Advancements in Immunotherapeutic Treatments for Hepatocellular Carcinoma: Potential of Combination Therapies
Abstract
:1. Introduction
2. Literature Search Strategy
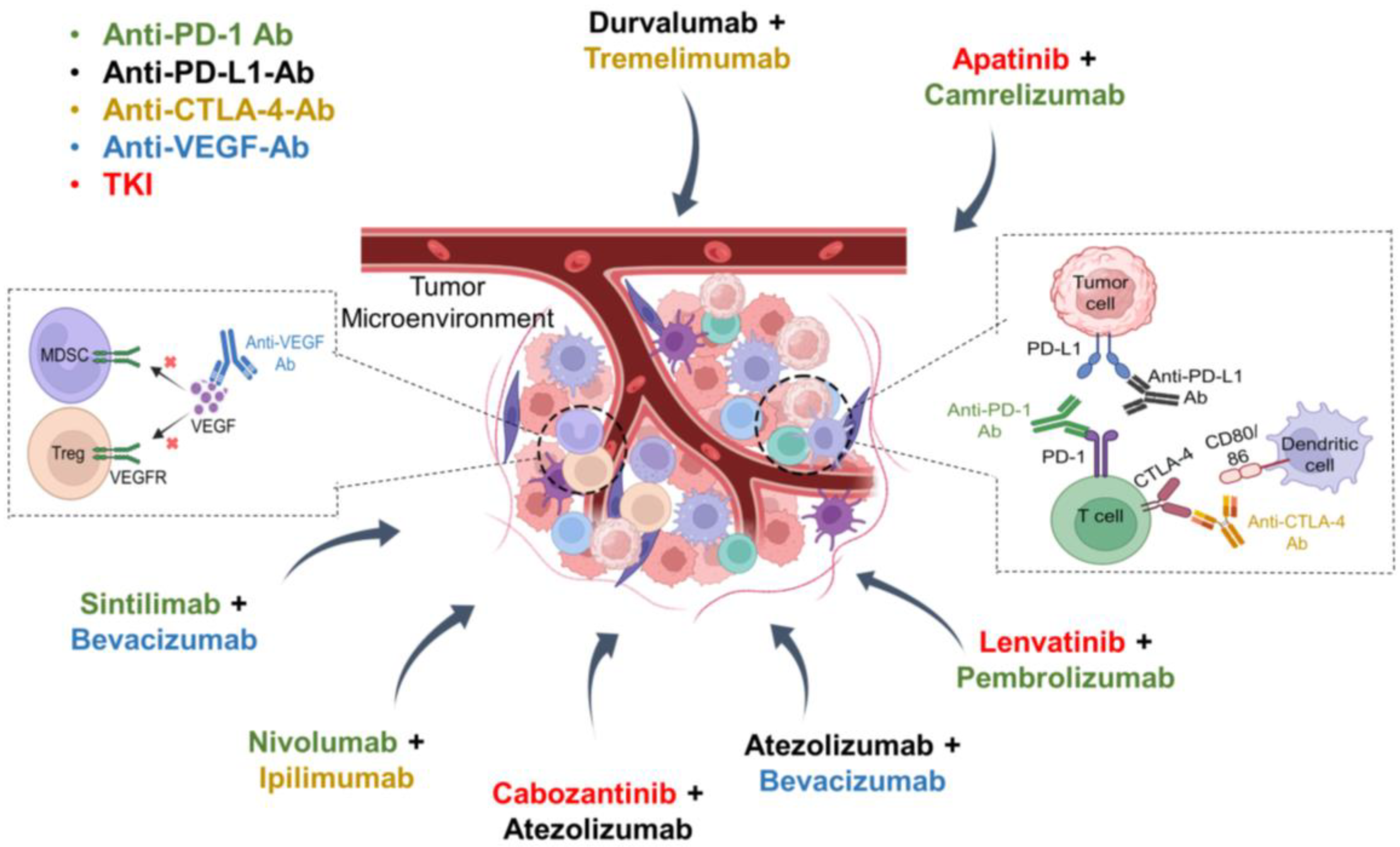
3. Immune Checkpoint Inhibition in HCC
3.1. PD-1/PD-L1 Inhibitors
3.2. CTLA-4 Inhibitors
4. Combinations of ICIs with TKIs or Anti-VEGF Antibodies
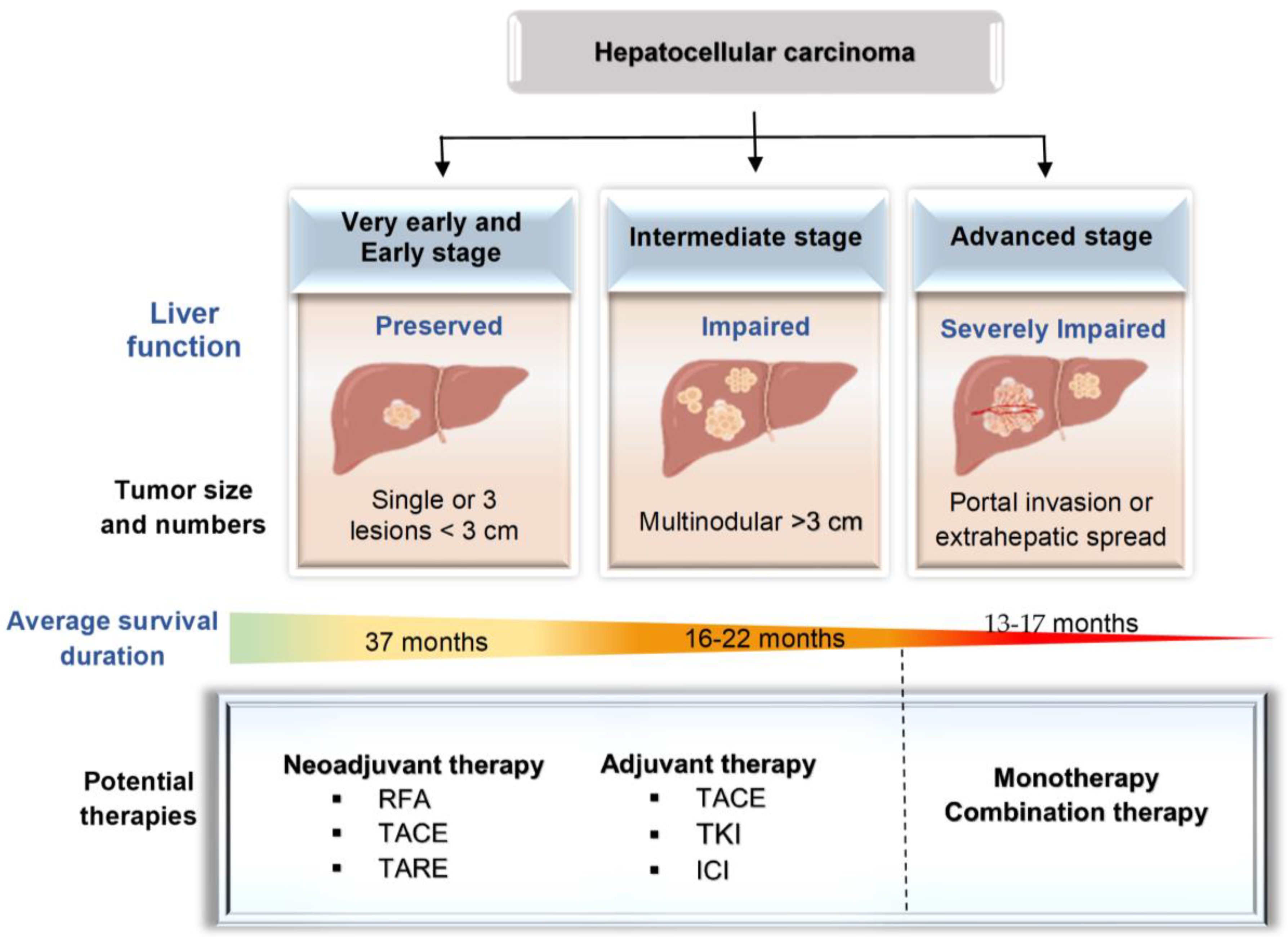
5. Immunotherapeutic Strategies for Different HCC Stages
5.1. Early- or Intermediate-Stage HCC
5.2. Advanced-Stage HCC
5.2.1. Monotherapy
5.2.2. Combination Therapy
6. Alternative Therapeutic Strategies Targeting Immune Cells for HCC
7. Limitations of Current Immunotherapies
8. Ongoing Clinical Studies
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toh, M.R.; Wong, E.Y.T.; Wong, S.H.; Ng, A.W.T.; Loo, L.-H.; Chow, P.K.-H.; Ngeow, J. Global epidemiology and genetics of hepatocellular carcinoma. Gastroenterology 2023, 164, 766–782. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Kanwal, F.; Llovet, J.M. Global trends in hepatocellular carcinoma epidemiology: Implications for screening, prevention and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 864–884. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-J.; Lee, J.; Choi, G.H.; Lee, M.W.; Park, D.A. A nationwide study on the current treatment status and natural prognosis of hepatocellular carcinoma in elderly. Sci. Rep. 2023, 13, 14584. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Jiang, X.; Li, M.; Luo, Y. Hepatitis virus and hepatocellular carcinoma: Recent advances. Cancers 2023, 15, 533. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Khaderi, S.; Singal, A.G.; Marrero, J.A.; Loo, N.; Asrani, S.K.; Amos, C.I.; Thrift, A.P.; Gu, X.; Luster, M. Risk factors for HCC in contemporary cohorts of patients with cirrhosis. Hepatology 2023, 77, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Song, B.G.; Choi, S.C.; Goh, M.J.; Kang, W.; Sinn, D.H.; Gwak, G.-Y.; Paik, Y.-H.; Choi, M.S.; Lee, J.H.; Paik, S.W. Metabolic dysfunction-associated fatty liver disease and the risk of hepatocellular carcinoma. JHEP Rep. 2023, 5, 100810. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Goh, B.-B.G.; Kam, J.-W.; Chang, P.-E.; Tan, C.-K. Comparisons between non-alcoholic steatohepatitis and alcohol-related hepatocellular carcinoma. Clin. Mol. Hepatol. 2020, 26, 196. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, Q.; Xu, X.; Wang, F. Clinical characteristics and risk factors of hepatitis B virus-related cirrhosis/hepatocellular carcinoma: A single-center retrospective study. Liver Res. 2023, 7, 237–243. [Google Scholar] [CrossRef]
- Kotsari, M.; Dimopoulou, V.; Koskinas, J.; Armakolas, A. Immune system and hepatocellular carcinoma (HCC): New insights into HCC progression. Int. J. Mol. Sci. 2023, 24, 11471. [Google Scholar] [CrossRef]
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv. Cancer Res. 2021, 149, 1–61. [Google Scholar]
- Dalzell, C.G.; Taylor, A.C.; White, S.B. New Insights on Liver-Directed Therapies in Hepatocellular Carcinoma. Cancer Metastasis Rev. 2023, 15, 5749. [Google Scholar] [CrossRef] [PubMed]
- Alawyia, B.; Constantinou, C. Hepatocellular carcinoma: A narrative review on current knowledge and future prospects. Curr. Treat. Options Oncol. 2023, 24, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Baloji, A.; Kalra, N.; Chaluvashetty, S.; Bhujade, H.; Chandel, K.; Duseja, A.; Taneja, S.; Gorsi, U.; Kumar, R.; Singh, H. Efficacy of Yttrium-90 Transarterial Radioembolisation in Advanced Hepatocellular Carcinoma: An Experience With Hybrid Angio-Computed Tomography and Glass Microspheres. J. Clin. Exp. Hepatol. 2024, 14, 101342. [Google Scholar] [CrossRef] [PubMed]
- Koulouris, A.; Tsagkaris, C.; Spyrou, V.; Pappa, E.; Troullinou, A.; Nikolaou, M. Hepatocellular carcinoma: An overview of the changing landscape of treatment options. J. Hepatocell. Carcinoma 2021, 8, 387–401. [Google Scholar] [CrossRef]
- Podlasek, A.; Abdulla, M.; Broering, D.; Bzeizi, K. Recent advances in locoregional therapy of hepatocellular carcinoma. Cancers 2023, 15, 3347. [Google Scholar] [CrossRef] [PubMed]
- Hojjat-Farsangi, M. Small-molecule inhibitors of the receptor tyrosine kinases: Promising tools for targeted cancer therapies. Int. J. Mol. Sci. 2014, 15, 13768–13801. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Y.; Ma, X.; Hu, H. The influence of cell cycle regulation on chemotherapy. Int. J. Mol. Sci. 2021, 22, 6923. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xia, F. Immunotherapy for hepatocellular carcinoma: Molecular pathogenesis and clinical research progress. Oncol. Transl. Med. 2023, 9, 206–212. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Yan, Z.; Tong, P.; Xia, Q.; He, K. Effect of infiltrating immune cells in tumor microenvironment on metastasis of hepatocellular carcinoma. Cell. Oncol. 2023, 46, 1595–1604. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, C.; Wang, H. Immune-checkpoint inhibitor resistance in cancer treatment: Current progress and future directions. Cancer Lett. 2023, 562, 216182. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Q.; Wang, N.; Liu, Z.; Zhang, B.; Zhao, Y. Research progresses of targeted therapy and immunotherapy for hepatocellular carcinoma. Curr. Med. Chem. 2021, 28, 3107–3146. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, S.; Zeng, S.; Shen, H. From bench to bed: The tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 396. [Google Scholar] [CrossRef] [PubMed]
- Giraud, J.; Chalopin, D.; Blanc, J.-F.; Saleh, M. Hepatocellular carcinoma immune landscape and the potential of immunotherapies. Front. Immunol. 2021, 12, 655697. [Google Scholar] [CrossRef]
- Rimassa, L.; Finn, R.S.; Sangro, B. Combination immunotherapy for hepatocellular carcinoma. J. Hepatol. 2023, 79, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Dong, C.; Li, X. Treatment Options for Hepatocellular Carcinoma Using Immunotherapy: Present and Future. J. Clin. Transl. Hepatol. 2024, 12, 389. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, B.; Ielasi, L.; Chen, R.; Abbati, C.; Tonnini, M.; Tovoli, F.; Granito, A. TKIs in combination with immunotherapy for hepatocellular carcinoma. Expert Rev. Anticancer Ther. 2023, 23, 279–291. [Google Scholar] [CrossRef]
- Cuesta, Á.M.; Palao, N.; Bragado, P.; Gutierrez-Uzquiza, A.; Herrera, B.; Sánchez, A.; Porras, A. New and Old Key Players in Liver Cancer. Int. J. Mol. Sci. 2023, 24, 17152. [Google Scholar] [CrossRef]
- Pinato, D.J.; Guerra, N.; Fessas, P.; Murphy, R.; Mineo, T.; Mauri, F.A.; Mukherjee, S.K.; Thursz, M.; Wong, C.N.; Sharma, R. Immune-based therapies for hepatocellular carcinoma. Oncogene Res. 2020, 39, 3620–3637. [Google Scholar] [CrossRef]
- Shi, F.-D.; Ljunggren, H.-G.; La Cava, A.; Van Kaer, L. Organ-specific features of natural killer cells. Nat. Rev. Immunol. 2011, 11, 658–671. [Google Scholar] [CrossRef]
- Pittet, M.J.; Di Pilato, M.; Garris, C.; Mempel, T.R. Dendritic cells as shepherds of T cell immunity in cancer. Immunity 2023, 56, 2218–2230. [Google Scholar] [CrossRef]
- Hao, X.; Sun, G.; Zhang, Y.; Kong, X.; Rong, D.; Song, J.; Tang, W.; Wang, X. Targeting immune cells in the tumor microenvironment of HCC: New opportunities and challenges. Front. Cell Dev. Biol. 2021, 9, 775462. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Mahmud, A.R.; Faijanur-Rob-Siddiquee, M.; Shahriar, A.; Biswas, P.; Shimul, M.E.K.; Ahmed, S.Z.; Ema, T.I.; Rahman, N.; Khan, M.A.J. Role of T cells in cancer immunotherapy: Opportunities and challenges. Cancer Pathog. Ther. 2023, 1, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alemohammad, H.; Najafzadeh, B.; Asadzadeh, Z.; Baghbanzadeh, A.; Ghorbaninezhad, F.; Najafzadeh, A.; Safarpour, H.; Bernardini, R.; Brunetti, O.; Sonnessa, M. The importance of immune checkpoints in immune monitoring: A future paradigm shift in the treatment of cancer. Biomed. Pharmacother. 2022, 146, 112516. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, M.; Xu, D.; Li, T.-E.; Zhang, Z.; Li, J.-H.; Wang, X.-Y.; Yang, X.; Lu, L.; Jia, H.-L. The combination of PD-1 blockade with interferon-α has a synergistic effect on hepatocellular carcinoma. Cell. Mol. Immunol. 2022, 19, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Yokosuka, T.; Takamatsu, M.; Kobayashi-Imanishi, W.; Hashimoto-Tane, A.; Azuma, M.; Saito, T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J. Exp. Med. 2012, 209, 1201–1217. [Google Scholar] [CrossRef]
- Peña-Asensio, J.; Calvo, H.; Torralba, M.; Miquel, J.; Sanz-de-Villalobos, E.; Larrubia, J.-R. Anti-Pd-1/Pd-L1 based combination immunotherapy to boost antigen-specific CD8+ T cell response in hepatocellular carcinoma. Cancers 2021, 13, 1922. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, J.; Rousseau, B.; Amaddeo, G.; Mercey, M.; Charpy, C.; Costentin, C.; Luciani, A.; Zafrani, E.S.; Laurent, A.; Azoulay, D. Programmed death ligand 1 expression in hepatocellular carcinoma: Relationship with clinical and pathological features. Hepatology 2016, 64, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.; Finn, R.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Han, K.-H.; Harding, J.; Merle, P. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann. Oncol. 2019, 30, 874. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.-Y.; Breder, V.V.; Edeline, J.; Chao, Y.; Ogasawara, S. Results of KEYNOTE-240: Phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J. Clin. Oncol. 2019, 37, 4004. [Google Scholar] [CrossRef]
- Azimnasab-Sorkhabi, P.; Soltani-Asl, M.; Kfoury Junior, J.R. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) as an undetermined tool in tumor cells. Hum. Cell 2023, 36, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, N.; Tardiel-Cyril, D.R.; Davtyan, A.; Generali, D.; Roudi, R.; Li, Y. CTLA-4 in regulatory T cells for cancer immunotherapy. Cancers 2021, 13, 1440. [Google Scholar] [CrossRef] [PubMed]
- Maker, A.V.; Attia, P.; Rosenberg, S.A. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J. Immunol. 2005, 175, 7746–7754. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Ma, X.; Zhang, X.; Cheng, B.; Wang, R.; Liu, Y.; Zhang, X. New techniques: A roadmap for the development of HCC immunotherapy. Front. Immunol. 2023, 14, 1121162. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Gomez-Martin, C.; de la Mata, M.; Iñarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J.I.; Larrea, E.; Alfaro, C.; Sarobe, P. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013, 59, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; He, X.; Zhang, X.; Zhao, X.; Zhang, Y.; Shi, Y.; Hua, S. Hepatocellular carcinoma: Signaling pathways, targeted therapy, and immunotherapy. MedComm 2024, 5, 474. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, H.; Zhang, L.; Zhu, A.X.; Bernards, R.; Qin, W.; Wang, C. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 203–222. [Google Scholar] [CrossRef]
- Nativel, B.; Ramin-Mangata, S.; Mevizou, R.; Figuester, A.; Andries, J.; Iwema, T.; Ikewaki, N.; Gasque, P.; Viranaïcken, W. CD 93 is a cell surface lectin receptor involved in the control of the inflammatory response stimulated by exogenous DNA. Immunology 2019, 158, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Kuai, J.; Jiang, Z.; Que, W.; Wang, P.; Huang, W.; Ding, W.; Zhong, L. CD93 overexpresses in liver hepatocellular carcinoma and represents a potential immunotherapy target. Front. Immunol. 2023, 14, 1158360. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Piani, F.; Borghi, C.; Marzioni, D. Role of CD93 in Health and Disease. Cells 2023, 12, 1778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, M.; Ding, Q.; Liu, M. CD93 correlates with immune infiltration and impacts patient immunotherapy efficacy: A pan-cancer analysis. Front. Cell Develop. Biol. 2022, 10, 817965. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Chen, J.-H.; Hsieh, F.-I. Major adverse cardiovascular events of vascular endothelial growth factor tyrosine kinase inhibitors among patients with different malignancy: A systemic review and network meta-analysis. J. Chin. Med. Assoc. 2024, 87, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Fulgenzi, C.A.M.; D’Alessio, A.; Ogunbiyi, O.; Demirtas, C.O.; Gennari, A.; Cortellini, A.; Sharma, R.; Pinato, D.J. Novel immunotherapy combinations in clinical trials for hepatocellular carcinoma: Will they shape the future treatment landscape? Expert Opin. Investig. Drugs. 2022, 31, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Qi, Q.; Qian, X.; Han, J.; Zhu, X.; Zhang, Q.; Xia, R. The role of PD-1/PD-L1 axis and macrophage in the progression and treatment of cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 2020, 38, 2960. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Ryoo, B.-Y.; Hsu, C.-H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.P.; Spahn, J.; Liu, B.; Abdullah, H. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020, 21, 808–820. [Google Scholar] [CrossRef]
- Yau, T.; Zagonel, V.; Santoro, A.; Acosta-Rivera, M.; Choo, S.P.; Matilla, A.; He, A.R.; Gracian, A.C.; El-Khoueiry, A.B.; Sangro, B. Nivolumab plus cabozantinib with or without ipilimumab for advanced hepatocellular carcinoma: Results from cohort 6 of the CheckMate 040 trial. J. Clin. Oncol. 2023, 41, 1747. [Google Scholar] [CrossRef]
- Nevola, R.; Ruocco, R.; Criscuolo, L.; Villani, A.; Alfano, M.; Beccia, D.; Imbriani, S.; Claar, E.; Cozzolino, D.; Sasso, F.C. Predictors of early and late hepatocellular carcinoma recurrence. World J. Gastroenterol. 2023, 29, 1243. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.-Y.; Hu, R.-H.; Ho, C.-M.; Wu, Y.-M.; Lee, P.-H.; Ho, M.-C. Surgical resection versus radiofrequency ablation for Barcelona Clinic Liver Cancer very early stage hepatocellular carcinoma: Long-term results of a single-center study. Am. J. Surg. 2020, 220, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Colagrande, S.; Inghilesi, A.L.; Aburas, S.; Taliani, G.G.; Nardi, C.; Marra, F. Challenges of advanced hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 7645. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Ramjeesingh, R.; Liu, D.; Tam, V.C.; Knox, J.J.; Card, P.B.; Meyers, B.M. Optimizing survival and the changing landscape of targeted therapy for intermediate and advanced hepatocellular carcinoma: A systematic review. J. Natl. Cancer Inst. 2021, 113, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Systemic therapy for hepatocellular carcinoma: Latest advances. Cancers 2018, 10, 412. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, L.; Xu, Y.; Lu, X.; Zhao, H.; Yang, H.; Sang, X. Neoadjuvant therapy and immunotherapy strategies for hepatocellular carcinoma. Am. J. Cancer Res. 2020, 10, 1658. [Google Scholar] [PubMed]
- Llovet, J.M.; De Baere, T.; Kulik, L.; Haber, P.K.; Greten, T.F.; Meyer, T.; Lencioni, R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293–313. [Google Scholar] [CrossRef]
- Kaseb, A.O.; Vence, L.; Blando, J.; Yadav, S.S.; Ikoma, N.; Pestana, R.C.; Vauthey, J.N.; Allison, J.P.; Sharma, P. Immunologic correlates of pathologic complete response to preoperative immunotherapy in hepatocellular carcinoma. Cancer Immunol. Res. 2019, 7, 1390–1395. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Chan, S.; Minami, T.; Chishina, H.; Aoki, T.; Takita, M.; Hagiwara, S.; Minami, Y.; Ida, H. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and Child–Pugh a liver function: A proof-of-concept study. Cancers 2019, 11, 1084. [Google Scholar] [CrossRef]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Marron, T.U.; Fiel, M.I.; Hamon, P.; Fiaschi, N.; Kim, E.; Ward, S.C.; Zhao, Z.; Kim, J.; Kennedy, P.; Gunasekaran, G. Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: A single-arm, open-label, phase 2 trial. Lancet Gastroenterol. Hepatol. 2022, 7, 219–229. [Google Scholar] [CrossRef]
- Chamseddine, S.; LaPelusa, M.; Kaseb, A.O. Systemic neoadjuvant and adjuvant therapies in the management of hepatocellular carcinoma—A narrative review. Cancers 2023, 15, 3508. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Chan, S.L.; Gu, S.; Bai, Y.; Ren, Z.; Lin, X.; Chen, Z.; Jia, W.; Jin, Y.; Guo, Y. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): A randomised, open-label, international phase 3 study. Lancet 2023, 402, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Sansonno, D.; Lauletta, G.; Russi, S.; Conteduca, V.; Sansonno, L.; Dammacco, F. Transarterial chemoembolization plus sorafenib: A sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: A randomized clinical trial. The Oncologist 2012, 17, 359–366. [Google Scholar] [CrossRef]
- Qin, S.; Chen, M.; Cheng, A.-L.; Kaseb, A.O.; Kudo, M.; Lee, H.C.; Yopp, A.C.; Zhou, J.; Wang, L.; Wen, X. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 402, 1835–1847. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xiang, Y.-J.; Yu, H.-M.; Cheng, Y.-Q.; Liu, Z.-H.; Qin, Y.-Y.; Shi, J.; Guo, W.-X.; Lu, C.-D.; Zheng, Y.-X. Adjuvant sintilimab in resected high-risk hepatocellular carcinoma: A randomized, controlled, phase 2 trial. Nat. Med. 2024, 30, 708–715. [Google Scholar] [CrossRef]
- Vogel, A.; Saborowski, A. Current strategies for the treatment of intermediate and advanced hepatocellular carcinoma. Cancer Treat. Rev. 2020, 82, 101946. [Google Scholar] [CrossRef]
- Cai, M.; Huang, W.; Liang, W.; Guo, Y.; Liang, L.; Lin, L.; Xie, L.; Zhou, J.; Chen, Y.; Cao, B. Lenvatinib, sintilimab plus transarterial chemoembolization for advanced stage hepatocellular carcinoma: A phase II study. Liver Int. 2024, 44, 920–930. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Melero, I.; Yau, T.C.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Choo, S.; Trojan, J.; Welling, T.; Meyer, T. Impact of antitumor activity on survival outcomes, and nonconventional benefit, with nivolumab (NIVO) in patients with advanced hepatocellular carcinoma (aHCC): Subanalyses of CheckMate-040. J. Clin. Oncol. 2018, 36, 475. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.-W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Chen, Z.; Fang, W.; Ren, Z.; Xu, R.; Ryoo, B.-Y.; Meng, Z.; Bai, Y.; Chen, X.; Liu, X. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): Phase 3 KEYNOTE-394 study. J. Clin. Oncol. 2022, 40, 383. [Google Scholar] [CrossRef]
- Ren, Z.; Qin, S.; Meng, Z.; Chen, Z.; Chai, X.; Xiong, J.; Bai, Y.; Yang, L.; Zhu, H.; Fang, W. A phase 2 study of camrelizumab for advanced hepatocellular carcinoma: Two-year outcomes and continued treatment beyond first RECIST-defined progression. Liver Cancer 2021, 10, 500–509. [Google Scholar] [CrossRef]
- Qin, S.; Kudo, M.; Meyer, T.; Finn, R.S.; Vogel, A.; Bai, Y.; Guo, Y.; Meng, Z.; Zhang, T.; Satoh, T. LBA36 Final analysis of RATIONALE-301: Randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann. Oncol. 2022, 33, 1402–1403. [Google Scholar] [CrossRef]
- Verset, G.; Borbath, I.; Karwal, M.; Verslype, C.; Van Vlierberghe, H.; Kardosh, A.; Zagonel, V.; Stal, P.; Sarker, D.; Palmer, D.H. Pembrolizumab monotherapy for previously untreated advanced hepatocellular carcinoma: Data from the open-label, phase II KEYNOTE-224 trial. Clin. Cancer Res. 2022, 28, 2547–2554. [Google Scholar] [CrossRef]
- Kelley, R.K.; Rimassa, L.; Cheng, A.-L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 995–1008. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study. Lancet Oncol. 2021, 22, 977–990. [Google Scholar] [CrossRef]
- Xu, J.; Shen, J.; Gu, S.; Zhang, Y.; Wu, L.; Wu, J.; Shao, G.; Zhang, Y.; Xu, L.; Yin, T. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): A nonrandomized, open-label, phase II trial. Clin. Cancer Res. 2021, 27, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kudo, M.; Merle, P.; Meyer, T.; Qin, S.; Ikeda, M.; Xu, R.; Edeline, J.; Ryoo, B.-Y.; Ren, Z. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1399–1410. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol. 2020, 6, 204564. [Google Scholar] [CrossRef]
- Kelley, R.K.; Sangro, B.; Harris, W.; Ikeda, M.; Okusaka, T.; Kang, Y.-K.; Qin, S.; Tai, D.W.-M.; Lim, H.Y.; Yau, T. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: Randomized expansion of a phase I/II study. J. Clin. Oncol. 2021, 39, 2991–3001. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Fan, W.; Zhu, B.; Wang, G.; Sun, J.; Xiao, C.; Huang, F.; Tang, R.; Cheng, Y.; Huang, Z. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: A phase III, randomized clinical trial (LAUNCH). J. Clin. Oncol. 2023, 41, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Chan, S.; Kelley, R.; Lau, G.; Kudo, M.; Sukeepaisarnjaroen, W.; De Toni, E.; Furuse, J.; Kang, Y.; Galle, P. SO-15 Four-year overall survival update from the phase 3 HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann. Oncol. 2023, 34, S168. [Google Scholar] [CrossRef]
- Hack, S.P.; Spahn, J.; Chen, M.; Cheng, A.-L.; Kaseb, A.; Kudo, M.; Lee, H.C.; Yopp, A.; Chow, P.; Qin, S. IMbrave 050: A Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020, 16, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cao, Q.; Wen, W.; Wang, H. Targeted therapy for hepatocellular carcinoma: Challenges and opportunities. Cancer Lett. 2019, 460, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Magen, A.; Hamon, P.; Fiaschi, N.; Soong, B.Y.; Park, M.D.; Mattiuz, R.; Humblin, E.; Troncoso, L.; D’souza, D.; Dawson, T. Intratumoral dendritic cell–CD4+ T helper cell niches enable CD8+ T cell differentiation following PD-1 blockade in hepatocellular carcinoma. Nat. Med. 2023, 29, 1389–1399. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Ding, Y.; Qin, Y. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front. Immunol. 2023, 14, 1133308. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Tak, W.Y.; Lee, Y.; Heo, M.-K.; Song, J.-S.; Kim, H.-Y.; Park, S.Y.; Bae, S.H.; Lee, J.H.; Heo, J. Adjuvant immunotherapy with autologous dendritic cells for hepatocellular carcinoma, randomized phase II study. Oncoimmunology 2017, 6, 1328335. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.H.; Midgley, R.S.; Mirza, N.; Torr, E.E.; Ahmed, F.; Steele, J.C.; Steven, N.M.; Kerr, D.J.; Young, L.S.; Adams, D.H. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology 2009, 49, 124–132. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Yu, Y.; Xu, Z.; Sun, Y.; Liu, H.; Cheng, J.; Liu, M.; Sha, B.; Li, L. Immunotherapy of patient with hepatocellular carcinoma using cytotoxic T lymphocytes ex vivo activated with tumor antigen-pulsed dendritic cells. J. Cancer 2018, 9, 275. [Google Scholar] [CrossRef]
- Takayama, T.; Sekine, T.; Makuuchi, M.; Yamasaki, S.; Kosuge, T.; Yamamoto, J.; Shimada, K.; Sakamoto, M.; Hirohashi, S.; Ohashi, Y. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: A randomised trial. Lancet 2000, 356, 802–807. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.-H.; Lim, Y.-S.; Yeon, J.E.; Song, T.-J.; Yu, S.J.; Gwak, G.-Y.; Kim, K.M.; Kim, Y.J.; Lee, J.W. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 2015, 148, 1383–1391.e6. [Google Scholar] [CrossRef]
- Shimizu, K.; Kotera, Y.; Aruga, A.; Takeshita, N.; Katagiri, S.; Ariizumi, S.-i.; Takahashi, Y.; Yoshitoshi, K.; Takasaki, K.; Yamamoto, M. Postoperative dendritic cell vaccine plus activated T-cell transfer improves the survival of patients with invasive hepatocellular carcinoma. Hum. Vaccin. Immunother. 2014, 10, 970–976. [Google Scholar] [CrossRef]
- Dal Bo, M.; De Mattia, E.; Baboci, L.; Mezzalira, S.; Cecchin, E.; Assaraf, Y.G.; Toffoli, G. New insights into the pharmacological, immunological, and CAR-T-cell approaches in the treatment of hepatocellular carcinoma. Drug Resist. Updat. 2020, 51, 100702. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Tong, C.; Shi, D.; Chen, M.; Guo, Y.; Chen, D.; Han, X.; Wang, H.; Wang, Y.; Shen, P. Efficacy and biomarker analysis of CD133-directed CAR T cells in advanced hepatocellular carcinoma: A single-arm, open-label, phase II trial. Oncoimmunol. 2020, 9, 1846926. [Google Scholar] [CrossRef]
- Cui, T.-M.; Liu, Y.; Wang, J.-B.; Liu, L.-X. Adverse effects of immune-checkpoint inhibitors in hepatocellular carcinoma. OncoTar. Ther. 2020, 13, 11725–11740. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Hsu, C.; Chan, S.L.; Choo, S.-P.; Kudo, M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J. Hepatol. 2020, 72, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhan, M.; Li, X.-Y.; Zhang, H.; Dauphars, D.J.; Jiang, J.; Yin, H.; Li, S.-Y.; Luo, S.; Li, Y. Clinically approved combination immunotherapy: Current status, limitations, and future perspective. Curr. Immunol. Rev. 2022, 3, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Peeraphatdit, T.; Wang, J.; Odenwald, M.A.; Hu, S.; Hart, J.; Charlton, M.R. Hepatotoxicity from immune checkpoint inhibitors: A systematic review and management recommendation. Hepatology 2020, 72, 315–329. [Google Scholar] [CrossRef]
- Xing, R.; Gao, J.; Cui, Q.; Wang, Q. Strategies to improve the antitumor effect of immunotherapy for hepatocellular carcinoma. Front. Immunol. 2021, 12, 783236. [Google Scholar] [CrossRef]
- Waidmann, O. Recent developments with immunotherapy for hepatocellular carcinoma. Expert Opin. Biol. Ther. 2018, 18, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.D.; Zhang, B.; Tywonek, K.; Meyers, B.M.; Serrano, P.E. Toxicity profiles of systemic therapies for advanced hepatocellular carcinoma: A systematic review and meta-analysis. JAMA Net. Open 2022, 5, 2222721. [Google Scholar] [CrossRef] [PubMed]
- Manzar, G.S.; De, B.S.; Abana, C.O.; Lee, S.S.; Javle, M.; Kaseb, A.O.; Vauthey, J.-N.; Tran Cao, H.S.; Koong, A.C.; Smith, G.L. Outcomes and toxicities of modern combined modality therapy with atezolizumab plus bevacizumab and radiation therapy for hepatocellular carcinoma. Cancers 2022, 14, 1901. [Google Scholar] [CrossRef] [PubMed]
- Sankar, K.; Ye, J.C.; Li, Z.; Zheng, L.; Song, W.; Hu-Lieskovan, S. The role of biomarkers in personalized immunotherapy. Biomark. Res. 2022, 10, 32. [Google Scholar] [CrossRef]
- Llovet, J.M.; Villanueva, A.; Marrero, J.A.; Schwartz, M.; Meyer, T.; Galle, P.R.; Lencioni, R.; Greten, T.F.; Kudo, M.; Mandrekar, S.J. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology 2021, 73, 158–191. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Cheng, Z.; Jiang, Z.; Gan, L.; Zhang, Z.; Xie, Z. Immunomodulatory Precision: A Narrative Review Exploring the Critical Role of Immune Checkpoint Inhibitors in Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 5490. [Google Scholar] [CrossRef]
- Ladd, A.D.; Duarte, S.; Sahin, I.; Zarrinpar, A. Mechanisms of drug resistance in HCC. Hepatology 2024, 79, 926–940. [Google Scholar] [CrossRef]
| Trial ID | Treatment | Trial Phase | No. of Patients | Drug Dose | Primary Outcomes at the End Point | Reference | ||
|---|---|---|---|---|---|---|---|---|
| ORR | PFS | OS | ||||||
| NCT03713593 | Lenvatinib + pembrolizumab | II | 100 | Lenvatinib 8 mg orally every day + pembrolizumab 200 mg intravenously (IV) on the first day of a 21-day cycle. | 46% | 9.3 months | 22 months | [58] |
| NCT01658878 | Cabozantinib + nivolumab (doublet) +/− ipilimumab (triplet) | I/II | 36 (doublet) and 35 (triplet) | Cabozantinib 40 mg orally per day with nivolumab 240 mg IV once in 14 days vs. cabozantinib 40 mg per day + nivolumab 3 mg/kg once after 14 days with ipilimumab 1 mg/kg IV once in 1.5 months | 17% vs. 29% | 5.1 months vs. 4.3 months | 20.1 months vs. 22.1 months | [60] |
| NCT04102098 | Atezolizumab + bevacizumab vs. sorafenib | III | 501 | Atezolizumab 1200 mg + bevacizumab 15 mg/kg IV on day one after every 21-day cycle | 27% vs. 12% | 6.8 months vs. 4.3 months | 19.2 months vs. 13.4 months | [94] |
| NCT01658878 | Nivolumab + ipilimumab | II | 148 | Nivolumab 1 mg/kg + ipilimumab 3 mg/kg IV once after every 21 days | 32% | N/A | 22.8 months | [90] |
| NCT02519348 | Durvalumab + tremelimumab | I–II | 332 | Durvalumab 1500 mg + tremelimumab 300 mg IV once every 30 days | 95% | N/A | 18.7 months | [91] |
| NCT03755791 | Cabozantinib + atezolizumab vs. sorafenib | III | 837 | Cabozantinib 40 mg orally once a day + atezolizumab 1200 mg IV every 21 days | N/A | 6.8 months vs. 4.2 months | 15.4 months vs. 15.5 months | [86] |
| NCT03794440 | Sintilimab + bevacizumab vs. sorafenib | II-III | 595 | Sintilimab 200 mg + bevacizumab 15 mg/kg IV on day 1 of every 21 days vs. sorafenib 400 mg orally twice daily | 25·0% | 4.6 months vs. 2.8 months | Longer OS for combination therapy compared to sorafenib monotherapy | [87] |
| NCT03905967 | Lenvatinib + chemoembolization vs. lenvatinib | III | 338 | Lenvatinib 8 mg orally once daily for patients < 60 kg, 12 mg for ≥ 60 kg | 54.1% vs. 25.0% | 10.6 months vs. 6.4 months | 17.8 months vs. 11.5 months | [92] |
| NCT02576509 | Nivolumab vs. sorafenib | III | 743 | Nivolumab 240 mg IV once after every 14 days vs. sorafenib 400 mg orally twice daily | 15% vs. 7% | 5.0 months vs. 4.0 months | 16·4 months vs. 14·7 months | [81] |
| NCT03062358 | Pembrolizumab vs. placebo | III | 453 | Pembrolizumab 200 mg IV once every 21 days | 12.7% vs. 1.3% | 2.6 months vs. 2.3 months | 14.6 months vs. 13 months | [82] |
| NCT03412773 | Tislelizumab vs. sorafenib | III | 672 | Tislelizumab 200 mg IV on day 1 of every 21-day cycle or sorafenib 400 mg twice a day | 14.3% vs. 5.4% | 2.2 months and 3.6 months | 15.9 months vs. 14.1 months | [84] |
| NCT03764293 | Camrelizumab + rivoceranib | III | 543 | Camrelizumab 200 mg IV on day 1 of every 14 days + rivoceranib 250 mg orally once a day | N/A | 5.3 months | 22.1 months | [74] |
| NCT03298451 | Tremelimumab + durvalumab vs. sorafenib | III1 | 1171 | Tremelimumab 300 mg + durvalumab 1500 mg IV on day 1 of every 14 days | N/A | N/A | 36-month OS was 30.7% for combination therapy and 19.8% for sorafenib monotherapy | [93] |
| NCT03463876 | Camrelizumab + apatinib | II | 120 | Camrelizumab 3 mg/kg IV on day 1 of every 14 days + daily oral dose of apatinib 250 mg | 34.3% | 5.7 months | N/A | [88] |
| NCT03713593 | Lenvatinib + pembrolizumab | III | 794 | Lenvatinib 8 mg/day orally with pembrolizumab 200 mg IV on day 1 of every 21 days | N/A | 8.2 months | 21.2 months | [89] |
| Trial ID | Treatment | Trial Phase | No. of Patients (Estimated) | Current Status |
|---|---|---|---|---|
| NCT04976634 | Pembrolizumab + lenvatinib with belzutifan | II | 730 | Ongoing |
| NCT05199285 | Nivolumab + ipilimumab | II | 40 | Ongoing |
| NCT05408221 | Rulonilimab + lenvatinib | II | 576 | Ongoing |
| NCT05412589 | Oxaplatin + camrelizumab and apatinib | II | 35 | Ongoing |
| NCT04777851 | Regorafenib + pembrolizumab vs. TACE/TARE | III | 496 | Ongoing |
| NCT05337137 | Nivolumab + relatlimab and bevacizumab | I | 162 | Ongoing |
| NCT04522544 | Durvalumab + tremelimumab with Y-90 SIRT | II | 55 | Ongoing |
| NCT05904886 | Atezolizumab + bevacizumab with or without tiragolumab | III | 650 | Ongoing |
| NCT05301842 | Durvalumab + tremelimumab +/− lenvatinib with TACE | III | 725 | Ongoing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarlashat, Y.; Mushtaq, H.; Pham, L.; Abbas, W.; Sato, K. Advancements in Immunotherapeutic Treatments for Hepatocellular Carcinoma: Potential of Combination Therapies. Int. J. Mol. Sci. 2024, 25, 6830. https://doi.org/10.3390/ijms25136830
Zarlashat Y, Mushtaq H, Pham L, Abbas W, Sato K. Advancements in Immunotherapeutic Treatments for Hepatocellular Carcinoma: Potential of Combination Therapies. International Journal of Molecular Sciences. 2024; 25(13):6830. https://doi.org/10.3390/ijms25136830
Chicago/Turabian StyleZarlashat, Yusra, Hassan Mushtaq, Linh Pham, Wasim Abbas, and Keisaku Sato. 2024. "Advancements in Immunotherapeutic Treatments for Hepatocellular Carcinoma: Potential of Combination Therapies" International Journal of Molecular Sciences 25, no. 13: 6830. https://doi.org/10.3390/ijms25136830








