Design Principles for Smart Linear Polymer Ligand Carriers with Efficient Transcellular Transport Capabilities
Abstract
1. Introduction
2. Results
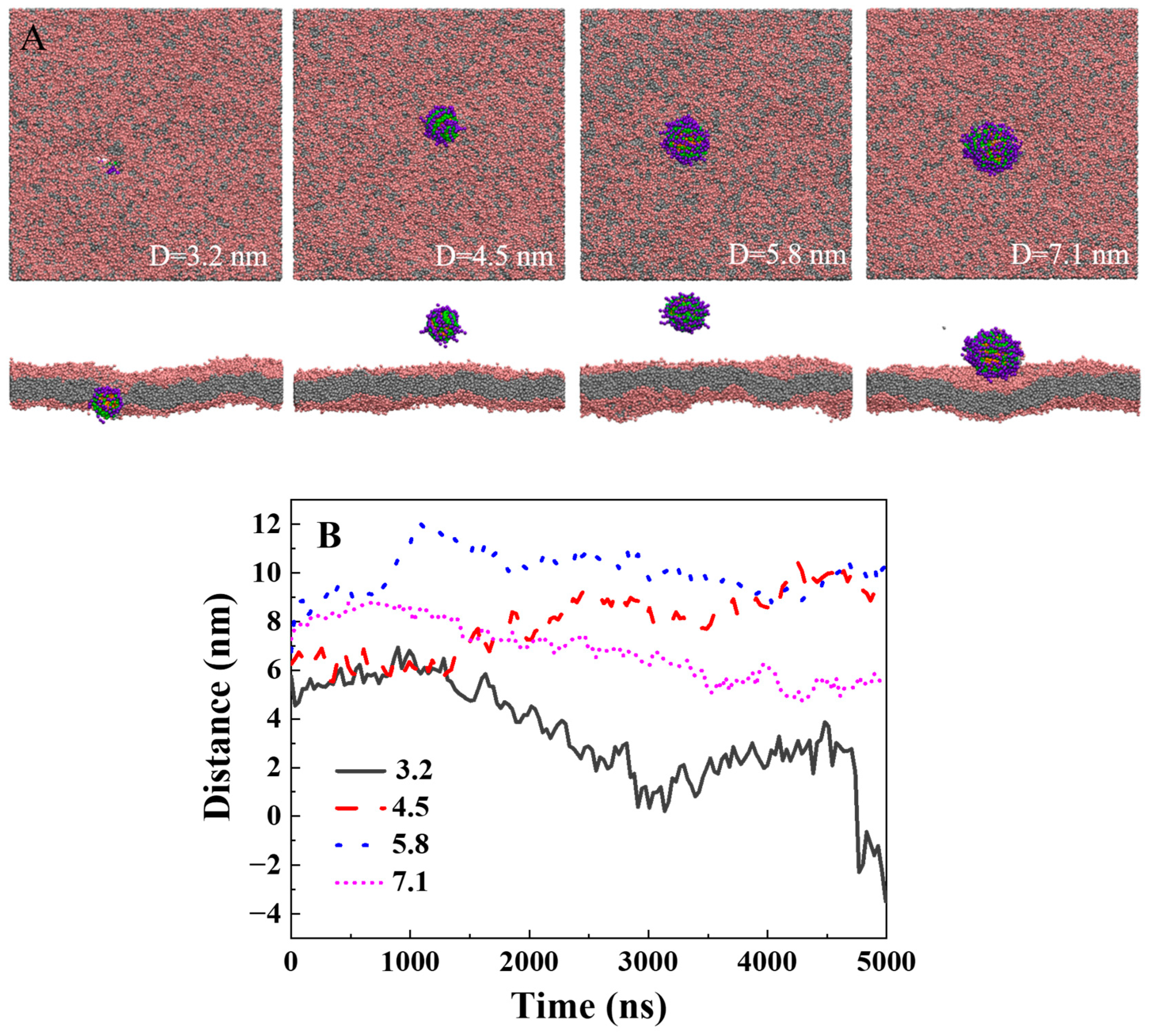
2.1. The Rigid Polymer Has a Higher Delivery Efficiency
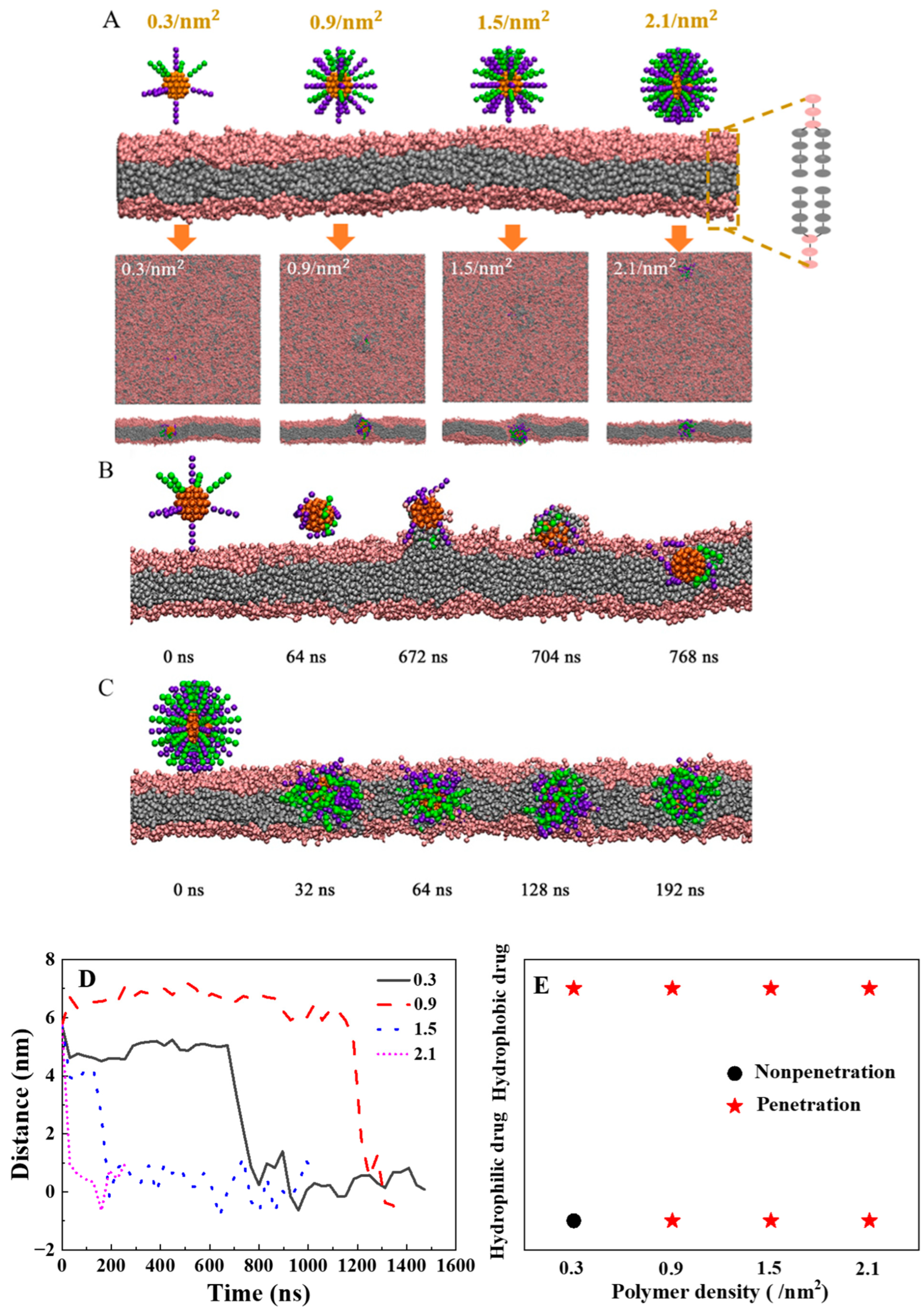
2.2. How to Design the Density of Polymers with Highly Efficient Transmembrane Ability
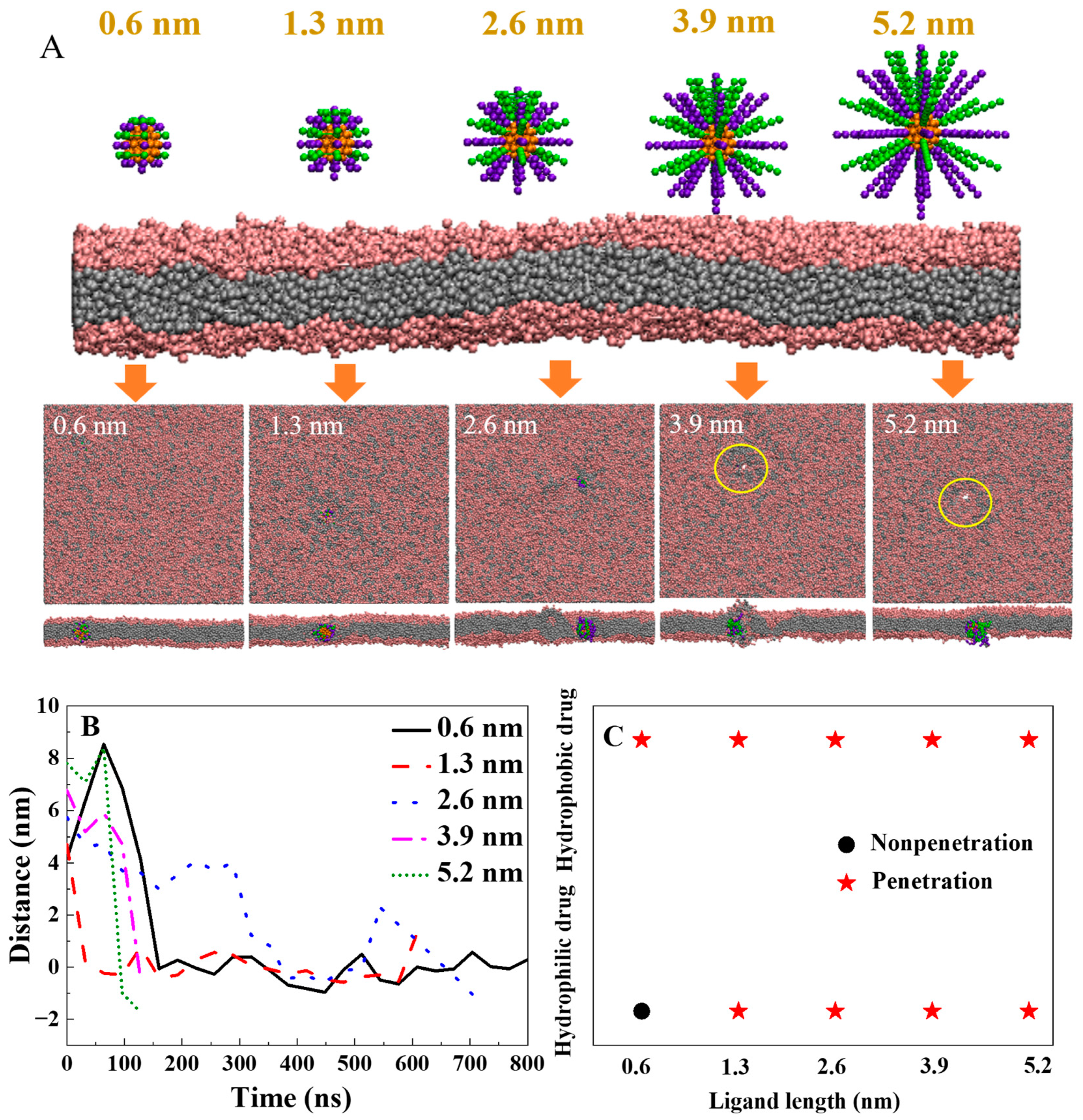
2.3. How to Design the Length of Polymers with Highly Efficient Transmembrane Ability and Low Cytotoxicity
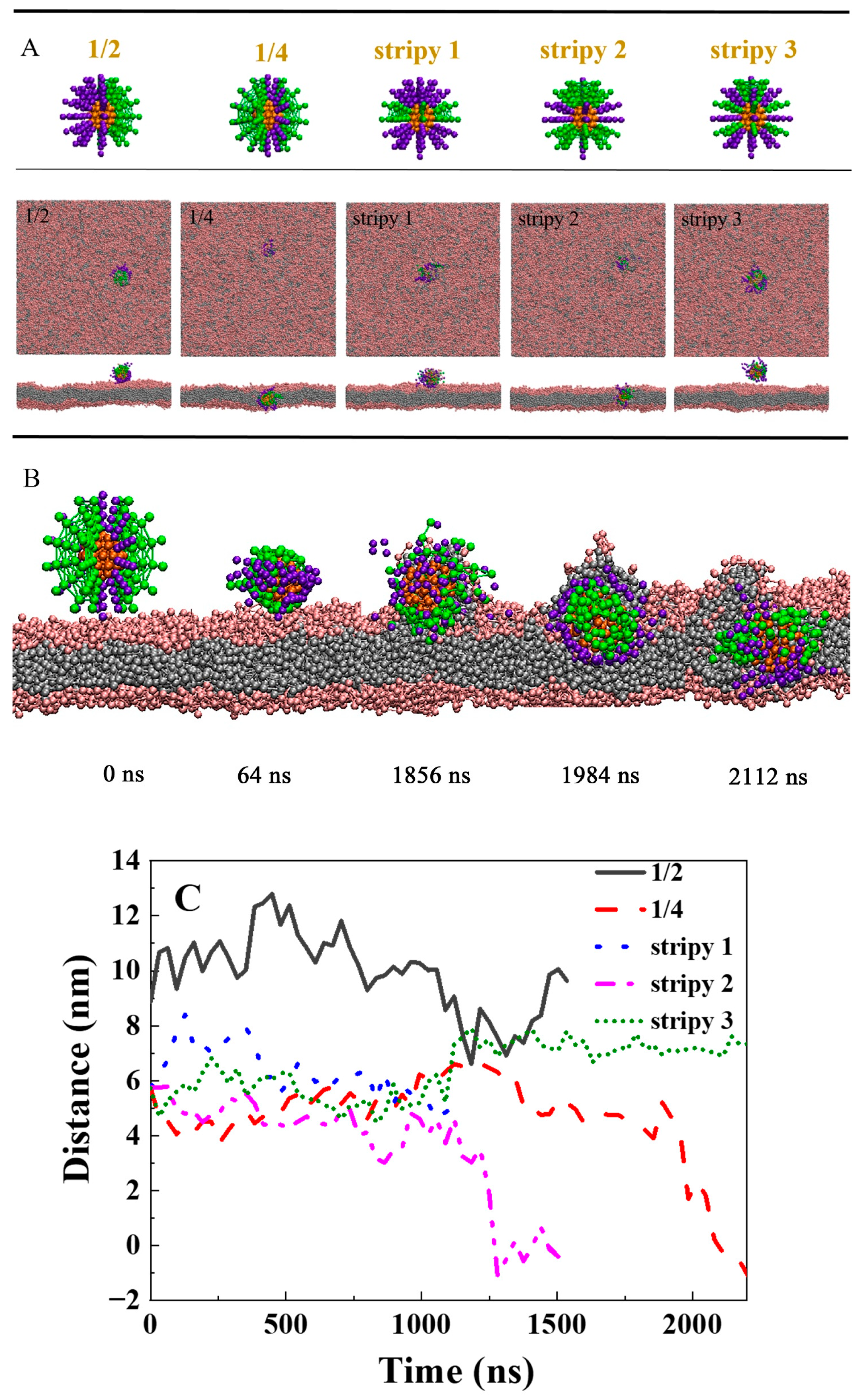
2.4. How the Distribution of the Polymer Influences Its Delivery Ability across the Lipid Membrane
3. Discussion
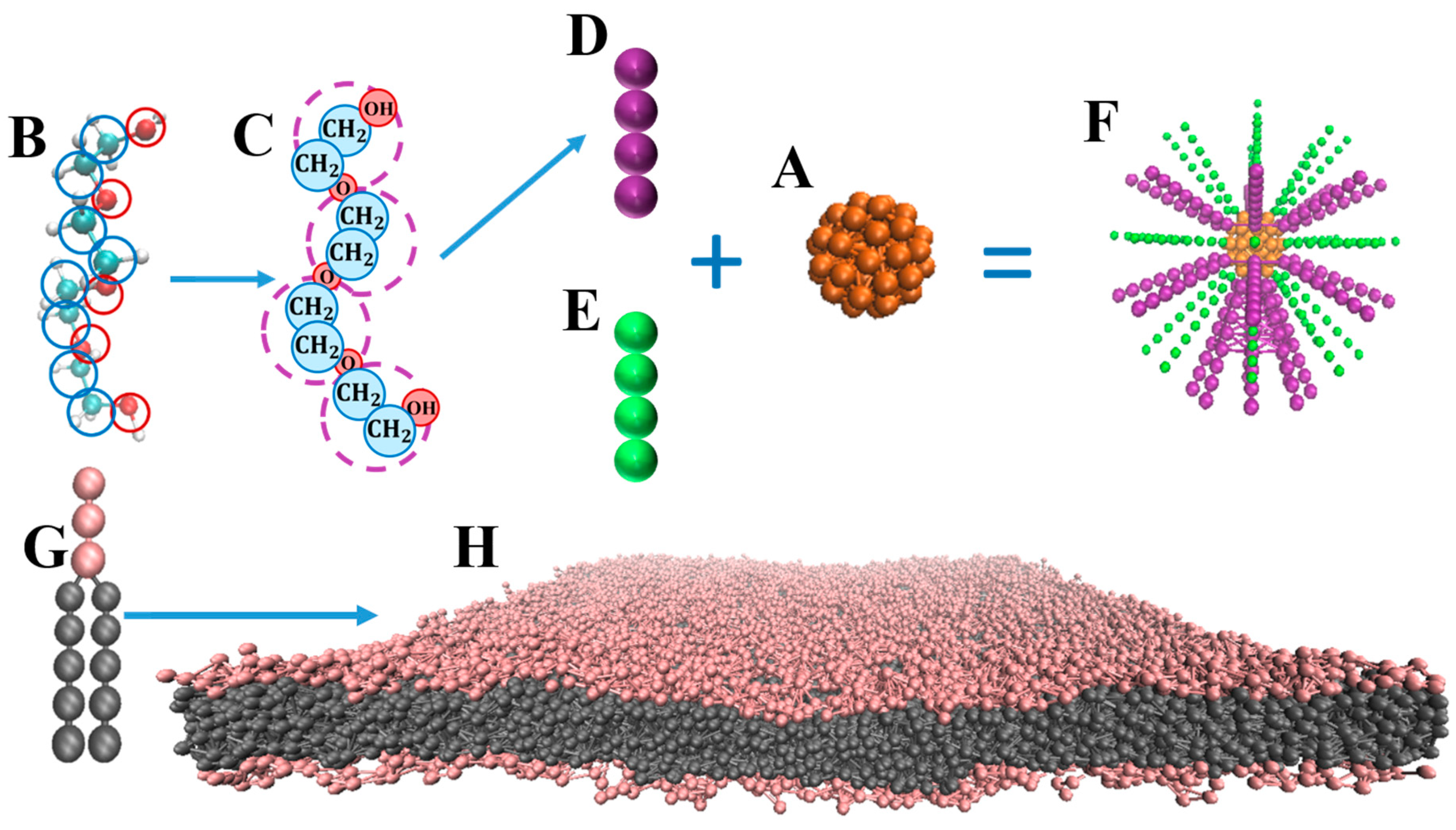
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, S.Y.; Li, D.D.; Lee, C.; Xie, J. Nanoparticle phototherapy in the era of cancer immunotherapy. Trends Chem. 2020, 2, 1082–1095. [Google Scholar] [CrossRef] [PubMed]
- Fabozzi, A.; Della Sala, F.; di Gennaro, M.; Barretta, M.; Longobardo, G.; Solimando, N.; Pagliuca, M.; Borzacchiello, A. Design of functional nanoparticles by microfluidic platforms as advanced drug delivery systems for cancer therapy. Lab Chip. 2023, 23, 1389–1409. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; Wang, S.Q.; Fontana, F.; Tapeinos, C.; Shahbazi, M.A.; Han, H.J.; Santos, H.A. Nanoparticles-based phototherapy systems for cancer treatment: Current status and clinical potential. Bioact. Mater. 2023, 23, 471–507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Centurion, F.; Misra, A.; Patel, S.; Gu, Z. Molecularly targeted nanomedicine enabled by inorganic nanoparticles for atherosclerosis diagnosis and treatment. Adv. Drug Deliv. Rev. 2023, 194, 22. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.T.; Wang, Y.T.; Lin, K.L.; Jiang, L. Nanoparticles modified by polydopamine: Working as “drug” carriers. Bioact. Mater. 2020, 5, 522–541. [Google Scholar] [CrossRef] [PubMed]
- Comegna, M.; Conte, G.; Falanga, A.P.; Marzano, M.; Cernera, G.; Di Lullo, A.M.; Amato, F.; Borbone, N.; D’Errico, S.; Ungaro, F.; et al. Assisting pna transport through cystic fibrosis human airway epithelia with biodegradable hybrid lipid-polymer nanoparticles. Sci. Rep. 2021, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Sun, C.Z.; Wang, C.; Jankovic, K.E.; Dong, Y.Z. Lipids and lipid derivatives for rna delivery. Chem. Rev. 2021, 121, 12181–12277. [Google Scholar] [CrossRef] [PubMed]
- Chali, S.P.; Ravoo, B.J. Polymer nanocontainers for intracellular delivery. Angew. Chem. Int. Ed. 2020, 59, 2962–2972. [Google Scholar] [CrossRef] [PubMed]
- Hamelmann, N.M.; Paulusse, J.M.J. Single-chain polymer nanoparticles in biomedical applications. J. Control. Release 2023, 356, 26–42. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Zhang, W.; Chu, X.Z.; Wang, A.R.; He, Z.L.; Si, C.L.; Hu, W.C. Dendritic cell-targeting polymer nanoparticle-based immunotherapy for cancer: A review. Int. J. Pharm. 2023, 635, 21. [Google Scholar] [CrossRef]
- Song, J.J.; Huang, S.J.; Zhang, Z.Z.; Jia, B.; Xie, H.; Kai, M.; Zhang, W. SPA: A peptide antagonist that acts as a cell-penetrating peptide for drug delivery. Drug Deliv. 2020, 27, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, S.; Milusheva, M.; Gledacheva, V.; Feizi-Dehnayebi, M.; Kaynarova, L.; Georgieva, D.; Delchev, V.; Stefanova, I.; Tumbarski, Y.; Mihaylova, R.; et al. Drug-delivery silver nanoparticles: A new perspective for phenindione as an anticoagulant. Biomedicines 2023, 11, 2201. [Google Scholar] [CrossRef] [PubMed]
- Kreitz, J.; Friedrich, M.J.; Guru, A.; Lash, B.; Saito, M.; Macrae, R.K.; Zhang, F. Programmable protein delivery with a bacterial contractile injection system. Nature 2023, 616, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Li, S.; Jiang, Z.D.; Tan, K.Q.; Meng, Y.L.; Zhang, D.Y.; Ma, X.L. Targeting lymph node delivery with nanovaccines for cancer immunotherapy: Recent advances and future directions. J. Nanobiotechnol. 2023, 21, 18. [Google Scholar] [CrossRef]
- Song, N.C.; Chu, Y.W.; Tang, J.P.; Yang, D.Y. Lipid-, inorganic-, polymer-, and DNA-based nanocarriers for delivery of the crispr/Cas9 system. Chembiochem 2023, 24, 10. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, K.; Prasad, B.L.V. Surface functionalization of inorganic nanoparticles with ligands: A necessary step for their utility. Chem. Soc. Rev. 2023, 52, 4121. [Google Scholar] [CrossRef] [PubMed]
- Henkel, C.; Wittmann, J.E.; Träg, J.; Will, J.; Stiegler, L.M.S.; Strohriegl, P.; Hirsch, A.; Unruh, T.; Zahn, D.; Halik, M.; et al. Mixed organic ligand shells: Controlling the nanoparticle surface morphology toward tuning the optoelectronic properties. Small 2020, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Stiegler, L.M.S.; Luchs, T.; Hirsch, A. Shell-by-shell functionalization of inorganic nanoparticles. Chem. A Eur. J. 2020, 26, 8483–8498. [Google Scholar] [CrossRef] [PubMed]
- Simonelli, F.; Bochicchio, D.; Ferrando, R.; Rossi, G. Monolayer-Protected Anionic Au Nanoparticles Walk into Lipid Membranes Step by Step. J. Phys. Chem. Lett. 2015, 6, 3175–3179. [Google Scholar] [CrossRef]
- Van Lehn, R.C.; Atukorale, P.U.; Carney, R.P.; Yang, Y.S.; Stellacci, F.; Irvine, D.J.; Alexander-Katz, A. Effect of particle diameter and surface composition on the spontaneous fusion of monolayer-protected gold nanoparticles with lipid bilayers. Nano Lett. 2013, 13, 4060–4067. [Google Scholar] [CrossRef]
- Han, X.X.; Gong, N.Q.; Xue, L.L.; Billingsley, M.M.; El-Mayta, R.; Shepherd, S.J.; Alameh, M.G.; Weissman, D.; Mitchell, M.J. Ligand-tethered lipid nanoparticles for targeted rna delivery to treat liver fibrosis. Nat. Commun. 2023, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, H.; Dobroff, A.S.; Driessen, W.H.P.; Cristini, V.; Brinker, L.M.; Staquicini, F.I.; Cardó-Vila, M.; D’Angelo, S.; Ferrara, F.; Proneth, B.; et al. Integrated nanotechnology platform for tumor-targeted multimodal imaging and therapeutic cargo release. Proc. Natl. Acad. Sci. USA 2016, 113, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Dirisala, A.; Uchida, S.; Li, J.J.; Van Guyse, J.F.R.; Hayashi, K.; Vummaleti, S.V.C.; Kaur, S.; Mochida, Y.; Fukushima, S.; Kataoka, K. Effective mrna protection by poly(l-ornithine) synergizes with endosomal escape functionality of a charge-conversion polymer toward maximizing mrna introduction efficiency. Macromol. Rapid Commun. 2022, 43, 9. [Google Scholar] [CrossRef] [PubMed]
- Mainini, F.; Eccles, M.R. Lipid and polymer-based nanoparticle sirna delivery systems for cancer therapy. Molecules 2020, 25, 2692. [Google Scholar] [CrossRef] [PubMed]
- Sunoqrot, S.; Abdel Gaber, S.A.; Abujaber, R.; Al-Majawleh, M.; Talhouni, S. Lipid- and polymer-based nanocarrier platforms for cancer vaccine delivery. ACS Appl. Bio Mater. 2024. [Google Scholar] [CrossRef] [PubMed]
- Swider, E.; Koshkina, O.; Tel, J.; Cruz, L.J.; de Vries, I.J.M.; Srinivas, M. Customizing poly(lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018, 73, 38–51. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.A.; Shegokar, R. Polyethylene glycol (peg): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Jafernik, K.; Ladniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiacek, A.E.; Szopa, A. Chitosan-based nanoparticles as effective drug delivery systems-a review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.B.; Guo, Y.Y.; Liu, S.; Hu, Y.; Yang, C.; Cheng, G.; Xue, C.Y.; Zuo, Y.Y.; Sun, B.B. Ph-mediated mucus penetration of zwitterionic polydopamine-modified silica nanoparticles. Nano Lett. 2023, 23, 7552–7560. [Google Scholar] [CrossRef]
- Kaczmarek, J.C.; Patel, A.K.; Kauffman, K.J.; Fenton, O.S.; Webber, M.J.; Heartlein, M.W.; DeRosa, F.; Anderson, D.G. Polymer-lipid nanoparticles for systemic delivery of mrna to the lungs. Angew. Chem. Int. Ed. 2016, 55, 13808–13812. [Google Scholar] [CrossRef]
- Dahlman, J.E.; Barnes, C.; Khan, O.F.; Thiriot, A.; Jhunjunwala, S.; Shaw, T.E.; Xing, Y.P.; Sager, H.B.; Sahay, G.; Speciner, L.; et al. In vivo endothelial sirna delivery using polymeric nanoparticles with low molecular weight. Nat. Nanotechnol. 2014, 9, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Eltoukhy, A.A.; Love, K.T.; Langer, R.; Anderson, D.G. Lipidoid-coated iron oxide nanoparticles for efficient DNA and sirna delivery. Nano Lett. 2013, 13, 1059–1064. [Google Scholar] [CrossRef]
- Li, H.Y.; Ye, X.X.; Guo, X.S.; Geng, Z.G.; Wang, G.Z. Effects of surface ligands on the uptake and transport of gold nanoparticles in rice and tomato. J. Hazard. Mater. 2016, 314, 188–196. [Google Scholar] [CrossRef]
- Chen, X.; Tieleman, D.P.; Liang, Q. Modulating interactions between ligand-coated nanoparticles and phase-separated lipid bilayers by varying the ligand density and the surface charge. Nanoscale 2018, 10, 2481–2491. [Google Scholar] [CrossRef]
- Noh, S.Y.; Nash, A.; Notman, R. The aggregation of striped nanoparticles in mixed phospholipid bilayers. Nanoscale 2020, 12, 4868–4881. [Google Scholar] [CrossRef]
- Li, Y.; Feng, D.W.; Zhang, X.R.; Cao, D.P. Design strategy of cell-penetrating copolymers for high efficient drug delivery. Biomaterials 2015, 52, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.Y.; Zhang, Y.; Farokhzad, R.A.; Mendes, B.B.; Conde, J.; Shi, J.J. ‘Passive’ nanoparticles for organ-selective systemic delivery: Design, mechanism and perspective. Chem. Soc. Rev. 2023, 52, 7579–7601. [Google Scholar] [CrossRef]
- Shen, Z.Q.; Nieh, M.P.; Li, Y. Decorating nanoparticle surface for targeted drug delivery: Opportunities and challenges. Polymers 2016, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Moss, K.H.; Popova, P.; Hadrup, S.R.; Astakhova, K.; Taskova, M. Lipid nanoparticles for delivery of therapeutic rna oligonucleotides. Mol. Pharm. 2019, 16, 2265–2277. [Google Scholar] [CrossRef]
- Maity, S.; Guchhait, R.; Sarkar, M.B.; Pramanick, K. Occurrence and distribution of micro/nanoplastics in soils and their phytotoxic effects: A review. Plant Cell Environ. 2022, 45, 1011–1028. [Google Scholar] [CrossRef]
- Khan, P.; Kaushik, R.; Jayaraj, A. Approaches and perspective of coarse-grained modeling and simulation for polymer-nanoparticle hybrid systems. Acs Omega 2022, 7, 47567–47586. [Google Scholar] [CrossRef] [PubMed]
- Binder, J.; Winkeljann, J.; Steinegger, K.; Trnovec, L.; Orekhova, D.; Zähringer, J.; Hörner, A.; Fell, V.; Tinnefeld, P.; Winkeljann, B.; et al. Closing the gap between experiment and simulation-a holistic study on the complexation of small interfering rnas with polyethylenimine. Mol. Pharm. 2024, 21, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.X.; Xu, D.; Dong, X.W.; Yang, K.; Yan, L.T. Insight into biophysicochemical principles of biopolymers through simulation and theory. Chin. J. Polym. Sci. 2023, 41, 1342–1354. [Google Scholar] [CrossRef]
- Frost, C.; Wiedbrauk, S.; Graham, E.; Yepuri, N.R.; Nelson, A.R.J.; Boase, N.R.B. Measuring the interactions and influence of amphipathic copolymers with lipid monolayers and bilayers as models of biological membranes. Macromol. Chem. Phys. 2023, 224, 2300315. [Google Scholar] [CrossRef]
- Wang, S.; Guo, H.; Li, Y.; Li, X. Penetration of nanoparticles across a lipid bilayer: Effects of particle stiffness and surface hydrophobicity. Nanoscale 2019, 11, 4025–4034. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Pang, W.; Gu, B.; Lin, X.; Ye, J. The stiffness-dependent tumor cell internalization of liquid metal nanoparticles. Nanoscale 2022, 14, 16902–16917. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.R.; Cao, D.P. A spontaneous penetration mechanism of patterned nanoparticles across a biomembrane. Soft. Matter. 2014, 10, 6844–6856. [Google Scholar] [CrossRef]
- Dirisala, A.; Uchida, S.; Toh, K.; Li, J.J.; Osawa, S.; Tockary, T.A.; Liu, X.Y.; Abbasi, S.; Hayashi, K.; Mochida, Y.; et al. Transient stealth coating of liver sinusoidal wall by anchoring two-armed peg for retargeting nanomedicines. Sci. Adv. 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Aramideh, A.; Ashjari, M.; Niazi, Z. Effects of natural polymers for enhanced silica-based mesoporous drug carrier. J. Drug Deliv. Sci. Technol. 2023, 81, 8. [Google Scholar] [CrossRef]
- Xie, L.Q.; Feng, J.W.; Tian, W.D.; Ma, Y.Q. Generation-Dependent Gel-Fluid Phase Transition of Membrane Caused by a PAMAM Dendrimer. Macromol. Theory Simul. 2014, 23, 531–536. [Google Scholar] [CrossRef]
- Balog, S.; de Almeida, M.S.; Taladriz-Blanco, P.; Rothen-Rutishauser, B.; Petri-Fink, A. Does the surface charge of the nanoparticles drive nanoparticle-cell membrane interactions? Curr. Opin. Biotechnol. 2024, 87, 7. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Wang, Z.W.; Ping, Y.H.; Miaol, Y.Y.; Xiao, Y.M.; Qu, L.B.; Zhang, L.; Hu, Y.S.; Wang, J.S. PEG/PEI-functionalized single-walled carbon nanotubes as delivery carriers for doxorubicin: Synthesis, characterization, and in vitro evaluation. Beilstein J. Nanotechnol. 2020, 11, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
- Su, G.X.; Zhou, X.F.; Zhou, H.Y.; Li, Y.; Zhang, X.R.; Liu, Y.; Cao, D.P.; Yan, B. Size-Dependent Facilitation of Cancer Cell Targeting by Proteins Adsorbed on Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 30037–30047. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Dkhar, D.S.; Kumari, R.; Divya; Mahapatra, S.; Srivastava, A.; Dubey, V.K.; Chandra, P. Ligand conjugated lipid-based nanocarriers for cancer theranostics. Biotechnol. Bioeng. 2022, 119, 3022–3043. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Li, W.J.; Xu, K.Y.; Xing, Y.T.; Shi, H.B.; Jing, Z.; Li, S.; Hong, Z.Y. Peg linker improves antitumor efficacy and safety of affibody-based drug conjugates. Int. J. Mol. Sci. 2021, 22, 1540. [Google Scholar] [CrossRef] [PubMed]
- Egorova, A.; Shtykalova, S.; Maretina, M.; Selutin, A.; Shved, N.; Deviatkin, D.; Selkov, S.; Baranov, V.; Kiselev, A. Polycondensed peptide carriers modified with cyclic rgd ligand for targeted suicide gene delivery to uterine fibroid cells. Int. J. Mol. Sci. 2022, 23, 1164. [Google Scholar] [CrossRef] [PubMed]
- Nanjaiah, H.; Moudgil, K.D. The utility of peptide ligand-functionalized liposomes for subcutaneous drug delivery for arthritis therapy. Int. J. Mol. Sci. 2023, 24, 6883. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, S.J.; Chowdhary, A.; Agrawal, G.P.; Mody, N.; Jain, A. Biodegradable concanavalin a functionalized polycaprolactone nanoparticle: A promising avenue for cancer therapy. J. Canc. Res. Ther. 2023, 19, S691–S700. [Google Scholar] [CrossRef] [PubMed]
- de Meyer, F.J.M.; Venturoli, M.; Smit, B. Molecular simulations of lipid-mediated protein-protein interactions. Biophys. J. 2008, 95, 1851–1865. [Google Scholar] [CrossRef]
- Grafmüller, A.; Shillcock, J.; Lipowsky, R. Pathway of membrane fusion with two tension-dependent energy barriers. Phys. Rev. Lett. 2007, 98, 4. [Google Scholar] [CrossRef]
- Grafmüller, A.; Shillcock, J.; Lipowsky, R. The fusion of membranes and vesicles: Pathway and energy barriers from dissipative particle dynamics. Biophys. J. 2009, 96, 2658–2675. [Google Scholar] [CrossRef]
- Venturoli, M.; Sperotto, M.M.; Kranenburg, M.; Smit, B. Mesoscopic models of biological membranes. Phys. Rep. -Rev. Sect. Phys. Lett. 2006, 437, 1–54. [Google Scholar] [CrossRef]
- Kranenburg, M.; Smit, B. Phase behavior of model lipid bilayers. J. Phys. Chem. B 2005, 109, 6553–6563. [Google Scholar] [CrossRef]
- Laradji, M.; Kumar, P.B.S. Dynamics of domain growth in self-assembled fluid vesicles. Phys. Rev. Lett. 2004, 93, 198105. [Google Scholar] [CrossRef]
- Li, C.Y.; Tang, Y.H.; Lin, H.H.; Zhang, C.H.; Liu, Z.; Yu, L.X.; Wang, X.L.; Lin, Y.K. Novel multiscale simulations on the membrane formation via hybrid induced phase separation process based on dissipative particle dynamics. Sep. Purif. Technol. 2023, 314, 16. [Google Scholar] [CrossRef]
- Li, Z.; Wei, Q.; Qi, Z.Q.; Zhou, L.X.; Li, J.W. In silico insights into the receptor-mediated endocytosis of virus-like nanoparticles. Chem. Phys. Lett. 2022, 790, 7. [Google Scholar] [CrossRef]
- Song, X.Y.; Ma, J.L.; Long, T.; Xu, X.F.; Zhao, S.L.; Liu, H.L. Mechanochemical cellular membrane internalization of nanohydrogels: A large-scale mesoscopic simulation. Acs Appl. Mater. Interfaces 2021, 13, 123–134. [Google Scholar] [CrossRef]
- Yang, K.; Ma, Y.Q. Computer simulation of the translocation of nanoparticles with different shapes across a lipid bilayer. Nat. Nanotechnol. 2010, 5, 579. [Google Scholar] [CrossRef]
- Bowman, C.; Chaplain, M.; Matzavinos, A. Dissipative particle dynamics simulation of critical pore size in a lipid bilayer membrane. R. Soc. Open Sci. 2019, 6, 9. [Google Scholar] [CrossRef]
- Groot, R.D.; Rabone, K.L. Mesoscopic simulation of cell membrane damage, morphology change and rupture by nonionic surfactants. Biophys. J. 2001, 81, 725–736. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, Z.; Zhang, Y.; Hu, J.; Fu, Y. Design Principles for Smart Linear Polymer Ligand Carriers with Efficient Transcellular Transport Capabilities. Int. J. Mol. Sci. 2024, 25, 6826. https://doi.org/10.3390/ijms25136826
Li Y, Zhang Z, Zhang Y, Hu J, Fu Y. Design Principles for Smart Linear Polymer Ligand Carriers with Efficient Transcellular Transport Capabilities. International Journal of Molecular Sciences. 2024; 25(13):6826. https://doi.org/10.3390/ijms25136826
Chicago/Turabian StyleLi, Ye, Zhun Zhang, Yezhuo Zhang, Jingcheng Hu, and Yujie Fu. 2024. "Design Principles for Smart Linear Polymer Ligand Carriers with Efficient Transcellular Transport Capabilities" International Journal of Molecular Sciences 25, no. 13: 6826. https://doi.org/10.3390/ijms25136826
APA StyleLi, Y., Zhang, Z., Zhang, Y., Hu, J., & Fu, Y. (2024). Design Principles for Smart Linear Polymer Ligand Carriers with Efficient Transcellular Transport Capabilities. International Journal of Molecular Sciences, 25(13), 6826. https://doi.org/10.3390/ijms25136826





