate how these onco-histones impact chromatin structure and remodeling. For instance, a dimer exchange assay facilitated by the nucleosome assembly protein (Nap1) demonstrated that histone mutations at the dimer–tetramer interface between histones H2B and H4 were destabilizing. These destabilizing mutations include E56 and R29 on histone H2A; D68, E71, and E76 on histone H2B; and E50 and E9 on histone H3. Additionally, some onco-histones inhibit the formation of stable octamers, such as H2BD68A/N, H2BE76K, H3E50K, and H3E97A/K [148,149,157,185]. These effects in vivo ultimately impact chromatin structure, resulting in significant consequences for gene expression.
7. Epigenetic Inhibitors and Clinical Trials
The incorporation of histone variants into chromatin can induce local and global changes in PTMs, ultimately affecting the histone interaction network as discussed in the previous sections. Therapeutic avenues that target several histone modifiers to alter acetylation and methylation pattern at anti-tumor gene promoters are being actively explored across different tumors. Several of these compounds have shown remarkable efficacy in treating breast cancer, both alone and in combination with other chemotherapeutic drugs and immunotherapy. We summarize some of these therapies below.
(a) DNA methyltransferase inhibitors (DNMTis): DNA methyltransferases (DNMTs) are enzymes that add a methyl group to the fifth carbon of cytosine residues in DNA for gene regulation. Aberrant DNA methylation patterns are linked to various diseases, including cancer. Targeting these patterns could help re-establish normal methylation and correct dysregulation. Currently, two types of DNMTis are used: 1. DNMTis that inhibit the enzymatic function of DNMTs and 2. cytosine analogs that incorporate into DNA and replace the carbon at position 5 of cytosine (C-5) with N-5, disabling DNMTs. These compounds reverse the DNA hypermethylation status of tumor suppressor gene promoters and reactivate their expression in cancerous cells [186,187]. These epi-drugs have been the focus of extensive research, including 5-azacytidine, SGI-110, hydralazine, the antisense oligonucleotide MG98, decitabine, and zebularine [188,189,190,191,192,193,194].
(b) Histone deacetylase inhibitors (HDACis) are a class of epigenetic anticancer therapeutics that target histone deacetylase enzymes. Deacetylation of histones at tumor suppressor genes can render the chromatin more compact and transcriptionally inactive. HDAC inhibitors can rectify the aberrant acetylation status of histones in cancers leading to reactivation of anti-oncogenes. Various cancer types, including breast cancers, have shown a positive response to HDACis because of their effectiveness in inhibiting tumor growth by increasing cell apoptosis. Several of these epigenetic compounds have shown promising outcomes for advanced breast cancer treatment during clinical trials, including entinostat, abexinostat, givinostat, and vorinostat [195,196,197,198,199,200].
(c) Histone methyltransferases inhibitors (HMTis) target histone lysine methyltransferases (KMTs) and arginine methyltransferases (PRMTs), which are enzymes that add methyl groups to lysine and arginine residues on histones. Various HMT inhibitors have shown significant anti-tumor effects in clinical studies. A significant number of these inhibitors target the function of EZH2, the catalytic subunit of polycomb repressive complex 2 (PRC2), which silences target gene expression by methylating histone 3 at lysine 27 (H3K27me3) in multiple cancers [201,202]. Some of these epi-enzyme inhibitors, such as pinometostat and tazemetostat, have been evaluated in clinical trials and showed promising results in breast tumors [203,204,205].
(d) Bromodomain and extra-terminal inhibitors (BETis) target a family of epigenetic readers that consist of two N-terminal tandem bromodomains and a C-terminal extra-terminal motif [206]. This family consists of bromodomain-containing proteins (BRD) such as BRD2, BRD3, BRD4, and the bromodomain testis associated protein (BRDT), which can form a complex with HDACs to control transcription through processes such as histone acetylation, chromatin remodeling, and recruitment of other transcriptional machinery [207,208,209]. One of the BETs that is garnering the most attention is BRD4, which is known to promote the transcriptional initiation and elongation by binding to hyper-acetylated promoters and super-enhancers to activate several oncogenes [210,211]. Several effective inhibitors that interfere with the binding of BETs to acetylated histones have shown promising outcomes, including but not limited to JQ1, I-BET762, TEN-010, and OTX-015 [212,213,214,215].
8. Conclusions and Future Perspective
This review focuses on histone variants implicated in breast cancer. Histone variants exhibit tissue- and time-dependent expression patterns and are incorporated into chromatin with the assistance of chaperones, often replacing canonical histones during or after cell division. Recent studies highlight significant dysregulation of histone variants in breast cancer and other diseases, underscoring their diverse cellular functions. Moreover, increasing evidence suggests varying mutation frequencies among histone variants within the same gene family. Surprisingly, even a single amino acid substitution in histone variants (including onco-histones) can markedly disrupt nucleosome stability, chromatin structure, DNA–nucleosome interactions, and DNA accessibility. Additionally, histone variants may carry distinct post-translational modifications that contribute to dysregulated chromatin remodeling networks and gene expression, thereby promoting tumorigenesis.
Fortunately, unlike genetic mutations, epigenetic aberrations have the potential to be reversible, providing new therapeutic avenues for cancer cell management. Indeed, the use of epigenetic targets in combination with conventional chemotherapeutic drugs is emerging as an effective technique for increasing anticancer activity, reducing drug resistance, and bolstering the host immune response [216,217,218,219]. Thus, we propose a deeper exploration of histone variants, their influence on nucleosome structure, and the downstream pathways involved in dysregulating cancer-related genes, especially in cases lacking evident DNA mutations in well-established breast cancer oncogenes.
Author Contributions
Conceptualization, H.D., W.N.S., D.C. and Y.N.F.-M.; methodology, H.D.; software, H.D.; validation, H.D., W.N.S., D.C. and Y.N.F.-M.; investigation, H.D. and Y.N.F.-M.; resources, H.D., W.N.S. and Y.N.F.-M.; writing—original draft preparation, H.D. and W.N.S.; writing—review and editing, H.D., W.N.S., D.C. and Y.N.F.-M.; visualization, H.D.; supervision, Y.N.F.-M.; project administration, Y.N.F.-M.; funding acquisition, Y.N.F.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Science Foundation grant MCB 2016515 (Y.N.F.-M.), by National Institute of Environmental Health Sciences (NIEHS) grants R01 ES024478 (Y.N.F.-M.), R01 ES034253 (Y.N.F.-M.), and 1R01 ES036051-01 (Y.N.F.-M.), and by the Van Andel Institution (VAI) support. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NSF, the NIEHS, or the VAI.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Acknowledgments
All graphics were created using Biorender.com, accessed on 12 June 2024.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Histone variant gene and protein names and their chromosomal locations.
Table A1.
Histone variant gene and protein names and their chromosomal locations.
| Chromosome | Name | Gene Name | Protein Name |
|---|---|---|---|
| 1q21.2 | H2A clustered histone 18 | H2AC18 | HIST2H2AA |
| 1q21.2 | H2A clustered histone 19 | H2AC19 | HIST2H2AA4 |
| 1q21.2 | H2A clustered histone 20 | H2AC20 | HIST2H2AC |
| 1q21.2 | H2A clustered histone 21 | H2AC21 | HIST2H2AB |
| 1q42.13 | H2A clustered histone 25 | H2AC25 | HIST3H2A |
| 1q21.2 | H2B clustered histone 18 | H2BC18 | HIST2H2BF |
| 1q21.2 | H2B clustered histone 19, pseudogene | H2BC19P | HIST2H2BD |
| 1q21.2 | H2B clustered histone 20, pseudogene | H2BC20P | HIST2H2BC |
| 1q21.2 | H2B clustered histone 21 | H2BC21 | HIST2H2BE |
| 1q42.13 | H2B clustered histone 26 | H2BC26 | HIST3H2BB |
| 1q42.13 | H2B clustered histone 27, pseudogene | H2BC27P | HIST3H2BA |
| 1q21.1 | H3.7 histone | H3-7 | HIST2H3PS2 |
| 1q21.2 | H3 clustered histone 13 | H3C13 | HIST2H3D |
| 1q21.2 | H3 clustered histone 14 | H3C14 | HIST2H3C |
| 1q21.2 | H3 histone clustered 15 | H3C15 | HIST2H3A |
| 1q42.12 | H3.3 histone A | H3-3A | H3F3A |
| 1q42.13 | H3.4 histone, cluster member | H3-4 | HIST3H3 |
| 1q21.2 | H4 clustered histone 14 | H4C14 | HIST2H4A |
| 1q21.2 | H4 clustered histone 15 | H4C15 | HIST2H4B |
| 2p23.3 | Centromere protein A | CENPA | CENP-A |
| 3q21.3 | H1.10 linker histone | H1-10 | H1FX |
| 3q22.1 | H1.8 linker histone | H1-8 | H1FOO |
| 4q23 | H2A.Z variant histone 1 | H2AZ1 | H2AFZ |
| 5q31.1 | macroH2A.1 histone | MACROH2A1 | H2AFY |
| 5q13.2 | H2B.L histone variant 1, pseudogene | H2BL1P | H2BP4 |
| 5p15.1 | H3.Y histone 1 | H3Y1 | H3.Y |
| 5p15.1 | H3.Y histone 2 | H3Y2 | H3.X |
| 6p22.2 | H1.1 linker histone, cluster member | H1-1 | HIST1H1A |
| 6p22.2 | H1.2 linker histone, cluster member | H1-2 | HIST1H1C |
| 6p22.2 | H1.3 linker histone, cluster member | H1-3 | HIST1H1D |
| 6p22.2 | H1.4 linker histone, cluster member | H1-4 | HIST1H1E |
| 6p22.1 | H1.5 linker histone, cluster member | H1-5 | HIST1H1B |
| 6p22.2 | H1.6 linker histone, cluster member | H1-6 | HIST1H1T |
| 6p22.2 | H1.12 linker histone, cluster member, pseudogene | H1-12P | HIST1H1PS1 |
| 6p22.2 | H2A clustered histone 1 | H2AC1 | HISTH2AA |
| 6p22.2 | H2A clustered histone 2, pseudogene | H2AC2P | HIST1H2APS1 |
| 6p22.2 | H2A clustered histone 3, pseudogene | H2AC3P | HIST1H2APS2 |
| 6p22.2 | H2A clustered histone 4 | H2AC4 | HIST1H2AB |
| 6p22.2 | H2A clustered histone 5, pseudogene | H2AC5P | HIST1H2APS5 |
| 6p22.2 | H2A clustered histone 6 | H2AC6 | HIST1H2AC |
| 6p22.2 | H2A clustered histone 7 | H2AC7 | HIST1H2AD |
| 6p22.2 | H2A clustered histone 8 | H2AC8 | HIST1H2AE |
| 6p22.2 | H2A clustered histone 9, pseudogene | H2AC9P | HIST1H2APS3 |
| 6p22.2 | H2A clustered histone 10, pseudogene | H2AC10P | HIST1H2APS4 |
| 6p22.1 | H2A clustered histone 11 | H2AC11 | HIST1H2AG |
| 6p22.1 | H2A clustered histone 12 | H2AC12 | HIST1H2AH |
| 6p22.1 | H2A clustered histone 13 | H2AC13 | HIST1H2AI |
| 6p22.1 | H2A clustered histone 14 | H2AC14 | HIST1H2AJ |
| 6p22.1 | H2A clustered histone 15 | H2AC15 | HIST1H2AK |
| 6p22.1 | H2A clustered histone 16 | H2AC16 | HIST1H2AL |
| 6p22.1 | H2A clustered histone 17 | H2AC17 | HIST1H2AM |
| 6p22.2 | H2B clustered histone 1 | H2BC1 | HIST1H2BA |
| 6p22.2 | H2B clustered histone 2, pseudogene | H2BC2P | HIST1H2BPS1 |
| 6p22.2 | H2B clustered histone 3 | H2BC3 | HIST1H2BB |
| 6p22.2 | H2B clustered histone 4 | H2BC4 | HIST1H2BC |
| 6p22.2 | H2B clustered histone 5 | H2BC5 | HIST1H2BD |
| 6p22.2 | H2B clustered histone 6 | H2BC6 | HIST1H2BE |
| 6p22.2 | H2B clustered histone 7 | H2BC7 | HIST1H2BF |
| 6p22.2 | H2B clustered histone 8 | H2BC8 | HIST1H2BG |
| 6p22.2 | H2B clustered histone 9 | H2BC9 | HIST1H2BH |
| 6p22.2 | H2B clustered histone 10 | H2BC10 | HIST1H2BI |
| 6p22.1 | H2B clustered histone 11 | H2BC11 | HIST1H2BJ |
| 6p22.1 | H2B clustered histone 12 | H2BC12 | HIST1H2BK |
| 6p22.1 | H2B clustered histone 13 | H2BC13 | HIST1H2BL |
| 6p22.1 | H2B clustered histone 14 | H2BC14 | HIST1H2BM |
| 6p22.1 | H2B clustered histone 15 | H2BC15 | HIST1H2BN |
| 6p22.1 | H2B clustered histone 16, pseudogene | H2BC16P | HIST1H2BPS2 |
| 6p22.1 | H2B clustered histone 17 | H2BC17 | HIST1H2BO |
| 6p22.2 | H3 clustered histone 1 | H3C1 | HIST1H3A |
| 6p22.2 | H3 clustered histone 2 | H3C2 | HIST1H3B |
| 6p22.2 | H3 clustered histone 3 | H3C3 | HIST1H3C |
| 6p22.2 | H3 clustered histone 4 | H3C4 | HIST1H3D |
| 6p22.2 | H3 clustered histone 5, pseudogene | H3C5P | |
| 6p22.2 | H3 clustered histone 6 | H3C6 | HIST1H3E |
| 6p22.2 | H3 clustered histone 7 | H3C7 | HIST1H3F |
| 6p22.2 | H3 clustered histone 8 | H3C8 | HIST1H3G |
| 6p22.2 | H3 clustered histone 9, pseudogene | H3C9P | HIST1H3PS1 |
| 6p22.1 | H3 clustered histone 10 | H3C10 | HIST1H3H |
| 6p22.1 | H3 clustered histone 11 | H3C11 | HIST1H3I |
| 6p22.1 | H3 clustered histone 12 | H3C12 | HIST1H3J |
| 6p22.2 | H4 clustered histone 1 | H4C1 | HIST1H4A |
| 6p22.2 | H3 clustered histone 2 | H4C2 | HIST1H4B |
| 6p22.2 | H3 clustered histone 3 | H4C3 | HIST1H4C |
| 6p22.2 | H3 clustered histone 4 | H4C4 | HIST1H4D |
| 6p22.2 | H3 clustered histone 5 | H4C5 | HIST1H4E |
| 6p22.2 | H3 clustered histone 6 | H4C6 | HIST1H4F |
| 6p22.2 | H3 clustered histone 7 | H4C7 | HIST1H4G |
| 6p22.2 | H3 clustered histone 8 | H4C8 | HIST1H4H |
| 6p22.1 | H3 clustered histone 9 | H4C9 | HIST1H4I |
| 6p22.1 | H3 clustered histone 10, pseudogene | H4C10P | HIST1H4PS1 |
| 6p22.1 | H3 clustered histone 11 | H4C11 | HIST1H4J |
| 6p22.1 | H3 clustered histone 12 | H4C12 | HIST1H4K |
| 6p22.1 | H3 clustered histone 13 | H4C13 | HIST1H4L |
| 7p13 | H2A.Z variant histone 2 | H2AZ2 | H2AFV |
| 7q36.1 | H2B.K variant histone 1 | H2BK1 | H2BE1 |
| 10q22.1 | macroH2A.2 histone | MACROH2A2 | H2AFY2 |
| 11q23.3 | H2A.X variant histone | H2AX | H2AFX |
| 12q13.11 | H1.7 linker histone | H1-7 | H1FNT |
| 12p12.3 | H2A.J histone | H2AJ | H2AFJ |
| 12p11.21 | H3.5 histone | H3-5 | H3F3C |
| 12p12.3 | H4 histone 16 | H4C16 | HIST4H4 |
| 17q21.33 | H1.9 linker histone, pseudogene | H1-9P | HILS1 |
| 17q11.2 | H2B.N variant histone | H2BN1 | H2B.N |
| 17q25.1 | H3.3 histone B | H3-3B | H3F3B |
| 21q22.3 | H2B clustered histone 12 like | H2BC12L | H2BFS |
| 22q13.1 | H1.0 linker histone | H1-0 | H1FV |
| Xq28 | H2A.B variant histone 1 | H2AB1 | H2AFB1 |
| Xq28 | H2A.B variant histone 2 | H2AB2 | H2AFB2 |
| Xq28 | H2A.B variant histone 3 | H2AB3 | H2AFB3 |
| Xp21.1 | H2A.L variant histone 1M, pseudogene | H2AL1MP | H2AL1SP |
| Xp21.1 | H2A.L variant histone 1Q | H2AL1Q | H2AL1QP |
| Xp11.4 | H2A.L variant histone 3 | H2AL3 | H2AL1RP |
| Xp11.4 | H2A.P histone | H2AP | HYPM (CXorf27) |
| Xq26.3 | H2A.Q variant histone 1, pseudogene | H2AQ1P | |
| Xq22.2 | H2B.W histone 1 | H2BW1 | H2BFWT |
| Xq22.2 | H2B.W histone 2 | H2BW2 | H2BFM |
| Xq22.2 | H2B.W histone 3, pseudogene | H2BW3P | |
| Xq22.2 | H2B.W histone 4, pseudogene | H2BW4P | H2BFXP |
References
- Chioda, M.; Eskeland, R.; Thompson, E.M. Histone Gene Complement, Variant Expression, and mRNA Processing in a Urochordate Oikopleura dioica that Undergoes Extensive Polyploidization. Mol. Biol. Evol. 2002, 19, 2247–2260. [Google Scholar] [CrossRef]
- Talbert, P.B.; Ahmad, K.; Almouzni, G.; Ausió, J.; Berger, F.; Bhalla, P.L.; Bonner, W.M.; Cande, W.Z.; Chadwick, B.P.; Chan, S.W.L.; et al. A unified phylogeny-based nomenclature for histone variants. Epigenet. Chromatin 2012, 5, 7. [Google Scholar] [CrossRef]
- Grau-Bové, X.; Navarrete, C.; Chiva, C.; Pribasnig, T.; Antó, M.; Torruella, G.; Galindo, L.J.; Lang, B.F.; Moreira, D.; López-Garcia, P.; et al. A phylogenetic and proteomic reconstruction of eukaryotic chromatin evolution. Nat. Ecol. Evol. 2022, 6, 1007–1023. [Google Scholar] [CrossRef]
- Zhou, B.R.; Feng, H.; Kale, S.; Fox, T.; Khant, H.; de Val, N.; Ghirlando, R.; Panchenko, A.R.; Bai, Y. Distinct Structures and Dynamics of Chromatosomes with Different Human Linker Histone Isoforms. Mol. Cell 2021, 81, 166–182.e6. [Google Scholar] [CrossRef]
- Phillips, E.O.N.; Gunjan, A. Histone variants: The unsung guardians of the genome. DNA Repair. 2022, 112, 103301. [Google Scholar] [CrossRef]
- Williamson, E.A.; Wray, J.W.; Bansal, P.; Hromas, R. Overview for the histone codes for DNA repair. Prog. Mol. Biol. Transl. Sci. 2012, 110, 207–227. [Google Scholar]
- Bhattacharya, S.; Reddy, D.; Jani, V.; Gadewal, N.; Shah, S.; Reddy, R.; Bose, K.; Sonavane, U.; Joshi, R.; Smoot, D.; et al. Histone isoform H2A1H promotes attainment of distinct physiological states by altering chromatin dynamics. Epigenet. Chromatin 2017, 10, 48. [Google Scholar] [CrossRef]
- Singh, R.; Mortazavi, A.; Telu, K.H.; Nagarajan, P.; Lucas, D.M.; Thomas-Ahner, J.M.; Clinton, S.K.; Byrd, J.C.; Freitas, M.A.; Parthun, M.R. Increasing the complexity of chromatin: Functionally distinct roles for replication-dependent histone H2A isoforms in cell proliferation and carcinogenesis. Nucleic Acids Res. 2013, 41, 9284–9295. [Google Scholar] [CrossRef][Green Version]
- Henikoff, S.; Smith, M.M. Histone variants and epigenetics. Cold Spring Harb. Perspect. Biol. 2015, 7, a019364. [Google Scholar] [CrossRef]
- Strahl, B.D.; Allis, C.D. The language of covalent histone modifications. Nature 2000, 403, 41–45. [Google Scholar] [CrossRef]
- Ramachandran, S.; Henikoff, S. Replicating Nucleosomes. Sci. Adv. 2015, 1, e1500587. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, J.; Li, Q. The replisome guides nucleosome assembly during DNA replication. Cell Biosci. 2020, 10, 37. [Google Scholar] [CrossRef]
- Korolev, N.; Vorontsova, O.V.; Nordenskiöld, L. Physicochemical analysis of electrostatic foundation for DNA–protein interactions in chromatin transformations. Progress. Biophys. Mol. Biol. 2007, 95, 23–49. [Google Scholar] [CrossRef]
- Rudnizky, S.; Khamis, H.; Ginosar, Y.; Goren, E.; Melamed, P.; Kaplan, A. Extended and dynamic linker histone-DNA Interactions control chromatosome compaction. Mol. Cell 2021, 81, 3410–3421.e4. [Google Scholar] [CrossRef]
- Burgess, R.J.; Zhang, Z. Histone chaperones in nucleosome assembly and human disease. Nat. Struct. Mol. Biol. 2013, 20, 14–22. [Google Scholar] [CrossRef]
- He, H.; Lee, M.-C.; Zheng, L.-L.; Zheng, L.; Luo, Y. Integration of the metabolic/redox state, histone gene switching, DNA replication and S-phase progression by moonlighting metabolic enzymes. Biosci. Rep. 2013, 33, e00018. [Google Scholar] [CrossRef]
- Paik, J.; Giovinazzi, S.; Gunjan, A. Coordination of DNA Replication and Histone Synthesis during S Phase. In The Initiation of DNA Replication in Eukaryotes; Kaplan, D.L., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 213–237. [Google Scholar]
- Mei, Q.; Huang, J.; Chen, W.; Tang, J.; Xu, C.; Yu, Q.; Cheng, Y.; Ma, L.; Yu, X.; Li, S. Regulation of DNA replication-coupled histone gene expression. Oncotarget 2017, 8, 95005. [Google Scholar] [CrossRef]
- Nelson, D.M.; Ye, X.; Hall, C.; Santos, H.; Ma, T.; Kao, G.D.; Yen, T.J.; Harper, J.W.; Adams, P.D. Coupling of DNA synthesis and histone synthesis in S phase independent of cyclin/cdk2 activity. Mol. Cell Biol. 2002, 22, 7459–7472. [Google Scholar] [CrossRef]
- Marzluff, W.F.; Wagner, E.J.; Duronio, R.J. Metabolism and regulation of canonical histone mRNAs: Life without a poly(A) tail. Nat. Rev. Genet. 2008, 9, 843–854. [Google Scholar] [CrossRef]
- Duronio, R.J.; Marzluff, W.F. Coordinating cell cycle-regulated histone gene expression through assembly and function of the Histone Locus Body. RNA Biol. 2017, 14, 726–738. [Google Scholar] [CrossRef]
- Kemp, J.P., Jr.; Yang, X.C.; Dominski, Z.; Marzluff, W.F.; Duronio, R.J. Superresolution light microscopy of the Drosophila histone locus body reveals a core-shell organization associated with expression of replication-dependent histone genes. Mol. Biol. Cell 2021, 32, 942–955. [Google Scholar] [CrossRef]
- Romeo, V.; Schümperli, D. Cycling in the nucleus: Regulation of RNA 3′ processing and nuclear organization of replication-dependent histone genes. Curr. Opin. Cell Biol. 2016, 40, 23–31. [Google Scholar] [CrossRef]
- Koreski, K.P.; Rieder, L.E.; McLain, L.M.; Chaubal, A.; Marzluff, W.F.; Duronio, R.J. Drosophila histone locus body assembly and function involves multiple interactions. Mol. Biol. Cell 2020, 31, 1525–1537. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, D.; DeRose, E.F.; Perera, L.; Dominski, Z.; Marzluff, W.F.; Tong, L.; Hall, T.M. Molecular mechanisms for the regulation of histone mRNA stem-loop-binding protein by phosphorylation. Proc. Natl. Acad. Sci. USA 2014, 111, E2937–E2946. [Google Scholar] [CrossRef]
- Wang, Z.F.; Whitfield, M.L.; Ingledue, T.C., 3rd; Dominski, Z.; Marzluff, W.F. The protein that binds the 3′ end of histone mRNA: A novel RNA-binding protein required for histone pre-mRNA processing. Genes. Dev. 1996, 10, 3028–3040. [Google Scholar] [CrossRef]
- Allard, P.; Yang, Q.; Marzluff, W.F.; Clarke, H.J. The stem-loop binding protein regulates translation of histone mRNA during mammalian oogenesis. Dev. Biol. 2005, 286, 195–206. [Google Scholar] [CrossRef]
- Sullivan, E.; Santiago, C.; Parker, E.D.; Dominski, Z.; Yang, X.; Lanzotti, D.J.; Ingledue, T.C.; Marzluff, W.F.; Duronio, R.J. Drosophila stem loop binding protein coordinates accumulation of mature histone mRNA with cell cycle progression. Genes. Dev. 2001, 15, 173–187. [Google Scholar] [CrossRef]
- Lanzotti, D.J.; Kupsco, J.M.; Yang, X.C.; Dominski, Z.; Marzluff, W.F.; Duronio, R.J. Drosophila stem-loop binding protein intracellular localization is mediated by phosphorylation and is required for cell cycle-regulated histone mRNA expression. Mol. Biol. Cell 2004, 15, 1112–1123. [Google Scholar] [CrossRef]
- Terme, J.-M.; Sesé, B.; Millán-Ariño, L.; Mayor, R.; Belmonte, J.C.I.; Barrero, M.J.; Jordan, A. Histone H1 Variants Are Differentially Expressed and Incorporated into Chromatin during Differentiation and Reprogramming to Pluripotency*. J. Biol. Chem. 2011, 286, 35347–35357. [Google Scholar] [CrossRef]
- Dzhondzhurov, L.; Yancheva, N.; Ivanova, E. Histones of terminally differentiated cells undergo continuous turnover. Biochemistry 1983, 22, 4095–4102. [Google Scholar] [CrossRef]
- Armstrong, C.; Spencer, S.L. Replication-dependent histone biosynthesis is coupled to cell-cycle commitment. Proc. Natl. Acad. Sci. USA 2021, 118, e2100178118. [Google Scholar] [CrossRef]
- Amatori, S.; Tavolaro, S.; Gambardella, S.; Fanelli, M. The dark side of histones: Genomic organization and role of oncohistones in cancer. Clin. Epigenet. 2021, 13, 71. [Google Scholar] [CrossRef]
- Kurumizaka, H.; Kujirai, T.; Takizawa, Y. Contributions of Histone Variants in Nucleosome Structure and Function. J. Mol. Biol. 2021, 433, 166678. [Google Scholar] [CrossRef]
- Talbert, P.B.; Henikoff, S. Histone variants at a glance. J. Cell Sci. 2021, 134, jcs244749. [Google Scholar] [CrossRef]
- Ferrand, J.; Rondinelli, B.; Polo, S.E. Histone Variants: Guardians of Genome Integrity. Cells 2020, 9, 2424. [Google Scholar] [CrossRef]
- Alonso-Curbelo, D.; Ho, Y.J.; Burdziak, C.; Maag, J.L.V.; Morris, J.P.t.; Chandwani, R.; Chen, H.A.; Tsanov, K.M.; Barriga, F.M.; Luan, W.; et al. A gene-environment-induced epigenetic program initiates tumorigenesis. Nature 2021, 590, 642–648. [Google Scholar] [CrossRef]
- Martire, S.; Banaszynski, L.A. The roles of histone variants in fine-tuning chromatin organization and function. Nat. Rev. Mol. Cell Biol. 2020, 21, 522–541. [Google Scholar] [CrossRef]
- Ballouz, S.; Pena, M.T.; Knight, F.M.; Adams, L.B.; Gillis, J.A. The transcriptional legacy of developmental stochasticity. bioRxiv 2019. [Google Scholar] [CrossRef]
- Hsu, C.J.; Meers, O.; Buschbeck, M.; Heidel, F.H. The Role of MacroH2A Histone Variants in Cancer. Cancers 2021, 13, 3003. [Google Scholar] [CrossRef]
- Kirkiz, E.; Meers, O.; Grebien, F.; Buschbeck, M. Histone Variants and Their Chaperones in Hematological Malignancies. HemaSphere 2023, 7, e927. [Google Scholar] [CrossRef]
- Johal, K.S.; Cheema, M.S.; Stefanelli, G. Histone Variants and Their Chaperones: An Emerging Epigenetic Mechanism in Neurodevelopment and Neurodevelopmental Disorders. JIN 2023, 22, 108. [Google Scholar] [CrossRef] [PubMed]
- Siddaway, R.; Milos, S.; Coyaud, É.; Yun, H.Y.; Morcos, S.M.; Pajovic, S.; Campos, E.I.; Raught, B.; Hawkins, C. The in vivo Interaction Landscape of Histones H3.1 and H3.3. Mol. Cell Proteom. 2022, 21, 100411. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhao, J.; Wang, Y.; Wang, M.; Long, H.; Liang, D.; Huang, L.; Wen, Z.; Li, W.; Li, X.; et al. H3.3 actively marks enhancers and primes gene transcription via opening higher-ordered chromatin. Genes. Dev. 2013, 27, 2109–2124. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Felsenfeld, G. Nucleosome stability mediated by histone variants H3.3 and H2A.Z. Genes. Dev. 2007, 21, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Tvardovskiy, A.; Schwämmle, V.; Kempf, S.J.; Rogowska-Wrzesinska, A.; Jensen, O.N. Accumulation of histone variant H3.3 with age is associated with profound changes in the histone methylation landscape. Nucleic Acids Res. 2017, 45, 9272–9289. [Google Scholar] [CrossRef]
- Loyola, A.; Almouzni, G. Marking histone H3 variants: How, when and why? Trends Biochem. Sci. 2007, 32, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Loyola, A.; Bonaldi, T.; Roche, D.; Imhof, A.; Almouzni, G. PTMs on H3 Variants before Chromatin Assembly Potentiate Their Final Epigenetic State. Mol. Cell 2006, 24, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wei, T.; Panchenko, A.R. Histone variant H2A.Z modulates nucleosome dynamics to promote DNA accessibility. Nat. Commun. 2023, 14, 769. [Google Scholar] [CrossRef]
- Rangasamy, D. Histone variant H2A.Z can serve as a new target for breast cancer therapy. Curr. Med. Chem. 2010, 17, 3155–3161. [Google Scholar] [CrossRef]
- Buschbeck, M.; Hake, S.B. Variants of core histones and their roles in cell fate decisions, development and cancer. Nat. Rev. Mol. Cell Biol. 2017, 18, 299–314. [Google Scholar] [CrossRef]
- Xia, W.; Jiao, J. Histone variant H3.3 orchestrates neural stem cell differentiation in the developing brain. Cell Death Differ. 2017, 24, 1548–1563. [Google Scholar] [CrossRef]
- Maze, I.; Noh, K.-M.; Soshnev, A.A.; Allis, C.D. Every amino acid matters: Essential contributions of histone variants to mammalian development and disease. Nat. Rev. Genet. 2014, 15, 259–271. [Google Scholar] [CrossRef]
- Gomes, A.P.; Ilter, D.; Low, V.; Rosenzweig, A.; Shen, Z.J.; Schild, T.; Rivas, M.A.; Er, E.E.; McNally, D.R.; Mutvei, A.P.; et al. Dynamic Incorporation of Histone H3 Variants into Chromatin Is Essential for Acquisition of Aggressive Traits and Metastatic Colonization. Cancer Cell 2019, 36, 402–417.e13. [Google Scholar] [CrossRef]
- Taguchi, H.; Xie, Y.; Horikoshi, N.; Maehara, K.; Harada, A.; Nogami, J.; Sato, K.; Arimura, Y.; Osakabe, A.; Kujirai, T.; et al. Crystal Structure and Characterization of Novel Human Histone H3 Variants, H3.6, H3.7, and H3.8. Biochemistry 2017, 56, 2184–2196. [Google Scholar] [CrossRef]
- Susano Pinto, D.M.; Flaus, A. The human canonical core histone catalogue. bioRxiv 2019. [Google Scholar] [CrossRef]
- Kurat, C.F.; Recht, J.; Radovani, E.; Durbic, T.; Andrews, B.; Fillingham, J. Regulation of histone gene transcription in yeast. Cell Mol. Life Sci. 2014, 71, 599–613. [Google Scholar] [CrossRef]
- Mariño-Ramírez, L.; Jordan, I.K.; Landsman, D. Multiple independent evolutionary solutions to core histone gene regulation. Genome Biol. 2006, 7, R122. [Google Scholar] [CrossRef]
- Singh, R.; Bassett, E.; Chakravarti, A.; Parthun, M.R. Replication-dependent histone isoforms: A new source of complexity in chromatin structure and function. Nucleic Acids Res. 2018, 46, 8665–8678. [Google Scholar] [CrossRef]
- Molden, R.C.; Bhanu, N.V.; LeRoy, G.; Arnaudo, A.M.; Garcia, B.A. Multi-faceted quantitative proteomics analysis of histone H2B isoforms and their modifications. Epigenet. Chromatin 2015, 8, 15. [Google Scholar] [CrossRef]
- Shah, S.; Verma, T.; Rashid, M.; Gadewal, N.; Gupta, S. Histone H2A isoforms: Potential implications in epigenome plasticity and diseases in eukaryotes. J. Biosci. 2020, 45, 4. [Google Scholar] [CrossRef]
- Heintz, N.; Zernik, M.; Roeder, R.G. The structure of the human histone genes: Clustered but not tandemly repeated. Cell 1981, 24, 661–668. [Google Scholar] [CrossRef]
- Marzluff, W.F.; Gongidi, P.; Woods, K.R.; Jin, J.; Maltais, L.J. The human and mouse replication-dependent histone genes. Genomics 2002, 80, 487–498. [Google Scholar] [CrossRef]
- Luense, L.J.; Wang, X.; Schon, S.B.; Weller, A.H.; Lin Shiao, E.; Bryant, J.M.; Bartolomei, M.S.; Coutifaris, C.; Garcia, B.A.; Berger, S.L. Comprehensive analysis of histone post-translational modifications in mouse and human male germ cells. Epigenet. Chromatin 2016, 9, 24. [Google Scholar] [CrossRef]
- Lyons, S.M.; Cunningham, C.H.; Welch, J.D.; Groh, B.; Guo, A.Y.; Wei, B.; Whitfield, M.L.; Xiong, Y.; Marzluff, W.F. A subset of replication-dependent histone mRNAs are expressed as polyadenylated RNAs in terminally differentiated tissues. Nucleic Acids Res. 2016, 44, 9190–9205. [Google Scholar] [CrossRef]
- Bonner, W.M.; Mannironi, C.; Orr, A.; Pilch, D.R.; Hatch, C.L. Histone H2A.X gene transcription is regulated differently than transcription of other replication-linked histone genes. Mol. Cell Biol. 1993, 13, 984–992. [Google Scholar]
- Mannironi, C.; Bonner, W.M.; Hatch, C.L. H2A.X. a histone isoprotein with a conserved C-terminal sequence, is encoded by a novel mRNA with both DNA replication type and polyA 3′ processing signals. Nucleic Acids Res. 1989, 17, 9113–9126. [Google Scholar] [CrossRef]
- Marzluff, W.F.; Koreski, K.P. Birth and Death of Histone mRNAs. Trends Genet. 2017, 33, 745–759. [Google Scholar] [CrossRef]
- Panyim, S.; Chalkley, R. A new histone found only in mammalian tissues with little cell division. Biochem. Biophys. Res. Commun. 1969, 37, 1042–1049. [Google Scholar] [CrossRef]
- Kari, V.; Karpiuk, O.; Tieg, B.; Kriegs, M.; Dikomey, E.; Krebber, H.; Begus-Nahrmann, Y.; Johnsen, S.A. A subset of histone H2B genes produces polyadenylated mRNAs under a variety of cellular conditions. PLoS ONE 2013, 8, e63745. [Google Scholar] [CrossRef]
- Sullivan, K.D.; Mullen, T.E.; Marzluff, W.F.; Wagner, E.J. Knockdown of SLBP results in nuclear retention of histone mRNA. RNA 2009, 15, 459–472. [Google Scholar] [CrossRef]
- Monteiro, F.L.; Vitorino, R.; Wang, J.; Cardoso, H.; Laranjeira, H.; Simões, J.; Caldas, M.; Henrique, R.; Amado, F.; Williams, C.; et al. The histone H2A isoform Hist2h2ac is a novel regulator of proliferation and epithelial–mesenchymal transition in mammary epithelial and in breast cancer cells. Cancer Lett. 2017, 396, 42–52. [Google Scholar] [CrossRef]
- Tyagi, M.; Khade, B.; Khan, S.A.; Ingle, A.; Gupta, S. Expression of histone variant, H2A.1 is associated with the undifferentiated state of hepatocyte. Exp. Biol. Med. 2014, 239, 1335–1339. [Google Scholar] [CrossRef]
- Singh, R.; Harshman, S.W.; Ruppert, A.S.; Mortazavi, A.; Lucas, D.M.; Thomas-Ahner, J.M.; Clinton, S.K.; Byrd, J.C.; Freitas, M.A.; Parthun, M.R. Proteomic profiling identifies specific histone species associated with leukemic and cancer cells. Clin. Proteom. 2015, 12, 22. [Google Scholar] [CrossRef][Green Version]
- Shah, S.G.; Rashid, M.; Natu, A.; Gupta, S. Differential expression of H2A isoforms contribute to tissue and lineage specificity with HIST2H2AC as a potential cancer biomarker. bioRxiv 2021. [Google Scholar] [CrossRef]
- Bowerman, S.; Wereszczynski, J. Effects of MacroH2A and H2A.Z on Nucleosome Dynamics as Elucidated by Molecular Dynamics Simulations. Biophys. J. 2016, 110, 327–337. [Google Scholar] [CrossRef]
- Kozlowski, M.; Corujo, D.; Hothorn, M.; Guberovic, I.; Mandemaker, I.K.; Blessing, C.; Sporn, J.; Gutierrez-Triana, A.; Smith, R.; Portmann, T.; et al. MacroH2A histone variants limit chromatin plasticity through two distinct mechanisms. EMBO Rep. 2018, 19, e44445. [Google Scholar] [CrossRef]
- Pinto, D.M.; Flaus, A. Structure and function of histone H2AX. Subcell. Biochem. 2010, 50, 55–78. [Google Scholar]
- Foster, E.R.; Downs, J.A. Histone H2A phosphorylation in DNA double-strand break repair. FEBS J. 2005, 272, 3231–3240. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, C.K.; Lee, S.B.; Lee, K.-H.; Cho, S.-W.; Ahn, J.-Y. Akt attenuates apoptotic death through phosphorylation of H2A under hydrogen peroxide-induced oxidative stress in PC12 cells and hippocampal neurons. Sci. Rep. 2016, 6, 21857. [Google Scholar] [CrossRef]
- Abuetabh, Y.; Wu, H.H.; Chai, C.; Al Yousef, H.; Persad, S.; Sergi, C.M.; Leng, R. DNA damage response revisited: The p53 family and its regulators provide endless cancer therapy opportunities. Exp. Mol. Med. 2022, 54, 1658–1669. [Google Scholar] [CrossRef]
- Walser, F.; Mulder, M.P.C.; Bragantini, B.; Burger, S.; Gubser, T.; Gatti, M.; Botuyan, M.V.; Villa, A.; Altmeyer, M.; Neri, D.; et al. Ubiquitin Phosphorylation at Thr12 Modulates the DNA Damage Response. Mol. Cell 2020, 80, 423–436.e9. [Google Scholar] [CrossRef]
- Banerjee, T.; Chakravarti, D. A peek into the complex realm of histone phosphorylation. Mol. Cell Biol. 2011, 31, 4858–4873. [Google Scholar] [CrossRef]
- Chen, C.; Ha, B.H.; Thévenin, A.F.; Lou, H.J.; Zhang, R.; Yip, K.Y.; Peterson, J.R.; Gerstein, M.; Kim, P.M.; Filippakopoulos, P.; et al. Identification of a major determinant for serine-threonine kinase phosphoacceptor specificity. Mol. Cell 2014, 53, 140–147. [Google Scholar] [CrossRef]
- Bönisch, C.; Hake, S.B. Histone H2A variants in nucleosomes and chromatin: More or less stable? Nucleic Acids Res. 2012, 40, 10719–10741. [Google Scholar] [CrossRef]
- Li, S.; Peng, Y.; Landsman, D.; Panchenko, A.R. DNA methylation cues in nucleosome geometry, stability and unwrapping. Nucleic Acids Res. 2022, 50, 1864–1874. [Google Scholar] [CrossRef] [PubMed]
- Huertas, J.; Schöler, H.R.; Cojocaru, V. Histone tails cooperate to control the breathing of genomic nucleosomes. PLoS Comput. Biol. 2021, 17, e1009013. [Google Scholar] [CrossRef]
- Chakraborty, K.; Loverde, S.M. Asymmetric breathing motions of nucleosomal DNA and the role of histone tails. J. Chem. Phys. 2017, 147, 065101. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Sakuraba, S.; Ishida, H. Free energy profiles for unwrapping the outer superhelical turn of nucleosomal DNA. PLoS Comput. Biol. 2018, 14, e1006024. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, H. Analysis of core histones by liquid chromatography–mass spectrometry and peptide mapping. J. Chromatogr. B 2003, 783, 173–179. [Google Scholar] [CrossRef]
- Tessarz, P.; Santos-Rosa, H.; Robson, S.C.; Sylvestersen, K.B.; Nelson, C.J.; Nielsen, M.L.; Kouzarides, T. Glutamine methylation in histone H2A is an RNA-polymerase-I-dedicated modification. Nature 2014, 505, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Mobley, R.J.; Abell, A.N. Controlling Epithelial to Mesenchymal Transition through Acetylation of Histone H2BK5. J. Nat. Sci. 2017, 3, e432. [Google Scholar]
- Huang, D.; Camacho, C.V.; Setlem, R.; Ryu, K.W.; Parameswaran, B.; Gupta, R.K.; Kraus, W.L. Functional Interplay between Histone H2B ADP-Ribosylation and Phosphorylation Controls Adipogenesis. Mol. Cell 2020, 79, 934–949.e14. [Google Scholar] [CrossRef] [PubMed]
- Bungard, D.; Fuerth, B.J.; Zeng, P.-Y.; Faubert, B.; Maas, N.L.; Viollet, B.; Carling, D.; Thompson, C.B.; Jones, R.G.; Berger, S.L. Signaling Kinase AMPK Activates Stress-Promoted Transcription via Histone H2B Phosphorylation. Science 2010, 329, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.A.; Um, S.H.; Lee, J.; Yoo, J.H.; Bang, S.Y.; Park, E.K.; Lee, M.G.; Nam, K.H.; Jeon, Y.J.; Park, J.W.; et al. S6K1 Phosphorylation of H2B Mediates EZH2 Trimethylation of H3: A Determinant of Early Adipogenesis. Mol. Cell 2016, 62, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Tachiwana, H.; Osakabe, A.; Shiga, T.; Miya, Y.; Kimura, H.; Kagawa, W.; Kurumizaka, H. Structures of human nucleosomes containing major histone H3 variants. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Hake, S.B.; Allis, C.D. Histone H3 variants and their potential role in indexing mammalian genomes: The “H3 barcode hypothesis”. Proc. Natl. Acad. Sci. USA 2006, 103, 6428–6435. [Google Scholar] [CrossRef] [PubMed]
- Hake, S.B.; Garcia, B.A.; Duncan, E.M.; Kauer, M.; Dellaire, G.; Shabanowitz, J.; Bazett-Jones, D.P.; Allis, C.D.; Hunt, D.F. Expression Patterns and Post-translational Modifications Associated with Mammalian Histone H3 Variants*. J. Biol. Chem. 2006, 281, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Tachiwana, H.; Kagawa, W.; Osakabe, A.; Kawaguchi, K.; Shiga, T.; Hayashi-Takanaka, Y.; Kimura, H.; Kurumizaka, H. Structural basis of instability of the nucleosome containing a testis-specific histone variant, human H3T. Proc. Natl. Acad. Sci. USA 2010, 107, 10454–10459. [Google Scholar] [CrossRef] [PubMed]
- Espiritu, D.; Gribkova, A.K.; Gupta, S.; Shaytan, A.K.; Panchenko, A.R. Molecular Mechanisms of Oncogenesis through the Lens of Nucleosomes and Histones. J. Phys. Chem. B 2021, 125, 3963–3976. [Google Scholar] [CrossRef]
- Dorigo, B.; Schalch, T.; Bystricky, K.; Richmond, T.J. Chromatin fiber folding: Requirement for the histone H4 N-terminal tail. J. Mol. Biol. 2003, 327, 85–96. [Google Scholar] [CrossRef]
- Ishida, H.; Kono, H. H4 Tails Potentially Produce the Diversity in the Orientation of Two Nucleosomes. Biophys. J. 2017, 113, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Kan, P.Y.; Caterino, T.L.; Hayes, J.J. The H4 tail domain participates in intra- and internucleosome interactions with protein and DNA during folding and oligomerization of nucleosome arrays. Mol. Cell Biol. 2009, 29, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Bendandi, A.; Patelli, A.S.; Diaspro, A.; Rocchia, W. The role of histone tails in nucleosome stability: An electrostatic perspective. Comput. Struct. Biotechnol. J. 2020, 18, 2799–2809. [Google Scholar] [CrossRef] [PubMed]
- Chakkalakal, S.A.; Heilig, J.; Baumann, U.; Paulsson, M.; Zaucke, F. Impact of Arginine to Cysteine Mutations in Collagen II on Protein Secretion and Cell Survival. Int. J. Mol. Sci. 2018, 19, 541. [Google Scholar] [CrossRef] [PubMed]
- Flaus, A.; Rencurel, C.; Ferreira, H.; Wiechens, N.; Owen-Hughes, T. Sin mutations alter inherent nucleosome mobility. EMBO J. 2004, 23, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.S.; Scorgie, J.K.; Dennehey, B.K.; Noone, S.; Tyler, J.K.; Churchill, M.E. The conformational flexibility of the C-terminus of histone H4 promotes histone octamer and nucleosome stability and yeast viability. Epigenet. Chromatin 2012, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, E.; Chmúrčiaková, N.; Cmarko, D. Human rDNA and Cancer. Cells 2021, 10, 3452. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Sun, X.; Shi, W.; Yanru, A.; Leung, S.T.C.; Ding, D.; Cheema, M.S.; MacPherson, N.; Nelson, C.J.; Ausio, J.; et al. A novel histone H4 variant H4G regulates rDNA transcription in breast cancer. Nucleic Acids Res. 2019, 47, 8399–8409. [Google Scholar] [CrossRef] [PubMed]
- Su, C.H.; Tzeng, T.Y.; Cheng, C.; Hsu, M.T. An H2A histone isotype regulates estrogen receptor target genes by mediating enhancer-promoter-3′-UTR interactions in breast cancer cells. Nucleic Acids Res. 2014, 42, 3073–3088. [Google Scholar] [CrossRef]
- Toda, H.; Seki, N.; Kurozumi, S.; Shinden, Y.; Yamada, Y.; Nohata, N.; Moriya, S.; Idichi, T.; Maemura, K.; Fujii, T.; et al. RNA-sequence-based microRNA expression signature in breast cancer: Tumor-suppressive miR-101-5p regulates molecular pathogenesis. Mol. Oncol. 2020, 14, 426–446. [Google Scholar] [CrossRef]
- Mamoor, S. Differential Expression of Histone Cluster 1, H2ag in Triple Negative Breast Cancer. 2021. Available online: https://osf.io/t8swk (accessed on 5 May 2024).
- Komatsu, M.; Yoshimaru, T.; Matsuo, T.; Kiyotani, K.; Miyoshi, Y.; Tanahashi, T.; Rokutan, K.; Yamaguchi, R.; Saito, A.; Imoto, S.; et al. Molecular features of triple negative breast cancer cells by genome-wide gene expression profiling analysis. Int. J. Oncol. 2013, 42, 478–506. [Google Scholar] [CrossRef]
- Stander, B.A.; Marais, S.; Vorster, C.J.; Joubert, A.M. In vitro effects of 2-methoxyestradiol on morphology, cell cycle progression, cell death and gene expression changes in the tumorigenic MCF-7 breast epithelial cell line. J. Steroid Biochem. Mol. Biol. 2010, 119, 149–160. [Google Scholar] [CrossRef]
- Malvia, S.; Bagadi, S.A.R.; Pradhan, D.; Chintamani, C.; Bhatnagar, A.; Arora, D.; Sarin, R.; Saxena, S. Study of Gene Expression Profiles of Breast Cancers in Indian Women. Sci. Rep. 2019, 9, 10018. [Google Scholar] [CrossRef]
- Mamoor, S. Differential Expression of Histone Cluster 3, H2a in Triple Negative Breast Cancer. 2021. Available online: https://osf.io/89ceb (accessed on 6 May 2024).
- Mamoor, S. HIST3H2A is Differentially Expressed in the Brain Metastases of Patients with Metastatic Breast Cancer. 2020. Available online: https://osf.io/nb4q9 (accessed on 6 May 2024).
- Nayak, S.R.; Harrington, E.; Boone, D.; Hartmaier, R.; Chen, J.; Pathiraja, T.N.; Cooper, K.L.; Fine, J.L.; Sanfilippo, J.; Davidson, N.E.; et al. A Role for Histone H2B Variants in Endocrine-Resistant Breast Cancer. Horm. Cancer 2015, 6, 214–224. [Google Scholar] [CrossRef]
- Manna, P.R.; Molehin, D.; Ahmed, A.U. Chapter Eleven—Dysregulation of Aromatase in Breast, Endometrial, and Ovarian Cancers: An Overview of Therapeutic Strategies. In Progress in Molecular Biology and Translational Science; Pruitt, K., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 144, pp. 487–537. [Google Scholar]
- Liu, Y.-R.; Jiang, Y.-Z.; Xu, X.-E.; Hu, X.; Yu, K.-D.; Shao, Z.-M. Comprehensive Transcriptome Profiling Reveals Multigene Signatures in Triple-Negative Breast Cancer. Clin. Cancer Res. 2016, 22, 1653–1662. [Google Scholar] [CrossRef]
- Di Benedetto, M.; Toullec, A.; Buteau-Lozano, H.; Abdelkarim, M.; Vacher, S.; Velasco, G.; Christofari, M.; Pocard, M.; Bieche, I.; Perrot-Applanat, M. MDA-MB-231 breast cancer cells overexpressing single VEGF isoforms display distinct colonisation characteristics. Br. J. Cancer 2015, 113, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Mamoor, S. Differential Expression of Histone Cluster1, H2bo in Triple Negatibe Breast Cancer. 2021. Available online: https://osf.io/aphrb (accessed on 6 May 2024).
- He, Y.; Cao, Y.; Wang, X.; Jisiguleng, W.; Tao, M.; Liu, J.; Wang, F.; Chao, L.; Wang, W.; Li, P.; et al. Identification of Hub Genes to Regulate Breast Cancer Spinal Metastases by Bioinformatics Analyses. Comput. Math. Methods Med. 2021, 2021, 5548918. [Google Scholar] [CrossRef] [PubMed]
- Mamoor, S. HIST1H2BO Is Differentially Expressed in the Brain Metastases of Patients with Metastatic Breast Cancer. 2020. Available online: https://osf.io/h8uaq (accessed on 8 May 2024).
- Xie, W.; Zhang, J.; Zhong, P.; Qin, S.; Zhang, H.; Fan, X.; Yin, Y.; Liang, R.; Han, Y.; Liao, Y.; et al. Expression and potential prognostic value of histone family gene signature in breast cancer. Exp. Ther. Med. 2019, 18, 4893–4903. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.; Maßberg, D.; Becker, C.; Altmüller, J.; Ubrig, B.; Bonatz, G.; Wölk, G.; Philippou, S.; Tannapfel, A.; Hatt, H.; et al. Olfactory Receptors as Biomarkers in Human Breast Carcinoma Tissues. Front. Oncol. 2018, 8, 33. [Google Scholar] [CrossRef]
- Fehniger, T.A.; Krysiak, K.; White, B.S.; Matlock, M.; Miller, C.; Fulton, R.; Kreisel, F.; Fronick, C.; Cook, L.; Veizer, J.; et al. Recurrent Somatic Genomic Alterations in Follicular NHL (FL) Revealed by Exome and Custom-Capture Next Generation Sequencing. Blood 2015, 126, 574. [Google Scholar] [CrossRef]
- Han, D.Y.; Fu, D.; Xi, H.; Li, Q.Y.; Feng, L.J.; Zhang, W.; Ji, G.; Xiao, J.C.; Wei, Q. Genomic expression profiling and bioinformatics analysis of pancreatic cancer. Mol. Med. Rep. 2015, 12, 4133–4140. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, R.; Gao, H.; Yang, Y.; Williams, B.R.G.; Gantier, M.P.; McMillan, N.A.J.; Xu, D.; Hu, Y.; Gao, Y. Identification of a histone family gene signature for predicting the prognosis of cervical cancer patients. Sci. Rep. 2017, 7, 16495. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.K.; Lehrer, J.; Alshalalfa, M.; Erho, N.; Davicioni, E.; Lotan, T.L. Gene expression signatures of neuroendocrine prostate cancer and primary small cell prostatic carcinoma. BMC Cancer 2017, 17, 759. [Google Scholar] [CrossRef]
- Zhong, L.K.; Gan, X.X.; Deng, X.Y.; Shen, F.; Feng, J.H.; Cai, W.S.; Liu, Q.Y.; Miao, J.H.; Zheng, B.X.; Xu, B. Potential five-mRNA signature model for the prediction of prognosis in patients with papillary thyroid carcinoma. Oncol. Lett. 2020, 20, 2302–2310. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Wang, X.; Yin, Y.; Zhang, Y.; Jia, J.; Lu, B.; Xue, W.; Qu, C.; Qi, J. Identification of a potentially functional circRNA-miRNA-mRNA ceRNA regulatory network in bladder cancer by analysis of microarray data. Transl. Androl. Urol. 2020, 10, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Pärssinen, J.; Alarmo, E.L.; Khan, S.; Karhu, R.; Vihinen, M.; Kallioniemi, A. Identification of differentially expressed genes after PPM1D silencing in breast cancer. Cancer Lett. 2008, 259, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Salhia, B.; Kiefer, J.; Ross, J.T.; Metapally, R.; Martinez, R.A.; Johnson, K.N.; DiPerna, D.M.; Paquette, K.M.; Jung, S.; Nasser, S.; et al. Integrated genomic and epigenomic analysis of breast cancer brain metastasis. PLoS ONE 2014, 9, e85448. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Li, W.; Wang, L.; Hu, J.; Song, Y.; Zhang, B.; Ren, X.; Ji, S.; Li, J.; Xu, P.; et al. Histone-Related Genes Are Hypermethylated in Lung Cancer and Hypermethylated HIST1H4F Could Serve as a Pan-Cancer Biomarker. Cancer Res. 2019, 79, 6101–6112. [Google Scholar] [CrossRef] [PubMed]
- Mamoor, S. HIST1H2BB Is a Differentially Expressed Gene in Human Metastatic Breast Cancer, in the Brain and in the Lymph Nodes. 2021. Available online: https://osf.io/cftxw (accessed on 8 May 2024).
- Zhang, L.; Zhang, W.; Sun, J.; Liu, K.N.; Gan, Z.X.; Liu, Y.Z.; Chang, J.F.; Yang, X.M.; Sun, F. Nucleotide variation in histone H2BL drives crossalk of histone modification and promotes tumour cell proliferation by upregulating c-Myc. Life Sci. 2021, 271, 119127. [Google Scholar] [CrossRef]
- Thakar, A.; Gupta, P.; Ishibashi, T.; Finn, R.; Silva-Moreno, B.; Uchiyama, S.; Fukui, K.; Tomschik, M.; Ausio, J.; Zlatanova, J. H2A.Z and H3.3 histone variants affect nucleosome structure: Biochemical and biophysical studies. Biochemistry 2009, 48, 10852–10857. [Google Scholar] [CrossRef]
- Yi Leong, H.J. Identification of Potentially Therapeutic Target Genes in Metastatic Breast Cancer via Integrative Network Analysis. Eurasian J. Med. Oncol. 2023, 7, 371–387. [Google Scholar] [CrossRef]
- Delaney, K.; Mailler, J.; Wenda, J.M.; Gabus, C.; Steiner, F.A. Differential Expression of Histone H3.3 Genes and Their Role in Modulating Temperature Stress Response in Caenorhabditis elegans. Genetics 2018, 209, 551–565. [Google Scholar] [CrossRef]
- Yamatani, Y.; Nakai, K. Comprehensive comparison of gene expression diversity among a variety of human stem cells. NAR Genom. Bioinform. 2022, 4, lqac087. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liang, Y.; Sun, C.; Zhai, Y.; Li, X.; Jiang, M.; Yang, R.; Li, X.; Shu, Q.; Kai, G.; et al. Dihydrotanshinone I Inhibits the Lung Metastasis of Breast Cancer by Suppressing Neutrophil Extracellular Traps Formation. Int. J. Mol. Sci. 2022, 23, 15180. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ye, Y.; Peng, K.; Zeng, Z.; Chen, L.; Zeng, Y. Histones: The critical players in innate immunity. Front. Immunol. 2022, 13, 1030610. [Google Scholar] [CrossRef] [PubMed]
- Fraschilla, I.; Amatullah, H.; Jeffrey, K.L. One genome, many cell states: Epigenetic control of innate immunity. Curr. Opin. Immunol. 2022, 75, 102173. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.Y.H.; Sun, X.; Ausió, J.; Ishibashi, T. Histone H4 variant, H4G, drives ribosomal RNA transcription and breast cancer cell proliferation by loosening nucleolar chromatin structure. J. Cell Physiol. 2020, 235, 9601–9608. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Nguyen, T.T.; Pang, M.Y.H.; Ishibashi, T. Primate-specific histone variants. Genome 2021, 64, 337–346. [Google Scholar] [CrossRef]
- Nacev, B.A.; Feng, L.; Bagert, J.D.; Lemiesz, A.E.; Gao, J.; Soshnev, A.A.; Kundra, R.; Schultz, N.; Muir, T.W.; Allis, C.D. The expanding landscape of ‘oncohistone’ mutations in human cancers. Nature 2019, 567, 473–478. [Google Scholar] [CrossRef]
- Bagert, J.D.; Mitchener, M.M.; Patriotis, A.L.; Dul, B.E.; Wojcik, F.; Nacev, B.A.; Feng, L.; Allis, C.D.; Muir, T.W. Oncohistone mutations enhance chromatin remodeling and alter cell fates. Nat. Chem. Biol. 2021, 17, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Vogler, C.; Huber, C.; Waldmann, T.; Ettig, R.; Braun, L.; Izzo, A.; Daujat, S.; Chassignet, I.; Lopez-Contreras, A.J.; Fernandez-Capetillo, O.; et al. Histone H2A C-terminus regulates chromatin dynamics, remodeling, and histone H1 binding. PLoS Genet. 2010, 6, e1001234. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.L.; Byrum, S.D.; Namjoshi, S.; Graves, H.K.; Dennehey, B.K.; Tackett, A.J.; Tyler, J.K. Mitotic phosphorylation of histone H3 threonine 80. Cell Cycle 2014, 13, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.A.; Sklenar, A.R.; Parthun, M.R. Application of mass spectrometry to the identification and quantification of histone post-translational modifications. J. Cell Biochem. 2004, 92, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Brewis, H.T.; Wang, A.Y.; Gaub, A.; Lau, J.J.; Stirling, P.C.; Kobor, M.S. What makes a histone variant a variant: Changing H2A to become H2A.Z. PLoS Genet. 2021, 17, e1009950. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.L.; Bele, A.; Small, E.C.; Will, C.M.; Nabet, B.; Oyer, J.A.; Huang, X.; Ghosh, R.P.; Grzybowski, A.T.; Yu, T.; et al. A Mutation in Histone H2B Represents a New Class of Oncogenic Driver. Cancer Discov. 2019, 9, 1438–1451. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.R.; Shah, T.; Torabifard, H. Histone H3 Orchestrates the Ubiquitination of Nucleosomal H2A by BRCA1/BARD1-UbcH5c Complex. bioRxiv 2024. [Google Scholar] [CrossRef]
- Eickbush, T.H.; Godfrey, J.E.; Elia, M.C.; Moudrianakis, E.N. H2a-specific proteolysis as a unique probe in the analysis of the histone octamer. J. Biol. Chem. 1988, 263, 18972–18978. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Strahl, B.D. Oncohistones: Corruption at the core. Nat. Chem. Biol. 2021, 17, 370–371. [Google Scholar] [CrossRef]
- Espinoza Pereira, K.N.; Shan, J.; Licht, J.D.; Bennett, R.L. Histone mutations in cancer. Biochem. Soc. Trans. 2023, 51, 1749–1763. [Google Scholar] [CrossRef]
- Kang, T.Z.E.; Zhu, L.; Yang, D.; Ding, D.; Zhu, X.; Wan, Y.C.E.; Liu, J.; Ramakrishnan, S.; Chan, L.L.; Chan, S.Y.; et al. The elevated transcription of ADAM19 by the oncohistone H2BE76K contributes to oncogenic properties in breast cancer. J. Biol. Chem. 2021, 296, 100374. [Google Scholar] [CrossRef] [PubMed]
- Messner, S.; Hottiger, M.O. Histone ADP-ribosylation in DNA repair, replication and transcription. Trends Cell Biol. 2011, 21, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Ogata, N.; Ueda, K.; Hayaishi, O. ADP-ribosylation of histone H2B. Identification of glutamic acid residue 2 as the modification site. J. Biol. Chem. 1980, 255, 7610–7615. [Google Scholar] [CrossRef] [PubMed]
- Kujirai, T.; Ehara, H.; Fujino, Y.; Shirouzu, M.; Sekine, S.I.; Kurumizaka, H. Structural basis of the nucleosome transition during RNA polymerase II passage. Science 2018, 362, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Tessadori, F.; Giltay, J.C.; Hurst, J.A.; Massink, M.P.; Duran, K.; Vos, H.R.; van Es, R.M.; Scott, R.H.; van Gassen, K.L.I.; Bakkers, J.; et al. Germline mutations affecting the histone H4 core cause a developmental syndrome by altering DNA damage response and cell cycle control. Nat. Genet. 2017, 49, 1642–1646. [Google Scholar] [CrossRef] [PubMed]
- Dao, H.T.; Pham, L.T.D. Acidic patch histone mutations and their effects on nucleosome remodeling. Biochem. Soc. Trans. 2022, 50, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Camacho, C.V.; Martire, S.; Nagari, A.; Setlem, R.; Gong, X.; Edwards, A.D.; Chiu, S.P.; Banaszynski, L.A.; Kraus, W.L. Oncohistone Mutations Occur at Functional Sites of Regulatory ADP-Ribosylation. Cancer Res. 2022, 82, 2361–2377. [Google Scholar] [CrossRef] [PubMed]
- Minic, Z.; Li, Y.; Hüttmann, N.; Uppal, G.K.; D’Mello, R.; Berezovski, M.V. Lysine Acetylome of Breast Cancer-Derived Small Extracellular Vesicles Reveals Specific Acetylation Patterns for Metabolic Enzymes. Biomedicines 2023, 11, 1076. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-M.; Chen, H.Y.; Davie, J.R. Effect of Estradiol on Histone Acetylation Dynamics in Human Breast Cancer Cells*. J. Biol. Chem. 2001, 276, 49435–49442. [Google Scholar] [CrossRef]
- Lu, C.; Ramirez, D.; Hwang, S.; Jungbluth, A.; Frosina, D.; Ntiamoah, P.; Healey, J.; Zhu, G.; Chen, W.; Klein, M.; et al. Histone H3K36M mutation and trimethylation patterns in chondroblastoma. Histopathology 2019, 74, 291–299. [Google Scholar] [CrossRef]
- Fang, J.; Huang, Y.; Mao, G.; Yang, S.; Rennert, G.; Gu, L.; Li, H.; Li, G.M. Cancer-driving H3G34V/R/D mutations block H3K36 methylation and H3K36me3-MutSα interaction. Proc. Natl. Acad. Sci. USA 2018, 115, 9598–9603. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Gong, J.; Yu, T.; Zou, Y.; Zhang, M.; Nie, L.; Chen, X.; Yue, Q.; Liu, Y.; Mao, Q.; et al. Diffuse Midline Gliomas With Histone H3 K27M Mutation in Adults and Children: A Retrospective Series of 164 Cases. Am. J. Surg. Pathol. 2022, 46, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.C.E.; Liu, J.; Chan, K.M. Histone H3 Mutations in Cancer. Curr. Pharmacol. Rep. 2018, 4, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Voon, H.P.J.; Hii, L.; Garvie, A.; Udugama, M.; Krug, B.; Russo, C.; Chüeh, A.C.; Daly, R.J.; Morey, A.; Bell, T.D.M.; et al. Pediatric glioma histone H3.3 K27M/G34R mutations drive abnormalities in PML nuclear bodies. Genome Biol. 2023, 24, 284. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhu, Y.; Feng, X.; Liu, X.; Zhou, K.; Wang, Q.; Zhang, H.; Shi, H. H3F3A K27M Mutation Promotes the Infiltrative Growth of High-Grade Glioma in Adults by Activating β-Catenin/USP1 Signaling. Cancers 2022, 14, 4836. [Google Scholar] [CrossRef] [PubMed]
- DiNapoli, S.E.; Martinez-McFaline, R.; Shen, H.; Doane, A.S.; Perez, A.R.; Verma, A.; Simon, A.; Nelson, I.; Balgobin, C.A.; Bourque, C.T.; et al. Histone 3 Methyltransferases Alter Melanoma Initiation and Progression Through Discrete Mechanisms. Front. Cell Dev. Biol. 2022, 10, 814216. [Google Scholar] [CrossRef] [PubMed]
- Lehnertz, B.; Zhang, Y.W.; Boivin, I.; Mayotte, N.; Tomellini, E.; Chagraoui, J.; Lavallée, V.P.; Hébert, J.; Sauvageau, G. H3(K27M/I) mutations promote context-dependent transformation in acute myeloid leukemia with RUNX1 alterations. Blood 2017, 130, 2204–2214. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.-T.; Piunti, A.; Qi, J.; Morgan, M.; Bartom, E.; Shilatifard, A.; Saratsis, A.M. Effects of H3.3G34V mutation on genomic H3K36 and H3K27 methylation patterns in isogenic pediatric glioma cells. Acta Neuropathol. Commun. 2020, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Farnung, L.; Dienemann, C.; Cramer, P. Structure of H3K36-methylated nucleosome-PWWP complex reveals multivalent cross-gyre binding. Nat. Struct. Mol. Biol. 2020, 27, 8–13. [Google Scholar] [CrossRef]
- Bhattarai, A.M.; Mainali, G.; Jha, P.; Karki, P.; Adhikari, A.; Pandit, A.; Bhattarai, A.M. Diffuse midline glioma H3K27M mutation in adult: A case report. Ann. Med. Surg. 2022, 76, 103567. [Google Scholar] [CrossRef]
- El-Hashash, A.H.K. Histone H3K27M Mutation in Brain Tumors. Adv. Exp. Med. Biol. 2021, 1283, 43–52. [Google Scholar] [PubMed]
- Nagaraja, S.; Quezada, M.A.; Gillespie, S.M.; Arzt, M.; Lennon, J.J.; Woo, P.J.; Hovestadt, V.; Kambhampati, M.; Filbin, M.G.; Suva, M.L.; et al. Histone Variant and Cell Context Determine H3K27M Reprogramming of the Enhancer Landscape and Oncogenic State. Mol. Cell 2019, 76, 965–980.e12. [Google Scholar] [CrossRef]
- Chen, H.; Lorton, B.; Gupta, V.; Shechter, D. A TGFβ-PRMT5-MEP50 axis regulates cancer cell invasion through histone H3 and H4 arginine methylation coupled transcriptional activation and repression. Oncogene 2017, 36, 373–386. [Google Scholar] [CrossRef]
- Pal, S.; Baiocchi, R.A.; Byrd, J.C.; Grever, M.R.; Jacob, S.T.; Sif, S. Low levels of miR-92b/96 induce PRMT5 translation and H3R8/H4R3 methylation in mantle cell lymphoma. EMBO J. 2007, 26, 3558–3569. [Google Scholar] [CrossRef]
- Tanikawa, C.; Espinosa, M.; Suzuki, A.; Masuda, K.; Yamamoto, K.; Tsuchiya, E.; Ueda, K.; Daigo, Y.; Nakamura, Y.; Matsuda, K. Regulation of histone modification and chromatin structure by the p53-PADI4 pathway. Nat. Commun. 2012, 3, 676. [Google Scholar] [CrossRef]
- Nozawa, K.; Takizawa, Y.; Pierrakeas, L.; Sogawa-Fujiwara, C.; Saikusa, K.; Akashi, S.; Luk, E.; Kurumizaka, H. Cryo-electron microscopy structure of the H3-H4 octasome: A nucleosome-like particle without histones H2A and H2B. Proc. Natl. Acad. Sci. USA 2022, 119, e2206542119. [Google Scholar] [CrossRef]
- Sahu, V.; Lu, C. Oncohistones: Hijacking the histone code. Annu. Rev. Cancer Biol. 2022, 6, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Dan, H.; Zhang, S.; Zhou, Y.; Guan, Q. DNA Methyltransferase Inhibitors: Catalysts For Antitumour Immune Responses. Onco Targets Ther. 2019, 12, 10903–10916. [Google Scholar] [CrossRef] [PubMed]
- Feehley, T.; O’Donnell, C.W.; Mendlein, J.; Karande, M.; McCauley, T. Drugging the epigenome in the age of precision medicine. Clin. Epigenet. 2023, 15, 6. [Google Scholar] [CrossRef]
- Christman, J.K. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: Mechanistic studies and their implications for cancer therapy. Oncogene 2002, 21, 5483–5495. [Google Scholar] [CrossRef]
- Daher-Reyes, G.S.; Merchan, B.M.; Yee, K.W.L. Guadecitabine (SGI-110): An investigational drug for the treatment of myelodysplastic syndrome and acute myeloid leukemia. Expert. Opin. Investig. Drugs 2019, 28, 835–849. [Google Scholar] [CrossRef] [PubMed]
- Papadatos-Pastos, D.; Yuan, W.; Pal, A.; Crespo, M.; Ferreira, A.; Gurel, B.; Prout, T.; Ameratunga, M.; Chénard-Poirier, M.; Curcean, A.; et al. Phase 1, dose-escalation study of guadecitabine (SGI-110) in combination with pembrolizumab in patients with solid tumors. J. Immunother. Cancer 2022, 10, e004495. [Google Scholar] [CrossRef]
- Kaufman, D.W.; Kelly, J.P.; Rosenberg, L.; Stolley, P.D.; Schottenfeld, D.; Shapiro, S. Hydralazine and breast cancer. J. Natl. Cancer Inst. 1987, 78, 243–246. [Google Scholar]
- Plummer, R.; Vidal, L.; Griffin, M.; Lesley, M.; de Bono, J.; Coulthard, S.; Sludden, J.; Siu, L.L.; Chen, E.X.; Oza, A.M.; et al. Phase I Study of MG98, an Oligonucleotide Antisense Inhibitor of Human DNA Methyltransferase 1, Given as a 7-Day Infusion in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2009, 15, 3177–3183. [Google Scholar] [CrossRef]
- Dahn, M.L.; Cruickshank, B.M.; Jackson, A.J.; Dean, C.; Holloway, R.W.; Hall, S.R.; Coyle, K.M.; Maillet, H.; Waisman, D.M.; Goralski, K.B.; et al. Decitabine Response in Breast Cancer Requires Efficient Drug Processing and Is Not Limited by Multidrug Resistance. Mol. Cancer Ther. 2020, 19, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Billam, M.; Sobolewski, M.D.; Davidson, N.E. Effects of a novel DNA methyltransferase inhibitor zebularine on human breast cancer cells. Breast Cancer Res. Treat. 2010, 120, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.S.; Chan, A.H.Y.; Ganesan, A. Thirty Years of HDAC Inhibitors: 2020 Insight and Hindsight. J. Med. Chem. 2020, 63, 12460–12484. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.S.; Parmigiani, R.B.; Marks, P.A. Histone deacetylase inhibitors: Molecular mechanisms of action. Oncogene 2007, 26, 5541–5552. [Google Scholar] [CrossRef] [PubMed]
- Lian, B.; Chen, X.; Shen, K. Inhibition of histone deacetylases attenuates tumor progression and improves immunotherapy in breast cancer. Front. Immunol. 2023, 14, 1164514. [Google Scholar] [CrossRef]
- Connolly, R.M.; Rudek, M.A.; Piekarz, R. Entinostat: A promising treatment option for patients with advanced breast cancer. Future Oncol. 2017, 13, 1137–1148. [Google Scholar] [CrossRef]
- Salvador, M.A.; Wicinski, J.; Cabaud, O.; Toiron, Y.; Finetti, P.; Josselin, E.; Lelièvre, H.; Kraus-Berthier, L.; Depil, S.; Bertucci, F.; et al. The histone deacetylase inhibitor abexinostat induces cancer stem cells differentiation in breast cancer with low Xist expression. Clin. Cancer Res. 2013, 19, 6520–6531. [Google Scholar] [CrossRef] [PubMed]
- Linden, H.M.; Kurland, B.F.; Link, J.; Novakova, A.; Chai, X.; Specht, J.M.; Gadi, V.K.; Gralow, J.; Schubert, E.K.; Peterson, L.; et al. A phase II clinical trial of HDACi (vorinostat) and AI therapy in breast cancer with molecular imaging correlates. J. Clin. Oncol. 2014, 32 (Suppl. S15), 556. [Google Scholar] [CrossRef]
- Duan, R.; Du, W.; Guo, W. EZH2: A novel target for cancer treatment. J. Hematol. Oncol. 2020, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Padi, S.K.R.; Tindall, D.J.; Guo, B. Polycomb protein EZH2 suppresses apoptosis by silencing the proapoptotic miR-31. Cell Death Dis. 2014, 5, e1486. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; Garcia-Manero, G.; Rizzieri, D.A.; Tibes, R.; Berdeja, J.G.; Savona, M.R.; Jongen-Lavrenic, M.; Altman, J.K.; Thomson, B.; Blakemore, S.J.; et al. The DOT1L inhibitor pinometostat reduces H3K79 methylation and has modest clinical activity in adult acute leukemia. Blood 2018, 131, 2661–2669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qu, J.; Qi, Y.; Duan, Y.; Huang, Y.-W.; Zhou, Z.; Li, P.; Yao, J.; Huang, B.; Zhang, S.; et al. EZH2 engages TGFβ signaling to promote breast cancer bone metastasis via integrin β1-FAK activation. Nat. Commun. 2022, 13, 2543. [Google Scholar] [CrossRef] [PubMed]
- Nassa, G.; Salvati, A.; Tarallo, R.; Gigantino, V.; Alexandrova, E.; Memoli, D.; Sellitto, A.; Rizzo, F.; Malanga, D.; Mirante, T.; et al. Inhibition of histone methyltransferase DOT1L silences ERα gene and blocks proliferation of antiestrogen-resistant breast cancer cells. Sci. Adv. 2019, 5, eaav5590. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chan, Y.-T.; Tan, H.-Y.; Li, S.; Wang, N.; Feng, Y. Epigenetic regulation in human cancer: The potential role of epi-drug in cancer therapy. Mol. Cancer 2020, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Cochran, A.G.; Conery, A.R.; Sims, R.J., 3rd. Bromodomains: A new target class for drug development. Nat. Rev. Drug Discov. 2019, 18, 609–628. [Google Scholar] [CrossRef]
- Andrikopoulou, A.; Liontos, M.; Koutsoukos, K.; Dimopoulos, M.A.; Zagouri, F. The emerging role of BET inhibitors in breast cancer. Breast 2020, 53, 152–163. [Google Scholar] [CrossRef]
- Qi, J. Bromodomain and extraterminal domain inhibitors (BETi) for cancer therapy: Chemical modulation of chromatin structure. Cold Spring Harb. Perspect. Biol. 2014, 6, a018663. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Park, Y.-K.; Park, S.; Jang, Y.; Waring, N.; Dey, A.; Ozato, K.; Lai, B.; Peng, W.; Ge, K. Brd4 binds to active enhancers to control cell identity gene induction in adipogenesis and myogenesis. Nat. Commun. 2017, 8, 2217. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Zhu, M.; Tan, X.; Zhang, Y.; Liu, X.; Yang, L. Super-enhancers and the super-enhancer reader BRD4: Tumorigenic factors and therapeutic targets. Cell Death Discov. 2023, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Deng, W.; Liu, Y.; Wang, C. General mechanism of JQ1 in inhibiting various types of cancer. Mol. Med. Rep. 2020, 21, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, C.-Y.; Wang, S. The Making of I-BET762, a BET Bromodomain Inhibitor Now in Clinical Development. J. Med. Chem. 2013, 56, 7498–7500. [Google Scholar] [CrossRef] [PubMed]
- Roboz, G.J.; Desai, P.; Lee, S.; Ritchie, E.K.; Winer, E.S.; DeMario, M.; Brennan, B.; Nüesch, E.; Chesne, E.; Brennan, L.; et al. A dose escalation study of RO6870810/TEN-10 in patients with acute myeloid leukemia and myelodysplastic syndrome. Leuk. Lymphoma 2021, 62, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, R.; Riveiro, M.E.; Astorgues-Xerri, L.; Odore, E.; Rezai, K.; Erba, E.; Panini, N.; Rinaldi, A.; Kwee, I.; Beltrame, L.; et al. The bromodomain inhibitor OTX015 (MK-8628) exerts anti-tumor activity in triple-negative breast cancer models as single agent and in combination with everolimus. Oncotarget 2017, 8, 7598–7613. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, N.; Sharma, A.R.; Baylin, S.B. Epigenetic Therapeutics: A New Weapon in the War Against Cancer. Annu. Rev. Med. 2016, 67, 73–89. [Google Scholar] [CrossRef]
- Fardi, M.; Solali, S.; Farshdousti Hagh, M. Epigenetic mechanisms as a new approach in cancer treatment: An updated review. Genes Dis 2018, 5, 304–311. [Google Scholar] [CrossRef]
- Yoo, C.B.; Jones, P.A. Epigenetic therapy of cancer: Past, present and future. Nat. Rev. Drug Discov. 2006, 5, 37–50. [Google Scholar] [CrossRef]
- Chomiak, A.A.; Tiedemann, R.L.; Liu, Y.; Kong, X.; Cui, Y.; Wiseman, A.K.; Thurlow, K.E.; Cornett, E.M.; Topper, M.J.; Baylin, S.B.; et al. Select EZH2 inhibitors enhance viral mimicry effects of DNMT inhibition through a mechanism involving NFAT:AP-1 signaling. Sci. Adv. 2024, 10, eadk4423. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).