The Effect of Physical Activity/Exercise on miRNA Expression and Function in Non-Communicable Diseases—A Systematic Review
Abstract
:1. Introduction
2. Results
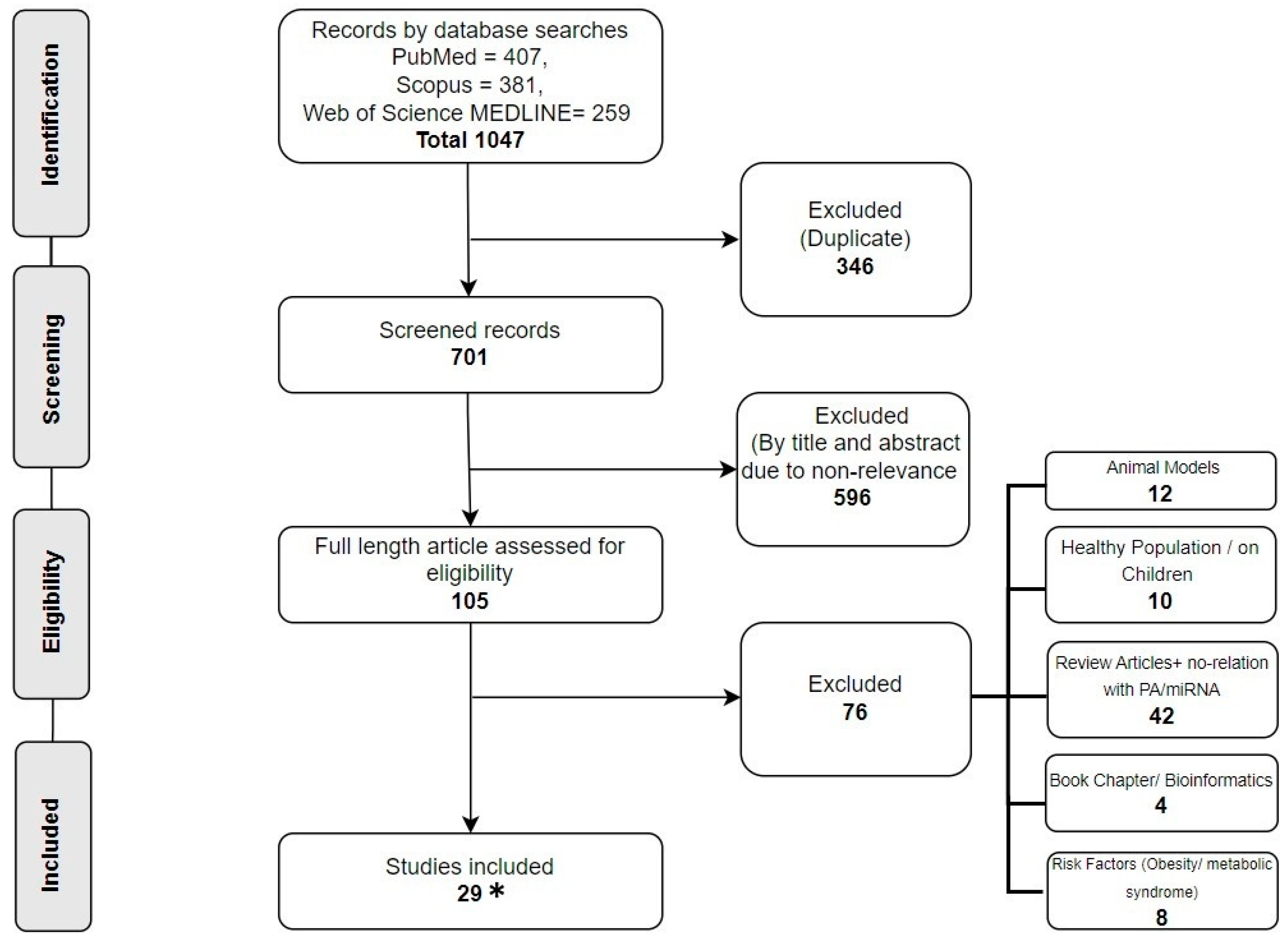
2.1. Research Selection
2.2. Study Characteristics
2.3. Cancer
2.4. Type 2 Diabetes Mellitus (T2DM)
2.5. Chronic Obstructive Pulmonary Disease (COPD)
2.6. Cardiovascular Diseases (CVDs)
3. Discussion
3.1. Cancers
3.2. Type 2 Diabetes Mellitus (T2DM)
3.3. Chronic Obstructive Pulmonary Disease (COPD)
3.4. Cardiovascular Diseases (CVDs)
4. Materials and Methods
4.1. Information Sources
4.2. Search Strategy
4.3. Eligibility Criteria
4.4. Data Collection
4.5. Data Extraction
4.6. Risk of Bias (Quality) Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jiménez-Avalos, J.A.; Fernández-Macías, J.C.; González-Palomo, A.K. Circulating exosomal MicroRNAs: New non-invasive biomarkers of non-communicable disease. Mol. Biol. Rep. 2021, 48, 961–967. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Horsburgh, S.; Robson-Ansley, P.; Adams, R.; Smith, C. Exercise and inflammation-related epigenetic modifications: Focus on DNA methylation. Exerc. Immunol. Rev. 2015, 21, 26–41. [Google Scholar]
- Bhome, R.; Del Vecchio, F.; Lee, G.-H.; Bullock, M.D.; Primrose, J.N.; Sayan, A.E.; Mirnezami, A.H. Exosomal microRNAs (exomiRs): Small molecules with a big role in cancer. Cancer Lett. 2018, 420, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ni, Y.-Q.; Xu, H.; Xiang, Q.-Y.; Zhao, Y.; Zhan, J.-K.; He, J.-Y.; Li, S.; Liu, Y.-S. Roles and mechanisms of exosomal non-coding RNAs in human health and diseases. Signal Transduct. Target. Ther. 2021, 6, 383. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.M.; Sanad, E.F.; Hamdy, N.M. MicroRNAs’ role in the environment-related non-communicable diseases and link to multidrug resistance, regulation, or alteration. Environ. Sci. Pollut. Res. Int. 2021, 28, 36984–37000. [Google Scholar] [CrossRef]
- Mathers, J.C.; Strathdee, G.; Relton, C.L. Induction of epigenetic alterations by dietary and other environmental factors. Adv. Genet. 2010, 71, 3–39. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Hernandez, P.; Castilla-Silgado, J.; Coto-Vilcapoma, A.; Fernández-Sanjurjo, M.; Fernández-García, B.; Tomás-Zapico, C.; Iglesias-Gutiérrez, E. Modulation of microRNAs through Lifestyle Changes in Alzheimer’s Disease. Nutrients 2023, 15, 3688. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Quattrocchi, A.; Agrifoglio, O.; Agodi, A. The Role of miRNAs as Biomarkers for Pregnancy Outcomes: A Comprehensive Review. Int. J. Genom. 2017, 2017, 8067972. [Google Scholar] [CrossRef]
- Witvrouwen, I.; Gevaert, A.B.; Possemiers, N.; Beckers, P.J.; Vorlat, A.; Heidbuchel, H.; Van Laere, S.J.; Van Craenenbroeck, A.H.; Van Craenenbroeck, E.M. Circulating microRNA as predictors for exercise response in heart failure with reduced ejection fraction. Eur. J. Prev. Cardiol. 2021, 28, 1673–1681. [Google Scholar] [CrossRef]
- Pheiffer, C.; Pheiffer, C.; Dias, S.; Dias, S.; Mendham, A.E.; Mendham, A.E.; Jack, B.; Jack, B.; Willmer, T.; Willmer, T.; et al. Changes in subcutaneous adipose tissue microRNA expression in response to exercise training in African women with obesity. Sci. Rep. 2022, 12, 18408. [Google Scholar] [CrossRef] [PubMed]
- Attwaters, M.; Hughes, S.M. Cellular and molecular pathways controlling muscle size in response to exercise. FEBS J. 2022, 289, 1428–1456. [Google Scholar] [CrossRef] [PubMed]
- Barber, J.L.; Zellars, K.N.; Barringhaus, K.G.; Bouchard, C.; Spinale, F.G.; Sarzynski, M.A. The Effects of Regular Exercise on Circulating Cardiovascular-related MicroRNAs. Sci. Rep. 2019, 9, 7527. [Google Scholar] [CrossRef] [PubMed]
- Momma, H.; Kawakami, R.; Honda, T.; Sawada, S.S. Muscle-strengthening activities are associated with lower risk and mortality in major non-communicable diseases: A systematic review and meta-analysis of cohort studies. Br. J. Sports Med. 2022, 56, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Ballin, M.; Nordström, P. Does exercise prevent major non-communicable diseases and premature mortality? A critical review based on results from randomized controlled trials. J. Intern. Med. 2021, 290, 1112–1129. [Google Scholar] [CrossRef] [PubMed]
- Ehtesham, N.; Shahrbanian, S.; Valadiathar, M.; Mowla, S.J. Modulations of obesity-related microRNAs after exercise intervention: A systematic review and bioinformatics analysis. Mol. Biol. Rep. 2021, 48, 2817–2831. [Google Scholar] [CrossRef] [PubMed]
- Sapp, R.M.; Shill, D.D.; Roth, S.M.; Hagberg, J.M. Circulating microRNAs in acute and chronic exercise: More than mere biomarkers. J. Appl. Physiol. 2017, 122, 702–717. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Vitucci, D.; Orlandella, F.M.; Terracciano, A.; Mariniello, R.M.; Imperlini, E.; Grazioli, E.; Orrù, S.; Krustrup, P.; Salvatore, G.; et al. Regular football training down-regulates miR-1303 muscle expression in veterans. Eur. J. Appl. Physiol. 2021, 121, 2903–2912. [Google Scholar] [CrossRef] [PubMed]
- Orlandella, F.M.; De Stefano, A.E.; Braile, M.; Luciano, N.; Mancini, A.; Franzese, M.; Buono, P.; Salvatore, G. Unveiling the miRNAs responsive to physical activity/exercise training in cancer: A systematic review. Crit. Rev. Oncol. Hematol. 2022, 180, 103844. [Google Scholar] [CrossRef]
- Fernandes-Silva, M.M.; Carvalho, V.O.; Guimarães, G.V.; Bacal, F.; Bocchi, E.A. Exercício físico e microRNAs: Novas fronteiras na insuficiência cardíaca. Arq. Bras. Cardiol. 2012, 98, 459–466. [Google Scholar] [CrossRef]
- Gazova, A.; Samakova, A.; Laczo, E.; Hamar, D.; Polakovicova, M.; Jurikova, M.; Kyselovic, J. Clinical utility of miRNA-1, miRNA-29g and miRNA-133s plasma levels in prostate cancer patients with high-intensity training after androgen-deprivation therapy. Physiol. Res. 2019, 68 (Suppl. S2), S139–S147. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.; Sheean, P.; Matthews, L.; Chitambar, C.R.; Banerjee, A.; Visotcky, A.; Bonini, M.; Stolley, M. Circulating miRNAs as early indicators of diet and physical activity response in women with metastatic breast cancer. Future Sci. OA 2021, 7, FSO694. [Google Scholar] [CrossRef] [PubMed]
- Hagstrom, A.D.; Denham, J. microRNAs in High and Low Responders to Resistance Training in Breast Cancer Survivors. Int. J. Sports Med. 2018, 39, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.; Isanejad, A.; Sadighi, S.; Khalighfard, S.; Alizadeh, A.M. Effect of a high-intensity interval training on serum microRNA levels in women with breast cancer undergoing hormone therapy. A single-blind randomized trial. Ann. Phys. Rehabil. Med. 2019, 62, 329–335. [Google Scholar] [CrossRef]
- Adams, B.D.; Arem, H.; Hubal, M.J.; Cartmel, B.; Li, F.; Harrigan, M.; Sanft, T.; Cheng, C.J.; Pusztai, L.; Irwin, M.L. Exercise and weight loss interventions and miRNA expression in women with breast cancer. Breast Cancer Res. Treat. 2018, 170, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Akbarinia, A.; Kargarfard, M.; Naderi, M. Aerobic training improves platelet function in type 2 diabetic patients: Role of microRNA-130a and GPIIb. Acta Diabetol. 2018, 55, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, I.; Silvestri, S.; Marcheggiani, F.; Olivieri, F.; Galeazzi, R.; Antonicelli, R.; Recchioni, R.; Marcheselli, F.; Bacchetti, T.; Tiano, L.; et al. Three Months Monitored Metabolic Fitness Modulates Cardiovascular Risk Factors in Diabetic Patients. Diabetes Metab. J. 2019, 43, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Morais Junior, G.S.; Souza, V.C.; Machado-Silva, W.; Henriques, A.D.; Alves, A.M.; Morais, D.B.; Nóbrega, O.T.; Brito, C.J.; Silva, R.J.d.S. Acute strength training promotes responses in whole blood circulating levels of miR-146a among older adults with type 2 diabetes mellitus. Clin. Interv. Aging 2017, 12, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Ghodrat, L.; Jahromi, I.R.; Jahromi, M.K.; Nemati, J. Effect of performing high-intensity interval training and resistance training on the same day vs. different days in women with type 2 diabetes. Eur. J. Appl. Physiol. 2022, 122, 2037–2047. [Google Scholar] [CrossRef]
- Olioso, D.; Dauriz, M.; Bacchi, E.; Negri, C.; Santi, L.; Bonora, E.; Moghetti, P. Effects of Aerobic and Resistance Training on Circulating Micro-RNA Expression Profile in Subjects with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 1119–1130. [Google Scholar] [CrossRef]
- Rowlands, D.S.; Page, R.A.; Sukala, W.R.; Giri, M.; Ghimbovschi, S.D.; Hayat, I.; Cheema, B.S.; Lys, I.; Leikis, M.; Sheard, P.W.; et al. Multi-omic integrated networks connect DNA methylation and miRNA with skeletal muscle plasticity to chronic exercise in Type 2 diabetic obesity. Physiol. Genom. 2014, 46, 747–765. [Google Scholar] [CrossRef]
- Simaitis, S.; Schulte-Körne, B.; Schiffer, T.; Bloch, W.; Predel, H.-G.; Brixius, K.; Brinkmann, C. Evidence for Training-Induced Changes in miRNA Levels in the Skeletal Muscle of Patients With Type 2 Diabetes Mellitus. Front. Physiol. 2020, 11, 599651. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Ahmadizad, S.; Naderi, M. Effects of endurance training on hsa-miR-223, P2RY12 receptor expression and platelet function in type 2 diabetic patients. Clin. Hemorheol. Microcirc. 2018, 68, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.; Kargarfard, M.; Braune, S.; Jung, F.; Naderi, M. Long-term aerobic exercise training in type two diabetic patients alters the expression of miRNA-223 and its corresponding target, the P2RY12 receptor, attenuating platelet function. Clin. Hemorheol. Microcirc. 2022, 80, 107–116. [Google Scholar] [CrossRef]
- Lewis, A.; Riddoch-Contreras, J.; Natanek, S.A.; Donaldson, A.; Man, W.D.-C.; Moxham, J.; Hopkinson, N.S.; Polkey, M.I.; Kemp, P.R. Downregulation of the serum response factor/miR-1 axis in the quadriceps of patients with COPD. Thorax 2012, 67, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, M.; Gao, H.; Han, S.; Liu, J.; Sun, X.; Zhao, L. Regulation of whole-transcriptome sequencing expression in COPD after personalized precise exercise training: A pilot study. Respir. Res. 2023, 24, 156. [Google Scholar] [CrossRef]
- Antunes-Correa, L.M.; Nobre, T.S.; Groehs, R.V.; Alves, M.J.N.N.; Fernandes, T.; Couto, G.K.; Rondon, M.U.P.B.; Oliveira, P.; Lima, M.; Mathias, W.; et al. Molecular basis for the improvement in muscle metaboreflex and mechanoreflex control in exercise-trained humans with chronic heart failure. Am. J. Physiol. Circ. Physiol. 2014, 307, H1655–H1666. [Google Scholar] [CrossRef] [PubMed]
- Taraldsen, M.D.; Wiseth, R.; Videm, V.; Bye, A.; Madssen, E. Associations between circulating microRNAs and coronary plaque characteristics: Potential impact from physical exercise. Physiol. Genom. 2022, 54, 129–140. [Google Scholar] [CrossRef]
- Witvrouwen, I.; Gevaert, A.B.; Possemiers, N.; Ectors, B.; Stoop, T.; Goovaerts, I.; Boeren, E.; Hens, W.; Beckers, P.J.; Vorlat, A.; et al. Plasma-Derived microRNAs Are Influenced by Acute and Chronic Exercise in Patients With Heart Failure With Reduced Ejection Fraction. Front. Physiol. 2021, 12, 736494. [Google Scholar] [CrossRef]
- Antunes-Correa, L.M.; Trevizan, P.F.; Bacurau, A.V.; Ferreira-Santos, L.; Gomes, J.L.; Urias, U.; Oliveira, P.A.; Alves, M.J.N.; de Almeida, D.R.; Brum, P.C.; et al. Effects of aerobic and inspiratory training on skeletal muscle microRNA-1 and downstream-associated pathways in patients with heart failure. J. Cachex-Sarcopenia Muscle 2019, 11, 89–102. [Google Scholar] [CrossRef]
- Mayr, B.; Müller, E.E.; Schäfer, C.; Droese, S.; Schönfelder, M.; Niebauer, J. Exercise-induced changes in miRNA expression in coronary artery disease. Clin. Chem. Lab. Med. 2021, 59, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, A.B.; Witvrouwen, I.; Van Craenenbroeck, A.H.; Van Laere, S.J.; Boen, J.R.A.; Van de Heyning, C.M.; Belyavskiy, E.; Mueller, S.; Winzer, E.; Duvinage, A.; et al. miR-181c level predicts response to exercise training in patients with heart failure and preserved ejection fraction: An analysis of the OptimEx-Clin trial. Eur. J. Prev. Cardiol. 2021, 28, 1722–1733. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Yang, X.-Y.; Wang, J.-S. MicroRNA-126 Level Increases During Exercise Rehabilitation of Heart Failure with a Preserved Ejection Fraction. Int. J. Gen. Med. 2021, 14, 3397–3404. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhou, Q.; Che, L.; Das, S.; Wang, L.; Jiang, J.; Li, G.; Xu, J.; Yao, J.; Wang, H.; et al. Circulating miR-21, miR-378, and miR-940 increase in response to an acute exhaustive exercise in chronic heart failure patients. Oncotarget 2016, 7, 12414–12425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ren, L.; Yu, M.; Liu, X.; Ma, W.; Huang, L.; Li, X.; Ye, X. S-equol inhibits proliferation and promotes apoptosis of human breast cancer MCF-7 cells via regulating miR-10a-5p and PI3K/AKT pathway. Arch. Biochem. Biophys. 2019, 672, 108064. [Google Scholar] [CrossRef] [PubMed]
- Riedel, S.; Radzanowski, S.; Bowen, T.S.; Werner, S.; Erbs, S.; Schuler, G.; Adams, V. Exercise training improves high-density lipoprotein-mediated transcription of proangiogenic microRNA in endothelial cells. Eur. J. Prev. Cardiol. 2015, 22, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Sieland, J.; Niederer, D.; Engeroff, T.; Vogt, L.; Troidl, C.; Schmitz-Rixen, T.; Banzer, W.; Troidl, K. Changes in miRNA expression in patients with peripheral arterial vascular disease during moderate- and vigorous-intensity physical activity. Eur. J. Appl. Physiol. 2023, 123, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Soerjomataram, I. The Changing Global Burden of Cancer: Transitions in Human Development and Implications for Cancer Prevention and Control. In Cancer: Disease Control Priorities, 3rd ed.; Gelband, H., Jha, P., Sankaranarayanan, R., Horton, S., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.-T.; Xu, W.-X.; Li, H.; Xia, M. Emerging roles of MiR-133a in human cancers. J. Cancer 2021, 12, 198–206. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, N.; Wicha, M.S.; Luo, M. The Roles of the Let-7 Family of MicroRNAs in the Regulation of Cancer Stemness. Cells 2021, 10, 2415. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, C.; Wu, Z.; Wang, Y.; Yin, W.; Lin, Y.; Zhou, L.; Lu, J. Upregulation of microRNA-1 inhibits proliferation and metastasis of breast cancer. Mol. Med. Rep. 2020, 22, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Humphries, B.; Yang, C.; Wang, Z. MiR-205 Dysregulations in Breast Cancer: The Complexity and Opportunities. Non-Coding RNA 2019, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Kousar, K.; Ahmad, T.; Abduh, M.S.; Kanwal, B.; Shah, S.S.; Naseer, F.; Anjum, S. miRNAs in Regulation of Tumor Microenvironment, Chemotherapy Resistance, Immunotherapy Modulation and miRNA Therapeutics in Cancer. Int. J. Mol. Sci. 2022, 23, 13822. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.-Y.; Xue, M.; Zhang, Q.-C.; Su, C.-F. In vivo and in vitro effects of microRNA-27a on proliferation, migration and invasion of breast cancer cells through targeting of SFRP1 gene via Wnt/β-catenin signaling pathway. Oncotarget 2017, 8, 15507–15519. [Google Scholar] [CrossRef]
- Roglic, G. WHO Global report on diabetes: A summary. Int. J. Noncommunicable Dis. 2016, 1, 3–8. [Google Scholar] [CrossRef]
- Wang, M.; Xie, K.; Zhao, S.; Jia, N.; Zong, Y.; Gu, W.; Cai, Y. Aerobic exercise improves cognitive impairment in mice with type 2 diabetes by regulating the MALAT1/miR-382-3p/BDNF signaling pathway in serum-exosomes. Mol. Med. 2023, 29, 130. [Google Scholar] [CrossRef]
- Kakouros, N.; Rade, J.J.; Kourliouros, A.; Resar, J.R. Platelet function in patients with diabetes mellitus: From a theoretical to a practical perspective. Int. J. Endocrinol. 2011, 2011, 742719. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, X.; Shan, P.-F. MicroRNAs and Cardiovascular Disease in Diabetes Mellitus. BioMed Res. Int. 2017, 2017, 4080364. [Google Scholar] [CrossRef]
- Zeitouni, M.; Clare, R.M.; Chiswell, K.; Abdulrahim, J.; Shah, N.; Pagidipati, N.P.; Shah, S.H.; Roe, M.T.; Patel, M.R.; Jones, W.S. Risk Factor Burden and Long-Term Prognosis of Patients with Premature Coronary Artery Disease. J. Am. Heart Assoc. 2020, 9, e017712. [Google Scholar] [CrossRef]
- Alipoor, B.; Ghaedi, H.; Meshkani, R.; Torkamandi, S.; Saffari, S.; Iranpour, M.; Omrani, M.D. Association of MiR-146a expression and type 2 diabetes mellitus: A meta-analysis. Int. J. Mol. Cell. Med. 2017, 6, 156–163. [Google Scholar] [CrossRef]
- Zhao, H.; Li, P.; Wang, J. The role of muscle-specific MicroRNAs in patients with chronic obstructive pulmonary disease and skeletal muscle dysfunction. Front. Physiol. 2022, 13, 954364. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Bi, C.; Song, J.; Wang, L.; Ge, C.; Liu, X.; Zhang, M. Upregulation of miR-142-5p in atherosclerotic plaques and regulation of oxidized low-density lipoprotein-induced apoptosis in macrophages. Mol. Med. Rep. 2015, 11, 3229–3234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, Y.; Xia, L.; Li, J.; Song, M.; Yang, C. Exercise-Induced ADAR2 Protects against Nonalcoholic Fatty Liver Disease through miR-34a. Nutrients 2022, 15, 121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, S.; Gu, Y.; Song, L.; Yu, S.; Feng, X. Tai Chi Improves Coronary Heart Disease Risk by Inactivating MAPK/ERK Pathway through Serum miR-126. Evid.-Based Complement. Altern. Med. 2020, 2020, 4565438. [Google Scholar] [CrossRef] [PubMed]
- Negrao, C.E.; Middlekauff, H.R.; Gomes-Santos, I.L.; Antunes-Correa, L.M.; Kagan, H.J.; Belekdanian, V.D.; Chen, J.; Backeris, P.; Hammoudi, N.; Turnbull, I.C.; et al. Effects of exercise training on neurovascular control and skeletal myopathy in systolic heart failure. Am. J. Physiol. Circ. Physiol. 2015, 308, H792–H802. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhong, J.; Zeng, Z.; Wang, H.; Li, J.; Liu, X.; Yang, X. MiR-181c protects cardiomyocyte injury by preventing cell apoptosis through PI3K/Akt signaling pathway. Cardiovasc. Diagn. Ther. 2020, 10, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Jankauskas, S.S.; Mone, P.; Avvisato, R.; Varzideh, F.; De Gennaro, S.; Salemme, L.; Macina, G.; Kansakar, U.; Cioppa, A.; Frullone, S.; et al. miR-181c targets Parkin and SMAD7 in human cardiac fibroblasts: Validation of differential microRNA expression in patients with diabetes and heart failure with preserved ejection fraction. Mech. Ageing Dev. 2023, 212, 111818. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
| Databases | Search Query |
|---|---|
| PubMed | ((cancer [Title/Abstract] OR cardiovascular disease [Title/Abstract] OR diabetes [Title/Abstract] OR chronic diseases [Title/Abstract] OR non-communicable diseases [Title/Abstract])) AND ((physical exercise [Title/Abstract] OR physical activity [Title/Abstract] OR exercise training [Title/Abstract] OR aerobic exercise [Title/Abstract] OR resistance exercise [Title/Abstract] AND ((miRNA expression [Title/Abstract] OR microRNA [Title/Abstract] OR miR- [Title/Abstract])) |
| Scopus | ( TITLE-ABS-KEY ( cancer ) OR TITLE-ABS-KEY ( cardiovascular AND diseases ) OR TITLE-ABS-KEY ( diabetes ) OR TITLE-ABS-KEY ( chronic AND diseases ) OR TITLE-ABS-KEY ( non AND communicable AND diseases ) AND TITLE-ABS-KEY ( physical AND exercise ) OR TITLE-ABS-KEY ( physical AND activity ) OR TITLE-ABS-KEY ( exercise AND training ) OR TITLE-ABS-KEY ( aerobic AND exercise ) OR TITLE-ABS-KEY ( resistance AND exercise ) AND TITLE-ABS-KEY ( miRNA AND expression ) OR TITLE-ABS-KEY ( microRNA ) OR TITLE-ABS-KEY ( miR- ) ) AND ( LIMIT-TO ( DOCTYPE , “ar” ) ) AND ( LIMIT-TO ( LANGUAGE , “English” ) ) |
| Web of Science (MEDLINE) | #4((((TS=(cancer)) OR TS=(cardiovascular disease)) OR TS=(diabetes )) OR TS=( chronic diseases)) OR TS=(non-communicable diseases) #5((((TS=(physical exercise )) OR TS=(physical activity)) OR TS=(exercise training)) OR TS=(aerobic exercise)) OR TS=(resistance exercise)#6((TS=(miRNA expression )) OR TS=(microRNA)) OR TS=( miR- )#6 AND #5 AND #4 and Journal Article (Publication Type) and English (Languages) |
| Author and Year | Study Population (Disease, Gender, and Age) | Sample Type Used for miRNA Extraction | Type, Frequency, and Duration of Exercise | Exercise Effects on miRNA Expression |
|---|---|---|---|---|
| Gazova et al. (2019) [21] | Prostate Cancer, 23 patients, 60–78-year-old males, randomized in only ADT vs. trained ADT | Blood plasma | Strength training (3 times/week for 16 weeks) | ↑ miR-1, miR-29b, miR-133a |
| Olson et al. (2021) [22] | 35 females, clinically stable breast cancer patients | Blood plasma | Moderate physical exercise and strength training (150 min, 2 times/week for 12 weeks) | ↑ miR-10a, miR-211, miR-205 |
| Hagstrom and Denham (2018) [23] | Females aged 49–50 years, 15 training BC survivors vs. 9 sedentary BC | Blood serum | Resistance training on leg and chest press (60 min, 3 times/week for 16 weeks) | ↑ miR-133a-3p miR-370–3p |
| Alizadeh et al. (2019) [24] | Breast cancer (BC), females, 15 healthy sedentary vs. 15 healthy HIIT vs. 26 BC sedentary patients in HT vs. 26 BC HIIT patients in HT | Blood serum | HIIT uphill walking (3 times/week for 12 weeks) | HIIT induces ↑ miR-206, miR-145, miR-143, miR9, let-7a ↓ miR-21, miR-155, miR-221, miR27a, miR-10b |
| Adams et al. (2018) [25] | Breast cancer, 100 adult female survivors in the LEAN trial, in comparison to 121 BC patients enrolled in the HOPE study | Blood serum | Diet + aerobic exercise (20 min/day for 24 weeks) | ↑ miR-191, miR-24, let-7b ↓ miR-106b, miR-27a, miR-92a |
| Author and Year | Study Population (Disease, Gender, and Age) | Sample Type Used for miRNA Extraction | Type, Frequency, and Duration of Exercise | Exercise Effects on miRNA Expression |
|---|---|---|---|---|
| Akbarinia et al., 2018 [26] | 24 females with T2DM (61.92 ± 3.63 years); AT vs. CG | Blood plasma | AT: 60–75% VO2peak; 3 times/week for 8 weeks | ↑ miR-130a expression in both groups. However, there were no significant differences between AT and CG. |
| Cirilli et al., 2019 [27] | 19 adults with T2DM (62 ± 2 years); HE, ME, and LE | Blood plasma | PA recorded for 12 weeks; HE, ME, and LE | ↑ miR-130a in ME; ↓ miR-146a in HE and LE |
| Morais Junior et al., 2017 [28] | 23 adults (13 with T2DM, 68.2 ± 5.3 years) | Blood serum | D1: Strength training (40 min) D2: walking 50 min (60–70% of heart rate reserve) | ↑ miR-146a in T2D after D1 |
| Ghodrat et al., 2022 [29] | 24 females with T2DM (45–65 years); RT+HIIT (SD; n = 7) RT+HIIT (DD; n = 6) CG (n = 8) | Blood serum | RT at 40–70% 1-RM + low-volume HIIT for 8 weeks | ↑ miR-146a in SD and DD; ↓ miR-29b in DD |
| Olioso et al., 2019 [30] | 24 adults with T2DM (55.8 ± 7.3 years); AT (n = 12) RT (n = 12) | Blood plasma | AT: 60–65% of heart rate reserve RT: 70–80% 1RM; 60 min, 3 times/week for 24 weeks | ↑ miR-423-3p, miR-451a, and miR-766-3p in AT and RT |
| Rowlands et al., 2014 [31] | 17 adults with T2DM (49 ± 5 years); AT (n = 8) RT (n = 9) | Skeletal muscle | AT: 40–60 min of cycle ergometer RT: 2–3 sets for 6–8 repetitions; 3 times/week for 16 weeks | ↓ miR-29a in AT; ↑ miR-23a and miR-195 in RT |
| Simaitis et al., 2020 [32] | 7 adults with T2DM (61 ± 10 years) | Skeletal muscle | AT: 70–80% of peak heart rate; 3 times/week for 12 weeks | ↓ miRNA-29b-3p ↓ miRNA-29c-3p ↓ miRNA-135a-5p |
| Taghizadeh et al., 2018 [33] | 20 females with T2DM (62.3 ± 4.0 years); AT (n = 10) CG (n = 10) | Blood plasma | AT: 60–75% of VO2peak; 3 times/week for 8 weeks | hsa-miR-223 expression was not significant between AT and CG |
| Taghizadeh et al., 2020 [34] | 24 adults with T2DM (60.0 ± 2.8 years); AT (n = 12) CG (n = 12) | Blood plasma | AT: 65–75% of VO2peak; 12 weeks | ↑ miRNA-223 in AT |
| Author and Year | Study Population (Disease, Gender, and Age) | Sample Type Used for miRNA Extraction | Type, Frequency, and Duration of Exercise | Exercise Effects on miRNA Expression |
|---|---|---|---|---|
| Lewis et al., 2012 [35] | 45 adults (31 with COPD, 65 ± 7 years) | Skeletal muscle | Daily PA | miR-133 and miR-206 expression negatively correlates with daily PA |
| Liu et al., 2023 [36] | 4 males with COPD (60–67 years) | Blood plasma | 12 weeks of individualized AT | ↓ miR-144-3p ↑ hsa-let-7c ↓ hsa-miR-1277 |
| Author and Year | Study Population (Disease, Gender, and Age) | Sample Type Used for miRNA Extraction | Type, Frequency, and Duration of Exercise | Exercise Effects on miRNA Expression |
|---|---|---|---|---|
| Witvrouwen et al. (2021) [10] | 18 Male HFrEF patients (50.7–65.4 years); 9 ER vs. 9 ENR | Blood serum | Aerobic exercise 3 times, 50 min/week for 4 months | ↑ Let-7b, miR-23a, miR-140, miR-146a, miR-191, miR-210, and miR-339-5p highly correlated with VO2 peak trainability in HFrEF |
| Taraldsen et al. (2022) [38] | 24 CAD patients, aerobic interval training (AIT) vs. moderate continuous training (MCT), compared to healthy individuals (CG) | Blood serum | AIT, 15 min, 3 times/week for 3 months; MCT, 46 min walk/week for 3 months | ↑ miR-146a-5p ↓ miR-15a-5p, miR-93-5p, and miR-451a |
| Witvrouwen et al., 2021b [39] | 25 males with HFrEF (55.6 ± 13.4 years) vs. 21 CG (60.0 ± 9.4 years) | Blood plasma | 3 times per week of combined strength and AT (at 90% of HR) for 15 weeks | ↓ miR-146a in HFrEF patients |
| Antunes-Correa et al. (2020) [40] | 33 (M + F) HFpEF patients (35–70 years); 11 IMT + 12 AET vs. 10 untrained CG | Muscle biopsies | Aerobic exercise (30 min, 5 times/week for 4 months) | ↑ miRNA-1 ↓ PI3K-AKT |
| Mayr et al. (2021) [41] | 20 (M + F) CAD patients (53–62 years) | Blood plasma | Maximum-cycle ergospirometry (12.44 ± 3.23 min) | ↑ miR338-3p ↓ miR101-3p no change in miR-1, miR-133, miR-208a, or miR-499 expression |
| Gevaert et al. (2021) [42] | 51 (M + F) HFpEF patients (70 ± 6 years); High responders (n = 30) Low responders (n = 21) vs. CG | Blood plasma | MCT: 35–50% of HRR, 200 min/week; HIIT: 20–50% of HRR, in 4 intervals of 200 min/week for 3 months | ↓ MiR-181c in low responders compared to high responders |
| Jin et al. (2021) [43] | 60 (M + F) HFpEF patients (55–70 years) exercise training vs. 30 healthy controls | Blood plasma | Aerobic exercise (30 min, 3 times/week for 3 months) | ↑ miR-126 in HFpEF patients with exercise, as compared to baseline |
| Xu et al., 2016 [44] | 28 males with HF (59.1 ± 1.8 years) | Blood serum | Incremental maximal cardiopulmonary exercise test | ↑ miR-21 ↑ miR-378 ↑ miR-940 |
| Zhang et al., 2020 [45] | 30 adults with CHD (61.0 ± 8.1 years); TG (n = 18) CG (n = 12) | Blood serum | Tai Chi training for 12 weeks | ↓ miR-126 in TG |
| Riedel et al. (2020) [46] | 8 (M + F) CHF patients (63 ± 3 years); ET vs. 8 healthy controls doing the same exercise | Blood serum | Aerobic exercise patients: 3–6 times daily for 5–20 min on a bicycle ergometer Healthy CG: 4 × 30 min/day, 5 times/week | ↓ miR-126, miR-21, and miR-222 No changes in miR-221 and miR-214 were observed |
| Sieland et al., 2023 [47] | 10 adults with PAD (72.0 ± 7.0 years) | Blood plasma | D1: incremental walking exercise until volitional exhaustion D2: 20 min of interval training | ↑ miRNA142-5p ↑ miRNA-424-5p in D2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzal, M.; Greco, F.; Quinzi, F.; Scionti, F.; Maurotti, S.; Montalcini, T.; Mancini, A.; Buono, P.; Emerenziani, G.P. The Effect of Physical Activity/Exercise on miRNA Expression and Function in Non-Communicable Diseases—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 6813. https://doi.org/10.3390/ijms25136813
Afzal M, Greco F, Quinzi F, Scionti F, Maurotti S, Montalcini T, Mancini A, Buono P, Emerenziani GP. The Effect of Physical Activity/Exercise on miRNA Expression and Function in Non-Communicable Diseases—A Systematic Review. International Journal of Molecular Sciences. 2024; 25(13):6813. https://doi.org/10.3390/ijms25136813
Chicago/Turabian StyleAfzal, Moomna, Francesca Greco, Federico Quinzi, Francesca Scionti, Samantha Maurotti, Tiziana Montalcini, Annamaria Mancini, Pasqualina Buono, and Gian Pietro Emerenziani. 2024. "The Effect of Physical Activity/Exercise on miRNA Expression and Function in Non-Communicable Diseases—A Systematic Review" International Journal of Molecular Sciences 25, no. 13: 6813. https://doi.org/10.3390/ijms25136813





