PRC1 Protein Subcomplexes Architecture: Focus on the Interplay between Distinct PCGF Subunits in Protein Interaction Networks
Abstract
1. Introduction
2. Results
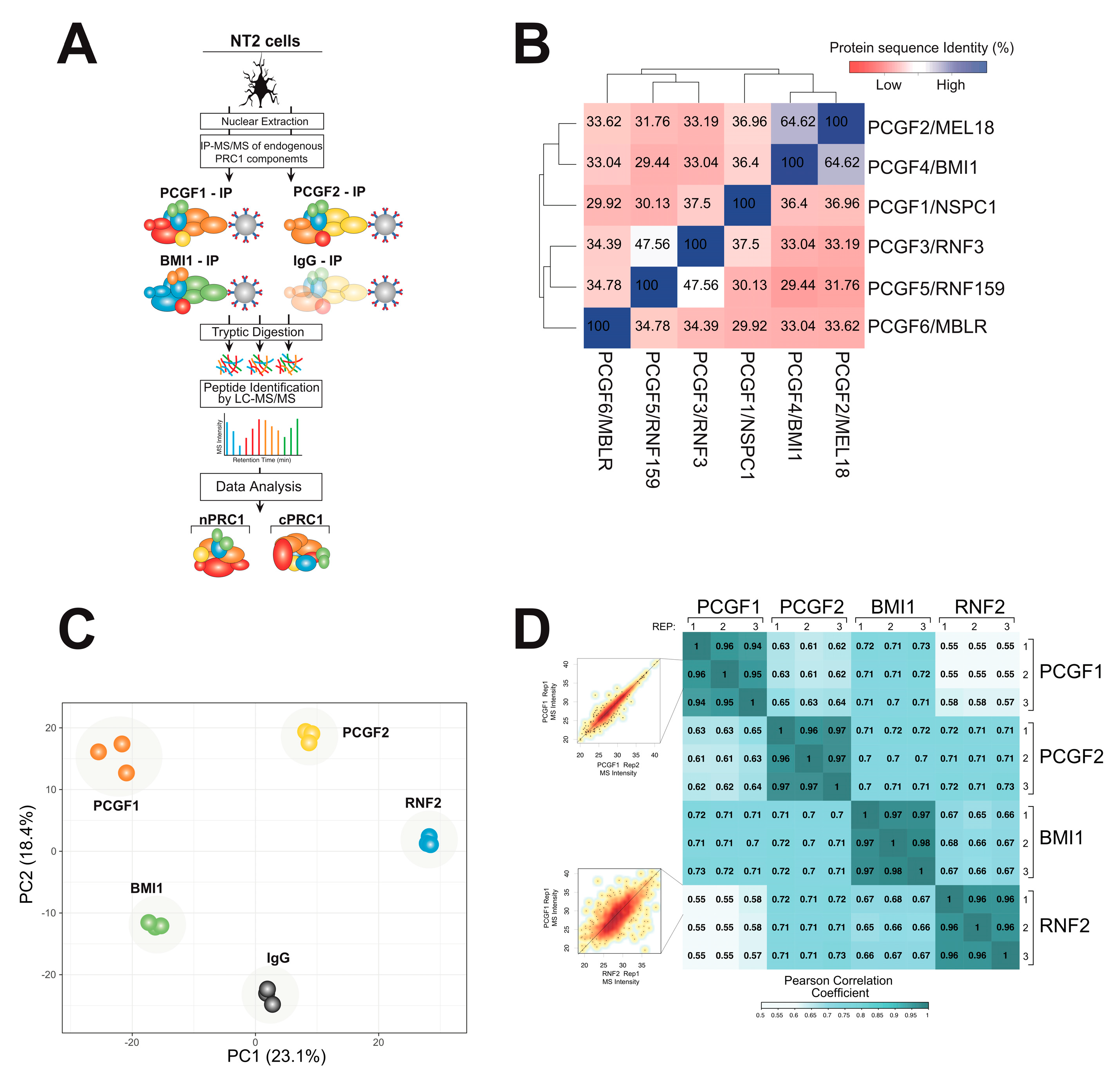
2.1. A Physical Interaction Screen for PRC1 Components Purified under Endogenous Conditions
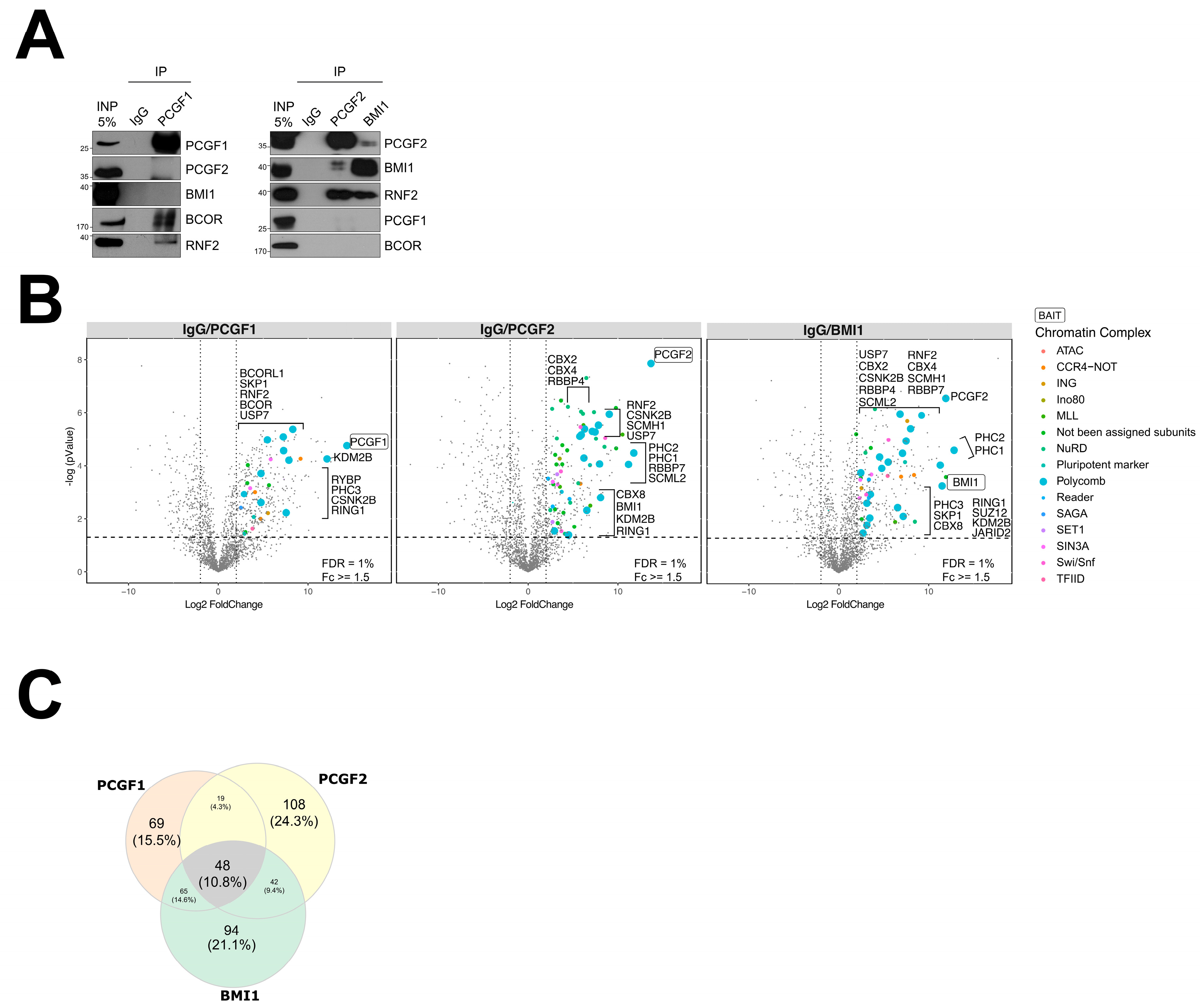
2.2. Affinity Purification (AP)-MS Screening Reveals Common and Distinct Interactomes among PRC1–PCGF Complexes
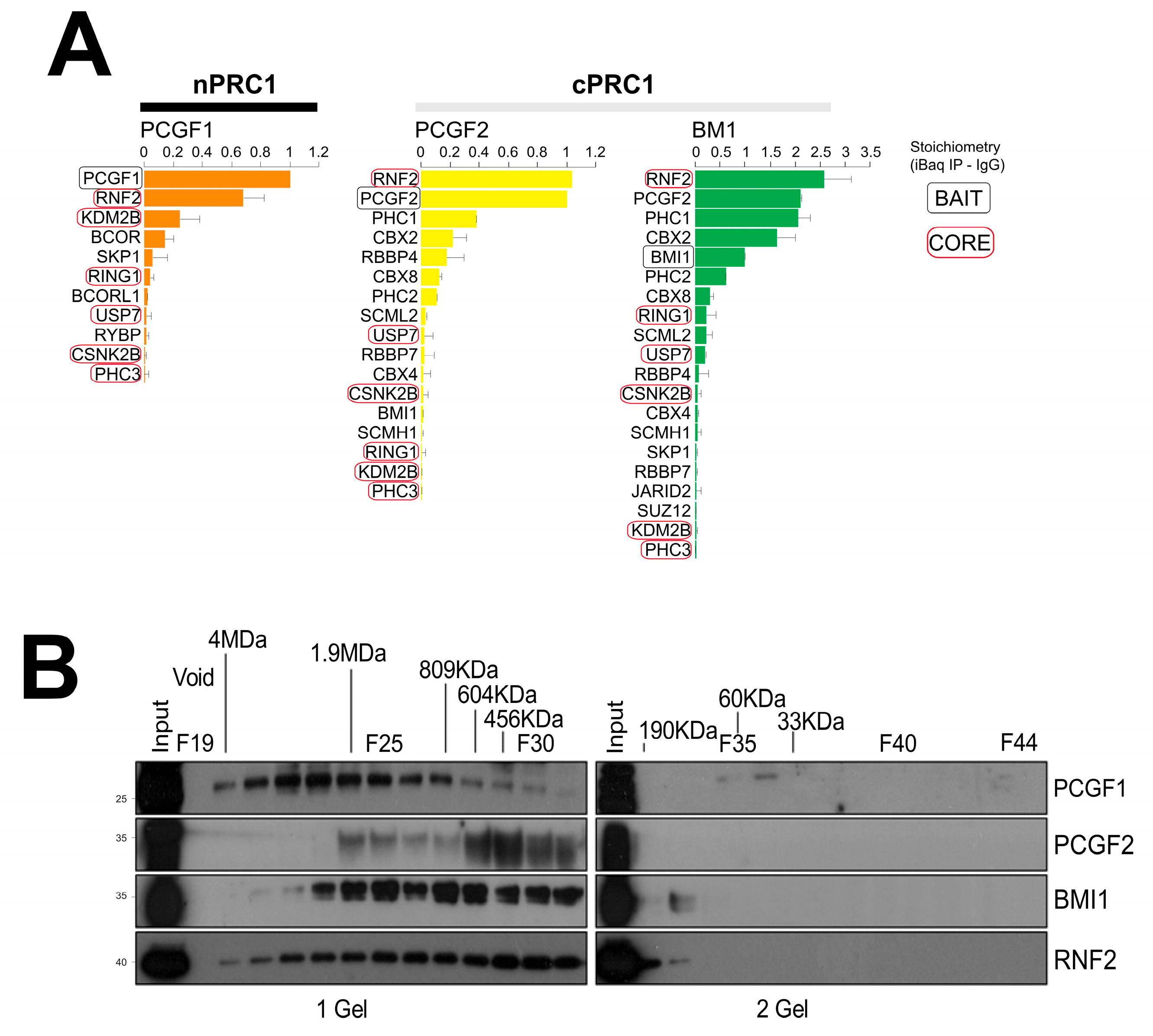
2.3. Relative Abundance Estimation and Molecular Mass of the Isolated PRC1 Complexes
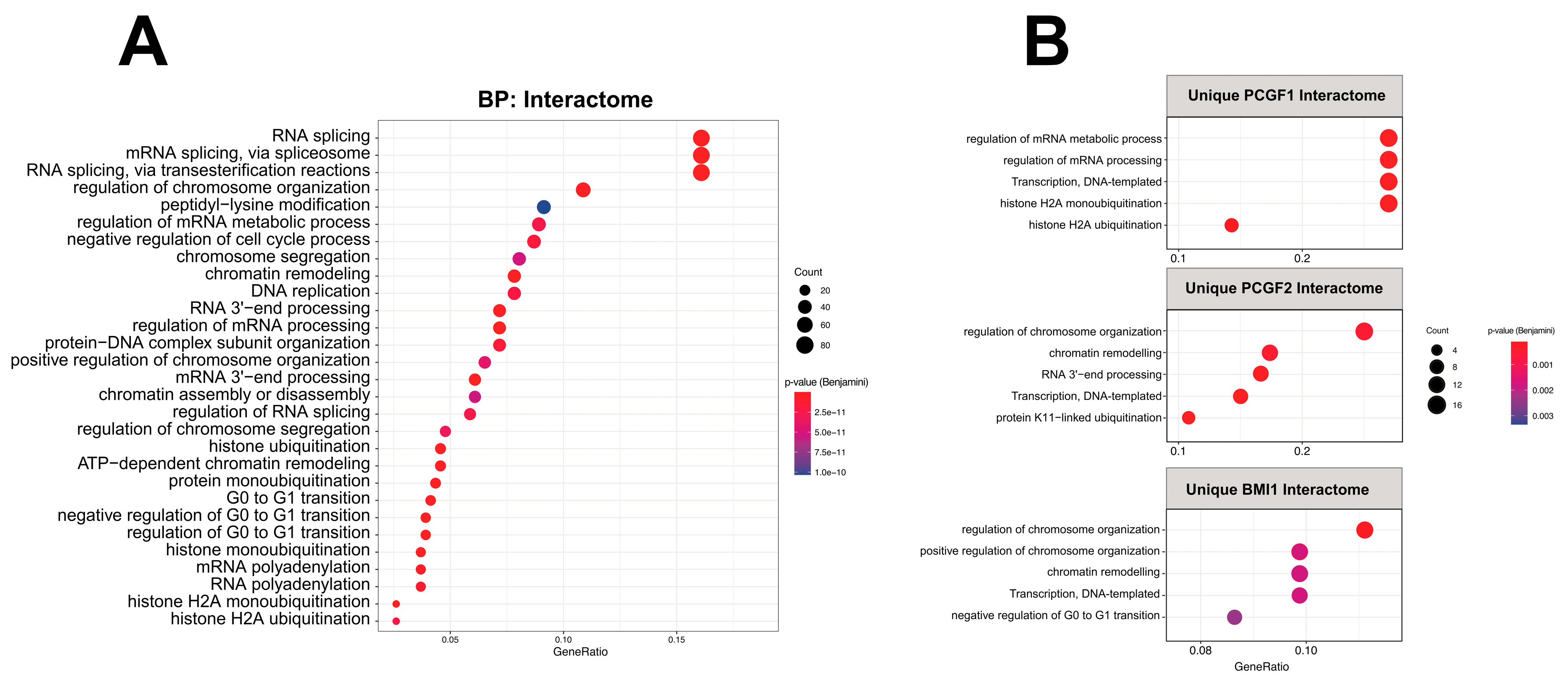
2.4. Functional Enrichment of PCGF Interactome Map to Multiple Pathways
2.5. The Role of PCGF Subunits in NT2 Cells and Mutual Regulation of PCGF Variants
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Isolation of Nuclei
4.3. Immunoprecipitation
4.4. Mass Spectrometry Analysis
4.5. Data Processing
4.6. Data and Statistical Analysis
4.7. Immunoblotting
4.8. Gel Filtration Column Chromatography
4.9. Real-Time Quantitative PCR
4.10. Cell Viability Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klemm, S.L.; Shipony, Z.; Greenleaf, W.J. Chromatin Accessibility and the Regulatory Epigenome. Nat. Rev. Genet. 2019, 20, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.D.; Allis, C.D.; Bernstein, E. Epigenetics: A Landscape Takes Shape. Cell 2007, 128, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Golbabapour, S.; Majid, N.A.; Hassandarvish, P.; Hajrezaie, M.; Abdulla, M.A.; Hadi, A.H.A. Gene Silencing and Polycomb Group Proteins: An Overview of Their Structure, Mechanisms and Phylogenetics. OMICS J. Integr. Biol. 2013, 17, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Entrevan, M.; Schuettengruber, B.; Cavalli, G. Regulation of Genome Architecture and Function by Polycomb Proteins. Trends Cell Biol. 2016, 26, 511–525. [Google Scholar] [CrossRef]
- Blackledge, N.P.; Rose, N.R.; Klose, R.J. Targeting Polycomb Systems to Regulate Gene Expression: Modifications to a Complex Story. Nat. Rev. Mol. Cell Biol. 2015, 16, 643–649. [Google Scholar] [CrossRef]
- Di Croce, L.; Helin, K. Transcriptional Regulation by Polycomb Group Proteins. Nat. Struct. Mol. Biol. 2013, 20, 1147–1155. [Google Scholar] [CrossRef]
- Aloia, L.; Di Stefano, B.; Di Croce, L. Polycomb Complexes in Stem Cells and Embryonic Development. Development 2013, 140, 2525–2534. [Google Scholar] [CrossRef]
- Conway, E.M.; Bracken, A.P. Chapter 4—Unraveling the Roles of Canonical and Noncanonical PRC1 Complexes. In Polycomb Group Proteins; Pirrotta, V., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 57–80. ISBN 978-0-12-809737-3. [Google Scholar]
- Piunti, A.; Shilatifard, A. The Roles of Polycomb Repressive Complexes in Mammalian Development and Cancer. Nat. Rev. Mol. Cell Biol. 2021, 22, 326–345. [Google Scholar] [CrossRef]
- Rodríguez-Paredes, M.; Lyko, F. The Importance of Non-Histone Protein Methylation in Cancer Therapy. Nat. Rev. Mol. Cell Biol. 2019, 20, 569–570. [Google Scholar] [CrossRef]
- Margueron, R.; Reinberg, D. The Polycomb Complex PRC2 and Its Mark in Life. Nature 2011, 469, 343–349. [Google Scholar] [CrossRef]
- van Mierlo, G.; Veenstra, G.J.C.; Vermeulen, M.; Marks, H. The Complexity of PRC2 Subcomplexes. Trends Cell Biol. 2019, 29, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Agius, S.C.; Flanigan, S.F.; Uckelmann, M.; Levina, V.; Owen, B.M.; Davidovich, C. PALI1 Facilitates DNA and Nucleosome Binding by PRC2 and Triggers an Allosteric Activation of Catalysis. Nat. Commun. 2021, 12, 4592. [Google Scholar] [CrossRef] [PubMed]
- Conway, E.; Jerman, E.; Healy, E.; Ito, S.; Holoch, D.; Oliviero, G.; Deevy, O.; Glancy, E.; Fitzpatrick, D.J.; Mucha, M.; et al. A Family of Vertebrate-Specific Polycombs Encoded by the LCOR/LCORL Genes Balance PRC2 Subtype Activities. Mol. Cell 2018, 70, 408–421.e8. [Google Scholar] [CrossRef] [PubMed]
- Scelfo, A.; Fernández-Pérez, D.; Tamburri, S.; Zanotti, M.; Lavarone, E.; Soldi, M.; Bonaldi, T.; Ferrari, K.J.; Pasini, D. Functional Landscape of PCGF Proteins Reveals Both RING1A/B-Dependent-and RING1A/B-Independent-Specific Activities. Mol. Cell 2019, 74, 1037–1052.e7. [Google Scholar] [CrossRef]
- Bernstein, E.; Duncan, E.M.; Masui, O.; Gil, J.; Heard, E.; Allis, C.D. Mouse Polycomb Proteins Bind Differentially to Methylated Histone H3 and RNA and Are Enriched in Facultative Heterochromatin. Mol. Cell Biol. 2006, 26, 2560–2569. [Google Scholar] [CrossRef]
- He, J.; Shen, L.; Wan, M.; Taranova, O.; Wu, H.; Zhang, Y. Kdm2b Maintains Murine Embryonic Stem Cell Status by Recruiting PRC1 Complex to CpG Islands of Developmental Genes. Nat. Cell Biol. 2013, 15, 373–384. [Google Scholar] [CrossRef]
- Blackledge, N.P.; Farcas, A.M.; Kondo, T.; King, H.W.; McGouran, J.F.; Hanssen, L.L.P.; Ito, S.; Cooper, S.; Kondo, K.; Koseki, Y.; et al. Variant PRC1 Complex-Dependent H2A Ubiquitylation Drives PRC2 Recruitment and Polycomb Domain Formation. Cell 2014, 157, 1445–1459. [Google Scholar] [CrossRef]
- Wu, X.; Johansen, J.V.; Helin, K. Fbxl10/Kdm2b Recruits Polycomb Repressive Complex 1 to CpG Islands and Regulates H2A Ubiquitylation. Mol. Cell 2013, 49, 1134–1146. [Google Scholar] [CrossRef]
- Chamberlain, S.J.; Yee, D.; Magnuson, T. Polycomb Repressive Complex 2 Is Dispensable for Maintenance of Embryonic Stem Cell Pluripotency. Stem Cells 2008, 26, 1496–1505. [Google Scholar] [CrossRef]
- Walker, E.; Chang, W.Y.; Hunkapiller, J.; Cagney, G.; Garcha, K.; Torchia, J.; Krogan, N.J.; Reiter, J.F.; Stanford, W.L. Polycomb-like 2 Associates with PRC2 and Regulates Transcriptional Networks during Mouse Embryonic Stem Cell Self-Renewal and Differentiation. Cell Stem Cell 2010, 6, 153–166. [Google Scholar] [CrossRef]
- Bracken, A.P.; Helin, K. Polycomb Group Proteins: Navigators of Lineage Pathways Led Astray in Cancer. Nat. Rev. Cancer 2009, 9, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Sauvageau, M.; Sauvageau, G. Polycomb Group Proteins: Multi-Faceted Regulators of Somatic Stem Cells and Cancer. Cell Stem Cell 2010, 7, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Kobayashi, S.; Hiratani, I. Epigenetic Differences between Naïve and Primed Pluripotent Stem Cells. Cell Mol. Life Sci. 2018, 75, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Boyer, L.A.; Plath, K.; Zeitlinger, J.; Brambrink, T.; Medeiros, L.A.; Lee, T.I.; Levine, S.S.; Wernig, M.; Tajonar, A.; Ray, M.K.; et al. Polycomb Complexes Repress Developmental Regulators in Murine Embryonic Stem Cells. Nature 2006, 441, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Abolpour Mofrad, S.; Kuenzel, K.; Friedrich, O.; Gilbert, D.F. Optimizing Neuronal Differentiation of Human Pluripotent NT2 Stem Cells in Monolayer Cultures. Dev. Growth Differ. 2016, 58, 664–676. [Google Scholar] [CrossRef]
- Tegenge, M.A.; Roloff, F.; Bicker, G. Rapid Differentiation of Human Embryonal Carcinoma Stem Cells (NT2) into Neurons for Neurite Outgrowth Analysis. Cell Mol. Neurobiol. 2011, 31, 635–643. [Google Scholar] [CrossRef]
- Stern, M.; Gierse, A.; Tan, S.; Bicker, G. Human Ntera2 Cells as a Predictive in Vitro Test System for Developmental Neurotoxicity. Arch. Toxicol. 2014, 88, 127–136. [Google Scholar] [CrossRef]
- Human NT2 Neurons Express a Large Variety of Neurotransmission Phenotypes in Vitro. J. Comp. Neurol. 2000, 422, 380–395. [CrossRef]
- Haile, Y.; Fu, W.; Shi, B.; Westaway, D.; Baker, G.; Jhamandas, J.; Giuliani, F. Characterization of the NT2-Derived Neuronal and Astrocytic Cell Lines as Alternative in Vitro Models for Primary Human Neurons and Astrocytes. J. Neurosci. Res. 2014, 92, 1187–1198. [Google Scholar] [CrossRef]
- Lee, V.M.; Andrews, P.W. Differentiation of NTERA-2 Clonal Human Embryonal Carcinoma Cells into Neurons Involves the Induction of All Three Neurofilament Proteins. J. Neurosci. 1986, 6, 514–521. [Google Scholar] [CrossRef]
- Oliviero, G.; Munawar, N.; Watson, A.; Streubel, G.; Manning, G.; Bardwell, V.; Bracken, A.P.; Cagney, G. The Variant Polycomb Repressor Complex 1 Component PCGF1 Interacts with a Pluripotency Sub-Network That Includes DPPA4, a Regulator of Embryogenesis. Sci. Rep. 2015, 5, 18388. Available online: https://www.nature.com/articles/srep18388 (accessed on 30 July 2024). [CrossRef] [PubMed]
- Krogan, N.J.; Cagney, G.; Yu, H.; Zhong, G.; Guo, X.; Ignatchenko, A.; Li, J.; Pu, S.; Datta, N.; Tikuisis, A.P.; et al. Global Landscape of Protein Complexes in the Yeast Saccharomyces Cerevisiae. Nature 2006, 440, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Gavin, A.-C.; Aloy, P.; Grandi, P.; Krause, R.; Boesche, M.; Marzioch, M.; Rau, C.; Jensen, L.J.; Bastuck, S.; Dümpelfeld, B.; et al. Proteome Survey Reveals Modularity of the Yeast Cell Machinery. Nature 2006, 440, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Carlson, A.; Sinitcyn, P.; Mann, M.; Cox, J. Visualization of LC-MS/MS Proteomics Data in MaxQuant. Proteomics 2015, 15, 1453–1456. Available online: https://pubmed.ncbi.nlm.nih.gov/25644178/ (accessed on 30 July 2024). [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, Scalable Generation of High-Quality Protein Multiple Sequence Alignments Using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Sanchez-Pulido, L.; Devos, D.; Sung, Z.R.; Calonje, M. RAWUL: A New Ubiquitin-like Domain in PRC1 Ring Finger Proteins That Unveils Putative Plant and Worm PRC1 Orthologs. BMC Genom. 2008, 9, 308. [Google Scholar] [CrossRef]
- Chittock, E.C.; Latwiel, S.; Miller, T.C.R.; Müller, C.W. Molecular Architecture of Polycomb Repressive Complexes. Biochem. Soc. Trans. 2017, 45, 193–205. [Google Scholar] [CrossRef]
- Junco, S.E.; Wang, R.; Gaipa, J.C.; Taylor, A.B.; Schirf, V.; Gearhart, M.D.; Bardwell, V.J.; Demeler, B.; Hart, P.J.; Kim, C.A. Structure of the Polycomb Group Protein PCGF1 in Complex with BCOR Reveals Basis for Binding Selectivity of PCGF Homologs. Structure 2013, 21, 665–671. [Google Scholar] [CrossRef]
- Thompson, J.D.; Poch, O. New Challenges and Strategies for Multiple Sequence Alignment in the Proteomics Era. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 475–492. ISBN 978-1-59259-890-8. [Google Scholar]
- de Napoles, M.; Mermoud, J.E.; Wakao, R.; Tang, Y.A.; Endoh, M.; Appanah, R.; Nesterova, T.B.; Silva, J.; Otte, A.P.; Vidal, M.; et al. Polycomb Group Proteins Ring1A/B Link Ubiquitylation of Histone H2A to Heritable Gene Silencing and X Inactivation. Dev. Cell 2004, 7, 663–676. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Erdjument-Bromage, H.; Vidal, M.; Tempst, P.; Jones, R.S.; Zhang, Y. Role of Histone H2A Ubiquitination in Polycomb Silencing. Nature 2004, 431, 873–878. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, J.; Bonasio, R.; Strino, F.; Sawai, A.; Parisi, F.; Kluger, Y.; Reinberg, D. PCGF Homologs, CBX Proteins, and RYBP Define Functionally Distinct PRC1 Family Complexes. Mol. Cell 2012, 45, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Hauri, S.; Comoglio, F.; Seimiya, M.; Gerstung, M.; Glatter, T.; Hansen, K.; Aebersold, R.; Paro, R.; Gstaiger, M.; Beisel, C. A High-Density Map for Navigating the Human Polycomb Complexome. Cell Rep. 2016, 17, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Wiederschain, D.; Chen, L.; Johnson, B.; Bettano, K.; Jackson, D.; Taraszka, J.; Wang, Y.K.; Jones, M.D.; Morrissey, M.; Deeds, J.; et al. Contribution of Polycomb Homologues Bmi-1 and Mel-18 to Medulloblastoma Pathogenesis. Mol. Cell Biol. 2007, 27, 4968–4979. [Google Scholar] [CrossRef] [PubMed]
- Morey, L.; Santanach, A.; Blanco, E.; Aloia, L.; Nora, E.P.; Bruneau, B.G.; Di Croce, L. Polycomb Regulates Mesoderm Cell Fate-Specification in Embryonic Stem Cells through Activation and Repression Mechanisms. Cell Stem Cell 2015, 17, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, Y.A.; Lennartsson, A.; Ehsani, R.; Kulakovskiy, I.V.; Vorontsov, I.E.; Panahandeh, P.; Khimulya, G.; Kasukawa, T.; FANTOM Consortium; Drabløs, F. EpiFactors: A Comprehensive Database of Human Epigenetic Factors and Complexes. Database 2015, 2015, bav067. Available online: https://academic.oup.com/database/article/doi/10.1093/database/bav067/2433200 (accessed on 30 July 2024). [CrossRef]
- Xu, Y.; Zhang, S.; Lin, S.; Guo, Y.; Deng, W.; Zhang, Y.; Xue, Y. WERAM: A Database of Writers, Erasers and Readers of Histone Acetylation and Methylation in Eukaryotes. Nucleic Acids Res. 2017, 45, D264–D270. [Google Scholar] [CrossRef]
- Oliviero, G.; Kovalchuk, S.; Rogowska-Wrzesinska, A.; Schwämmle, V.; Jensen, O.N. Distinct and Diverse Chromatin Proteomes of Ageing Mouse Organs Reveal Protein Signatures That Correlate with Physiological Functions. eLife 2022, 11, e73524. Available online: https://elifesciences.org/articles/73524 (accessed on 30 July 2024). [CrossRef]
- Albert, T.K.; Hanzawa, H.; Legtenberg, Y.I.A.; de Ruwe, M.J.; van den Heuvel, F.A.J.; Collart, M.A.; Boelens, R.; Timmers, H.T.M. Identification of a Ubiquitin–Protein Ligase Subunit within the CCR4–NOT Transcription Repressor Complex. EMBO J. 2002, 21, 355–364. [Google Scholar] [CrossRef]
- Vissers, L.E.L.M.; Kalvakuri, S.; de Boer, E.; Geuer, S.; Oud, M.; van Outersterp, I.; Kwint, M.; Witmond, M.; Kersten, S.; Polla, D.L.; et al. De Novo Variants in CNOT1, a Central Component of the CCR4-NOT Complex Involved in Gene Expression and RNA and Protein Stability, Cause Neurodevelopmental Delay. Am. J. Hum. Genet. 2020, 107, 164–172. [Google Scholar] [CrossRef]
- Gibbons, R.J.; McDowell, T.L.; Raman, S.; O’Rourke, D.M.; Garrick, D.; Ayyub, H.; Higgs, D.R. Mutations in ATRX, Encoding a SWI/SNF-like Protein, Cause Diverse Changes in the Pattern of DNA Methylation. Nat. Genet. 2000, 24, 368–371. [Google Scholar] [CrossRef]
- Sokpor, G.; Xie, Y.; Rosenbusch, J.; Tuoc, T. Chromatin Remodeling BAF (SWI/SNF) Complexes in Neural Development and Disorders. Front. Mol. Neurosci. 2017, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Smits, A.H.; Jansen, P.W.T.C.; Poser, I.; Hyman, A.A.; Vermeulen, M. Stoichiometry of Chromatin-Associated Protein Complexes Revealed by Label-Free Quantitative Mass Spectrometry-Based Proteomics. Nucleic Acids Res. 2013, 41, e28. [Google Scholar] [CrossRef] [PubMed]
- Taherbhoy, A.M.; Huang, O.W.; Cochran, A.G. BMI1-RING1B Is an Autoinhibited RING E3 Ubiquitin Ligase. Nat. Commun. 2015, 6, 7621. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, T.; van Lohuizen, M.; van der Lugt, N.; Mizutani-Koseki, Y.; Kanno, M.; Taniguchi, M.; Vidal, M.; Alkema, M.; Berns, A.; Koseki, H. Mice Doubly Deficient for the Polycomb Group Genes Mel18 and Bmi1 Reveal Synergy and Requirement for Maintenance but Not Initiation of Hox Gene Expression. Development 2001, 128, 1587–1597. [Google Scholar] [CrossRef]
- Bracken, A.P.; Kleine-Kohlbrecher, D.; Dietrich, N.; Pasini, D.; Gargiulo, G.; Beekman, C.; Theilgaard-Mönch, K.; Minucci, S.; Porse, B.T.; Marine, J.C.; et al. The Polycomb Group Proteins Bind throughout the INK4A-ARF Locus and Are Disassociated in Senescent Cells. Genes Dev. 2007, 21, 525–530. [Google Scholar] [CrossRef]
- Maertens, G.N.; El Messaoudi-Aubert, S.; Racek, T.; Stock, J.K.; Nicholls, J.; Rodriguez-Niedenführ, M.; Gil, J.; Peters, G. Several Distinct Polycomb Complexes Regulate and Co-Localize on the INK4a Tumor Suppressor Locus. PLOS ONE 2009, 4, e6380. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0006380 (accessed on 30 July 2024). [CrossRef]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular Senescence and Tumor Suppressor Gene P16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Souroullas, G.P.; Diekman, B.O.; Krishnamurthy, J.; Hall, B.M.; Sorrentino, J.A.; Parker, J.S.; Sessions, G.A.; Gudkov, A.V.; Sharpless, N.E. Cells Exhibiting Strong p16INK4a Promoter Activation in Vivo Display Features of Senescence. Proc. Natl. Acad. Sci. USA 2019, 116, 2603–2611. [Google Scholar] [CrossRef]
- Scelfo, A.; Piunti, A.; Pasini, D. The Controversial Role of the Polycomb Group Proteins in Transcription and Cancer: How Much Do We Not Understand Polycomb Proteins? FEBS J. 2014, 282, 1703–1722. Available online: https://febs.onlinelibrary.wiley.com/doi/full/10.1111/febs.13112 (accessed on 30 July 2024). [CrossRef]
- Tamburri, S.; Conway, E.; Pasini, D. Polycomb-Dependent Histone H2A Ubiquitination Links Developmental Disorders with Cancer. Trends Genet. 2022, 38, 333–352. [Google Scholar] [CrossRef]
- Fursova, N.A.; Blackledge, N.P.; Nakayama, M.; Ito, S.; Koseki, Y.; Farcas, A.M.; King, H.W.; Koseki, H.; Klose, R.J. Synergy between Variant PRC1 Complexes Defines Polycomb-Mediated Gene Repression. Mol. Cell 2019, 74, 1020–1036.e8. [Google Scholar] [CrossRef] [PubMed]
- Moqri, M.; Cipriano, A.; Simpson, D.J.; Rasouli, S.; Murty, T.; de Jong, T.A.; Nachun, D.; de Sena Brandine, G.; Ying, K.; Tarkhov, A.; et al. PRC2-AgeIndex as a Universal Biomarker of Aging and Rejuvenation. Nat. Commun. 2024, 15, 5956. [Google Scholar] [CrossRef] [PubMed]
- Strübbe, G.; Popp, C.; Schmidt, A.; Pauli, A.; Ringrose, L.; Beisel, C.; Paro, R. Polycomb Purification by in Vivo Biotinylation Tagging Reveals Cohesin and Trithorax Group Proteins as Interaction Partners. Proc. Natl. Acad. Sci. USA 2011, 108, 5572–5577. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.S.; Weiss, A.; Erdjument-Bromage, H.; Shao, Z.; Tempst, P.; Kingston, R.E. The Core of the Polycomb Repressive Complex Is Compositionally and Functionally Conserved in Flies and Humans. Mol. Cell Biol. 2002, 22, 6070–6078. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Lin, Q.; Ensenat-Waser, R.; Rose-John, S.; Zenke, M. Polycomb Group Protein Bmi1 Promotes Hematopoietic Cell Development from Embryonic Stem Cells. Stem Cells Dev. 2012, 21, 121–132. [Google Scholar] [CrossRef]
- Mai, J.; Peng, X.-D.; Tang, J.; Du, T.; Chen, Y.-H.; Wang, Z.-F.; Zhang, H.-L.; Huang, J.-H.; Zhong, Z.-Y.; Yang, D.; et al. AKT-Mediated Regulation of Chromatin Ubiquitylation and Tumorigenesis through Mel18 Phosphorylation. Oncogene 2021, 40, 2422–2436. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Jang, K.-S.; Shin, D.-H.; Oh, M.-Y.; Kim, H.-J.; Kim, Y.; Kong, G. Mel-18 Negatively Regulates INK4a/ARF-Independent Cell Cycle Progression via Akt Inactivation in Breast Cancer. Cancer Res. 2008, 68, 4201–4209. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Scheijen, B.; Voncken, J.W.; Kieboom, K.; Berns, A.; van Lohuizen, M. Bmi-1 Collaborates with c-Myc in Tumorigenesis by Inhibiting c-Myc-Induced Apoptosis via INK4a/ARF. Genes. Dev. 1999, 13, 2678–2690. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Kieboom, K.; Marino, S.; DePinho, R.A.; van Lohuizen, M. The Oncogene and Polycomb-Group Gene Bmi-1 Regulates Cell Proliferation and Senescence through the Ink4a Locus. Nature 1999, 397, 164–168. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal Sample Preparation Method for Proteome Analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant Enables High Peptide Identification Rates, Individualized p.p.b.-Range Mass Accuracies and Proteome-Wide Protein Quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Mellacheruvu, D.; Wright, Z.; Couzens, A.L.; Lambert, J.-P.; St-Denis, N.A.; Li, T.; Miteva, Y.V.; Hauri, S.; Sardiu, M.E.; Low, T.Y.; et al. The CRAPome: A Contaminant Repository for Affinity Purification-Mass Spectrometry Data. Nat. Methods 2013, 10, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus Computational Platform for Comprehensive Analysis of (Prote)Omics Data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate Proteome-Wide Label-Free Quantification by Delayed Normalization and Maximal Peptide Ratio Extraction, Termed MaxLFQ. Mol. Cell Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimpoor, M.; Goeman, J.J. Inflated False Discovery Rate Due to Volcano Plots: Problem and Solutions. Brief. Bioinform. 2021, 22, bbab053. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munawar, N.; Wynne, K.; Oliviero, G. PRC1 Protein Subcomplexes Architecture: Focus on the Interplay between Distinct PCGF Subunits in Protein Interaction Networks. Int. J. Mol. Sci. 2024, 25, 9809. https://doi.org/10.3390/ijms25189809
Munawar N, Wynne K, Oliviero G. PRC1 Protein Subcomplexes Architecture: Focus on the Interplay between Distinct PCGF Subunits in Protein Interaction Networks. International Journal of Molecular Sciences. 2024; 25(18):9809. https://doi.org/10.3390/ijms25189809
Chicago/Turabian StyleMunawar, Nayla, Kieran Wynne, and Giorgio Oliviero. 2024. "PRC1 Protein Subcomplexes Architecture: Focus on the Interplay between Distinct PCGF Subunits in Protein Interaction Networks" International Journal of Molecular Sciences 25, no. 18: 9809. https://doi.org/10.3390/ijms25189809
APA StyleMunawar, N., Wynne, K., & Oliviero, G. (2024). PRC1 Protein Subcomplexes Architecture: Focus on the Interplay between Distinct PCGF Subunits in Protein Interaction Networks. International Journal of Molecular Sciences, 25(18), 9809. https://doi.org/10.3390/ijms25189809





