Integrative Analyses of Circulating Proteins and Metabolites Reveal Sex Differences in the Associations with Cardiac Function among DCM Patients
Abstract
1. Introduction
2. Results
2.1. Characteristics of the DCM Patients in the Discovery Cohort
2.2. Lack of Association between LVEDD and Proteins or Metabolites
2.3. Associations between LVEF and Proteins or Metabolites
2.4. Associations between LVEF and Proteins in Men
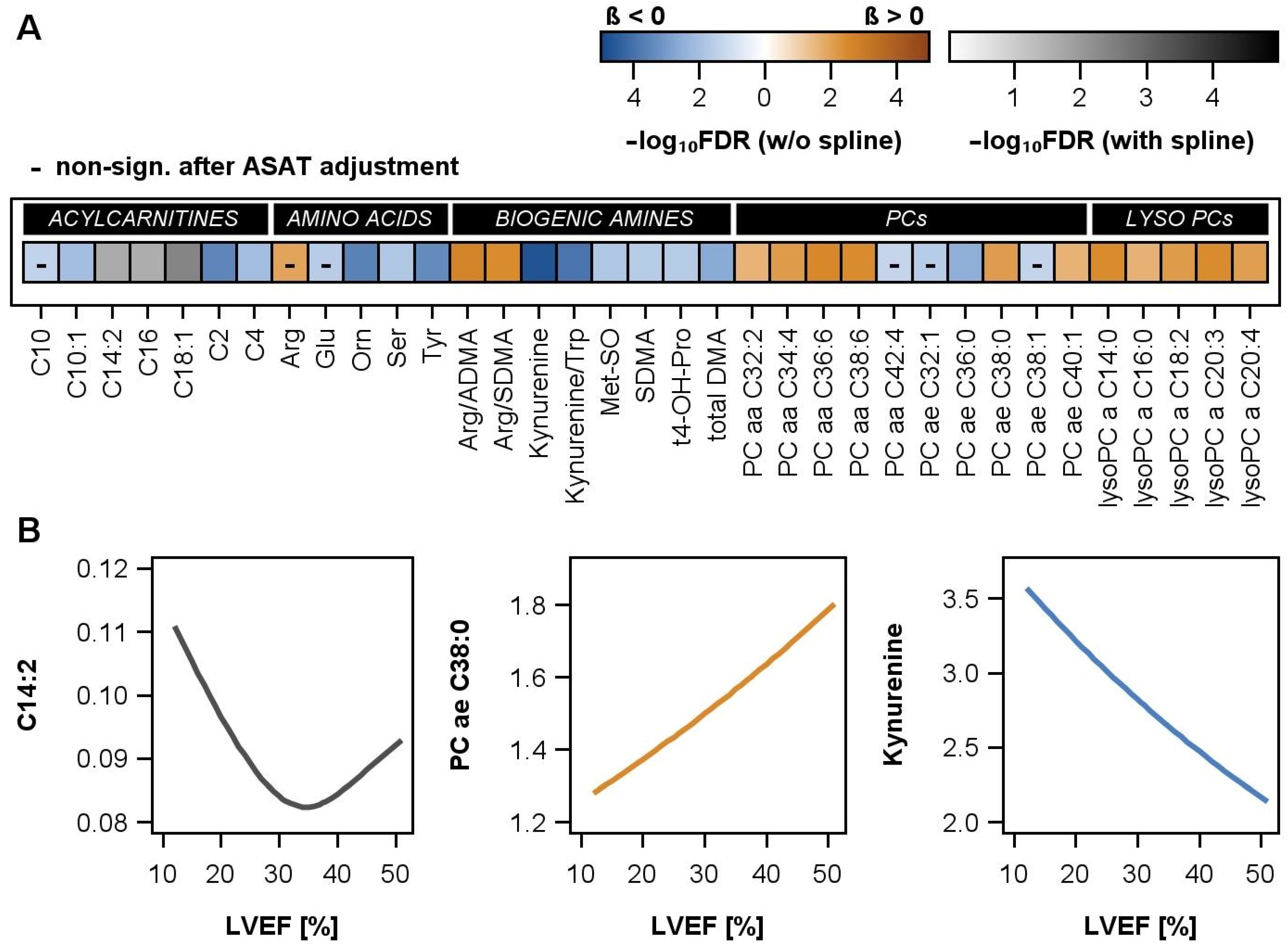
2.5. Associations between LVEF and Metabolites in Men
2.6. Associations between LVEF and Metabolites in the Validation Cohort
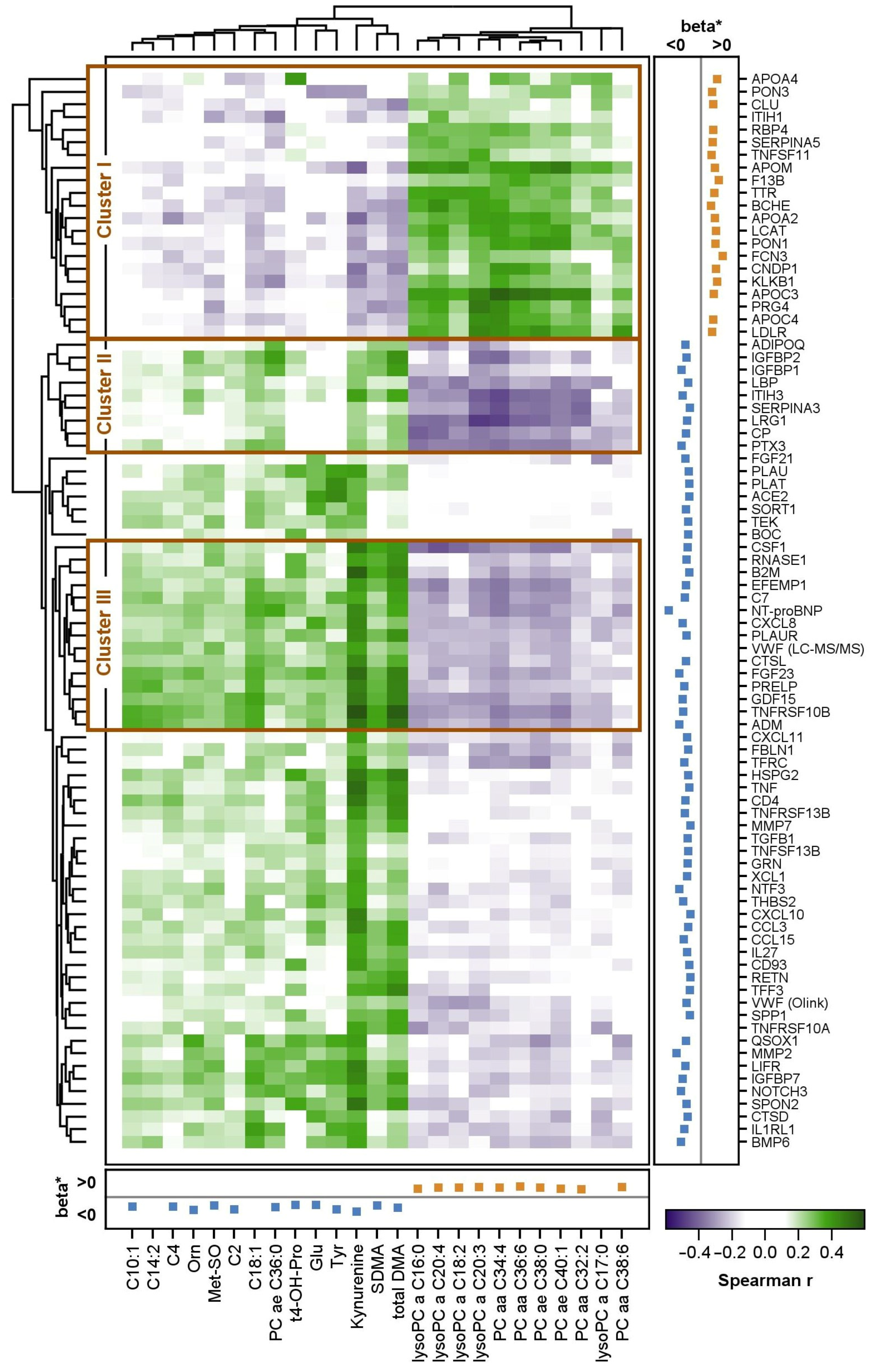
2.7. Correlation between Proteins and Metabolites
3. Discussion
3.1. Integration of Proteomic and Metabolomic Results
3.2. Strengths and Limitations
3.3. Conclusions
4. Materials and Methods
4.1. Discovery Cohort
4.2. Validation Cohort
4.3. Echocardiography
4.4. Global Protein Profiling
4.5. Targeted Protein Analysis by Proximity Extension Assay
4.6. Verification of Protein Measurements
4.7. Functional Categorization of Proteins and Nomenclature
4.8. Targeted Metabolomic Profiling
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [PubMed]
- Schultheiss, H.P.; Fairweather, D.; Caforio, A.L.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Primers 2019, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; De Groote, P.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Polovina, M.; Bauersachs, J.; Arad, M.; Ben Gal, T.; Lund, L.H.; Felix, S.B.; Arbustini, E.; Caforio, A.L.P.; Farmakis, D.; et al. Heart failure in cardiomyopathies: A position paper from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 553–576. [Google Scholar] [CrossRef] [PubMed]
- Merlo, M.; Cannatà, A.; Gobbo, M.; Stolfo, D.; Elliott, P.M.; Sinagra, G. Evolving concepts in dilated cardiomyopathy. Eur. J. Heart Fail. 2018, 20, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Ampong, I. Metabolic and Metabolomics Insights into Dilated Cardiomyopathy. Ann. Nutr. Metab. 2022, 78, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Palstrom, N.B.; Matthiesen, R.; Rasmussen, L.M.; Beck, H.C. Recent Developments in Clinical Plasma Proteomics-Applied to Cardiovascular Research. Biomedicines 2022, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Tayal, U.; Verdonschot, J.A.; Hazebroek, M.R.; Howard, J.; Gregson, J.; Newsome, S.; Gulati, A.; Pua, C.J.; Halliday, B.P.; Lota, A.S.; et al. Precision Phenotyping of Dilated Cardiomyopathy Using Multidimensional Data. J. Am. Coll. Cardiol. 2022, 79, 2219–2232. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.; She, R.; Luzum, J.; Li, J.; Bryson, T.D.; Pinto, Y.; Sabbah, H.N.; Williams, L.K.; Lanfear, D.E. Plasma Proteomic Profile Predicts Survival in Heart Failure with Reduced Ejection Fraction. Circ. Genom. Precis. Med. 2021, 14, e003140. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.; Frese, K.S.; Sedaghat-Hamedani, F.; Kayvanpour, E.; Tappu, R.; Nietsch, R.; Tugrul, O.F.; Wisdom, M.; Dietrich, C.; Amr, A.; et al. Energy Metabolites as Biomarkers in Ischemic and Dilated Cardiomyopathy. Int. J. Mol. Sci. 2021, 22, 1999. [Google Scholar] [CrossRef]
- Liu, C.; Li, R.; Liu, Y.; Li, Z.; Sun, Y.; Yin, P.; Huang, R. Characteristics of Blood Metabolic Profile in Coronary Heart Disease, Dilated Cardiomyopathy and Valvular Heart Disease Induced Heart Failure. Front. Cardiovasc. Med. 2020, 7, 622236. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, S.; Jing, R.; Jin, H.; Hu, Y.; Wang, J.; Gu, M.; Niu, H.; Zhang, S.; Chen, L.; et al. Plasma Metabolomic Profiles Differentiate Patients with Dilated Cardiomyopathy and Ischemic Cardiomyopathy. Front. Cardiovasc. Med. 2020, 7, 597546. [Google Scholar] [CrossRef]
- Mueller-Hennessen, M.; Düngen, H.-D.; Lutz, M.; Trippel, T.D.; Kreuter, M.; Sigl, J.; Müller, O.J.; Tahirovic, E.; Witt, H.; Ternes, P.; et al. A Novel Lipid Biomarker Panel for the Detection of Heart Failure with Reduced Ejection Fraction. Clin. Chem. 2017, 63, 267–277. [Google Scholar] [CrossRef]
- Ameling, S.; Herda, L.R.; Hammer, E.; Steil, L.; Teumer, A.; Trimpert, C.; Dörr, M.; Kroemer, H.K.; Klingel, K.; Kandolf, R.; et al. Myocardial gene expression profiles and cardiodepressant autoantibodies predict response of patients with dilated cardiomyopathy to immunoadsorption therapy. Eur. Heart J. 2013, 34, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Todd, N.; Lai, Y.C. Current Understanding of Circulating Biomarkers in Pulmonary Hypertension Due to Left Heart Disease. Front. Med. 2020, 7, 570016. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Norton, N.; Bruno, K.A.; Cooper, L.T.; Atwal, P.S.; Fairweather, D. Sex Differences, Genetic and Environmental Influences on Dilated Cardiomyopathy. J. Clin. Med. 2021, 10, 2289. [Google Scholar] [CrossRef]
- Fairweather, D.; Beetler, D.J.; Musigk, N.; Heidecker, B.; Lyle, M.A.; Cooper, L.T.; Bruno, K.A. Sex and gender differences in myocarditis and dilated cardiomyopathy: An update. Front. Cardiovasc. Med. 2023, 10, 1129348. [Google Scholar] [CrossRef] [PubMed]
- Argirò, A.; Ho, C.; Day, S.M.; van der Velden, J.; Cerbai, E.; Saberi, S.; Tardiff, J.C.; Lakdawala, N.K.; Olivotto, I. Sex-Related Differences in Genetic Cardiomyopathies. J. Am. Heart Assoc. 2022, 11, e024947. [Google Scholar] [CrossRef]
- St Pierre, S.R.; Peirlinck, M.; Kuhl, E. Sex Matters: A Comprehensive Comparison of Female and Male Hearts. Front. Physiol. 2022, 13, 831179. [Google Scholar] [CrossRef]
- Michelhaugh, S.A.; Januzzi, J.L., Jr. Finding a Needle in a Haystack: Proteomics in Heart Failure. JACC Basic Transl. Sci. 2020, 5, 1043–1053. [Google Scholar] [CrossRef]
- Adamo, L.; Yu, J.; Rocha-Resende, C.; Javaheri, A.; Head, R.D.; Mann, D.L. Proteomic Signatures of Heart Failure in Relation to Left Ventricular Ejection Fraction. J. Am. Coll. Cardiol. 2020, 76, 1982–1994. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lian, H.; Yu, J.; Wu, J.; Chen, X.; Wang, P.; Tian, L.; Yang, Y.; Yang, J.; Li, D.; et al. Study on diverse pathological characteristics of heart failure in different stages based on proteomics. J. Cell Mol. Med. 2022, 26, 1169–1182. [Google Scholar] [CrossRef]
- Feig, M.A.; Pop, C.; Bhardwaj, G.; Sappa, P.K.; Dörr, M.; Ameling, S.; Weitmann, K.; Nauck, M.; Lehnert, K.; Beug, D.; et al. Global plasma protein profiling reveals DCM characteristic protein signatures. J. Proteom. 2019, 209, 103508. [Google Scholar] [CrossRef]
- Rehulkova, H.; Rehulka, P.; Fucikova, A.M.; Stulik, J.; Pudil, R. Identification of novel biomarker candidates for hypertrophic cardiomyopathy and other cardiovascular diseases leading to heart failure. Physiol. Res. 2016, 65, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.J.; Hasegawa, K.; Kochav, S.M.; Mohajer, P.; Jung, J.; Maurer, M.S.; Reilly, M.P.; Fifer, M.A. Application of Proteomics Profiling for Biomarker Discovery in Hypertrophic Cardiomyopathy. J. Cardiovasc. Transl. Res. 2019, 12, 569–579. [Google Scholar] [CrossRef]
- Ritterhoff, J.; Tian, R. Metabolism in cardiomyopathy: Every substrate matters. Cardiovasc. Res. 2017, 113, 411–421. [Google Scholar] [CrossRef]
- The HDL Proteome Watch Database. Available online: https://homepages.uc.edu/~davidswm/HDLproteome.html (accessed on 4 October 2023).
- Feingold, K.R. Introduction to Lipids and Lipoproteins. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Yang, K.; Wang, J.; Xiang, H.; Ding, P.; Wu, T.; Ji, G. LCAT- targeted therapies: Progress, failures and future. Biomed. Pharmacother. 2022, 147, 112677. [Google Scholar] [CrossRef]
- Nelson, V.L.; Eadie, A.L.; Brunt, K.R. A step toward a unifying preclinical model of dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H1028–H1031. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, C.J.; Lamichhane, S.; Connolly, J.A.; Soehnlen, S.M.; Khalaf, F.K.; Malhotra, D.; Haller, S.T.; Isailovic, D.; Kennedy, D.J. A PON for All Seasons: Comparing Paraoxonase Enzyme Substrates, Activity and Action including the Role of PON3 in Health and Disease. Antioxidants 2022, 11, 590. [Google Scholar] [CrossRef]
- Van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859 Pt B, 1558–1572. [Google Scholar] [CrossRef]
- Cheng, M.L.; Wang, C.H.; Shiao, M.S.; Liu, M.H.; Huang, Y.Y.; Huang, C.Y.; Mao, C.T.; Lin, J.F.; Ho, H.Y.; Yang, N.I. Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure: Diagnostic and prognostic value of metabolomics. J. Am. Coll. Cardiol. 2015, 65, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz-Siemion, M.; Ciborowski, M.; Ptaszynska-Kopczynska, K.; Szpakowicz, A.; Lisowska, A.; Jasiewicz, M.; Waszkiewicz, E.; Kretowski, A.; Musial, W.; Kaminski, K. LC-MS-based serum fingerprinting reveals significant dysregulation of phospholipids in chronic heart failure. J. Pharm. Biomed. Anal. 2018, 154, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, X.; Chen, W.; Zhong, L.; Cui, M. Comprehensive plasma metabolomic and lipidomic analyses reveal potential biomarkers for heart failure. Mol. Cell Biochem. 2021, 476, 3449–3460. [Google Scholar] [CrossRef] [PubMed]
- Ala, M.; Eftekhar, S.P. The Footprint of Kynurenine Pathway in Cardiovascular Diseases. Int. J. Tryptophan Res. 2022, 15, 11786469221096643. [Google Scholar] [CrossRef] [PubMed]
- Dschietzig, T.B.; Kellner, K.-H.; Sasse, K.; Boschann, F.; Klüsener, R.; Ruppert, J.; Armbruster, F.P.; Bankovic, D.; Meinitzer, A.; Mitrovic, V.; et al. Plasma Kynurenine Predicts Severity and Complications of Heart Failure and Associates with Established Biochemical and Clinical Markers of Disease. Kidney Blood Press. Res. 2019, 44, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, J.; Yu, K.; Nie, D.; Zhao, C.; Jiao, L.; Wang, Z.; Zhou, L.; Wang, F.; Yu, Q.; et al. Indoleamine 2,3-Dioxygenase 1 Deletion-Mediated Kynurenine Insufficiency Inhibits Pathological Cardiac Hypertrophy. Hypertension 2023, 80, 2099–2111. [Google Scholar] [CrossRef] [PubMed]
- Law, S.-H.; Chan, M.-L.; Marathe, G.K.; Parveen, F.; Chen, C.-H.; Ke, L.-Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; Sung, M.M.; Ezekowitz, J.; Mandal, R.; Han, B.; Bjorndahl, T.C.; Bouatra, S.; Anderson, T.; Oudit, G.Y.; Wishart, D.S.; et al. Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS ONE 2015, 10, e0124844. [Google Scholar] [CrossRef] [PubMed]
- Maisch, B.; Pankuweit, S. Inflammatory dilated cardiomyopathy: Etiology and clinical management. Herz 2020, 45, 221–229. [Google Scholar] [CrossRef]
- Hazebroek, M.R.; Henkens, M.T.; Raafs, A.G.; Verdonschot, J.A.; Merken, J.J.; Dennert, R.M.; Eurlings, C.; Hamid, M.A.A.; Wolffs, P.F.; Winkens, B.; et al. Intravenous immunoglobulin therapy in adult patients with idiopathic chronic cardiomyopathy and cardiac parvovirus B19 persistence: A prospective, double-blind, randomized, placebo-controlled clinical trial. Eur. J. Heart Fail. 2021, 23, 302–309. [Google Scholar] [CrossRef]
- Harding, D.; Chong, M.H.A.; Lahoti, N.; Bigogno, C.M.; Prema, R.; Mohiddin, S.A.; Marelli-Berg, F. Dilated cardiomyopathy and chronic cardiac inflammation: Pathogenesis, diagnosis and therapy. J. Intern. Med. 2023, 293, 23–47. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.M.; Zhang, Q.; Jin, L. Association of beta-2-microglobulin with cardiovascular and all-cause mortality in the general and non-CKD population. Medicine 2023, 102, e33202. [Google Scholar] [CrossRef] [PubMed]
- Kakareko, K.; Rydzewska-Rosołowska, A.; Zbroch, E.; Hryszko, T. TRAIL and Cardiovascular Disease-A Risk Factor or Risk Marker: A Systematic Review. J. Clin. Med. 2021, 10, 1252. [Google Scholar] [CrossRef] [PubMed]
- Velissaris, D.; Zareifopoulos, N.; Koniari, I.; Karamouzos, V.; Bousis, D.; Gerakaris, A.; Platanaki, C.; Kounis, N. Soluble Urokinase Plasminogen Activator Receptor as a Diagnostic and Prognostic Biomarker in Cardiac Disease. J. Clin. Med. Res. 2021, 13, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Dambrova, M.; Makrecka-Kuka, M.; Kuka, J.; Vilskersts, R.; Nordberg, D.; Attwood, M.M.; Smesny, S.; Sen, Z.D.; Guo, A.C.; Oler, E.; et al. Acylcarnitines: Nomenclature, Biomarkers, Therapeutic Potential, Drug Targets, and Clinical Trials. Pharmacol. Rev. 2022, 74, 506–551. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.R.; De la Rosa, M.V.G.; Rosania, G.R.; Stringer, K.A. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.; Li, S.; Lv, J. Endothelial Dysfunction and Diabetic Cardiomyopathy. Front. Endocrinol. 2022, 13, 851941. [Google Scholar] [CrossRef] [PubMed]
- Strobel, J.; Mieth, M.; Endreß, B.; Auge, D.; König, J.; Fromm, M.F.; Maas, R. Interaction of the cardiovascular risk marker asymmetric dimethylarginine (ADMA) with the human cationic amino acid transporter 1 (CAT1). J. Mol. Cell. Cardiol. 2012, 53, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Paulus, W.J.; Vermeulen, M.A.; Richir, M.C.; Davids, M.; Wisselink, W.; de Mol, B.A.; van Leeuwen, P.A. role of asymmetric dimethylarginine and arginine in the failing heart and its vasculature. Eur. J. Heart Fail. 2010, 12, 1274–1281. [Google Scholar] [CrossRef]
- Zernecke, A.; Preissner, K.T. Extracellular Ribonucleic Acids (RNA) Enter the Stage in Cardiovascular Disease. Circ. Res. 2016, 118, 469–479. [Google Scholar] [CrossRef]
- Cabrera-Fuentes, H.A.; Ruiz-Meana, M.; Simsekyilmaz, S.; Kostin, S.; Inserte, J.; Saffarzadeh, M.; Galuska, S.P.; Vijayan, V.; Barba, I.; Barreto, G.; et al. RNase1 prevents the damaging interplay between extracellular RNA and tumour necrosis factor-alpha in cardiac ischaemia/reperfusion injury. Thromb. Haemost. 2014, 112, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Fuentes, H.A.; Niemann, B.; Grieshaber, P.; Wollbrueck, M.; Gehron, J.; Preissner, K.T.; Böning, A. RNase1 as a potential mediator of remote ischaemic preconditioning for cardioprotection†. Eur. J. Cardiothorac. Surg. 2015, 48, 732–737; discussion 737. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [PubMed]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef] [PubMed]
- García-Rivas, G.; Castillo, E.C.; Gonzalez-Gil, A.M.; Maravillas-Montero, J.L.; Brunck, M.; Torres-Quintanilla, A.; Elizondo-Montemayor, L.; Torre-Amione, G. The role of B cells in heart failure and implications for future immunomodulatory treatment strategies. ESC Heart Fail. 2020, 7, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Henry, W.L.; Gardin, J.M.; Ware, J.H. Echocardiographic measurements in normal subjects from infancy to old age. Circulation 1980, 62, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Mestroni, L.; Maisch, B.; McKenna, W.J.; Schwartz, K.; Charron, P.; Rocco, C.; Tesson, F.; Richter, R.; Wilke, A.; Komajda, M. Guidelines for the study of familial dilated cardiomyopathies. Collaborative Research Group of the European Human and Capital Mobility Project on Familial Dilated Cardiomyopathy. Eur. Heart J. 1999, 20, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, M.; Eriksson, A.; Tran, B.; Assarsson, E.; Fredriksson, S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 2011, 39, e102. [Google Scholar] [CrossRef]
- Eick, C.; Klinger-König, J.; Zylla, S.; Hannemann, A.; Budde, K.; Henning, A.K.; Pietzner, M.; Nauck, M.; Völzke, H.; Grabe, H.J.; et al. Broad Metabolome Alterations Associated with the Intake of Oral Contraceptives Are Mediated by Cortisol in Premenopausal Women. Metabolites 2021, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Stone, C.; Koo, C. Additive splines in statistics. In Proceedings of the Statistical Computing Section, Las Vegas, NV, USA, 5–8 August 1985; American Statistical Association: Washington, DC, USA, 1985. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]

| Characteristics | Total Sample (n = 368) | Male (n = 297) | Female (n = 71) |
|---|---|---|---|
| Age, years | 54.8 (47.9–63.7) | 54.9 (48.2–63.5) | 54.4 (46.8–65.7) |
| BMI, kg/m2 | 28.0 (25.1–31.2) | 28.4 (25.6–31.6) | 26.0 (22.8–29.4) |
| Diabetes mellitus, % | 17.1 | 18.2 | 12.7 |
| Current smoking, % * | 30.8 | 30.7 | 31.0 |
| LVEF, % | 30.8 (25.0–37.0) | 30.0 (25.0–36.0) | 33.0 (27.0–37.5) |
| HFrEF, % * | 76.6 | 76.8 | 75.8 |
| LVEDDmeasured, mm * | 68.0 (63.0–73.0) | 69.0 (64.0–74.0) | 63.0 (60.0–69.0) |
| LVEDDacc. to HENRY, % * | 139 (129–149) | 140 (130–150) | 135 (127–146) |
| NYHA Class, % | |||
| I | 14.1 | 14.5 | 12.7 |
| II | 34.0 | 32.7 | 39.4 |
| III | 40.2 | 40.7 | 38.0 |
| IV | 3.30 | 3.37 | 2.80 |
| missing | 8.40 | 8.80 | 7.00 |
| MAGGIC score | 17.0 (13.0–21.0) | 17.0 (13.0–21.0) | 15.0 (11.0–19.0) |
| Symptom time, days * | 113 (33–489) | 112 (33–459) | 131 (35–804) |
| Myocardial inflammation, % | 41.8 | 40.1 | 49.3 |
| Medication intake, % | |||
| ACE inhibitors or AT1 antagonists | 98.8 | 99.7 | 95.8 |
| Beta blockers | 97.0 | 98.3 | 91.6 |
| Aldosterone antagonists | 53.8 | 55.2 | 47.9 |
| Diuretics | 84.0 | 86.9 | 71.8 |
| Digitalis | 22.3 | 23.9 | 15.5 |
| History of, % | |||
| Stroke | 6.00 | 7.10 | 1.40 |
| Atrial fibrillation | 24.5 | 27.6 | 11.3 |
| Cancer | 4.90 | 4.70 | 5.60 |
| COPD * | 8.17 | 8.50 | 7.00 |
| hsCRP, mg/L * | 6.92 (6.58–7.31) | 6.91 (6.57–7.31) | 7.05 (6.60–7.33) |
| ASAT, µkatal/L * | 0.43 (0.32–0.62) | 0.44 (0.34–0.63) | 0.34 (0.27–0.59) |
| Total cholesterol, mmol/L * | 5.10 (4.20–6.00) | 5.00 (4.20–5.90) | 5.35 (4.50–6.20) |
| HDL-cholesterol, mmol/L * | 1.05 (0.85–1.30) | 1.00 (0.80–1.23) | 1.34 (1.15–1.56) |
| LDL-cholesterol, mmol/L * | 3.15 (2.50–3.89) | 3.13 (2.48–3.90) | 3.19 (2.62–3.87) |
| eGFR, mL/min/1.73 m2 | 83.9 (69.2–98.0) | 83.7 (70.2–96.8) | 89.2 (66.3–103.1) |
| Chronic kidney disease, % | |||
| mild to moderate | 15.2 | 14.5 | 18.3 |
| severe | 0.27 | 0.34 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannemann, A.; Ameling, S.; Lehnert, K.; Dörr, M.; Felix, S.B.; Nauck, M.; Al-Noubi, M.N.; Schmidt, F.; Haas, J.; Meder, B.; et al. Integrative Analyses of Circulating Proteins and Metabolites Reveal Sex Differences in the Associations with Cardiac Function among DCM Patients. Int. J. Mol. Sci. 2024, 25, 6827. https://doi.org/10.3390/ijms25136827
Hannemann A, Ameling S, Lehnert K, Dörr M, Felix SB, Nauck M, Al-Noubi MN, Schmidt F, Haas J, Meder B, et al. Integrative Analyses of Circulating Proteins and Metabolites Reveal Sex Differences in the Associations with Cardiac Function among DCM Patients. International Journal of Molecular Sciences. 2024; 25(13):6827. https://doi.org/10.3390/ijms25136827
Chicago/Turabian StyleHannemann, Anke, Sabine Ameling, Kristin Lehnert, Marcus Dörr, Stephan B. Felix, Matthias Nauck, Muna N. Al-Noubi, Frank Schmidt, Jan Haas, Benjamin Meder, and et al. 2024. "Integrative Analyses of Circulating Proteins and Metabolites Reveal Sex Differences in the Associations with Cardiac Function among DCM Patients" International Journal of Molecular Sciences 25, no. 13: 6827. https://doi.org/10.3390/ijms25136827
APA StyleHannemann, A., Ameling, S., Lehnert, K., Dörr, M., Felix, S. B., Nauck, M., Al-Noubi, M. N., Schmidt, F., Haas, J., Meder, B., Völker, U., Friedrich, N., & Hammer, E. (2024). Integrative Analyses of Circulating Proteins and Metabolites Reveal Sex Differences in the Associations with Cardiac Function among DCM Patients. International Journal of Molecular Sciences, 25(13), 6827. https://doi.org/10.3390/ijms25136827






