Eco-Friendly Lithium Separators: A Frontier Exploration of Cellulose-Based Materials
Abstract
:1. Introduction
2. Lithium-Ion Battery Separators Based on Cellulose and Its Derivatives
2.1. Physically Modified Cellulose
2.1.1. Microcrystal Cellulose
2.1.2. Micro-Fibrillated Cellulose
2.1.3. Nanocellulose
2.2. Chemically Modified Cellulose
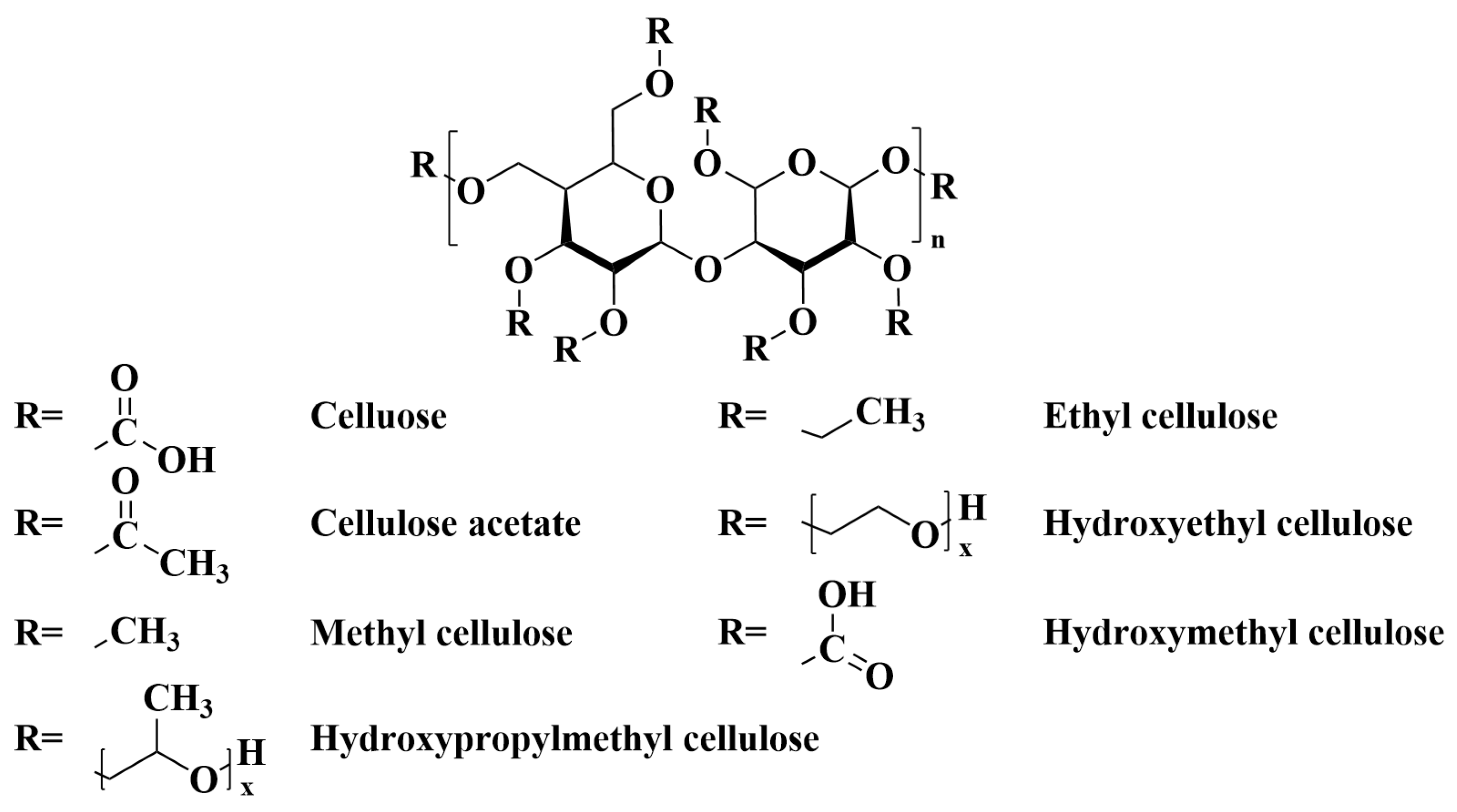
2.2.1. Cellulose Acetate
2.2.2. Methyl Cellulose
2.2.3. Carboxymethyl Cellulose
2.2.4. Ethyl Cellulose
2.2.5. Hydroxy Cellulose
3. Preparation Method of Cellulose-Based Lithium Battery Separator
3.1. Wet Process Membrane Production
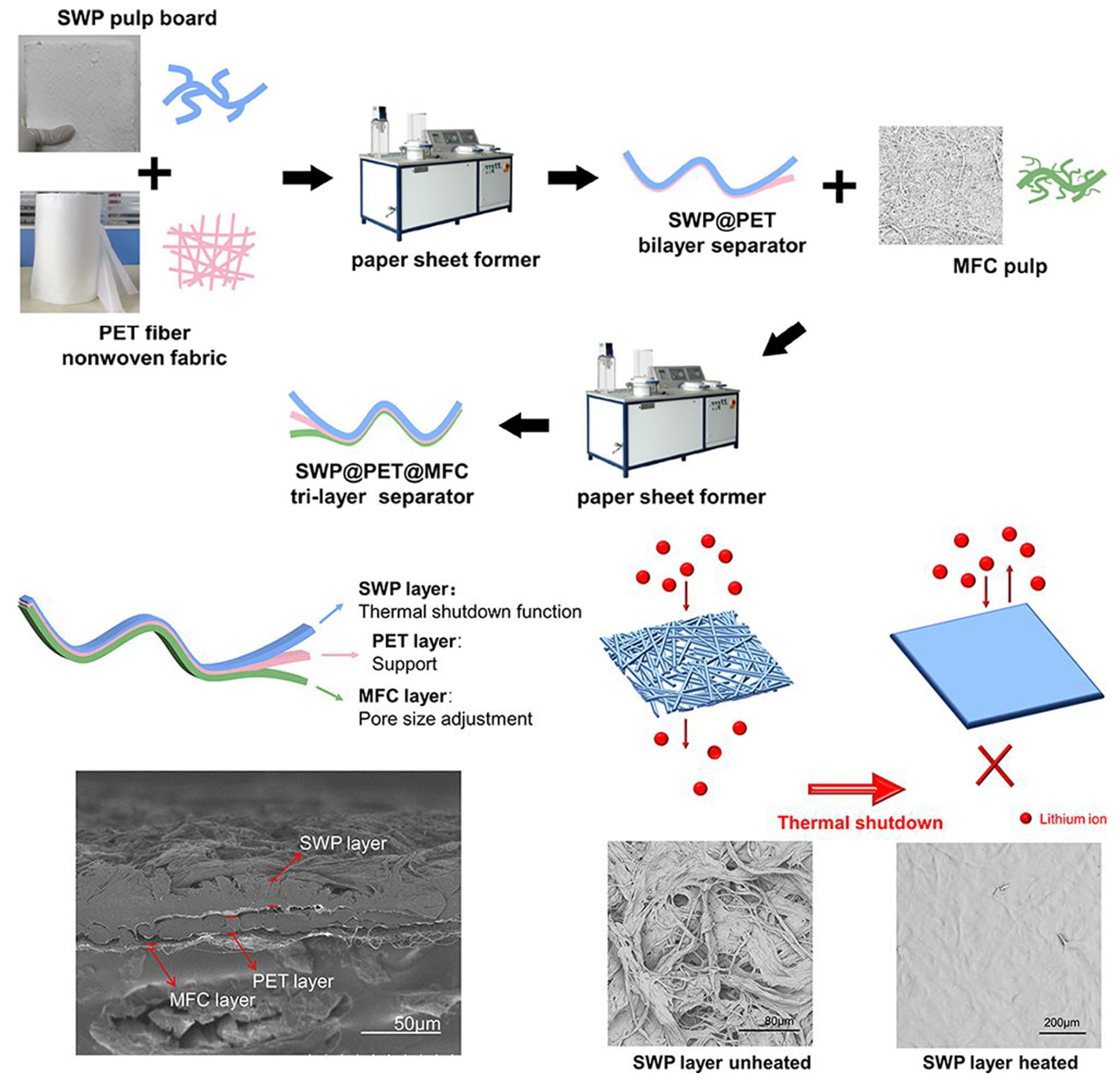
3.1.1. Papermaking Method
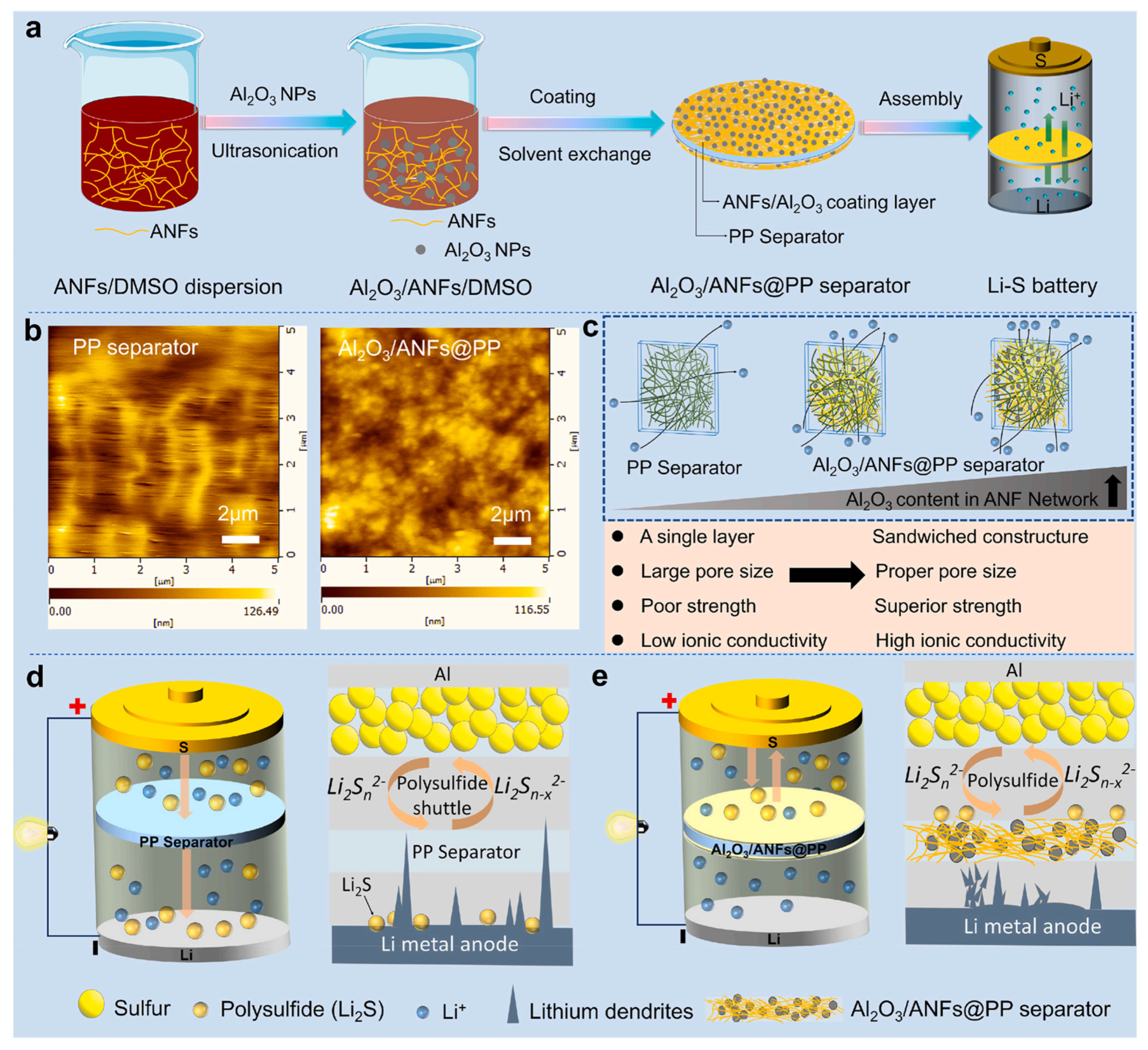
3.1.2. Coating Method
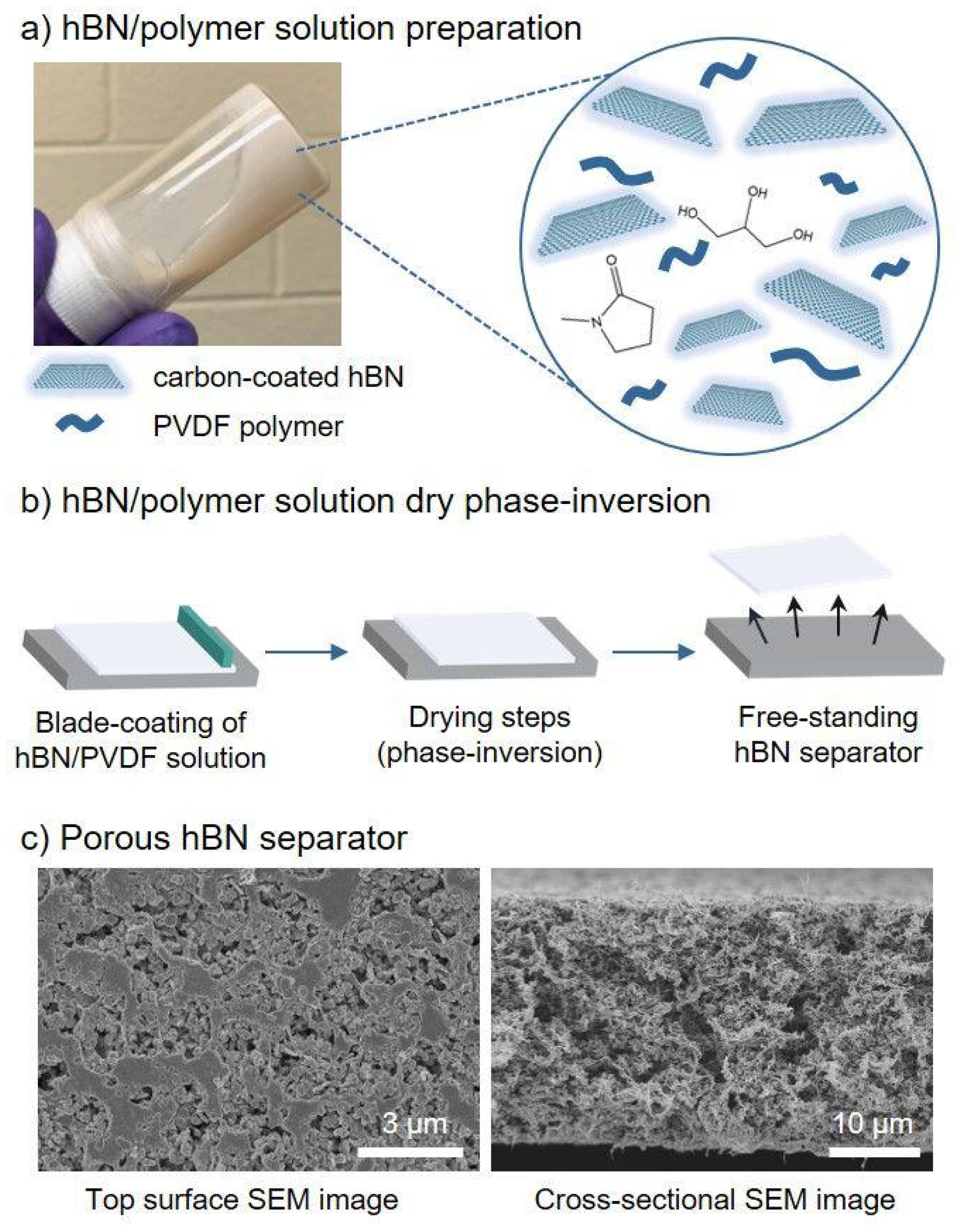
3.1.3. Casting Method
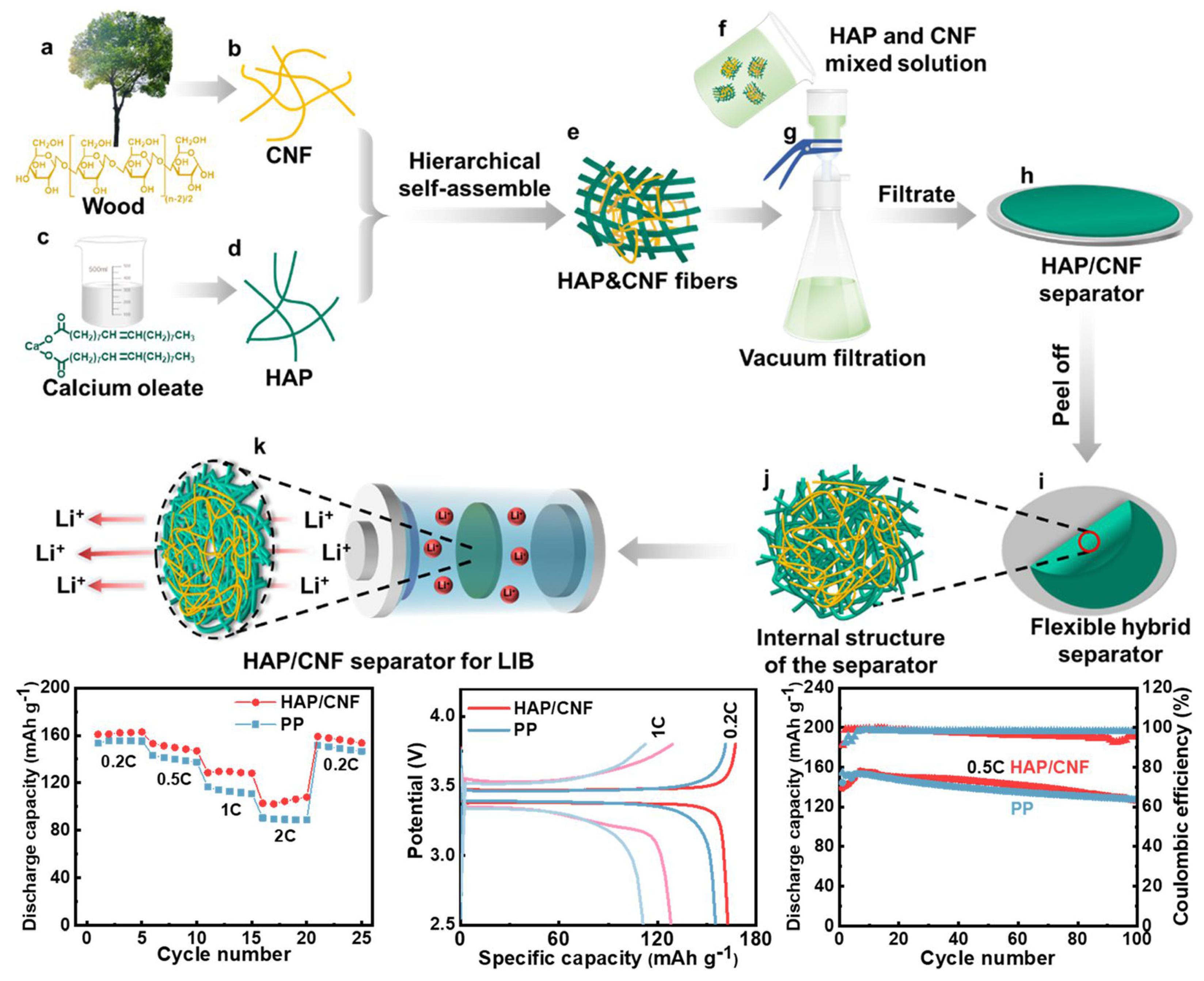
3.1.4. Vacuum Filtration Method
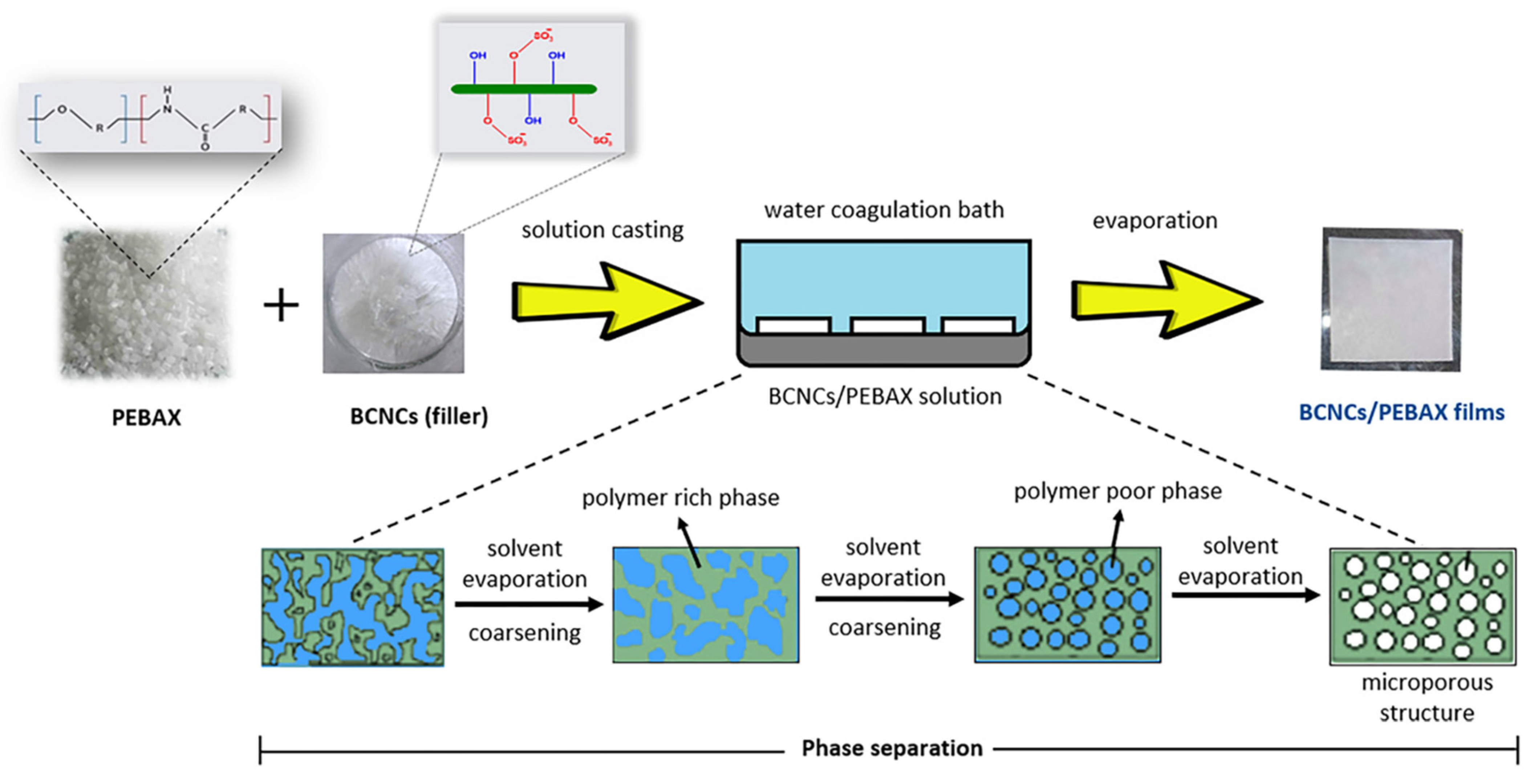
3.1.5. Phase Separation Method
3.2. Dry Membrane Production
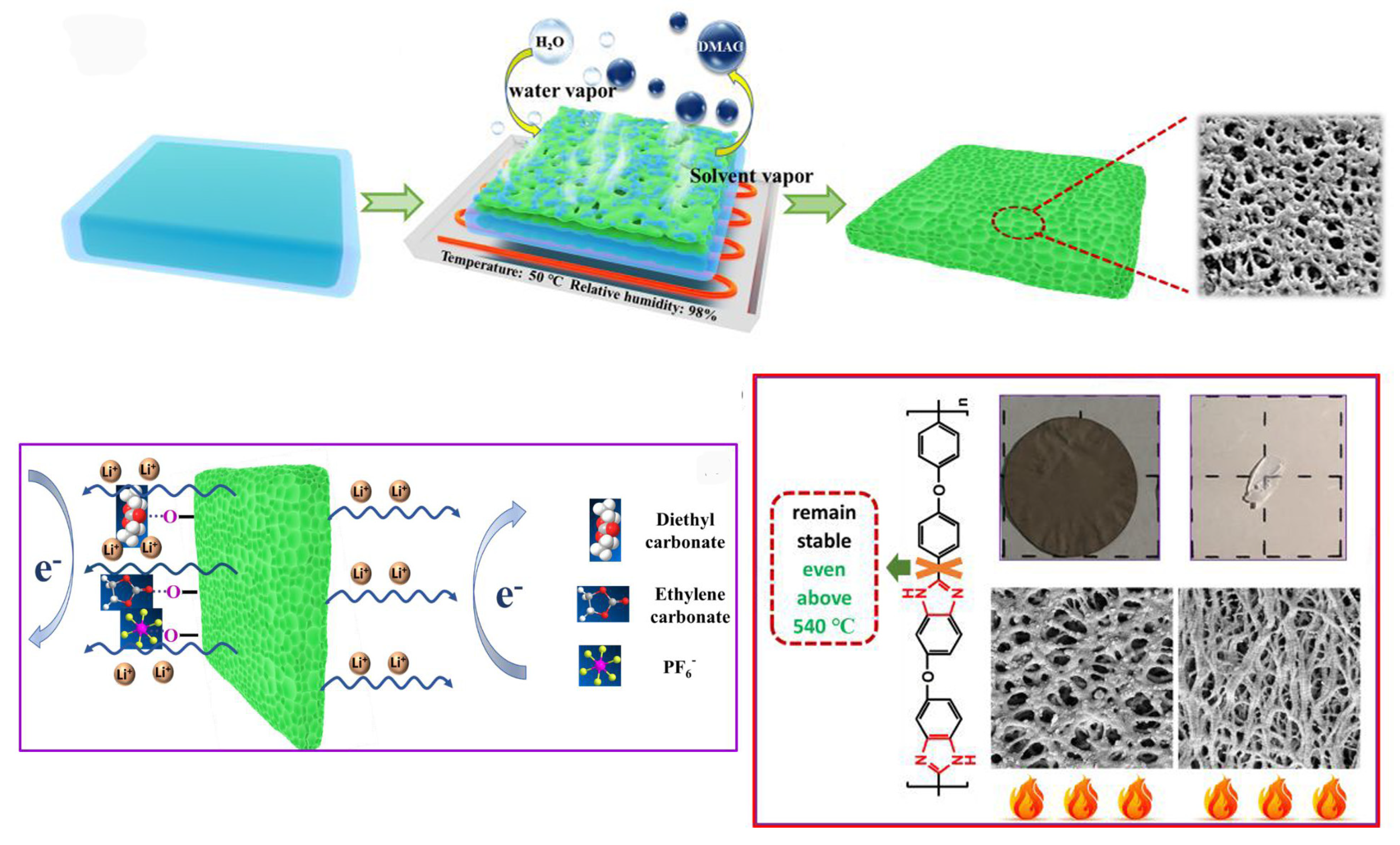
3.2.1. Aerogel Method
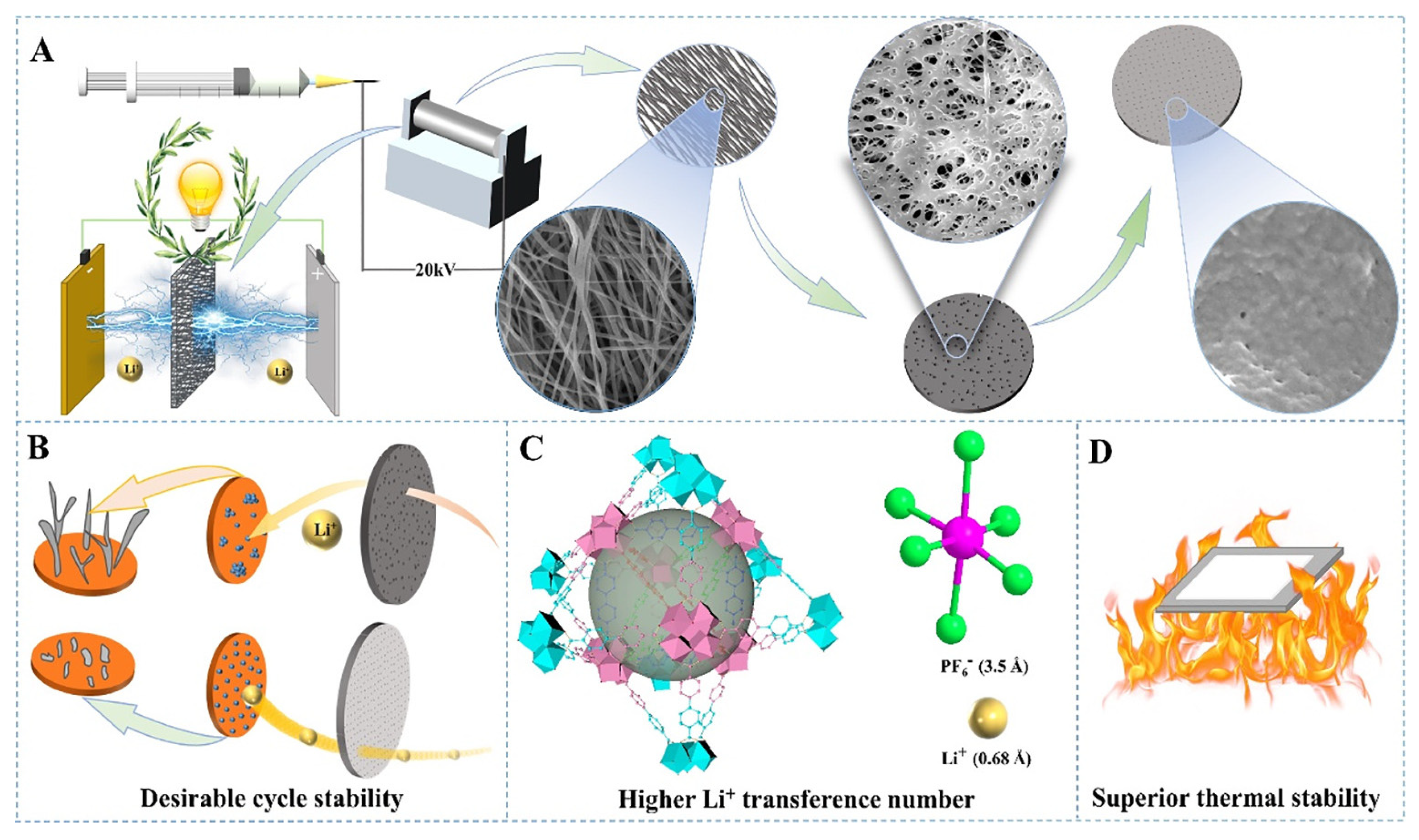
3.2.2. Electrospinning Method
| Preparation Method | Cellulose and Its Derivatives | Thickness (um) | Thermal Stability (°C) | Tensile Strength (MPa) | Ionic Conductivity (mS cm−1) | Porosity (%) | Cyclic Performance (%) | Electrolyte Absorption (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Papermaking method | CMC | 67 | 300 | 27.7 | / | 75 | 150 cycles 95.6 | 286 | [166] |
| BC/ATP | / | 350 | 27.09 | 1.75 | 59.17 | 300 cycles 94.5 | 470 | [211] | |
| CF/ANF | 40 | 200 | 33 | 0.75 | 49.5 | 100 cycles 89.6 | 157 | [212] | |
| Coating method | PVDF/EC | / | 347 | 73 | 0.79 | / | 200 cycles 95.7 | / | [34] |
| GF/CNF | 30 | / | / | 1.7 | 66 | 200 cycles 80 | / | [213] | |
| Casting method | BCNC/ PEBAX | / | 150 | 14.9 | 9.79 | 56.8 | / | 101.4 | [77] |
| Vacuum filtration method | ECM | 12 | 290 | / | 0.35 | 53 | 100 cycles 78.6 | 281 | [214] |
| BC/CS | 57.4 | 260 | 9.9 | 2.9 | 65.8 | 100 cycles 90 | 358 | [215] | |
| ZIF-8/BC | / | 325 | 50.1 | 1.12 | 73.2 | 100 cycles 89.2 | 340.5 | [216] | |
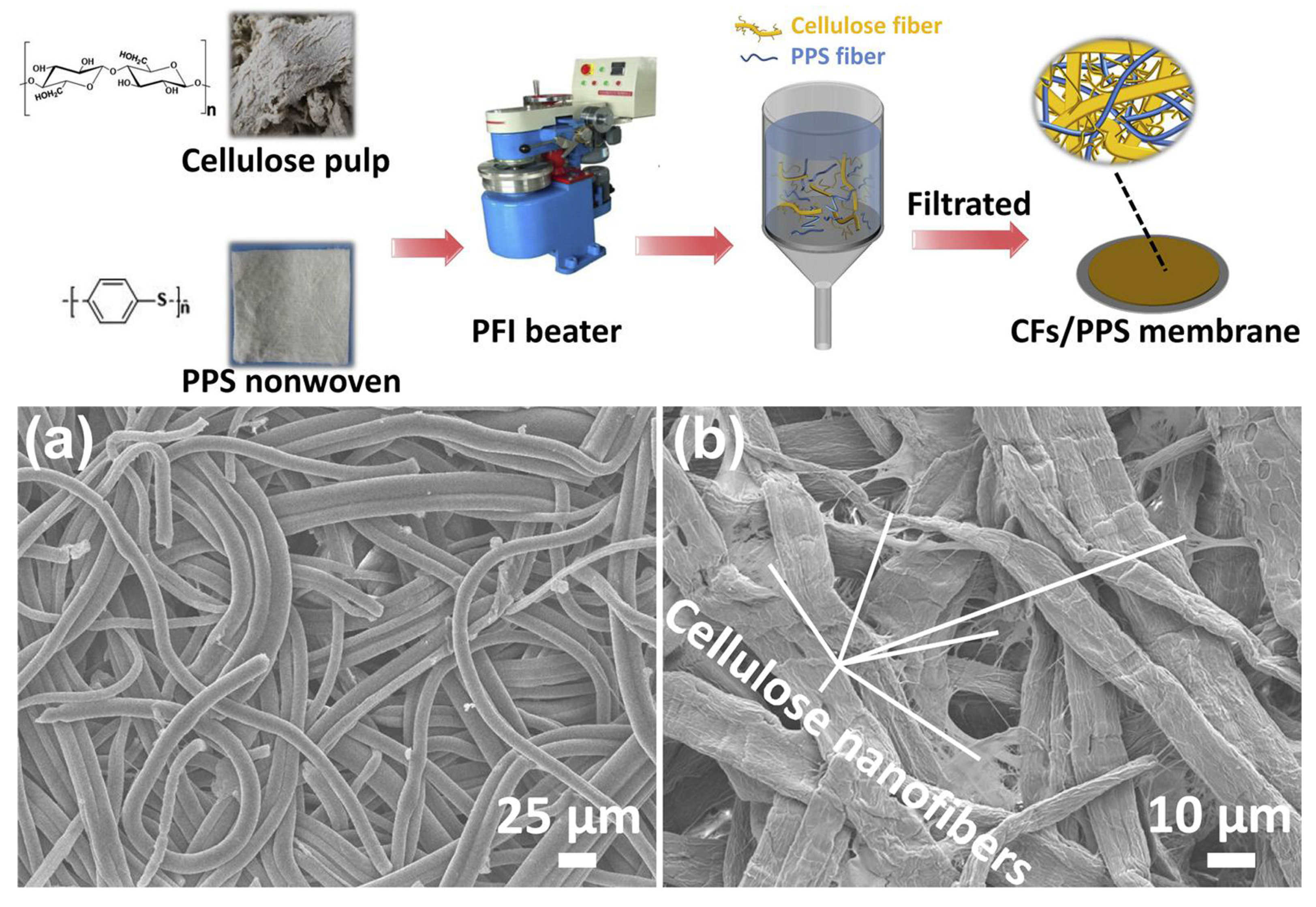
| CFs/PPS-1/1 | / | 200 | 20.52 | 1.26 | 61.1 | 100 cycles 90.3 | 259.6 | [182] | |
| Electrospinning | PVDF/CA | 30 | 450 | 7.6 | 1.36 | 86.5 | 50 cycles 91.8 | 311 | [217] |
| PVDF/TPP/CA | / | 170 | 8.5 | 4.4 | 90 | 100 cycles 86.9 | 301 | [218] | |
| PVDF/HFP/CA | / | 200 | 34.1 | 6.16 | 66 | 100 cycles 75.4 | 355 | [219] | |
| Phase separation method | PVA/CNF | 25 | 275 | 18 | 1.1 | 60 | 50 cycles 93 | 230 | [220] |
| PVDF/CNF/CEC | 50 | 170 | 14.3 | 1.26 | 60.2 | 50 cycles 98.96 | 370 | [221] | |
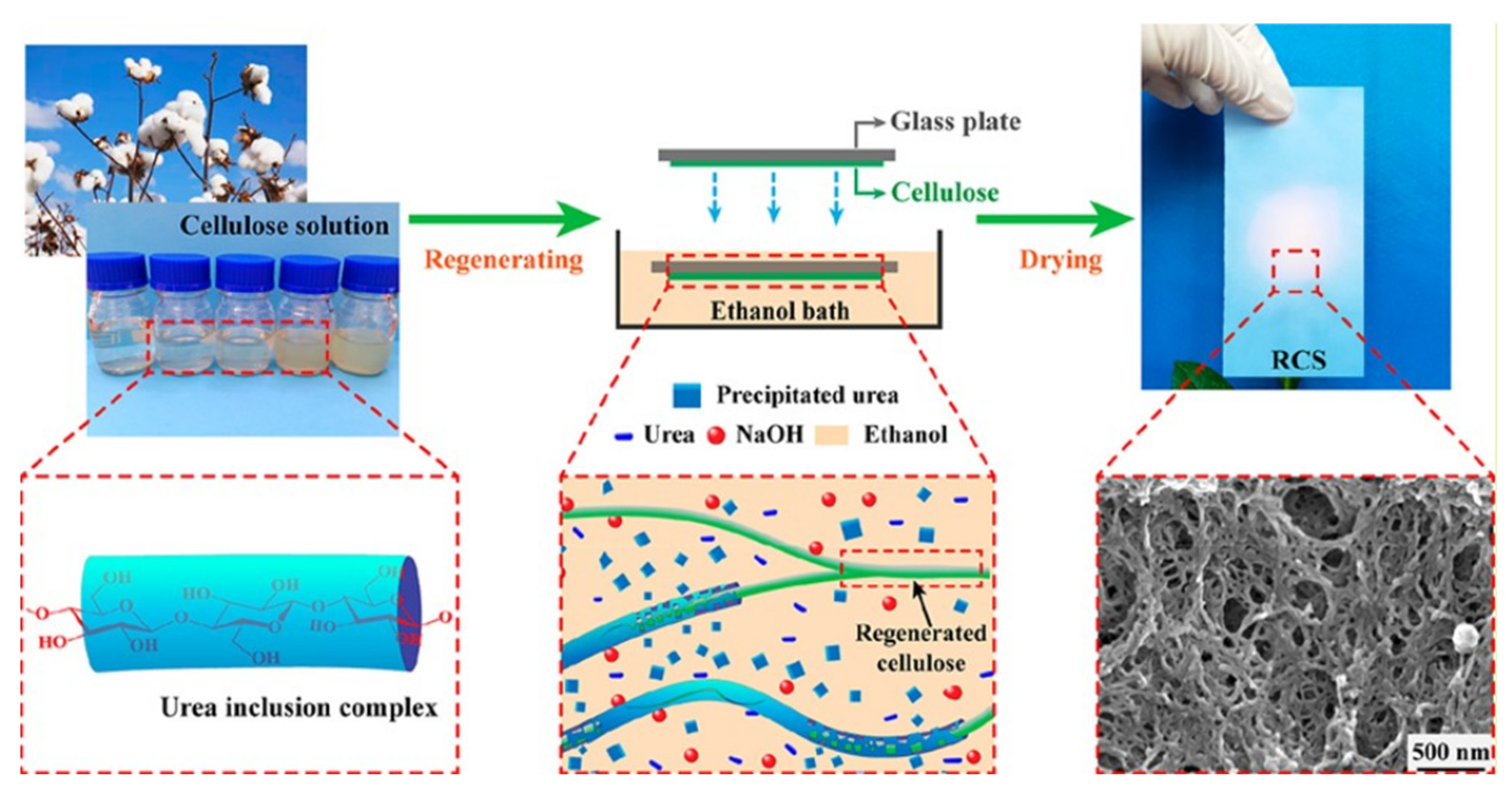
| RCS | 19.74 | 260 | / | 1.25 | 61 | 100 cycles 81 | 436 | [196] | |
| Aerogel method | CNF/AG | 160 | 150 | / | 2.64 | 99.5 | 200 cycles 41 | 12,000 | [200] |
| ANFs | / | 300 | / | 1.04 | 92.6 | 200 cycles 70 | 695 | [222] | |
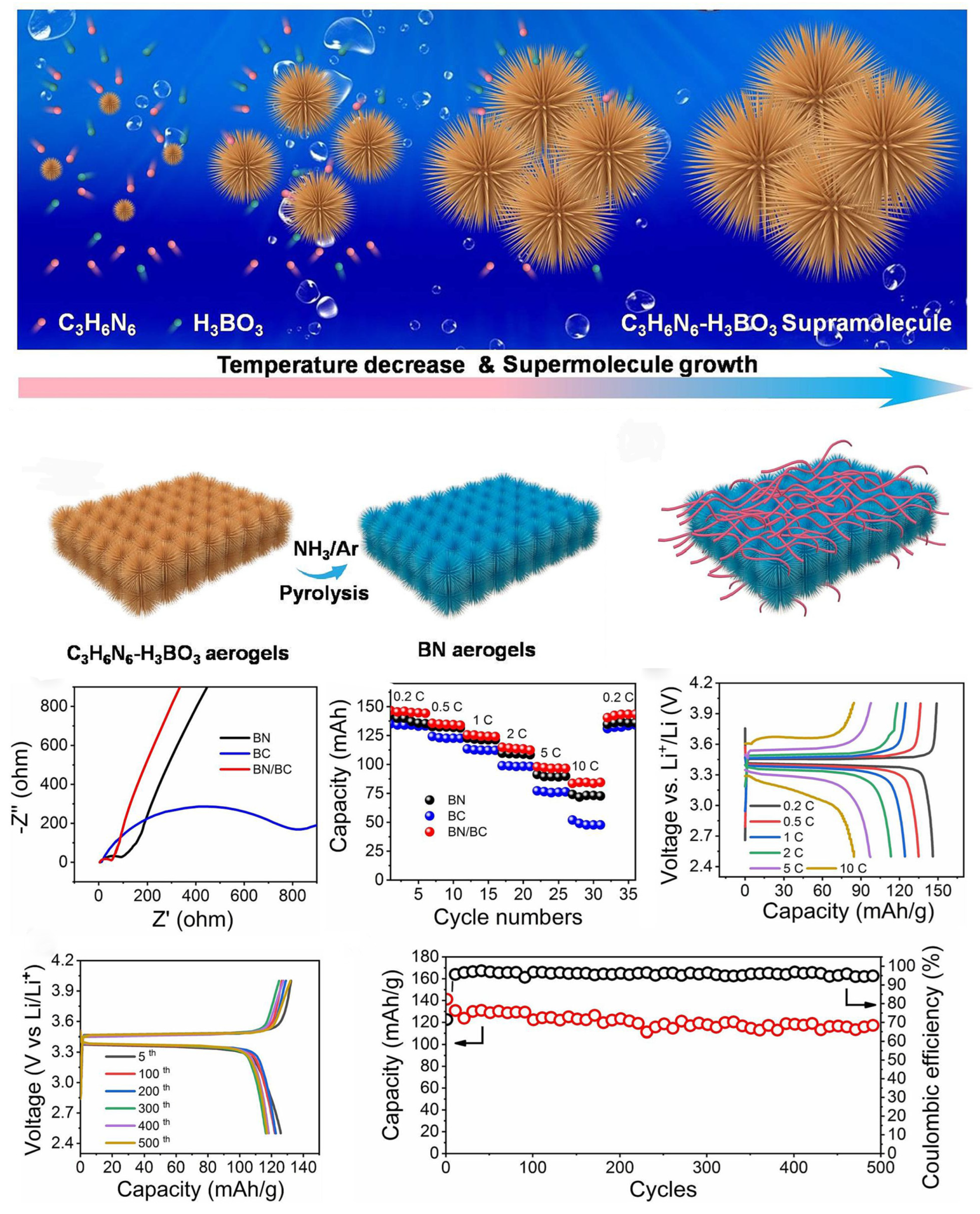
| BN/BC | 100 | 300 | 0.2 | / | / | 500 cycles 94 | / | [201] |
4. Conclusions and Outlook
4.1. Conclusions
4.2. Outlook
Funding
Conflicts of Interest
References
- Taberna, P.L.; Mitra, S.; Poizot, P.; Simon, P.; Tarascon, J.M. High rate capabilities Fe3O4-based Cu nano-architectured electrodes for lithium-ion battery applications. Nat. Mater. 2006, 5, 567–573. [Google Scholar] [CrossRef]
- Yang, M.; Hou, J. Membranes in Lithium Ion Batteries. Membranes 2012, 2, 367–383. [Google Scholar] [CrossRef]
- Li, S.; Zhu, W.; Tang, Q.; Huang, Z.; Yu, P.; Gui, X.; Lin, S.; Hu, J.; Tu, Y. Mini Review on Cellulose-Based Composite Separators for Lithium-Ion Batteries: Recent Progress and Perspectives. Energy Fuels 2021, 35, 12938–12947. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Kefan, L.; Chengyuan, Y.; Xiaogang, L.; Nanxi, D.; Bingxue, L.; Guofeng, T.; Shengli, Q.; Dezhen, W. Controllable Coaxial Coating of Boehmite on the Surface of Polyimide Nanofiber Membrane and Its Application as a Separator for Lithium-Ion Batteries. Energy Technol. 2022, 10, 2100982. [Google Scholar] [CrossRef]
- Lizundia, E.; Costa, C.M.; Alves, R.; Lanceros-Méndez, S. Cellulose and its derivatives for lithium ion battery separators: A review on the processing methods and properties. Carbohydr. Polym. Technol. Appl. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Hu, H.; Xue, W.; Jiang, P.; Li, Y. Polyimide-Based Materials for Lithium-Ion Battery Separator Applications: A Bibliometric Study. Int. J. Polym. Sci. 2022, 2022, 6740710. [Google Scholar] [CrossRef]
- Xia, Y.; Li, X.; Zhuang, J.; Wang, W.; Abbas, S.C.; Fu, C.; Zhang, H.; Chen, T.; Yuan, Y.; Zhao, X.; et al. Exploitation of function groups in cellulose materials for lithium-ion batteries applications. Carbohydr. Polym. 2024, 325, 121570. [Google Scholar] [CrossRef]
- Luo, W.; Cheng, S.; Wu, M.; Zhang, X.; Yang, D.; Rui, X. A review of advanced separators for rechargeable batteries. J. Power Sources 2021, 509, 230372. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R.; Zhao, R.; Qi, X.; Li, K.; Sun, Q.; Ma, M.; Qie, L.; Huang, Y. A “dendrite-eating” separator for high-areal-capacity lithium-metal batteries. Energy Storage Mater. 2020, 31, 181–186. [Google Scholar] [CrossRef]
- Li, C.; Liu, R.; Xiao, Y.; Cao, F.; Zhang, H. Recent progress of separators in lithium-sulfur batteries. Energy Storage Mater. 2021, 40, 439–460. [Google Scholar] [CrossRef]
- Song, Y.-H.; Wu, K.-J.; Zhang, T.-W.; Lu, L.-L.; Guan, Y.; Zhou, F.; Wang, X.-X.; Yin, Y.-C.; Tan, Y.-H.; Li, F.; et al. A Nacre-Inspired Separator Coating for Impact-Tolerant Lithium Batteries. Adv. Mater. 2019, 31, 1905711. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pan, R.; Ruan, C.; Edström, K.; Strømme, M.; Nyholm, L. Redox-Active Separators for Lithium-Ion Batteries. Adv. Sci. 2018, 5, 1700663. [Google Scholar] [CrossRef]
- Waqas, M.; Ali, S.; Feng, C.; Chen, D.; Han, J.; He, W. Recent Development in Separators for High-Temperature Lithium-Ion Batteries. Small 2019, 15, 1901689. [Google Scholar] [CrossRef]
- Huang, X.; He, R.; Li, M.; Chee, M.O.L.; Dong, P.; Lu, J. Functionalized separator for next-generation batteries. Mater. Today 2020, 41, 143–155. [Google Scholar] [CrossRef]
- Huang, X. Separator technologies for lithium-ion batteries. J. Solid State Electrochem. 2011, 15, 649–662. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Dai, J.; Shi, C.; Li, C.; Shen, X.; Peng, L.; Wu, D.; Sun, D.; Zhang, P.; Zhao, J. A rational design of separator with substantially enhanced thermal features for lithium-ion batteries by the polydopamine–ceramic composite modification of polyolefin membranes. Energy Environ. Sci. 2016, 9, 3252–3261. [Google Scholar] [CrossRef]
- Wang, C.; Wu, K.; Cui, J.; Fang, X.; Li, J.; Zheng, N. Robust Room-Temperature Sodium-Sulfur Batteries Enabled by a Sandwich-Structured MXene@C/Polyolefin/MXene@C Dual-functional Separator. Small 2022, 18, 2106983. [Google Scholar] [CrossRef]
- Wang, E.; Chiu, C.-H.; Chou, P.-H. Safety assessment of polyolefin and nonwoven separators used in lithium-ion batteries. J. Power Sources 2020, 461, 228148. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, C.; Dong, C.; Shen, C.; Shuai, B.; Li, C.; Li, Y.; An, Q.; Xu, X.; Mai, L. Polydopamine-assisted in-situ formation of dense MOF layer on polyolefin separator for synergistic enhancement of lithium-sulfur battery. Nano Res. 2022, 15, 8048–8055. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Meng, G.; Zhang, J. Function-directed design of battery separators based on microporous polyolefin membranes. J. Mater. Chem. A 2022, 10, 14137–14170. [Google Scholar] [CrossRef]
- Kim, M.; Shon, J.-Y.; Nho, Y.C.; Lee, T.-W.; Park, J.H. Positive Effects of E-Beam Irradiation in Inorganic Particle Based Separators for Lithium-Ion Battery. J. Electrochem. Soc. 2010, 157, A31. [Google Scholar] [CrossRef]
- Yoneda, H.; Nishimura, Y.; Doi, Y.; Fukuda, M.; Kohno, M. Development of microporous PE films to improve lithium ion batteries. Polym. J. 2010, 42, 425–437. [Google Scholar] [CrossRef]
- Cho, T.-H.; Tanaka, M.; Onishi, H.; Kondo, Y.; Nakamura, T.; Yamazaki, H.; Tanase, S.; Sakai, T. Battery performances and thermal stability of polyacrylonitrile nano-fiber-based nonwoven separators for Li-ion battery. J. Power Sources 2008, 181, 155–160. [Google Scholar] [CrossRef]
- Cho, T.-H.; Tanaka, M.; Ohnishi, H.; Kondo, Y.; Yoshikazu, M.; Nakamura, T.; Sakai, T. Composite nonwoven separator for lithium-ion battery: Development and characterization. J. Power Sources 2010, 195, 4272–4277. [Google Scholar] [CrossRef]
- Liang, X.; Yang, Y.; Jin, X.; Cheng, J. Polyethylene Oxide-Coated Electrospun Polyimide Fibrous Seperator for High-Performance Lithium-Ion Battery. J. Mater. Sci. Technol. 2016, 32, 200–206. [Google Scholar] [CrossRef]
- Gu, Q.-Q.; Xue, H.-J.; Li, Z.-W.; Song, J.-C.; Sun, Z.-Y. High-performance polyethylene separators for lithium-ion batteries modified by phenolic resin. J. Power Sources 2021, 483, 229155. [Google Scholar] [CrossRef]
- Li, Y.; Yu, L.; Hu, W.; Hu, X. Thermotolerant separators for safe lithium-ion batteries under extreme conditions. J. Mater. Chem. A 2020, 8, 20294–20317. [Google Scholar] [CrossRef]
- Liao, C.; Wang, W.; Han, L.; Mu, X.; Wu, N.; Wang, J.; Gui, Z.; Hu, Y.; Kan, Y.; Song, L. A flame retardant sandwiched separator coated with ammonium polyphosphate wrapped by SiO2 on commercial polyolefin for high performance safety lithium metal batteries. Appl. Mater. Today 2020, 21, 100793. [Google Scholar] [CrossRef]
- Yu, J.; Dong, N.; Liu, B.; Tian, G.; Qi, S.; Wu, D. A newly-developed heat-resistance polyimide microsphere coating to enhance the thermal stability of commercial polyolefin separators for advanced lithium-ion battery. Chem. Eng. J. 2022, 442, 136314. [Google Scholar] [CrossRef]
- Zhao, P.; Yang, J.; Shang, Y.; Wang, L.; Fang, M.; Wang, J.; He, X. Surface modification of polyolefin separators for lithium ion batteries to reduce thermal shrinkage without thickness increase. J. Energy Chem. 2015, 24, 138–144. [Google Scholar] [CrossRef]
- Li, W.; Yang, B.; Pang, R.; Zhang, M. Sandwiched aramid nanofiber/Al2O3-coated polyolefin separators for advanced lithium–sulfur batteries. Compos. Commun. 2023, 38, 101489. [Google Scholar] [CrossRef]
- Zuo, X.; Wu, J.; Ma, X.; Deng, X.; Cai, J.; Chen, Q.; Liu, J.; Nan, J. A poly(vinylidene fluoride)/ethyl cellulose and amino-functionalized nano-SiO2 composite coated separator for 5 V high-voltage lithium-ion batteries with enhanced performance. J. Power Sources 2018, 407, 44–52. [Google Scholar] [CrossRef]
- Ajkidkarn, P.; Manuspiya, H. Solution plasma synthesis of bacterial cellulose acetate derived from nata de coco waste incorporated with polyether block amide. Int. J. Biol. Macromol. 2022, 209, 1486–1497. [Google Scholar] [CrossRef]
- Casas, X.; Niederberger, M.; Lizundia, E. A Sodium-Ion Battery Separator with Reversible Voltage Response Based on Water-Soluble Cellulose Derivatives. ACS Appl. Mater. Interfaces 2020, 12, 29264–29274. [Google Scholar] [CrossRef]
- Chen, W.; Shi, L.; Wang, Z.; Zhu, J.; Yang, H.; Mao, X.; Chi, M.; Sun, L.; Yuan, S. Porous cellulose diacetate-SiO2 composite coating on polyethylene separator for high-performance lithium-ion battery. Carbohydr. Polym. 2016, 147, 517–524. [Google Scholar] [CrossRef]
- Huang, C.; Ji, H.; Yang, Y.; Guo, B.; Luo, L.; Meng, Z.; Fan, L.; Xu, J. TEMPO-oxidized bacterial cellulose nanofiber membranes as high-performance separators for lithium-ion batteries. Carbohydr. Polym. 2020, 230, 115570. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Fan, Z.; Li, S.; Bernussi, A.; Newman, N. Functionalized bacterial cellulose as a separator to address polysulfides shuttling in lithium–sulfur batteries. Mater. Today Energy 2021, 21, 100813. [Google Scholar] [CrossRef]
- Mittal, N.; Ojanguren, A.; Cavasin, N.; Lizundia, E.; Niederberger, M. Transient Rechargeable Battery with a High Lithium Transport Number Cellulosic Separator. Adv. Funct. Mater. 2021, 31, 2101827. [Google Scholar] [CrossRef]
- Xia, Y.; Li, X.; Zhuang, J.; Yuan, Y.; Wang, W. Cellulose microspheres enhanced polyvinyl alcohol separator for high-performance lithium-ion batteries. Carbohydr. Polym. 2023, 300, 120231. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, L.; Yan, X.; Ding, X. Recent Advances of Cellulose-Based Materials and Their Promising Application in Sodium-Ion Batteries and Capacitors. Small 2018, 14, 1802444. [Google Scholar] [CrossRef]
- Rafique, A.; Sequeira, I.; Bento, A.S.; Moniz, M.P.; Carmo, J.; Oliveira, E.; Oliveira, J.P.; Marques, A.; Ferreira, I.; Baptista, A.C. A facile blow spinning technique for green cellulose acetate/polystyrene composite separator for flexible energy storage devices. Chem. Eng. J. 2023, 464, 142515. [Google Scholar] [CrossRef]
- Zhu, K.; Li, L.; Xue, P.; Pu, J.; Wu, L.; Guo, G.; Wang, R.; Zhang, Y.; Peng, H.; Hong, G.; et al. “Three-in-one” strategy: Heat regulation and conversion enhancement of a multifunctional separator for safer lithium–sulfur batteries. Carbon Energy 2023, 5, e352. [Google Scholar] [CrossRef]
- Yang, J.-L.; Zhao, X.-X.; Zhang, W.; Ren, K.; Luo, X.-X.; Cao, J.-M.; Zheng, S.-H.; Li, W.-L.; Wu, X.-L. “Pore-Hopping” Ion Transport in Cellulose-Based Separator Towards High-Performance Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2023, 62, e202300258. [Google Scholar] [CrossRef]
- Zhang, F.; Lan, X.; Peng, H.; Hu, X.; Zhao, Q. A “Trojan Horse” Camouflage Strategy for High-Performance Cellulose Paper and Separators. Adv. Funct. Mater. 2020, 30, 2002169. [Google Scholar] [CrossRef]
- Jabbour, L.; Bongiovanni, R.; Chaussy, D.; Gerbaldi, C.; Beneventi, D. Cellulose-based Li-ion batteries: A review. Cellulose 2013, 20, 1523–1545. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, Z.; Xiang, Y.; Liu, D.; Xie, Z.; Qu, D.; Sun, M.; Tang, H.; Li, J. Cellulose-based material in lithium-sulfur batteries: A review. Carbohydr. Polym. 2021, 255, 117469. [Google Scholar] [CrossRef]
- Sheng, J.; Wang, R.; Yang, R. Physicochemical Properties of Cellulose Separators for Lithium Ion Battery: Comparison with Celgard2325. Materials 2019, 12, 2. [Google Scholar] [CrossRef]
- Sheng, J.; Tong, S.; He, Z.; Yang, R. Recent developments of cellulose materials for lithium-ion battery separators. Cellulose 2017, 24, 4103–4122. [Google Scholar] [CrossRef]
- Rezayan, A.; Nie, R.; Wang, J.; Lu, T.; Xu, C.C.; Zhang, Y. Efficient one-pot synthesis of 5-hydroxymethylfurfural from functionalized microcrystalline cellulose: An alternative approach to strong acidic/basic catalysis. Chem. Eng. J. 2023, 462, 142219. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Khimeche, K. Tetrazole-functionalized microcrystalline cellulose: A promising biopolymer for advanced energetic materials. Chem. Eng. J. 2020, 400, 125960. [Google Scholar] [CrossRef]
- Singh, M.; Pahal, V.; Ahuja, D. Isolation and characterization of microfibrillated cellulose and nanofibrillated cellulose with “biomechanical hotspots”. Carbohydr. Polym. 2020, 234, 115827. [Google Scholar] [CrossRef]
- Siqueira, I.S.D.; Dweck, J.; Toledo Filho, R.D. Effect of microcrystalline and microfibrillated cellulose on the evolution of hydration of cement pastes by thermogravimetry. J. Therm. Anal. Calorim. 2020, 142, 1413–1428. [Google Scholar] [CrossRef]
- Perondi, D.; Bassanesi, G.R.; Manera, C.; Lazzari, L.K.; Godinho, M.; Zattera, A.J.; Dotto, G.L. From cellulose to graphene-like porous carbon nanosheets. Microporous Mesoporous Mater. 2021, 323, 111217. [Google Scholar] [CrossRef]
- van de Ven, T.G.M.; Sheikhi, A. Hairy cellulose nanocrystalloids: A novel class of nanocellulose. Nanoscale 2016, 8, 15101–15114. [Google Scholar] [CrossRef]
- Lv, X.; Han, J.; Liu, M.; Yu, H.; Liu, K.; Yang, Y.; Sun, Y.; Pan, P.; Liang, Z.; Chang, L.; et al. Overview of preparation, modification, and application of tunicate-derived nanocellulose. Chem. Eng. J. 2023, 452, 139439. [Google Scholar] [CrossRef]
- Abdellah Ali, S.F.; William, L.A.; Fadl, E.A. Cellulose acetate, cellulose acetate propionate and cellulose acetate butyrate membranes for water desalination applications. Cellulose 2020, 27, 9525–9543. [Google Scholar] [CrossRef]
- Chen, G.-J.; Lee, D.-J. Synthesis of asymmetrical cellulose acetate/cellulose triacetate forward osmosis membrane: Optimization. J. Taiwan Inst. Chem. Eng. 2019, 96, 299–304. [Google Scholar] [CrossRef]
- Elkony, Y.; Mansour, E.-S.; Elhusseiny, A.; Ebrahim, S. Effect of cellulose acetate/cellulose triacetate ratio on reverse osmosis blend membrane performance. Polym. Eng. Sci. 2020, 60, 2852–2863. [Google Scholar] [CrossRef]
- Akram, M.; Taha, I.; Ghobashy, M.M. Low temperature pyrolysis of carboxymethylcellulose. Cellulose 2016, 23, 1713–1724. [Google Scholar] [CrossRef]
- Chagas, R.; Gericke, M.; Ferreira, R.B.; Heinze, T.; Ferreira, L.M. Synthesis and characterization of dicarboxymethyl cellulose. Cellulose 2020, 27, 1965–1974. [Google Scholar] [CrossRef]
- Lakshmi, D.S.; Trivedi, N.; Reddy, C.R.K. Synthesis and characterization of seaweed cellulose derived carboxymethyl cellulose. Carbohydr. Polym. 2017, 157, 1604–1610. [Google Scholar] [CrossRef]
- Candido, R.G.; Gonçalves, A.R. Synthesis of cellulose acetate and carboxymethylcellulose from sugarcane straw. Carbohydr. Polym. 2016, 152, 679–686. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, R.; Cranston, E.D.; Pelton, R.H. Stable Aqueous Foams from Cellulose Nanocrystals and Methyl Cellulose. Biomacromolecules 2016, 17, 4095–4099. [Google Scholar] [CrossRef]
- Lyytikäinen, J.; Laukala, T.; Backfolk, K. Temperature-dependent interactions between hydrophobically modified ethyl(hydroxyethyl)cellulose and methyl nanocellulose. Cellulose 2019, 26, 7079–7087. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Zhang, C.; Feng, F.; Zhang, H. Sequential electrospinning of multilayer ethylcellulose/gelatin/ethylcellulose nanofibrous film for sustained release of curcumin. Food Chem. 2020, 308, 125599. [Google Scholar] [CrossRef] [PubMed]
- Niamsap, T.; Lam, N.T.; Sukyai, P. Production of hydroxyapatite-bacterial nanocellulose scaffold with assist of cellulose nanocrystals. Carbohydr. Polym. 2019, 205, 159–166. [Google Scholar] [CrossRef]
- Seoane, I.T.; Manfredi, L.B.; Cyras, V.P. Bilayer biocomposites based on coated cellulose paperboard with films of polyhydroxybutyrate/cellulose nanocrystals. Cellulose 2018, 25, 2419–2434. [Google Scholar] [CrossRef]
- Pan, R.; Cheung, O.; Wang, Z.; Tammela, P.; Huo, J.; Lindh, J.; Edström, K.; Strømme, M.; Nyholm, L. Mesoporous Cladophora cellulose separators for lithium-ion batteries. J. Power Sources 2016, 321, 185–192. [Google Scholar] [CrossRef]
- Li, Y.; Long, J.; Liang, Y.; Hu, J. Tri-Layer Nonwoven Separator with Thermal Shutdown Function Fabricated through a Facile Papermaking Method for High-Safety Lithium-Ion Battery. ACS Appl. Polym. Mater. 2023, 5, 5305–5313. [Google Scholar] [CrossRef]
- Thiangtham, S.; Runt, J.; Saito, N.; Manuspiya, H. Fabrication of biocomposite membrane with microcrystalline cellulose (MCC) extracted from sugarcane bagasse by phase inversion method. Cellulose 2020, 27, 1367–1384. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Liang, Y. Preparation and characterization of a Lithium-ion battery separator from cellulose nanofibers. Heliyon 2015, 1, e00032. [Google Scholar] [CrossRef]
- Wang, N.; Liu, W.; Liao, H.; Li, Z.; Chen, Y.; Zeng, G. Pure cellulose nanofiber separator with high ionic conductivity and cycling stability for lithium-ion batteries. Int. J. Biol. Macromol. 2023, 250, 126078. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Li, C.; Liang, Z.; Hu, X.; Liu, H.; Zhang, Z.; Cui, M.; Chen, G.; Wan, J.; et al. Highly Thermally Stable, Highly Electrolyte-Wettable Hydroxyapatite/Cellulose Nanofiber Hybrid Separators for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2023, 6, 3862–3871. [Google Scholar] [CrossRef]
- Gonçalves, R.; Lizundia, E.; Silva, M.M.; Costa, C.M.; Lanceros-Méndez, S. Mesoporous Cellulose Nanocrystal Membranes as Battery Separators for Environmentally Safer Lithium-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 3749–3761. [Google Scholar] [CrossRef]
- Ajkidkarn, P.; Manuspiya, H. Novel bacterial cellulose nanocrystals/polyether block amide microporous membranes as separators for lithium-ion batteries. Int. J. Biol. Macromol. 2020, 164, 3580–3588. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, J.; Qiu, S.; Jia, Y.; Wang, L.; Wang, H. Tailoring the pore size of polyphenylene sulfide nonwoven with bacterial cellulose (BC) for heat-resistant and high-wettability separator in lithium-ion battery. Compos. Commun. 2021, 24, 100659. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, H.; Huang, H.; Wei, Z.; Zhao, Y. Water-soluble ammonium polyphosphate synchronously enables mechanically robust and flame-retardant cellulose composite separator for high safety lithium batteries. J. Power Sources 2023, 558, 232627. [Google Scholar] [CrossRef]
- Chen, W.; Wang, X.; Liang, J.; Chen, Y.; Ma, W.; Zhang, S. A High Performance Polyacrylonitrile Composite Separator with Cellulose Acetate and Nano-Hydroxyapatite for Lithium-Ion Batteries. Membranes 2022, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lv, S.; Zhang, M.; He, J.; Ni, P. UV-photopolymerized cellulose acetate-acrylate membranes for lithium-ion battery separator. Colloids Surf. A 2023, 667, 131359. [Google Scholar] [CrossRef]
- Arundati, A.H.; Ratri, C.R.; Chalid, M.; Aqoma, H.; Nugraha, A.F. A combination of nonsolvent and thermally induced phase separation (N-TIPS) technique for the preparation of highly porous cellulose acetate membrane as lithium-ion battery separators. Ionics 2024, 30, 123–133. [Google Scholar] [CrossRef]
- Liao, H.; Hong, H.; Zhang, H.; Li, Z. Preparation of hydrophilic polyethylene/methylcellulose blend microporous membranes for separator of lithium-ion batteries. J. Membr. Sci. 2016, 498, 147–157. [Google Scholar] [CrossRef]
- Ndruru, S.T.C.L.; Pramono, E.; Wahyuningrum, D.; Bundjali, B.; Arcana, I.M. Preparation and characterization of biopolymer blend electrolyte membranes based on derived celluloses for lithium-ion batteries separator. Bull. Mater. Sci. 2021, 44, 104. [Google Scholar] [CrossRef]
- Kennedy, S.; Kim, J.; Kim, J.; Phiri, I.; Ryou, S.-Y. Water-based dual polymer ceramic-coated composite separator for high-energy-density lithium secondary batteries. J. Ind. Eng. Chem. 2024, 130, 638–647. [Google Scholar] [CrossRef]
- Xiong, M.; Tang, H.; Wang, Y.; Pan, M. Ethylcellulose-coated polyolefin separators for lithium-ion batteries with improved safety performance. Carbohydr. Polym. 2014, 101, 1140–1146. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Li, M.X.; Chang, Z.; Wang, Y.F.; Gao, J.; Zhu, Y.S.; Wu, Y.P.; Huang, W. A Sandwich PVDF/HEC/PVDF Gel Polymer Electrolyte for Lithium Ion Battery. Electrochim. Acta 2017, 245, 752–759. [Google Scholar] [CrossRef]
- Francis, C.F.J.; Kyratzis, I.L.; Best, A.S.J.A.M. Lithium-Ion Battery Separators for Ionic-Liquid Electrolytes: A Review. Adv. Mater. 2020, 32, 1904205. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, G.; Yang, X.; Wen, W.; Zhang, B.; Du, W.; Li, X.; Xie, H. H-Bond Cross-Linked Polyimide Nanofiber-Modified Polyethylene Composite Separators for Lithium-Ion Batteries. Energy Fuels 2023, 37, 6770–6777. [Google Scholar] [CrossRef]
- Nomura, K.; Terwilliger, P. Self-dual Leonard pairs. Spec. Matrices 2018, 7, 1–19. [Google Scholar] [CrossRef]
- Gu, Q.-Q.; Fu, C.-L.; Sun, Z.-Y. Resin-silica composite nanoparticle grafted polyethylene membranes for lithium ion batteries. J. Appl. Polym. Sci. 2021, 138, 50713. [Google Scholar] [CrossRef]
- Martinez-Cisneros, C.; Antonelli, C.; Levenfeld, B.; Varez, A.; Sanchez, J.Y. Evaluation of polyolefin-based macroporous separators for high temperature Li-ion batteries. Electrochim. Acta 2016, 216, 68–78. [Google Scholar] [CrossRef]
- Li, D.; Xu, H.; Liu, Y.; Jiang, Y.; Li, F.; Xue, B. Fabrication of diatomite/polyethylene terephthalate composite separator for lithium-ion battery. Ionics 2019, 25, 5341–5351. [Google Scholar] [CrossRef]
- Cai, B.-R.; Cao, J.-H.; Liang, W.-H.; Yang, L.-Y.; Liang, T.; Wu, D.-Y. Ultraviolet-Cured Al2O3-Polyethylene Terephthalate/Polyvinylidene Fluoride Composite Separator with Asymmetric Design and Its Performance in Lithium Batteries. ACS Appl. Energy Mater. 2021, 4, 5293–5303. [Google Scholar] [CrossRef]
- Liang, T.; Liang, W.-H.; Cao, J.-H.; Wu, D.-Y. Enhanced Performance of High Energy Density Lithium Metal Battery with PVDF-HFP/LAGP Composite Separator. ACS Appl. Energy Mater. 2021, 4, 2578–2585. [Google Scholar] [CrossRef]
- Javadi, O.; Fathollahi Zonouz, A.; Soltanieh, M.; Mousavi, S.A. PVDF/PU blend membrane separator for lithium-ion batteries via non-solvent-induced phase separation (NIPS). J. Solid State Electrochem. 2021, 25, 2385–2394. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Y.; Yuan, B.; Guang, Z.; Liu, J.; Dong, L.; Ji, Y.; Li, Q.; Liang, Y.; Dong, Y.; et al. Ethyl cyanoacrylate reinforced polyvinylidene fluoride separators for robust lithium ion batteries. Mater. Chem. Front. 2021, 5, 2434–2441. [Google Scholar] [CrossRef]
- Yoo, G.; Pyo, S.; Gong, Y.J.; Cho, J.; Kim, H.; Kim, Y.S.; Yoo, J. Highly reliable quinone-based cathodes and cellulose nanofiber separators: Toward eco-friendly organic lithium batteries. Cellulose 2020, 27, 6707–6717. [Google Scholar] [CrossRef]
- Gou, J.; Liu, W.; Tang, A. A novel method to prepare a highly porous separator based on nanocellulose with multi-scale pore structures and its application for rechargeable lithium ion batteries. J. Membr. Sci. 2021, 639, 119750. [Google Scholar] [CrossRef]
- Tkachenko, T.; Sheludko, Y.; Yevdokymenko, V.; Kamenskyh, D.; Khimach, N.; Povazhny, V.; Filonenko, M.; Aksylenko, M.; Kashkovsky, V. Physico-chemical properties of flax microcrystalline cellulose. Appl. Nanosci. 2022, 12, 1007–1020. [Google Scholar] [CrossRef]
- Cho, B.-G.; Mun, S.-B.; Lim, C.-R.; Kang, S.B.; Cho, C.-W.; Yun, Y.-S. Adsorption modeling of microcrystalline cellulose for pharmaceutical-based micropollutants. J. Hazard. Mater. 2022, 426, 128087. [Google Scholar] [CrossRef]
- Deng, S.; Binauld, S.; Mangiante, G.; Frances, J.M.; Charlot, A.; Bernard, J.; Zhou, X.; Fleury, E. Microcrystalline cellulose as reinforcing agent in silicone elastomers. Carbohydr. Polym. 2016, 151, 899–906. [Google Scholar] [CrossRef]
- Kian, L.K.; Jawaid, M.; Ariffin, H.; Karim, Z. Isolation and characterization of nanocrystalline cellulose from roselle-derived microcrystalline cellulose. Int. J. Biol. Macromol. 2018, 114, 54–63. [Google Scholar] [CrossRef]
- Lidén, A.; Karna, N.K.; Mattsson, T.; Theliander, H. Dewatering microcrystalline cellulose: The influence of ionic strength. Sep. Purif. Technol. 2021, 264, 118245. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Chen, M.; Goff, H.D.; Zhong, F.; Sharif, H.R.; Li, Y. Functionality and nutritional aspects of microcrystalline cellulose in food. Carbohydr. Polym. 2017, 172, 159–174. [Google Scholar] [CrossRef]
- Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R.; Montero, B. Processing and characterization of polyols plasticized-starch reinforced with microcrystalline cellulose. Carbohydr. Polym. 2016, 149, 83–93. [Google Scholar] [CrossRef]
- Bangar, S.P.; Esua, O.J.; Nickhil, C.; Whiteside, W.S. Microcrystalline cellulose for active food packaging applications: A review. Food Packag. Shelf Life 2023, 36, 101048. [Google Scholar] [CrossRef]
- Rasheed, M.; Jawaid, M.; Karim, Z.; Abdullah, L.C. Morphological, Physiochemical and Thermal Properties of Microcrystalline Cellulose (MCC) Extracted from Bamboo Fiber. Molecules 2020, 25, 2824. [Google Scholar] [CrossRef]
- Alavi, M. Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications. e-Polymers 2019, 19, 103–119. [Google Scholar] [CrossRef]
- Bhandari, K.; Adaval, A.; Bhattacharyya, A.R.; Maulik, S.R. Synthesis of Microcrystalline Cellulose from Carpenter Waste and Its Characterizations. J. Nat. Fibers 2022, 19, 1975–1989. [Google Scholar] [CrossRef]
- Santos, D.; Iop, G.D.; Bizzi, C.A.; Mello, P.A.; Mesko, M.F.; Balbinot, F.P.; Flores, E.M.M. A single step ultrasound-assisted nitrocellulose synthesis from microcrystalline cellulose. Ultrason. Sonochem. 2021, 72, 105453. [Google Scholar] [CrossRef]
- Kanbua, C.; Rattanawongwiboon, T.; Khamlue, R.; Ummartyotin, S. Green synthesis of sulfonated cellulose/polyether block amide/polyethylene glycol diacrylate (SC/PEBAX/PEGDA) composite membrane by gamma radiation and sulfonation techniques for battery application. Int. J. Biol. Macromol. 2023, 248, 125844. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.; MacNaughtan, W.; Foster, T.J. Interactions between microfibrillar cellulose and carboxymethyl cellulose in an aqueous suspension. Carbohydr. Polym. 2018, 185, 112–119. [Google Scholar] [CrossRef]
- Agarwal, D.; MacNaughtan, W.; Ibbett, R.; Foster, T.J. Effect of moisture content on thermal and water absorption properties of microfibrillar cellulose with polymeric additives. Carbohydr. Polym. 2019, 211, 91–99. [Google Scholar] [CrossRef]
- Hettegger, H.; Beaumont, M.; Potthast, A.; Rosenau, T. Aqueous Modification of Nano- and Microfibrillar Cellulose with a Click Synthon. ChemSusChem 2016, 9, 75–79. [Google Scholar] [CrossRef]
- Jeoh, T.; Santa-Maria, M.C.; O’Dell, P.J. Assessing cellulose microfibrillar structure changes due to cellulase action. Carbohydr. Polym. 2013, 97, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Sourty, E.; Ryan, D.H.; Marchessault, R.H. Ferrite-Loaded Membranes of Microfibrillar Bacterial Cellulose Prepared by in Situ Precipitation. Chem. Mater. 1998, 10, 1755–1757. [Google Scholar] [CrossRef]
- Spence, K.L.; Venditti, R.A.; Habibi, Y.; Rojas, O.J.; Pawlak, J.J. The effect of chemical composition on microfibrillar cellulose films from wood pulps: Mechanical processing and physical properties. Bioresour. Technol. 2010, 101, 5961–5968. [Google Scholar] [CrossRef]
- Yu, H.; Liu, R.; Shen, D.; Wu, Z.; Huang, Y. Arrangement of cellulose microfibrils in the wheat straw cell wall. Carbohydr. Polym. 2008, 72, 122–127. [Google Scholar] [CrossRef]
- Zhao, Y.; Moser, C.; Henriksson, G. Transparent Composites Made from Tunicate Cellulose Membranes and Environmentally Friendly Polyester. ChemSusChem 2018, 11, 1728–1735. [Google Scholar] [CrossRef]
- Bolloli, M.; Antonelli, C.; Molméret, Y.; Alloin, F.; Iojoiu, C.; Sanchez, J.-Y. Nanocomposite poly(vynilidene fluoride)/nanocrystalline cellulose porous membranes as separators for lithium-ion batteries. Electrochim. Acta 2016, 214, 38–48. [Google Scholar] [CrossRef]
- Xu, Q.; Kong, Q.; Liu, Z.; Zhang, J.; Wang, X.; Liu, R.; Yue, L.; Cui, G. Polydopamine-coated cellulose microfibrillated membrane as high performance lithium-ion battery separator. RSC Adv. 2014, 4, 7845–7850. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Huang, C.; Luo, L.; Deng, Z.; Guo, W.; Xu, J.; Meng, Z. A novel cellulose membrane from cattail fibers as separator for Li-ion batteries. Cellulose 2021, 28, 9309–9321. [Google Scholar] [CrossRef]
- Hiltunen, S.; Heiskanen, I.; Backfolk, K. Effect of hydrothermal treatment of microfibrillated cellulose on rheological properties and formation of hydrolysis products. Cellulose 2018, 25, 4653–4662. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Sabu, A.; Tiwari, S.K. Materials chemistry and the futurist eco-friendly applications of nanocellulose: Status and prospect. J. Saudi Chem. Soc. 2018, 22, 949–978. [Google Scholar] [CrossRef]
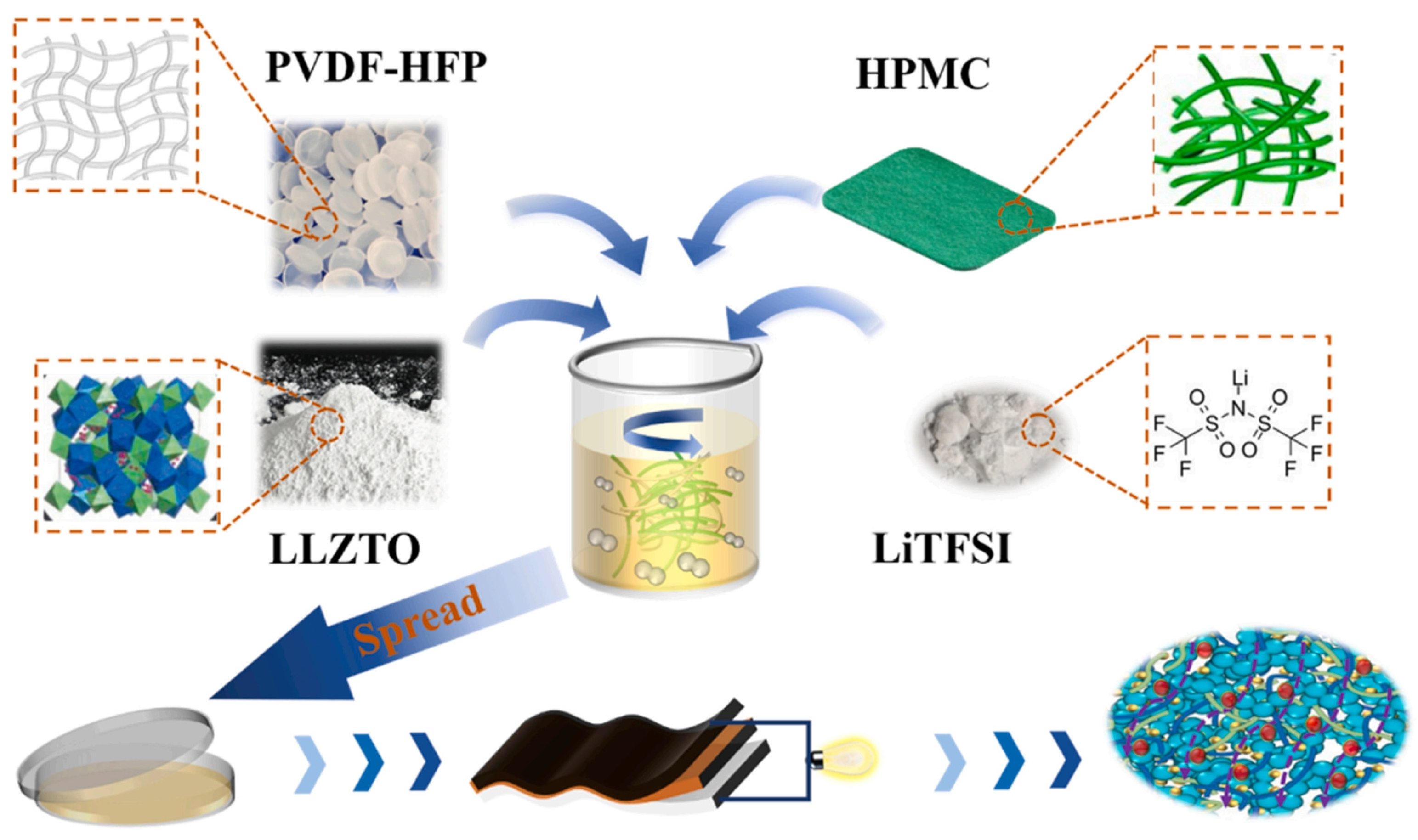
- Zhou, K.; Zhang, M.; Zhang, X.; Wang, T.; Wang, H.; Wang, Z.; Tang, X.; Bai, M.; Li, S.; Wang, Z.; et al. A cellulose reinforced polymer composite electrolyte for the wide-temperature-range solid lithium batteries. Chem. Eng. J. 2023, 464, 142537. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Liu, X.; Chen, C.; Feng, X.; Zhang, Z.; Liu, P. Composite solid electrolyte with Li+ conducting 3D porous garnet-type framework for all-solid-state lithium batteries. Mater. Chem. Front. 2022, 6, 1672–1680. [Google Scholar] [CrossRef]
- Su, M.; Huang, G.; Wang, S.; Wang, Y.; Wang, H. High safety separators for rechargeable lithium batteries. Sci. China Chem. 2021, 64, 1131–1156. [Google Scholar] [CrossRef]
- Xu, R.; Sun, Y.; Wang, Y.; Huang, J.; Zhang, Q. Two-dimensional vermiculite separator for lithium sulfur batteries. Chin. Chem. Lett. 2017, 28, 2235–2238. [Google Scholar] [CrossRef]
- Zhai, P.; Liu, K.; Wang, Z.; Shi, L.; Yuan, S. Multifunctional separators for high-performance lithium ion batteries. J. Power Sources 2021, 499, 229973. [Google Scholar] [CrossRef]
- Djafari Petroudy, S.R.; Rasooly Garmaroody, E.; Rudi, H. Oriented Cellulose Nanopaper (OCNP) based on bagasse cellulose nanofibrils. Carbohydr. Polym. 2017, 157, 1883–1891. [Google Scholar] [CrossRef]
- Hai, L.V.; Kim, H.C.; Kafy, A.; Zhai, L.; Kim, J.W.; Kim, J. Green all-cellulose nanocomposites made with cellulose nanofibers reinforced in dissolved cellulose matrix without heat treatment. Cellulose 2017, 24, 3301–3311. [Google Scholar] [CrossRef]
- Hu, C.; Zhou, Y.; Zhang, T.; Jiang, T.; Meng, C.; Zeng, G. Morphological, Thermal, Mechanical, and Optical Properties of Hybrid Nanocellulose Film Containing Cellulose Nanofiber and Cellulose Nanocrystals. Fibers Polym. 2021, 22, 2187–2193. [Google Scholar] [CrossRef]
- Chen, J.-H.; Liu, J.-G.; Yuan, T.-Q.; Sun, R.-C. Comparison of cellulose and chitin nanocrystals for reinforcing regenerated cellulose fibers. J. Appl. Polym. Sci. 2017, 134, 44880. [Google Scholar] [CrossRef]
- Nascimento, E.S.; Barros, M.O.; Cerqueira, M.A.; Lima, H.L.; Borges, M.d.F.; Pastrana, L.M.; Gama, F.M.; Rosa, M.F.; Azeredo, H.M.C.; Gonçalves, C. All-cellulose nanocomposite films based on bacterial cellulose nanofibrils and nanocrystals. Food Packag. Shelf Life 2021, 29, 100715. [Google Scholar] [CrossRef]
- Penttilä, P.A.; Imai, T.; Sugiyama, J. Fibrillar assembly of bacterial cellulose in the presence of wood-based hemicelluloses. Int. J. Biol. Macromol. 2017, 102, 111–118. [Google Scholar] [CrossRef]
- Skvortsova, Z.N.; Gromovykh, T.I.; Grachev, V.S.; Traskin, V.Y. Physicochemical Mechanics of Bacterial Cellulose. Colloid J. 2019, 81, 366–376. [Google Scholar] [CrossRef]
- Uetani, K.; Koga, H.; Nogi, M. Estimation of the Intrinsic Birefringence of Cellulose Using Bacterial Cellulose Nanofiber Films. ACS Macro Lett. 2019, 8, 250–254. [Google Scholar] [CrossRef]
- Islam, M.S.; Chen, L.; Sisler, J.; Tam, K.C. Cellulose nanocrystal (CNC)–inorganic hybrid systems: Synthesis, properties and applications. J. Mater. Chem. B 2018, 6, 864–883. [Google Scholar] [CrossRef]
- Kelley, J.; Simonsen, J.; Ding, J. Poly(vinylidene fluoride-co-hexafluoropropylene) nanocomposites incorporating cellulose nanocrystals with potential applications in lithium ion batteries. J. Appl. Polym. Sci. 2013, 127, 487–493. [Google Scholar] [CrossRef]
- Gregory, D.A.; Tripathi, L.; Fricker, A.T.R.; Asare, E.; Orlando, I.; Raghavendran, V.; Roy, I. Bacterial cellulose: A smart biomaterial with diverse applications. Mater. Sci. Eng. R Rep. 2021, 145, 100623. [Google Scholar] [CrossRef]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Rahimian Koloor, S.S.; Petrů, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Abral, H.; Chairani, M.K.; Rizki, M.D.; Mahardika, M.; Handayani, D.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.M.; Ilyas, R.A. Characterization of compressed bacterial cellulose nanopaper film after exposure to dry and humid conditions. J. Mater. Res. Technol. 2021, 11, 896–904. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, C.; Gao, G.; Hu, C.; Luo, L.; Xu, J. Aramid nanofiber/bacterial cellulose composite separators for lithium-ion batteries. Carbohydr. Polym. 2020, 247, 116702. [Google Scholar] [CrossRef] [PubMed]
- Atila, D.; Keskin, D.; Tezcaner, A. Cellulose acetate based 3-dimensional electrospun scaffolds for skin tissue engineering applications. Carbohydr. Polym. 2015, 133, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Xu, F.; Alcoutlabi, M.; Mao, Y.; Lozano, K. Fibrous cellulose membrane mass produced via forcespinning® for lithium-ion battery separators. Cellulose 2015, 22, 1311–1320. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.; Zhang, M.; He, J.; Ni, P. A separator based on cross-linked nano-SiO2 and cellulose acetate for lithium-ion batteries. Electrochim. Acta 2020, 334, 135585. [Google Scholar] [CrossRef]
- Deng, L.; Wang, Y.; Cai, C.; Wei, Z.; Fu, Y. 3D-cellulose acetate-derived hierarchical network with controllable nanopores for superior Li+ transference number, mechanical strength and dendrites hindrance. Carbohydr. Polym. 2021, 274, 118620. [Google Scholar] [CrossRef]
- Nam Kung, D.C.; Kang, S.W. Stabilized cellulose separator based on NaCl and glycerol with uniform structure. Polymer 2023, 281, 126111. [Google Scholar] [CrossRef]
- Morozova, S. Methylcellulose fibrils: A mini review. Polym. Int. 2020, 69, 125–130. [Google Scholar] [CrossRef]
- Xiao, S.Y.; Yang, Y.Q.; Li, M.X.; Wang, F.X.; Chang, Z.; Wu, Y.P.; Liu, X. A composite membrane based on a biocompatible cellulose as a host of gel polymer electrolyte for lithium ion batteries. J. Power Sources 2014, 270, 53–58. [Google Scholar] [CrossRef]
- Bol, M.; Dobos, M.A.; Lebioda, S.; Saake, B.; Mischnick, P. Methanolysis of carboxymethyl cellulose: A comprehensive study. Cellulose 2019, 26, 383–397. [Google Scholar] [CrossRef]
- Casaburi, A.; Montoya Rojo, Ú.; Cerrutti, P.; Vázquez, A.; Foresti, M.L. Carboxymethyl cellulose with tailored degree of substitution obtained from bacterial cellulose. Food Hydrocoll. 2018, 75, 147–156. [Google Scholar] [CrossRef]
- Karataş, M.; Arslan, N. Flow behaviours of cellulose and carboxymethyl cellulose from grapefruit peel. Food Hydrocoll. 2016, 58, 235–245. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, P.; Chen, L.; Yang, P.; Zhao, J. Effect of a thin ceramic-coating layer on thermal and electrochemical properties of polyethylene separator for lithium-ion batteries. J. Power Sources 2014, 270, 547–553. [Google Scholar] [CrossRef]
- Kim, P.J.; Pol, V.G. High Performance Lithium Metal Batteries Enabled by Surface Tailoring of Polypropylene Separator with a Polydopamine/Graphene Layer. Adv. Energy Mater. 2018, 8, 1802665. [Google Scholar] [CrossRef]
- Bergmann, J.B.; Redondo, A.; Steiner, U.; Wilts, B.D.; Moatsou, D. Insect Antiadhesive Surfaces Using Electrosprayed Wrinkled Ethyl Cellulose Particles. ACS Appl. Mater. Interfaces 2021, 13, 9232–9238. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.A.; Quinchia, L.A.; Spikes, H.A.; Gallegos, C. Suitability of ethyl cellulose as multifunctional additive for blends of vegetable oil-based lubricants. J. Clean. Prod. 2017, 151, 1–9. [Google Scholar] [CrossRef]
- Kim, J.; Han, J. Bio-based process for the catalytic production of ethyl levulinate from cellulose. Appl. Energy 2021, 300, 117430. [Google Scholar] [CrossRef]
- Su, X.; Yang, Z.; Tan, K.B.; Chen, J.; Huang, J.; Li, Q. Preparation and characterization of ethyl cellulose film modified with capsaicin. Carbohydr. Polym. 2020, 241, 116259. [Google Scholar] [CrossRef]
- Yan, H.-X.; Zhang, S.-S.; He, J.-H.; Liu, J.-P. Application of ethyl cellulose, microcrystalline cellulose and octadecanol for wax based floating solid dispersion pellets. Carbohydr. Polym. 2016, 148, 143–152. [Google Scholar] [CrossRef]
- Chen, Q.; Zuo, X.; Liang, H.; Zhu, T.; Zhong, Y.; Liu, J.; Nan, J. A Heat-Resistant Poly(oxyphenylene benzimidazole)/Ethyl Cellulose Blended Polymer Membrane for Highly Safe Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 637–645. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, H.; Hong, H.; Li, Z.; Qin, G.; Zhu, H.; Lin, Y. Novel cellulose aerogel coated on polypropylene separators as gel polymer electrolyte with high ionic conductivity for lithium-ion batteries. J. Membr. Sci. 2016, 514, 332–339. [Google Scholar] [CrossRef]
- Gao, C.; Li, X.; Wei, G.; Wang, S.; Zhao, X.; Kong, F. Flexible PVDF-HFP based hybrid composite solid electrolyte membrane co-filled with hydroxypropyl methyl cellulose and Li6.4La3Zr1.4Ta0.6O12 for high-performance solid-state lithium batteries. Ind. Crops Prod. 2023, 195, 116426. [Google Scholar] [CrossRef]
- Zolin, L.; Destro, M.; Chaussy, D.; Penazzi, N.; Gerbaldi, C.; Beneventi, D. Aqueous processing of paper separators by filtration dewatering: Towards Li-ion paper batteries. J. Mater. Chem. A 2015, 3, 14894–14901. [Google Scholar] [CrossRef]
- Zhang, J.; Yue, L.; Kong, Q.; Liu, Z.; Zhou, X.; Zhang, C.; Xu, Q.; Zhang, B.; Ding, G.; Qin, B.; et al. Sustainable, heat-resistant and flame-retardant cellulose-based composite separator for high-performance lithium ion battery. Sci. Rep. 2014, 4, 3935. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, H.; Wu, F.; Xiong, X.; Han, K. Natural Halloysite Nanotubes Coated Commercial Paper or Waste Newspaper as Highly-Thermal-Stable Separator for Lithium-Ion Batteries. Adv. Mater. Technol. 2022, 7, 2200075. [Google Scholar] [CrossRef]
- Pan, R.; Xu, X.; Sun, R.; Wang, Z.; Lindh, J.; Edström, K.; Strømme, M.; Nyholm, L. Nanocellulose Modified Polyethylene Separators for Lithium Metal Batteries. Small 2018, 14, 1704371. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, J.; Lei, J.; Liu, D.; Xie, Z.; Qu, D.; Li, K.; Deng, T.; Tang, H. Advanced Separators for Lithium-Ion and Lithium–Sulfur Batteries: A Review of Recent Progress. ChemSusChem 2016, 9, 3023–3039. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, Q.; Zhou, H.; Qi, L. Binder-Free TiO2-Coated Polypropylene Separators for Advanced Lithium-Ion Batteries. Energy Technol. 2020, 8, 2000228. [Google Scholar] [CrossRef]
- Xu, Q.; Wei, C.; Fan, L.; Peng, S.; Xu, W.; Xu, J. A bacterial cellulose/Al2O3 nanofibrous composite membrane for a lithium-ion battery separator. Cellulose 2017, 24, 1889–1899. [Google Scholar] [CrossRef]
- Cao, D.; Shen, X.; Wang, Y.; Liu, J.; Shi, H.; Gao, X.; Liu, X.; Fu, L.; Wu, Y.; Chen, Y. Conductive Polymer Coated Cathodes in Li–O2 Batteries. ACS Appl. Energy Mater. 2020, 3, 951–956. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, C.; Song, Z.; Wang, Q.; Lan, Y.; Luo, J.; Bo, L.; Yue, Z.; Sun, F.; Li, X. Application of PVDF Organic Particles Coating on Polyethylene Separator for Lithium Ion Batteries. Materials 2019, 12, 3125. [Google Scholar] [CrossRef] [PubMed]
- Gandhimathi, S.; Krishnan, H.; Paradesi, D. New series of organic–inorganic polymer nanocomposite membranes for fuel cell applications. High Perform. Polym. 2019, 32, 296–305. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, Y.; Li, Z.; Wang, Y. Facile fabrication of a low-cost and environmentally friendly inorganic-organic composite membrane for aquatic dye removal. J. Environ. Manag. 2020, 256, 109969. [Google Scholar] [CrossRef]
- Su, Z.; He, Y.; Liu, S.; Li, J.; Xiao, X.; Nan, J.; Zuo, X. Uniform Lithium Deposition Achieved by SnO2/Hydroxypropyl Methyl Cellulose Composite Separator toward Ultrastable Lithium Metal Batteries. ACS Appl. Energy Mater. 2022, 5, 10264–10275. [Google Scholar] [CrossRef]
- de Moraes, A.C.M.; Hyun, W.J.; Luu, N.S.; Lim, J.-M.; Park, K.-Y.; Hersam, M.C. Phase-Inversion Polymer Composite Separators Based on Hexagonal Boron Nitride Nanosheets for High-Temperature Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 8107–8114. [Google Scholar] [CrossRef]
- Singh, S.K.; Shalu; Balo, L.; Gupta, H.; Singh, V.K.; Tripathi, A.K.; Verma, Y.L.; Singh, R.K. Improved electrochemical performance of EMIMFSI ionic liquid based gel polymer electrolyte with temperature for rechargeable lithium battery. Energy 2018, 150, 890–900. [Google Scholar] [CrossRef]
- Lv, D.; Chai, J.; Wang, P.; Zhu, L.; Liu, C.; Nie, S.; Li, B.; Cui, G. Pure cellulose lithium-ion battery separator with tunable pore size and improved working stability by cellulose nanofibrils. Carbohydr. Polym. 2021, 251, 116975. [Google Scholar] [CrossRef]
- Huang, C.; Ji, H.; Guo, B.; Luo, L.; Xu, W.; Li, J.; Xu, J. Composite nanofiber membranes of bacterial cellulose/halloysite nanotubes as lithium ion battery separators. Cellulose 2019, 26, 6669–6681. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, J.; Xu, J.; Yin, X.; Wu, J.; Chen, S.; Zhu, Z.; Wang, L.; Wang, H. Facile fabrication of cellulose/polyphenylene sulfide composite separator for lithium-ion batteries. Carbohydr. Polym. 2020, 248, 116753. [Google Scholar] [CrossRef]
- Li, Y.; Pu, H. Facile fabrication of multilayer separators for lithium-ion battery via multilayer coextrusion and thermal induced phase separation. J. Power Sources 2018, 384, 408–416. [Google Scholar] [CrossRef]
- Wu, Q.-Y.; Liang, H.-Q.; Gu, L.; Yu, Y.; Huang, Y.-Q.; Xu, Z.-K. PVDF/PAN blend separators via thermally induced phase separation for lithium ion batteries. Polymer 2016, 107, 54–60. [Google Scholar] [CrossRef]
- Yang, B.; Yang, Y.; Xu, X.; Ke, Y.; Pan, Y.; Su, L.; Wang, Y.; Wang, S.; Qian, J.; Xia, R.; et al. Hierarchical microstructure and performance of PVDF/PMMA/SiO2 lithium battery separator fabricated by thermally-induced phase separation (TIPS). J. Mater. Sci. 2022, 57, 11274–11288. [Google Scholar] [CrossRef]
- Dong, T.; Arifeen, W.U.; Choi, J.; Yoo, K.; Ko, T. Surface-modified electrospun polyacrylonitrile nano-membrane for a lithium-ion battery separator based on phase separation mechanism. Chem. Eng. J. 2020, 398, 125646. [Google Scholar] [CrossRef]
- Manly, A.J.; Tenhaeff, W.E. One-step fabrication of robust lithium ion battery separators by polymerization-induced phase separation. J. Mater. Chem. A 2022, 10, 10557–10568. [Google Scholar] [CrossRef]
- Liang, H.-Q.; Li, H.-N.; Yu, H.-H.; Zhou, Y.-T.; Xu, Z.-K. Polysulfone membranes via thermally induced phase separation. Chin. J. Polym. Sci. 2017, 35, 846–856. [Google Scholar] [CrossRef]
- Pathak, B.; Xavier, P.; Bose, S.; Basu, S. Thermally induced phase separation in levitated polymer droplets. Phys. Chem. Chem. Phys. 2016, 18, 32477–32485. [Google Scholar] [CrossRef] [PubMed]
- Rusakov, D.; Menner, A.; Bismarck, A. High-Performance Polymer Foams by Thermally Induced Phase Separation. Macromol. Rapid Commun. 2020, 41, 2000110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-Q.; Hao, S.; Xiao, J.; Jia, Z.-Q. Preparation of Poly(4-methyl-1-pentene) Membranes by Low-Temperature Thermally Induced Phase Separation. ACS Appl. Polym. Mater. 2023, 5, 1998–2005. [Google Scholar] [CrossRef]
- Li, J.; Yu, Y.; Zhou, P.; Li, H.; Liu, Y. Dependency of polyacrylonitrile membrane structures on Hansen solubility parameters during non-solvent induced phase separation. J. Appl. Polym. Sci. 2022, 139, e52383. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Qin, Y.; Huang, Y.; Xiao, C. Oriented structure design and evaluation of Fe3O4/o-MWCNTs/PVC composite membrane assisted by magnetic field. J. Taiwan Inst. Chem. Eng. 2021, 120, 278–290. [Google Scholar] [CrossRef]
- Rarima, R.; Unnikrishnan, G. Poly(lactic acid)/gelatin foams by non-solvent induced phase separation for biomedical applications. Polym. Degrad. Stab. 2020, 177, 109187. [Google Scholar] [CrossRef]
- Yushkin, A.; Basko, A.; Balynin, A.; Efimov, M.; Lebedeva, T.; Ilyasova, A.; Pochivalov, K.; Volkov, A. Effect of Acetone as Co-Solvent on Fabrication of Polyacrylonitrile Ultrafiltration Membranes by Non-Solvent Induced Phase Separation. Polymers 2022, 14, 4603. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Sheng, J.; Zhu, H.; Yang, R. Nanoporous Regenerated Cellulose Separator for High-Performance Lithium Ion Batteries Prepared by Nonsolvent-Induced Phase Separation. ACS Sustain. Chem. Eng. 2021, 9, 14756–14765. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, C.-E.; Zhou, M.-Y.; John, A.E.; Zhu, B.-K. High thermal resistance polyimide separators prepared via soluble precusor and non-solvent induced phase separation process for lithium ion batteries. Electrochim. Acta 2016, 187, 125–133. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Zou, R.; Huang, Y.; Lai, H.; Chen, Z.; Peng, X. Cellulose nanofiber-derived carbon aerogel for advanced room-temperature sodium–sulfur batteries. Carbon Energy 2023, 5, e203. [Google Scholar] [CrossRef]
- Zhu, S.; Gong, L.; Pan, Y.; Deng, Y.; Zhou, Y.; Cheng, X.; Zhang, H. Coral-like interconnected carbon aerogel modified separator for advanced lithium-sulfur batteries. Electrochim. Acta 2020, 354, 136637. [Google Scholar] [CrossRef]
- Raafat, L.; Wicklein, B.; Majer, G.; Jahnke, T.; Diem, A.M.; Bill, J.; Burghard, Z. Shape-Conformable, Eco-Friendly Cellulose Aerogels as High-Performance Battery Separators. ACS Appl. Energy Mater. 2021, 4, 763–774. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z.; Lv, T.; Dong, K.; Liu, Y.; Qi, Y.; Cao, S.; Chen, T. Ultrafast self-assembly of supramolecular hydrogels toward novel flame-retardant separator for safe lithium ion battery. J. Colloid Interface Sci. 2023, 649, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cao, H.; Zhou, Z.; Mei, X.; Yu, L.; Chen, X.; He, G.; Zhao, Y.; Wu, D.; Sun, D. Concentrated Multi-nozzle Electrospinning. Fibers Polym. 2019, 20, 1180–1186. [Google Scholar] [CrossRef]
- Cai, M.; Yuan, D.; Zhang, X.; Pu, Y.; Liu, X.; He, H.; Zhang, L.; Ning, X. Lithium ion battery separator with improved performance via side-by-side bicomponent electrospinning of PVDF-HFP/PI followed by 3D thermal crosslinking. J. Power Sources 2020, 461, 228123. [Google Scholar] [CrossRef]
- Hao, J.; Lei, G.; Li, Z.; Wu, L.; Xiao, Q.; Wang, L. A novel polyethylene terephthalate nonwoven separator based on electrospinning technique for lithium ion battery. J. Membr. Sci. 2013, 428, 11–16. [Google Scholar] [CrossRef]
- Liao, C.; Han, L.; Wu, N.; Mu, X.; Hu, Y.; Kan, Y.; Song, L. Fabrication of a necklace-like fiber separator by the electrospinning technique for high electrochemical performance and safe lithium metal batteries. Mater. Chem. Front. 2021, 5, 5033–5043. [Google Scholar] [CrossRef]
- Qian, Y.; Chen, K.; Feng, Z.; Ouyang, Y.; Lan, Q.; Zhang, C.; Feng, W.; Miao, Y.-E.; Liu, T. A Fluorinated-Polyimide-Based Composite Nanofibrous Separator with Homogenized Pore Size for Wide-Temperature Lithium Metal Batteries. Small Struct. 2023, 4, 2200383. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, L.; Shen, J.; Liu, F.; Chen, G.; Tao, R.; Ma, S.; Peng, Y.; Lu, Y. Anion-Sorbent Composite Separators for High-Rate Lithium-Ion Batteries. Adv. Mater. 2019, 31, 1808338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, G.; Li, X.; Cui, S.; Ying, S.; Feng, X.; Mi, L.; Chen, W. Synergism of surface group transfer and in-situ growth of silica-aerogel induced high-performance modified polyacrylonitrile separator for lithium/sodium-ion batteries. J. Membr. Sci. 2019, 577, 137–144. [Google Scholar] [CrossRef]
- Boriboon, D.; Vongsetskul, T.; Limthongkul, P.; Kobsiriphat, W.; Tammawat, P. Cellulose ultrafine fibers embedded with titania particles as a high performance and eco-friendly separator for lithium-ion batteries. Carbohydr. Polym. 2018, 189, 145–151. [Google Scholar] [CrossRef]
- Deng, J.; Cao, D.; Yang, X.; Zhang, G. Cross-linked cellulose/carboxylated polyimide nanofiber separator for lithium-ion battery application. Chem. Eng. J. 2022, 433, 133934. [Google Scholar] [CrossRef]
- Liao, C.; Mu, X.; Han, L.; Li, Z.; Zhu, Y.; Lu, J.; Wang, H.; Song, L.; Kan, Y.; Hu, Y. A flame-retardant, high ionic-conductivity and eco-friendly separator prepared by papermaking method for high-performance and superior safety lithium-ion batteries. Energy Storage Mater. 2022, 48, 123–132. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, J.; Du, M.; Hui, H.; Sun, Z. Safety and cycling stability enhancement of cellulose paper-based lithium-ion battery separator by aramid nanofibers. Eur. Polym. J. 2022, 171, 111222. [Google Scholar] [CrossRef]
- Pan, R.; Sun, R.; Wang, Z.; Lindh, J.; Edström, K.; Strømme, M.; Nyholm, L. Double-sided conductive separators for lithium-metal batteries. Energy Storage Mater. 2019, 21, 464–473. [Google Scholar] [CrossRef]
- Liu, A.; Jiang, Z.; Li, S.; Du, J.; Tao, Y.; Lu, J.; Cheng, Y.; Wang, H. A degradable membrane based on lignin-containing cellulose for high-energy lithium-ion batteries. Int. J. Biol. Macromol. 2022, 213, 690–698. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, R.; Wang, Y.; Fu, D.; Sheng, J.; Guo, X. A bacterial cellulose-based separator with tunable pore size for lithium-ion batteries. Carbohydr. Polym. 2023, 304, 120489. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, J.; Du, M.; Zhang, F.; He, X. Highly porous zeolitic imidazolate framework-8@bacterial cellulose composite separator with enhanced electrolyte absorption capability for lithium-ion batteries. Cellulose 2022, 29, 5163–5176. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Shao, Z.; Liu, S. Cellulosic materials-enhanced sandwich structure-like separator via electrospinning towards safer lithium-ion battery. Carbohydr. Polym. 2019, 214, 328–336. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, L.; Ma, X.; Dong, L.; Jin, Z.; Xia, G.; Du, P.; Xiong, J. Electrospun cellulose polymer nanofiber membrane with flame resistance properties for lithium-ion batteries. Carbohydr. Polym. 2020, 234, 115907. [Google Scholar] [CrossRef]
- Huang, F.; Xu, Y.; Peng, B.; Su, Y.; Jiang, F.; Hsieh, Y.-L.; Wei, Q. Coaxial Electrospun Cellulose-Core Fluoropolymer-Shell Fibrous Membrane from Recycled Cigarette Filter as Separator for High Performance Lithium-Ion Battery. ACS Sustain. Chem. Eng. 2015, 3, 932–940. [Google Scholar] [CrossRef]
- Liu, C.; Shao, Z.; Wang, J.; Lu, C.; Wang, Z. Eco-friendly polyvinyl alcohol/cellulose nanofiber–Li+ composite separator for high-performance lithium-ion batteries. RSC Adv. 2016, 6, 97912–97920. [Google Scholar] [CrossRef]
- Li, L.; Yu, M.; Jia, C.; Liu, J.; Lv, Y.; Liu, Y.; Zhou, Y.; Liu, C.; Shao, Z. Cellulosic Biomass-Reinforced Polyvinylidene Fluoride Separators with Enhanced Dielectric Properties and Thermal Tolerance. ACS Appl. Mater. Interfaces 2017, 9, 20885–20894. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-C.; Chen, H.-J.; Wu, G.; Wang, X.-L.; Wang, Y.-Z. Multifunctional robust aerogel separator towards high-temperature, large-rate, long-cycle lithium-ion batteries. Chin. Chem. Lett. 2023, 34, 107546. [Google Scholar] [CrossRef]
- Ali, Y.; Iqbal, N.; Lee, S. Simultaneous effect of particle size and location on stress development in the electrodes of lithium-ion batteries. Int. J. Energy Res. 2020, 44, 12145–12157. [Google Scholar] [CrossRef]
- Jeon, D.H. Enhancing electrode wettability in lithium-ion battery via particle-size ratio control. Appl. Mater. Today 2021, 22, 100976. [Google Scholar] [CrossRef]
- Jana, K.K.; Lue, S.J.; Huang, A.; Soesanto, J.F.; Tung, K.-L. Separator Membranes for High Energy-Density Batteries. ChemBioEng Rev. 2018, 5, 346–371. [Google Scholar] [CrossRef]
- Lin, G.; Jia, K.; Bai, Z.; Liu, C.; Liu, S.; Huang, Y.; Liu, X. Metal-Organic Framework Sandwiching Porous Super-Engineering Polymeric Membranes as Anionphilic Separators for Dendrite-free Lithium Metal Batteries. Adv. Funct. Mater. 2022, 32, 2207969. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, W.; Hua, J.; Wang, Y.; Yi, H.; Zhao, W.; Zhao, Q.; Jia, H.; Fei, B.; Pan, F. An Anionic-MOF-Based Bifunctional Separator for Regulating Lithium Deposition and Suppressing Polysulfides Shuttle in Li–S Batteries. Small Methods 2020, 4, 2000082. [Google Scholar] [CrossRef]
- Yang, L.-Y.; Cao, J.-H.; Cai, B.-R.; Liang, T.; Wu, D.-Y. Electrospun MOF/PAN composite separator with superior electrochemical performances for high energy density lithium batteries. Electrochim. Acta 2021, 382, 138346. [Google Scholar] [CrossRef]
- Mao, H.; Da, X.; Shen, H.; Zhao, W.; Li, X.; Su, Y.; Ding, S.; Yu, W. Cellulose-based separator woven by double-layer-configuration fibers for high S-load Li-S batteries. Electrochim. Acta 2024, 492, 144363. [Google Scholar] [CrossRef]
- Li, Z.; Qian, P.; Li, H.; Xiao, H.; Chen, J.; Li, G. Phosphorylated cellulose nanofibers establishing reliable ion-sieving barriers for durable lithium-sulfur batteries. J. Energy Chem. 2024, 92, 619–628. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, L.; Wang, J.; Chai, Q.; Sun, S.; Chen, X.; Wang, X.; Zhang, Y.; Yan, W. Phosphorus flame retardant modified aramid nanofiber separator for advanced safety lithium-sulfur batteries. J. Energy Storage 2024, 92, 112030. [Google Scholar] [CrossRef]
- Fu, J.; Wang, H.; Xiao, P.; Zeng, C.; Sun, Q.; Li, H. A high strength, anti-corrosion and sustainable separator for aqueous zinc-based battery by natural bamboo cellulose. Energy Storage Mater. 2022, 48, 191–191.f6. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Zhang, Y.; Deng, J.; Chen, N.; Xie, S.; Ma, Y.; Wang, Z. Designing Anti-Swelling Nanocellulose Separators with Stable and Fast Ion Transport Channels for Efficient Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2304280. [Google Scholar] [CrossRef]




| Property | Cellulose-Based Separator | Polypropylene (PP) Separator and Polyethylene (PE) Separator |
|---|---|---|
| Environmental | Renewable, biodegradable | Nondegradable, long-term environmental impact |
| Thermal stability | High, does not shrink or melt at high temperatures | Shrinks or melts at high temperatures, increasing thermal runaway risk |
| Electrolyte wettability | Good, strong hydrophilicity | Poor, strong hydrophobicity, requires additional treatment |
| Mechanical strength | High, effectively prevents short circuits | Lower, easily damaged, increasing short circuit risk |
| Electrochemical stability | Good compatibility with electrolyte and electrode materials, fewer side reactions | Poor compatibility, may have side reactions |
| Production cost | Potentially higher, requires improved processes for large-scale commercialization | Lower, already mass-produced |
| Commercial maturity | In research stage, further validation needed for commercial use | Mature, widely used in current lithium-ion batteries |
| Kind | Materials | Preparation Method | Thickness (μm) | Tensile Strength (Mpa) | Thermal Stability (°C) | Electrolyte Absorption (%) | Porosity (%) | Ionic Conductivity (mS⋅cm−1) | Cost (¥) | Cyclic Performance (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Physically modified cellulose | MFC | Papermaking process | 35 | 61 | 150 | 93 | 46 | 0.4 | 140 | 50 cycles 99.5 | [70] |
| SWP@PET@MFC | Papermaking process | 51.3 | / | 200 | 348.6 | / | 0.77 | 269 | 200 cycles 80.2 | [71] | |
| MCC/PLA/PBS | Phase separation method | 30 | 3–6 | 135 | 138 | / | 2.06 | 525 | / | [72] | |
| CNF/PET | Papermaking process | 30 | 3 | 257 | 250 | 70 | / | 220 | 100 cycles 91.7 | [73] | |
| TBA-FD | Vacuum filtration method | / | 13 | 160 | 296 | 70.8 | 1.90 | 120 | 100 cycles 96.4 | [74] | |
| HAP/CNF | Vacuum filtration method | 28 | 9.94 | 250 | 162 | 76 | 0.81 | 160 | 100 cycles 67.1 | [75] | |
| MCNC | Phase separation method | 150 | / | / | 275 | 75.3 | 2.7 | 235 | 50 cycles 99.8 | [76] | |
| BCNCs/PEBAX | Phase separation method | / | 14.9 | / | 101.4 | 56.8 | 9.79 | 260 | / | [77] | |
| BC/PBS | Suction filtration method | 60 | 3 | 285 | 216 | 62.7 | 1.55 | 430 | 100 cycles 92 | [78] | |
| BLA | Casting method | 85 | 12.9 | 210 | 446 | 70 | 2.45 | 315 | 100 cycles 97 | [79] | |
| Chemically modified cellulose | CA/PAN/HAP | Electrospinning | 46 | 11.18 | 270 | 281 | 61 | 3.02 | 330 | 50 cycles 99.48 | [80] |
| CPT20 | UV-induced polymerization | 15 | / | 230 | 190 | / | 4.17 | 430 | 100 cycles 98.2 | [81] | |
| CA55 | Phase separation | / | / | 250 | 331 | 62 | 0.0307 | 240 | / | [82] | |
| HDPE/MC | Coating method | / | 3.5 | 130 | 130 | 68 | 1.01 | 250 | 50 cycles 96 | [83] | |
| CMC | Microwave method | / | 11.02 | 202 | / | / | 0.12 | 180 | / | [84] | |
| Al2O3 CCSs | / | 26 | / | 130 | 125.5 | / | 0.982 | 190 | 200 cycles 96.5 | [85] | |
| PP/EC/PE/PP | Coating method | 28 | / | 300 | / | / | 0.13 | 325 | 100 cycles 99 | [86] | |
| PVDF/HEC/PVDF | Coating method | 58 | 21.5 | 290 | 135.4 | / | 0.88 | 240 | 145 cycles 100 | [87] | |
| Polyolefin | PE | Coating method | 24 | 102.24 | 135 | 186.7 | 65.64 | 0.206 | 62 | 450 cycles 72 | [28] |
| PP | Coating method | 25 | 13.24 | 90 | / | 55 | / | 62 | / | [88] | |
| PE | Coating method | 12 | 82 | 140 | 123 | 42 | 0.12 | 50 | 200 cycles 78.4 | [89] | |
| PP Celgard 2400 | Coating method | 25 | / | 170 | 91 | / | / | 62 | 100 cycles 75 | [90] | |
| PE | Coating method | 24.8 | 94.3 | 140 | 119 | 43.5 | 0.212 | 65 | 200 cycles 83.1 | [91] | |
| PP | Coating method | 25 | 127 | 140 | 88 | 44 | 0.108 | 62 | 60 cycles 66 | [92] | |
| Synthetics | PVDF/PET | Coating method | 22 | 18 | 160 | 72 | 26.3 | 0.003 | 110 | / | [93] |
| PET | Coating method | 22 | 36.1 | 255 | 89 | / | 1.34 | 120 | 300 cycles 43.5 | [94] | |
| PVDF/HFP | Electrospinning | 58 | 2.74 | 200 | 350 | 92 | 2.05 | 230 | 500 cycles 84.5 | [95] | |
| PVDF/PU | Phase separation | 27 | 7.83 | 140 | 201.8 | 66.3 | 1.34 | 240 | 60 cycles 74 | [96] | |
| PVDF/EVA | Coating method | 25 | 70 | 200 | / | / | 0.9 | 210 | 100 cycles 98.4 | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, T.; Xiao, P.; Luo, M.; Nie, S.; Li, F.; Liu, Y. Eco-Friendly Lithium Separators: A Frontier Exploration of Cellulose-Based Materials. Int. J. Mol. Sci. 2024, 25, 6822. https://doi.org/10.3390/ijms25136822
Zhao T, Xiao P, Luo M, Nie S, Li F, Liu Y. Eco-Friendly Lithium Separators: A Frontier Exploration of Cellulose-Based Materials. International Journal of Molecular Sciences. 2024; 25(13):6822. https://doi.org/10.3390/ijms25136822
Chicago/Turabian StyleZhao, Tian, Pengcheng Xiao, Mingliang Luo, Saiqun Nie, Fuzhi Li, and Yuejun Liu. 2024. "Eco-Friendly Lithium Separators: A Frontier Exploration of Cellulose-Based Materials" International Journal of Molecular Sciences 25, no. 13: 6822. https://doi.org/10.3390/ijms25136822
APA StyleZhao, T., Xiao, P., Luo, M., Nie, S., Li, F., & Liu, Y. (2024). Eco-Friendly Lithium Separators: A Frontier Exploration of Cellulose-Based Materials. International Journal of Molecular Sciences, 25(13), 6822. https://doi.org/10.3390/ijms25136822






