STAT5 Is Necessary for the Metabolic Switch Induced by IL-2 in Cervical Cancer Cell Line SiHa
Abstract
:1. Introduction
2. Results
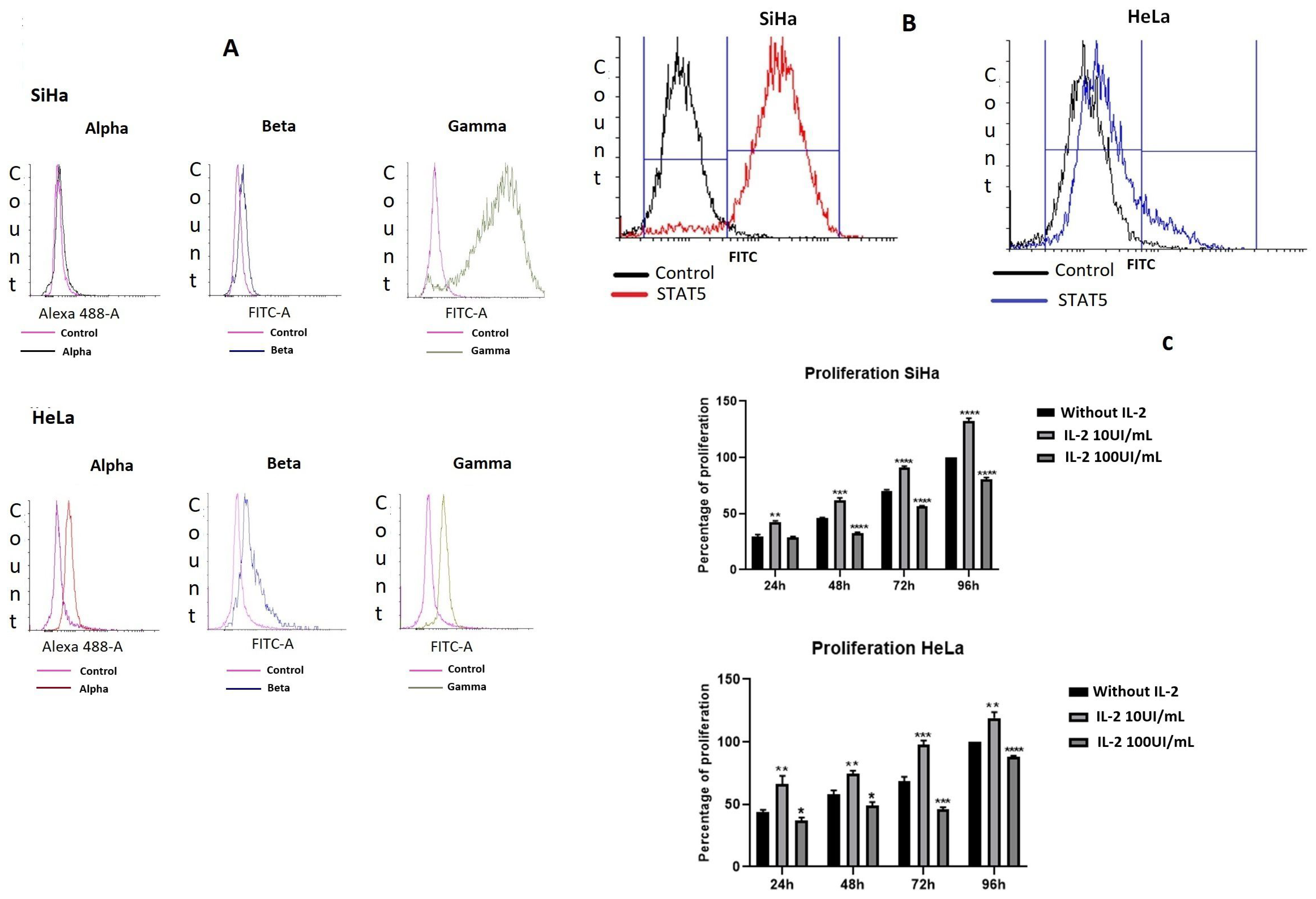
2.1. Cervical Cancer Cell Lines Express the Different Subunits of the IL-2 Receptor and Proliferate in Response to IL-2
2.2. IL-2 Induced Tyrosine Phosphorylation of STAT5
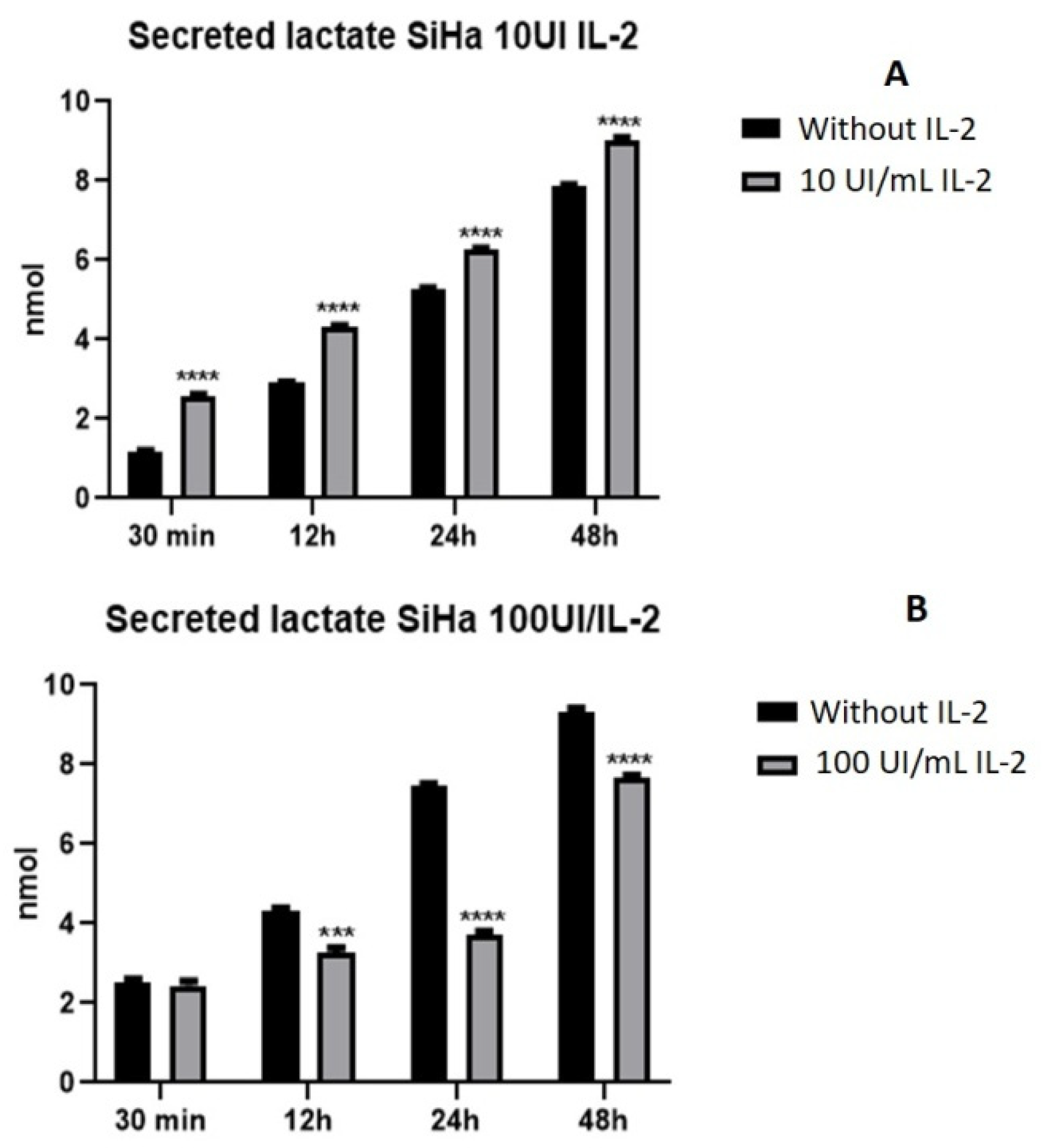
2.3. IL-2 Modulates Lactate Secretion in a Dose-Dependent Manner in SiHa Cells
2.4. IL-2 Induced an Increase in the NAD+/NADH Ratio in SiHa Cells
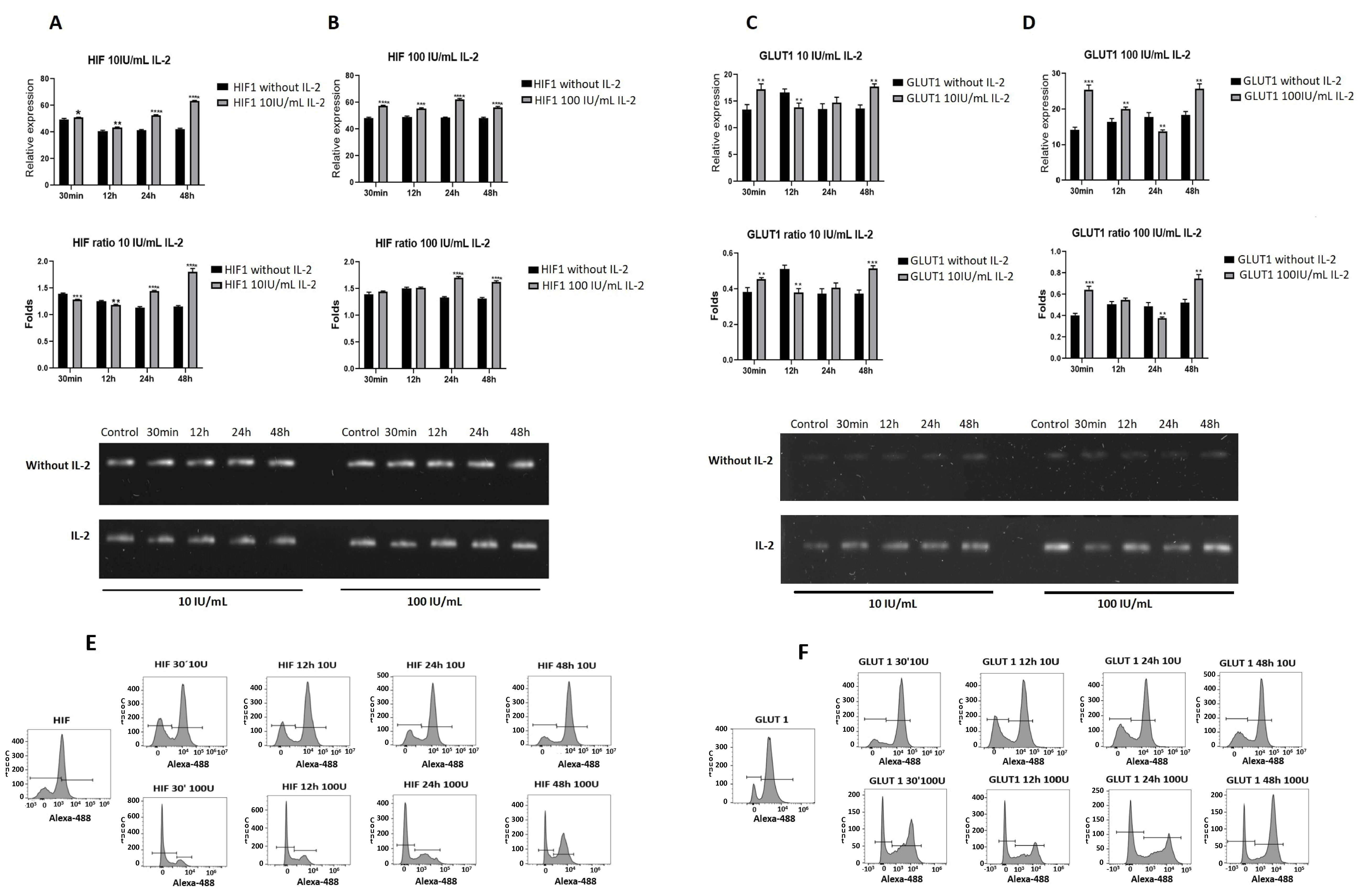
2.5. IL-2 Modified the Expression of Genes Related to Energy Metabolism
2.6. STAT5 Silencing Decreases Cell Proliferation
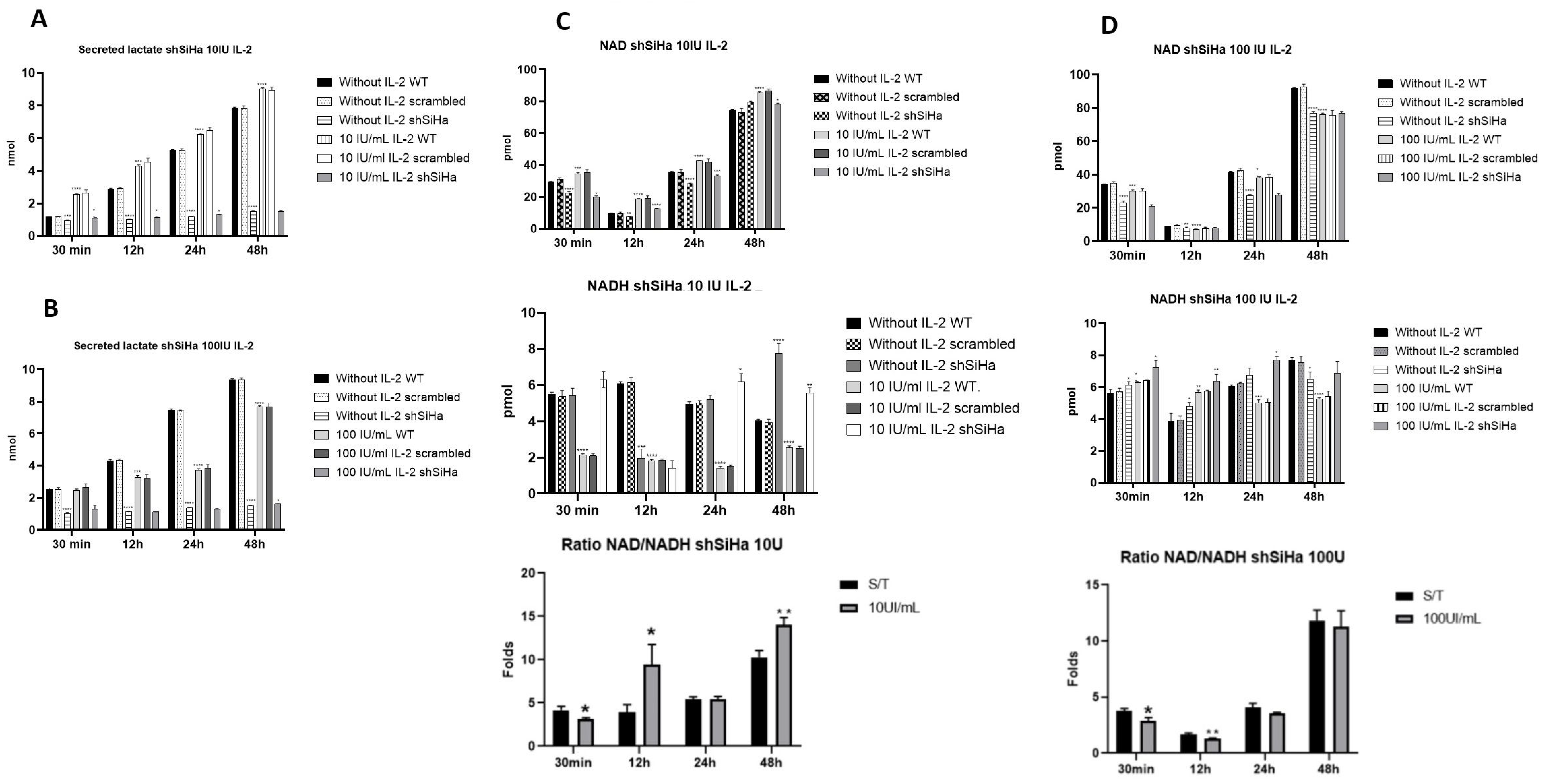
2.7. STAT5 Modulates the Production of Lactate and the NAD+/NADH Ratio in SiHa Cells
2.8. STAT5 Regulates the Expression of Genes Related to the Metabolic Switch in Cervical Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture Conditions
4.2. Flow Cytometry
4.3. Lactate Quantitation
4.4. Determination of NAD+ and NADH Levels
4.5. Silencing of STAT5
4.6. Determination of Cell Proliferation
4.7. Lysis and Immunoprecipitation
4.8. Immunoprecipitation and Immunoblotting
4.9. PCR
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Warburg, O.; Posener, K.; Negelein, E. Über den Stoffwechsel der Tumoren. Biochem. Z. 1924, 152, 319–344. [Google Scholar]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef]
- Dang, C.V. Links between metabolism and cancer. Genes Dev. 2012, 26, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.A.; Harris, I.; McCracken, S.; Mak, T.W. Cancer cell metabolism. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 299–311. [Google Scholar] [CrossRef]
- Kroemer, G.; Pouyssegur, J. Tumor cell metabolism: Cancer’s Achilles’ heel. Cancer Cell 2008, 13, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Voss, S.D.; Leary, T.P.; Sondel, P.M.; Robb, R.J. Identification of a direct interaction between interleukin 2 and the p64 interleukin 2 receptor gamma chain. Proc. Natl. Acad. Sci. USA 1993, 90, 2428–2432. [Google Scholar] [CrossRef]
- Rimoldi, D.; Salvi, S.; Hartmann, F.; Schreyer, M.; Blum, S.; Zografos, L.; Plaisance, S.; Azzarone, B.; Carrel, S. Expression of IL-2 receptors in human melanoma cells. Anticancer Res. 1993, 13, 555–564. [Google Scholar]
- Yasumura, S.; Lin, W.C.; Weidmann, E.; Hebda, P.; Whiteside, T.L. Expression of interleukin 2 receptors on human carcinoma cell lines and tumor growth inhibition by interleukin 2. Int. J. Cancer 1994, 59, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.S.; Jin, Y.; Graham, D.K.; Witthuhn, B.A.; Ihle, J.N.; Liu, E.T. A kinase-deficient splice variant of the human JAK3 is expressed in hematopoietic and epithelial cancer cells. J. Biol. Chem. 1995, 270, 25028–25036. [Google Scholar] [CrossRef] [PubMed]
- Miyasaki, T.; Kawahara, A.; Fujii, H.; Nakagawa, Y.; Minami, Y.; Liu, Z.J.; Oishi, I.; Silvennoinen, O.; Witthuhn, B.A.; Ihle, J.N.; et al. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science 1994, 266, 1045–1047. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.A.; Kawamura, M.; Kirken, R.A.; Chen, Y.Q.; Blake, T.B.; Shibuya, K.; Ortaldo, J.R.; McVicar, D.W.; O’Shea, J.J. Phosphorylation and activation of the Jak-3 Janus kinase in response to inteleukin-2. Nature 1994, 370, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.; Bacon, C.M.; Finbloom, D.S.; Rees, R.C.; Kaplan, D.; Shibuya, K.; Ortaldo, J.R.; Gupta, S.; Chen, Y.Q.; Giri, J.D.; et al. Tyrosine phosphorylation and activation of STAT5, STAT3, and Janus kinases by interleukins 2 and 15. Proc. Natl. Acad. Sci. USA 1995, 92, 8705–8709. [Google Scholar] [CrossRef] [PubMed]
- Chueh, F.Y.; Leong, K.F.; Yu, C.L. Mitochondrial translocation of signal transducer and activator of transcription 5 (STAT5) in leukemic T cells and cytokine-stimulated cells. Biochem. Biophys. Res. Commun. 2010, 402, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Seki, S.; Jenks, J.A.; Kohli, A.; Kawli, T.; Martin, D.P.; Snyder, M.; Baccheta, R.; Nadeau, K.C. Identification of STAT5A and STAT5B target genes in Human T cells. PLoS ONE 2014, 9, e86790. [Google Scholar] [CrossRef]
- Wofford, J.A.; Wieman, H.L.; Jacobs, S.R.; Zhao, Y.; Rathmell, J.C. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood 2008, 111, 2101–2111. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.J.; Wang, L.Y.; Chodosh, L.A.; Keith, B.; Simon, M.C. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol. Cell. Biol. 2003, 23, 9361–9374. [Google Scholar] [CrossRef]
- Wang, V.; Davis, D.A.; Haque, M.; Huang, L.E.; Yarchoan, R. Differential gene upregulation by hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha in HEK293T cells. Cancer Res. 2005, 65, 3299–3306. [Google Scholar] [CrossRef]
- Lagunas-Cruz, M.C.; Valle-Mendiola, A.; Trejo-Huerta, J.; Rocha-Zavaleta, L.; Mora-García, M.L.; Gutiérrez-Hoya, A.; Weiss-Steider, B.; Soto-Cruz, I. IL-2 Induces Transient Arrest in the G1 Phase to Protect Cervical Cancer Cells from Entering Apoptosis. J. Oncol. 2019, 2019, 7475295. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Cirilo, L.; Torres-Corioriles, E.I.; Rangel-Corona, R.; Corona Ortega, M.T.; Weiss-Steider, B. Evidence that the viral oncoproteins E6 and E7 of HPV induce the expression of a functional IL-2R on cervical cancer cells. Cytokine 2021, 148, 155592. [Google Scholar] [CrossRef] [PubMed]
- Valle-Mendiola, A.; Weiss-Steider, B.; Rocha-Zavaleta, L.; Soto-Cruz, I. IL-2 enhances cervical cancer cells proliferation and JAK3/STAT5 phosphorylation at low doses, while at high doses IL-2 has opposite effects. Cancer Investig. 2014, 32, 115–125. [Google Scholar] [CrossRef]
- Rocha-Zavaleta, L.; Huitron, C.; Cáceres-Cortés, J.R.; Alvarado-Moreno, J.A.; Valle-Mendiola, A.; Soto-Cruz, I.; Weiss-Steider, B.; Rangel-Corona, R. Interleukin-2 (IL-2) receptor-betagamma signalling is activated by c-Kit in the absence of IL-2, or by exogenous IL-2 via JAK3/STAT5 in human papillomavirus-associated cervical cancer. Cell Signal. 2004, 16, 1239–1247. [Google Scholar] [CrossRef]
- Halim, C.E.; Deng, S.; Ong, M.S.; Yap, C.T. Involvement of STAT5 in Oncogenesis. Biomedicines 2020, 8, 316. [Google Scholar] [CrossRef]
- Hong, S.; Laimins, L.A. The JAK-STAT Transcriptional Regulator, STAT-5, Activates the ATM DNA Damage Pathway to Induce HPV 31 Genome Amplification upon Epithelial Dierentiation. PLoS Pathog. 2013, 9, e1003295. [Google Scholar] [CrossRef]
- Zhang, W.; Hong, S.; Maniar, K.P.; Cheng, S.; Jie, C.; Rademaker, A.W.; Krensky, A.M.; Clayberger, C. KLF13 regulates the diferentiation-dependent human papillomavirus life cycle in keratinocytes through STAT5 and IL-8. Oncogene 2016, 35, 5565–5575. [Google Scholar] [CrossRef]
- Morgan, E.L.; Macdonald, A. JAK2 Inhibition Impairs Proliferation and Sensitises Cervical Cancer Cells to Cisplatin-Induced Cell Death. Cancers 2019, 11, 1934. [Google Scholar] [CrossRef]
- Sobti, R.C.; Singh, N.; Hussain, S.; Suri, V.; Bharadwaj, M.; Das, B.C. Deregulation of STAT-5 isoforms in the development of HPV-mediated cervical carcinogenesis. J. Recept. Signal Transduct. Res. 2010, 30, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Chou, C.Y.; Wu, Y.H.; Hsueh, W.T.; Hsu, C.H.; Guo, H.R.; Lee, W.Y.; Su, W.C. Constitutive STAT5 activation correlates with better survival in cervical cancer patients treated with radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 658–666. [Google Scholar] [CrossRef]
- Yin, C.; Qie, S.; Sang, N. Carbon soured metabolism and its regulation in cancer cells. Crit. Rev. Eukaryot Gene Expr. 2012, 22, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Valle-Mendiola, A.; Soto-Cruz, I. Energy Metabolism in Cancer: The Roles of STAT3 and STAT5 in the Regulation of Metabolism-Related Genes. Cancers 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Walenta, S.; Schroeder, T.; Mueller-Klieser, W. Lactate in Solid Malignant Tumors: Potential Basis of a Metabolic Classification in Clinical Oncology. Curr. Med. Chem. 2004, 11, 2195–2204. [Google Scholar] [CrossRef]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Zhan, L.; Guo, J.Y.; et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Li, K.Y.; Cai, L.; Hensley, C.T.; Kim, J.; Zacharias, L.G.; Yang, C.; Do, Q.N.; Doucette, S.; Burguete, D.; et al. Lactate metabolism in human lung tumors. Cell 2017, 171, 358–371.e9. [Google Scholar] [CrossRef] [PubMed]
- Kindt, N.; Descamps, G.; Lechien, J.R.; Remmelink, M.; Colet, J.M.; Wattiez, R.; Berchem, G.; Journe, F.; Saussez, S. Involvement of HPV Infection in the Release of Macrophage Migration Inhibitory Factor in Head and Neck Squamous Cell Carcinoma. J. Clin. Med. 2019, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Yabu, M.; Shime, H.; Hara, H.; Saito, T.; Matsumoto, M.; Seya, T.; Akazawa, T.; Inoue, N. IL-23-dependent and -independent enhancement pathways of IL-17A production by lactic acid. Int. Immunol. 2011, 23, 29–41. [Google Scholar] [CrossRef]
- Dietl, K.; Renner, K.; Dettmer, K.; Timischl, B.; Eberhart, K.; Dorn, C.; Hellerbrand, C.; Kastenberger, M.; Kunz-Schughart, L.A.; Oefner, P.J.; et al. Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes. J. Immunol. 2010, 184, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Hoffmann, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 2007, 109, 3812–3819. [Google Scholar] [CrossRef]
- Wagner, W.; Ciszewski, W.M.; Kania, K.D. L- and D-lactate enhance DNA repair and modulate the resistance of cervical carcinoma cells to anticancer drugs via histone deacetylase inhibition and hydroxycarboxylic acid receptor 1 activation. Cell Commun. Signal 2015, 13, 36. [Google Scholar] [CrossRef]
- Li, B.; Sui, L. Metabolic reprogramming in cervical cancer and metabolomics perspectives. Nutr. Metab. 2021, 18, 93. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Cell–cell and intracellular lactate shuttles. J. Physiol. 2009, 587, 5591–5600. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, L.; Morandi, A.; Giannoni, E.; Chiarugi, P. Lactate: A metabolic drives in the tumor landscape. Trends Biochem. Sci. 2019, 44, 153–166. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Amjad, S.; Nisar, S.; Bhat, A.A.; Frenneaux, M.P.; Fakhro, K.; Haris, M.; Reddy, R.; Patay, Z.; Baur, J.; Bagga, P. Role of NAD+ in regulating cellular and metabolic signaling pathways. Mol. Metab. 2021, 49, 101195. [Google Scholar] [CrossRef] [PubMed]
- Fa-Xing, Y.; Ru-Ping, D.; Shuang-Ru, G.; Lei, Z.; Yan, L. Logic of a mammalian metabolic cycle: An oscillated NAD+/NADH redox signaling regulates coordinated histone expression and S-phase progression. Cell Cycle 2009, 8, 773–779. [Google Scholar]
- Veiga Moreira, J.; Hamraz, M.; Abolhassani, M.; Bigan, E.; Pérès, S.; Paulevé, L.; Nogueira, M.L.; Steyaert, J.M.; Schwartz, L. The Redox Status of Cancer Cells Supports Mechanisms behind the Warburg Effect. Metabolites 2016, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Chueh, F.Y.; Leong, K.F.; Cronk, R.J.; Venkitachalam, S.; Pabich, S.; Yu, C.L. Nuclear localization of pyruvate dehydrogenase complex-E2 (PDC-E2), a mitochondrial enzyme, and its role in signal transducer and activator of transcription 5 (STAT5)-dependent gene transcription. Cell Signal 2011, 23, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Nishimura, N.; Hazama, A. Cesium Treatment Depresses Glycolysis Pathway in HeLa Cell. Cell Physiol. Biochem. 2021, 55, 477–488. [Google Scholar]
- Cheng, Y.; Chen, G.; Hong, L.; Zhou, L.; Hu, M.; Li, B.; Huang, J.; Xia, L.; Li, C. How does hypoxia inducible factor-1α participate in enhancing the glycolysis activity in cervical cancer? Ann. Diagn. Pathol. 2013, 17, 305–311. [Google Scholar] [CrossRef]
- Łuczak, M.; Roszak, A.; Pawlik, P.; Kedzia, H.; Lianeri, M.; Jagodziński, P.P. Increased expression of HIF-1A and its implication in the hypoxia pathway in primary advanced uterine cervical carcinoma. Oncol. Rep. 2011, 26, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Cluff, E.; Magdaleno, C.C.; Fernandez, E.; House, T.; Swaminathan, S.; Varadaraj, A.; Rajasekaran, N. Hypoxia-inducible factor-1 alpha expression is induced by IL-2 via the PI3K/mTOR pathway in hypoxic NK cells and supports effector functions in NKL cells and ex vivo expanded NK cells. Cancer Immunol. Immunother. 2022, 71, 1989–2005. [Google Scholar] [CrossRef] [PubMed]
- Cammann, C.; Rath, A.; Reichl, U.; Lingel, H.; Brunner-Weinzierl, M.; Simeoni, L. Early changes in the metabolic profile of activated CD8(+) T cells. BMC Cell Biol. 2016, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Chang, J.H. Differential effect of GLUT1 overexpression on survival and tumor immune microenvironment of human papilloma virus type 16-positive and -negative cervical cancer. Sci. Rep. 2019, 9, 13301. [Google Scholar] [CrossRef] [PubMed]
- Priego-Hernández, V.D.; Arizmendi-Izazaga, A.; Soto-Flores, D.G.; Santiago-Ramón, N.; Feria-Valadez, M.D.; Navarro-Tito, N.; Jiménez-Wences, H.; Martínez-Carrillo, D.N.; Salmerón-Bárcenas, E.G.; Leyva-Vázquez, M.A.; et al. Expression of HIF-1 and Genes Involved in Glucose Metabolism Is Increased in Cervical Cancer and HPV-16-Positive Cell Lines. Pathogens 2023, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.H.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Villarino, A.V.; Laurence, A.D.; Davis, F.P.; Nivelo, L.; Brooks, S.R.; Sun, H.W. A central role for STAT5 in the transcriptional programing of T helper cell metabolism. Sci. Immunol. 2022, 7, eabl9467. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Vincent, E.E.; Cronin, J.G.; Panetti, S.; Chambers, M.; Holm, S.R. Akt and STAT5 mediate naïve human CD4+ T-cell early metabolic response to TCR stimulation. Nat. Commun. 2019, 10, 2042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Liu, Y.; Zhang, P.; Nie, J.; Zhao, R.; Shi, Q.; Sun, H.; Jiao, D.; Chen, Y.; et al. Mitochondrial STAT5A promotes metabolic remodeling and the Warburg effect by inactivating the pyruvate dehydrogenase complex. Cell Death Dis. 2021, 12, 634. [Google Scholar] [CrossRef]
- Hwang, Y.C.; Shaw, S.; Kaneko, M.; Redd, H.; Marrero, M.B.; Ramasamy, R. Aldose reductase pathway mediates JAK-STAT signaling: A novel axis in myocardial ischemic injury. FASEB J. 2005, 19, 795–797. [Google Scholar] [CrossRef]
- Reddy, M.M.; Fernandes, M.S.; Deshpande, A.; Weisberg, E.; Inguilizian, H.V.; Abdel-Wahab, O. The JAK2V617F oncogene requires expression of inducible phosphofructokinase/fructose-bisphosphatase 3 for cell growth and increased metabolic activity. Leukemia 2012, 26, 481–489. [Google Scholar] [CrossRef]
- Vora, M.; Alattia, L.A.; Ansari, J.; Ong, M.; Cotelingam, J.; Coppola, D. Shackelford. Nicotinamide Phosphoribosyl Transferase a Reliable Marker of Progression in Cervical Dysplasia. Anticancer Res. 2017, 37, 4821–4825. [Google Scholar] [PubMed]
- Shackelford, R.E.; Mayhall, K.; Maxwell, N.M.; Kandil, E.; Coppola, D. Nicotinamide phosphoribosyltransferase in malignancy: A review. Genes Cancer 2013, 4, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Rongvaux, A.; Shea, R.J.; Mulks, M.H.; Gigot, D.; Urbain, J.; Leo, O.; Andris, F. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur. J. Immunol. 2002, 32, 3225–3234. [Google Scholar] [CrossRef] [PubMed]
- Nowell, M.A.; Richards, P.J.; Fielding, C.A.; Ognjanovic, S.; Topley, N.; Williams, A.S. Regulation of pre-B cell colony enhancing factor by STAT-3- dependent inter-leukin-6 trans-signaling: Implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum 2006, 54, 2084–2095. [Google Scholar] [CrossRef]
- Sun, X.; Elangovan, V.R.; Mapes, B.; Camp, S.M.; Sammani, S.; Saadat, L. The NAMPT promoter is regulated by mechanical stress, signal transducer and activator of transcription 5, and acute respiratory distress syndrome-associated genetic variants. Am. J. Respir. Cell Mol. Biol. 2014, 51, 660–667. [Google Scholar] [CrossRef]
- Ohanna, M.; Cerezo, M.; Nottet, N.; Bille, K.; Didier, R.; Beranger, G. Pivotal role of NAMPT in the switch of melanoma cells toward an invasive and drug-resistant phenotype. Genes Dev. 2018, 32, 448–461. [Google Scholar] [CrossRef]
- Lemoine, M.; Derenzini, E.; Buglio, D.; Medeiros, L.J.; Davis, R.E.; Zhang, J. The pan-deacetylase inhibitor panobinostat induces cell death and synergizes with everolimus in Hodgkin lymphoma cell lines. Blood 2012, 119, 4017–4025. [Google Scholar] [CrossRef] [PubMed]
- Belmokhtar, K.; Bourguignon, T.; Worou, M.E.; Khamis, G.; Bonnet, P.; Domenech, J.; Eder, V. Regeneration of three layers vascular wall by using BMP2-treated MSC involving HIF-1α and Id1 expressions through JAK/STAT pathways. Stem. Cell Rev. Rep. 2011, 7, 847–859. [Google Scholar] [CrossRef]
- Koop, L.K.; Rafalkiaer, U.; Gjerdrum, L.M.R.; Helvad, R.; Pedersen, I.H.; Litman, T. STAT5-mediated expression of oncogenic miR-155 in cutaneous T-cell Lymphoma. Cell Cycle 2013, 12, 1939–1947. [Google Scholar] [CrossRef]
- Bruning, U.; Cerone, L.; Neufeld, Z.; Fitzpatrick, S.F.; Cheong, A.; Scholz, C.C. MicroRNA-155 promotes resolution of hypoxia-inducible factor1 alpha activity during prolonged hypoxia. Mol. Cell. Biol. 2011, 31, 4087–4096. [Google Scholar] [CrossRef] [PubMed]
- Lao, G.; Liu, P.; Wu, Q.; Zhang, W.; Liu, Y.; Yang, L.; Ma, C. Mir-155 promotes cervical cancer proliferation through suppression of its target gene gene LKB1. Tumour Biol. 2014, 35, 11933–11938. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.O.; Tavares, V.; Gil da Costa, R.M.; Medeiros, R. MiR-150 and miR-155 expression predicts survival of cervical cancer patients: A translational approach to novel prognostic biomarkers. Biomarkers 2023, 28, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.J.; Wang, L.; Zhang, Y.; Zheng, J.D.; Liu, L. LncRNA UCA1 regulates cervical cancer survival and EMT occurrence by targeting miR-155. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9869–9879. [Google Scholar] [PubMed]
- Feng, J.; Xie, G.; Zhan, Y.; Lu, J.; Xu, L.; Fan, S. Elevated HSP90 associates with expression of HIF-1α and p-AKT and is predictive of poor prognosis in nasopharyngeal carcinoma. Histopathology 2019, 75, 202–212. [Google Scholar] [CrossRef]
- Loboda, A.; Jozkowicz, A.; Dulak, J. HIF-1 and HIF-2 transcription factors—Similar but not identical. Mol. Cells 2010, 29, 435–442. [Google Scholar] [CrossRef]
- Liu, Y.V.; Baek, J.H.; Zhang, H.; Diez, R.; Cole, R.N.; Semenza, G.L. RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol. Cells 2007, 25, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.V.; Semenza, G.L. RACK1 vs. HSP90 competition for HIF-1 alpha degradation vs. stabilization. Cell Cycle 2007, 6, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, S.; Tu, J.; Cai, W.; Xu, Q. HSP90 inhibits apoptosis and promotes growth by regulating HIF-1α abundance in hepatocellular carcinoma. Int. J. Mol. Med. 2016, 37, 825–835. [Google Scholar]
- Xu, W.; Yu, F.; Yan, M.; Lu, L.; Zou, W.; Sun, L. Geldanamycin, a heat shock protein 90-binding agent, disrupts Stat5 activation in IL-2-stimulated cells. J. Cell Physiol. 2004, 198, 188–196. [Google Scholar] [CrossRef]
- Jego, G.; Hermetet, F.; Girodon, F.; Garrido, C. Chaperoning STAT3/5 by heat shock proteins: Interest of their targeting in cancer therapy. Cancers 2020, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Moulick, K.; Ahn, J.H.; Zong, H.; Rodina, A.; Cerchietti, L.; Gomes DaGama, E.M.; Caldas-Lopes, E.; Beebe, K.; Perna, F.; Hatzi, K.; et al. Affinity-based proteomics reveal cancer-specific networks coordinated by HSP90. Nat. Chem. Biol. 2011, 7, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Schwock, J.; Pham, N.A.; Cao, M.P.; Hedley, D.W. Efficacy of Hsp90 inhibition for induction of apoptosis and inhibition of growth in cervical carcinoma cells in vitro and in vivo. Cancer Chemother. Pharmacol. 2008, 61, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; He, S.L.; Li, L.J.; Wang, C. Hsp90 up-regulates PD-L1 to promote HPV-positive cervical cancer via HER2/PI3K/AKT pathway. Mol. Med. 2021, 27, 130. [Google Scholar] [CrossRef]
- Xu, D.; Dong, P.; Xiong, Y.; Yue, J.; Konno, Y.; Ihira, K.; Kobayashi, N.; Todo, Y.; Watari, H. MicroRNA-361-mediated inhibition of HSP90 expression and EMT in cervical cáncer is counteracted by oncogenic lncRNA NEAT1. Cells 2020, 9, 632. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valle-Mendiola, A.; Rocha-Zavaleta, L.; Maldonado-Lagunas, V.; Morelos-Laguna, D.; Gutiérrez-Hoya, A.; Weiss-Steider, B.; Soto-Cruz, I. STAT5 Is Necessary for the Metabolic Switch Induced by IL-2 in Cervical Cancer Cell Line SiHa. Int. J. Mol. Sci. 2024, 25, 6835. https://doi.org/10.3390/ijms25136835
Valle-Mendiola A, Rocha-Zavaleta L, Maldonado-Lagunas V, Morelos-Laguna D, Gutiérrez-Hoya A, Weiss-Steider B, Soto-Cruz I. STAT5 Is Necessary for the Metabolic Switch Induced by IL-2 in Cervical Cancer Cell Line SiHa. International Journal of Molecular Sciences. 2024; 25(13):6835. https://doi.org/10.3390/ijms25136835
Chicago/Turabian StyleValle-Mendiola, Arturo, Leticia Rocha-Zavaleta, Vilma Maldonado-Lagunas, Diego Morelos-Laguna, Adriana Gutiérrez-Hoya, Benny Weiss-Steider, and Isabel Soto-Cruz. 2024. "STAT5 Is Necessary for the Metabolic Switch Induced by IL-2 in Cervical Cancer Cell Line SiHa" International Journal of Molecular Sciences 25, no. 13: 6835. https://doi.org/10.3390/ijms25136835




