Functional and Structural Properties of Cytoplasmic Tropomyosin Isoforms Tpm1.8 and Tpm1.9
Abstract
:1. Introduction
2. Results
2.1. Properties of Isolated Tpm Molecules
2.1.1. Differential Scanning Calorimetry Experiments
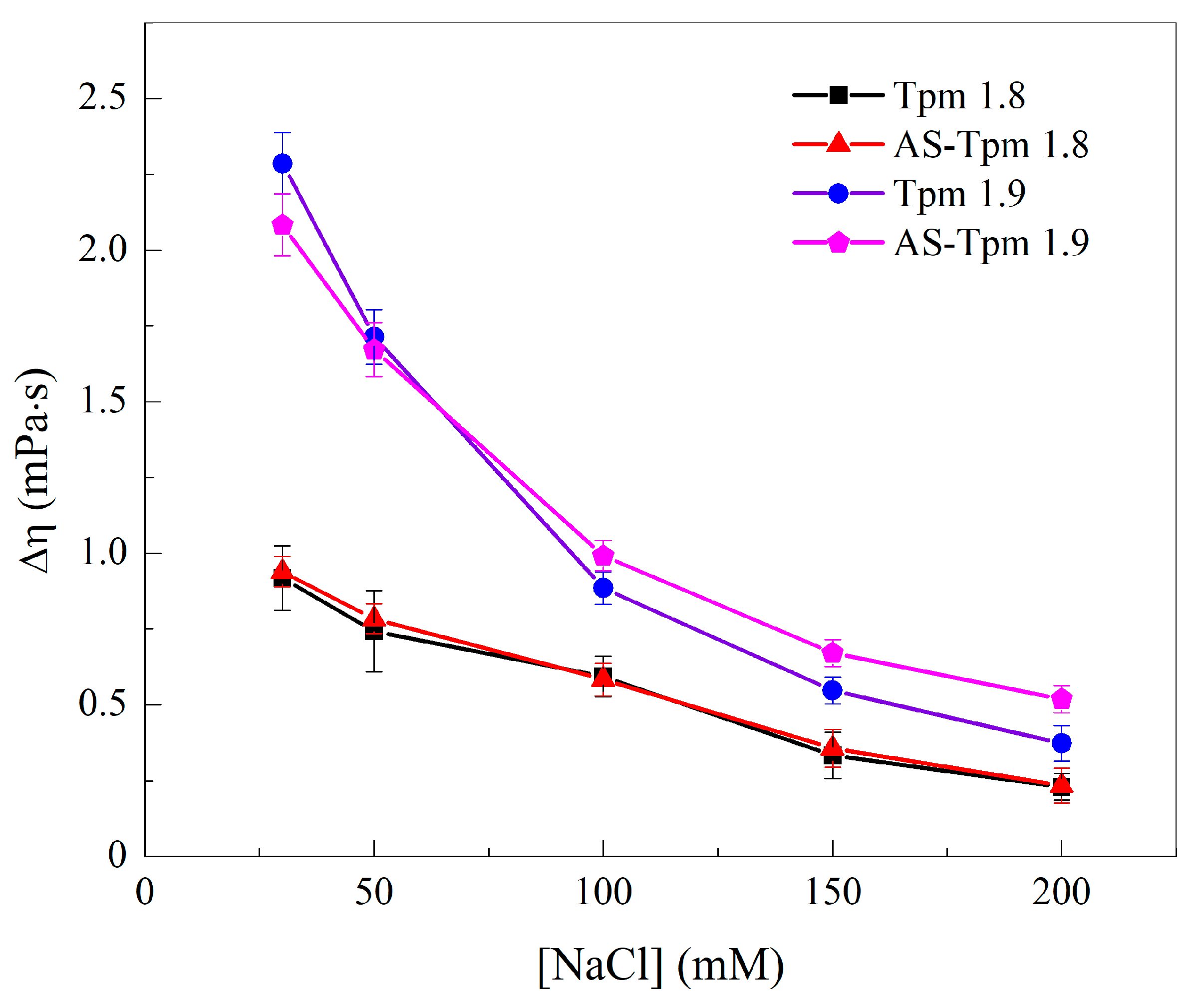
2.1.2. Viscosimetry of Tpm Solutions
2.2. Affinity of Tpm to F-Actin and Stability of Its Complexes
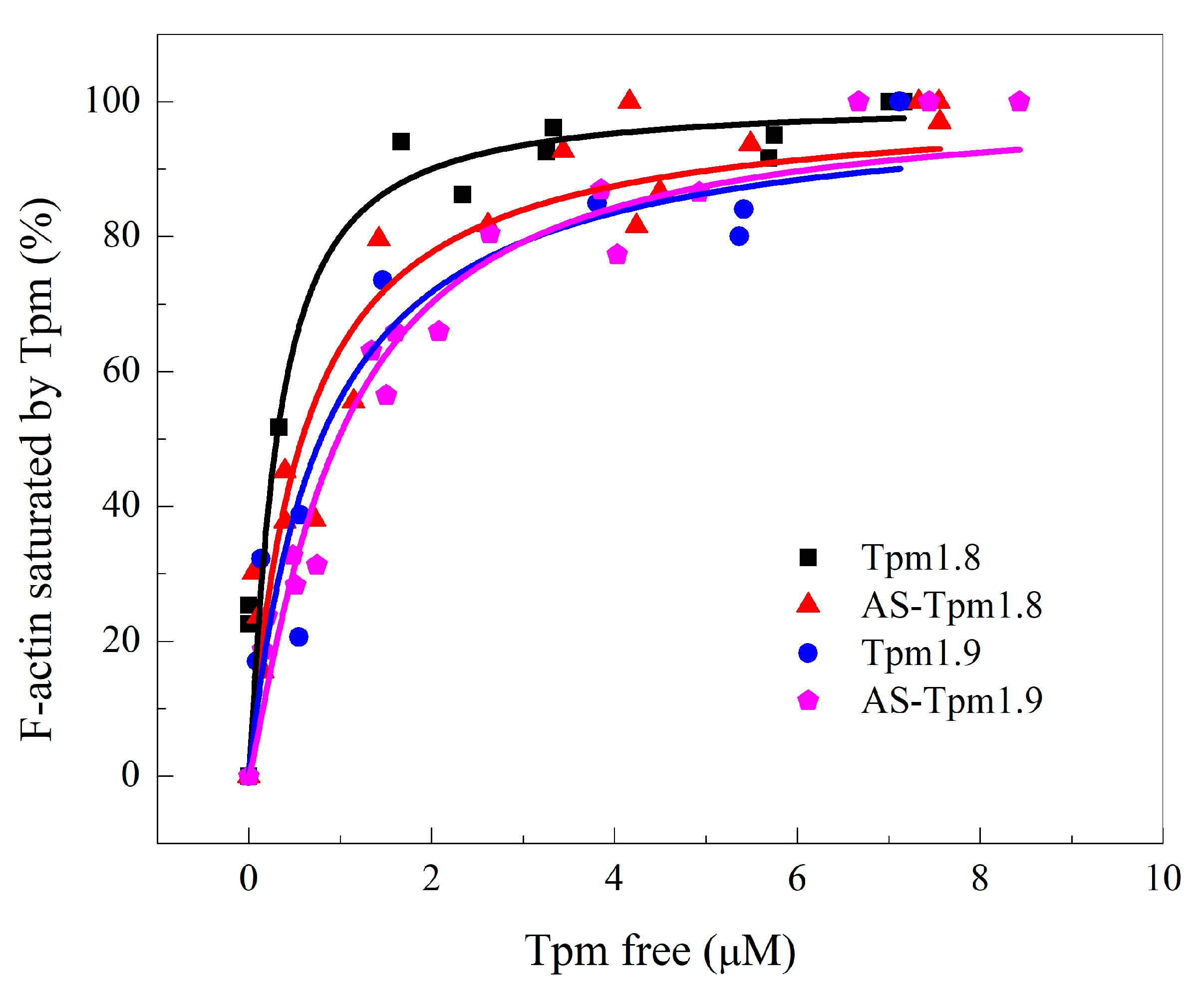
2.2.1. Co-Sedimentation Assay
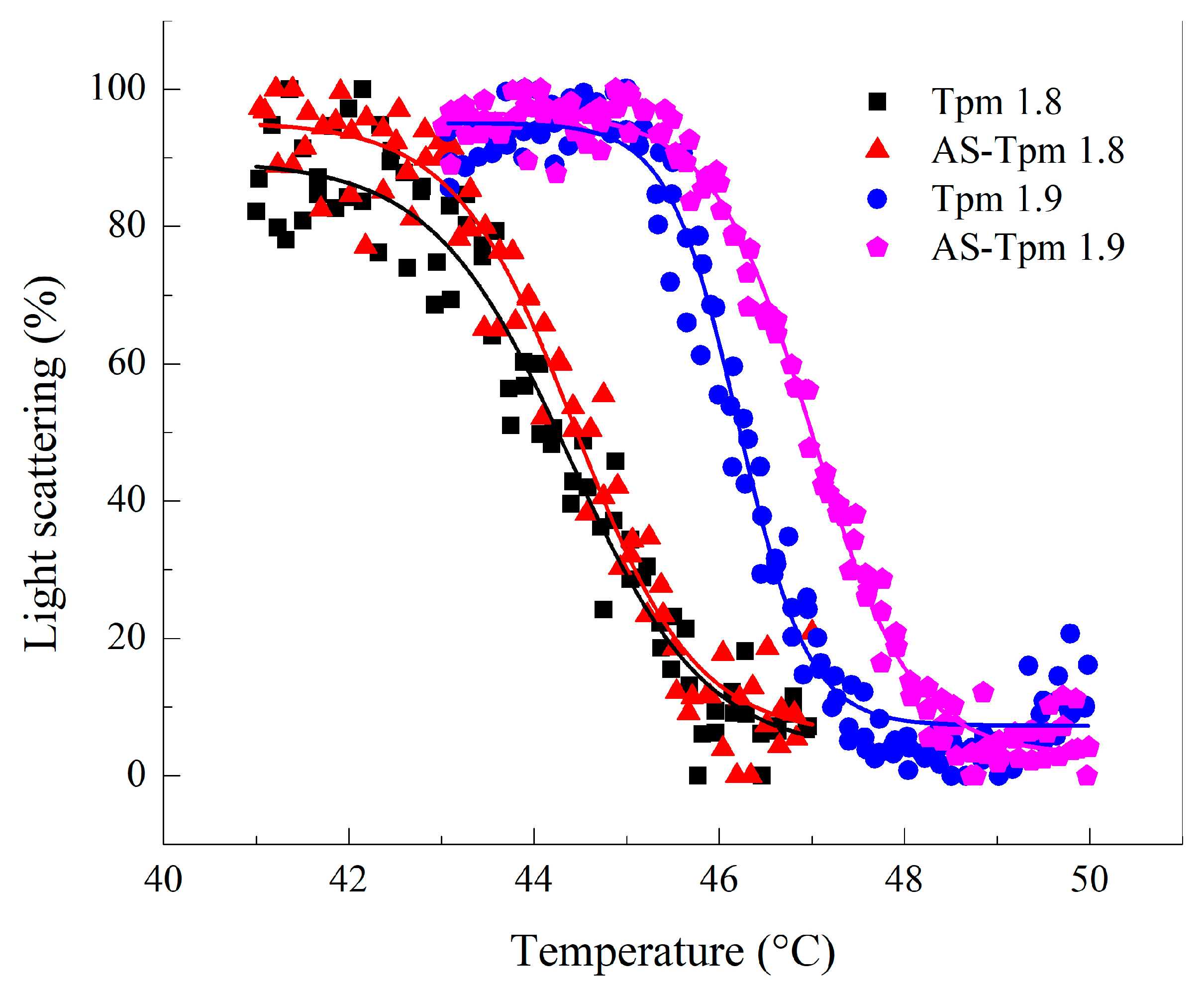
2.2.2. Thermally Induced Dependences of the Light Scattering
2.3. Functional Properties of Tpm–F-Actin Complexes
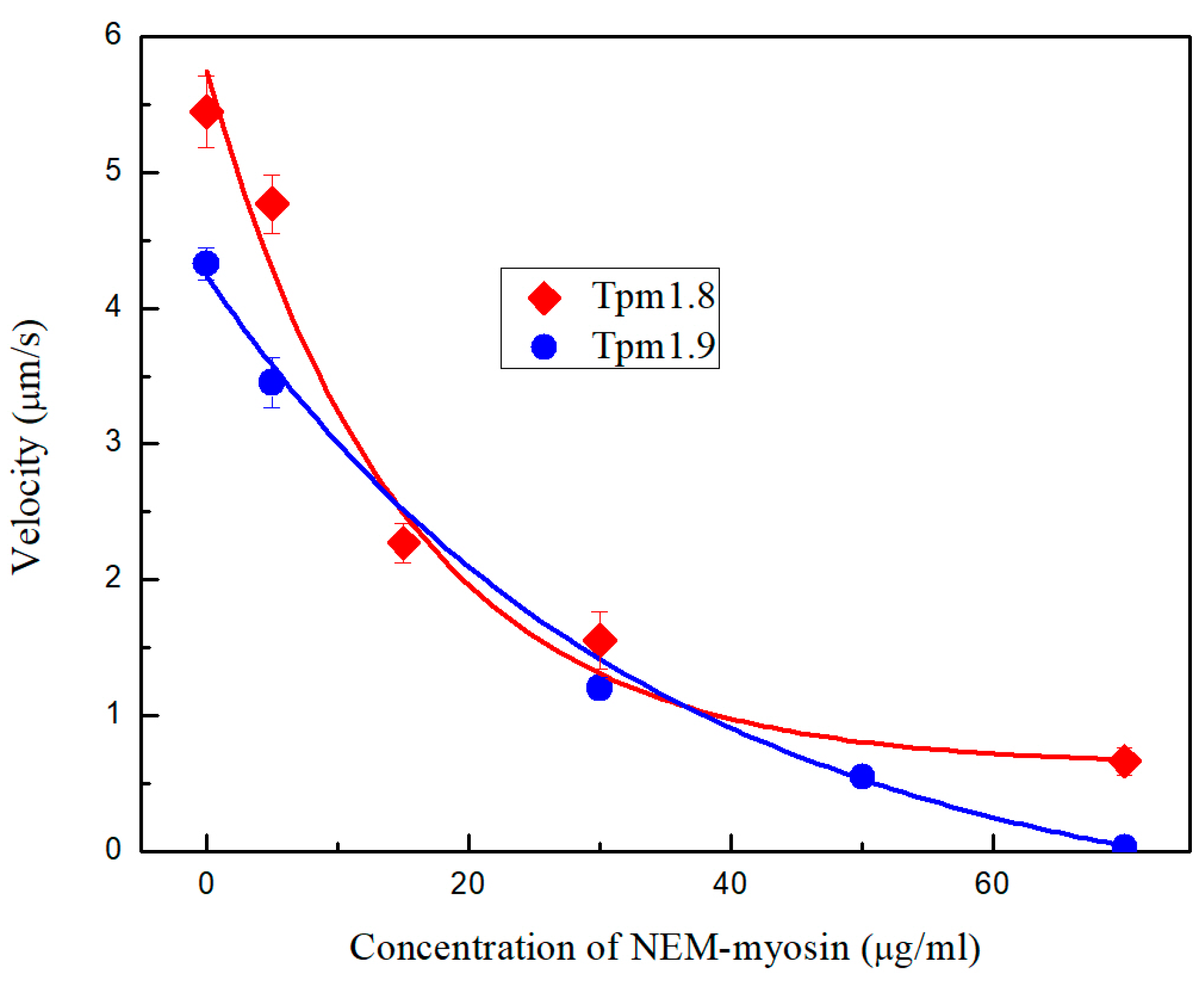
2.3.1. Load Measurements
2.3.2. Bending Stiffness
3. Discussion
3.1. Structural Properties of Tpm1.8 and Tpm1.9 Isoforms
3.2. Interaction between F-Actin and Tpm1.8 and Tpm1.9
3.3. Functional Peculiarities of Tpm1.8 and Tpm1.9 Complexes with Fibrillar Actin
3.4. Influence of Ala-Ser Extension on the Properties of Tpm1.8 and Tpm1.9 Isoforms
3.5. Possible Relationship between the Properties of Tpm1.8 and Tpm1.9 Isoforms and Their Functions in the Cell
4. Materials and Methods
4.1. Protein Preparations
4.2. Differential Scanning Calorimetry (DSC)
4.3. Viscosity Measurements
4.4. Measuring Tpm Affinity to F-Actin
4.5. Stability of Tpm–F-Actin Complexes Measured by Light Scattering
4.6. In Vitro Motility Assay with NEM-Myosin
4.7. Bending Stiffness Measurements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perrin, B.J.; Ervasti, J.M. The Actin Gene Family: Function Follows Isoform. Cytoskeleton 2010, 67, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, E.S.; Higgs, H.N. The Many Faces of Actin: Matching Assembly Factors with Cellular Structures. Nat. Cell Biol. 2007, 9, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Hardeman, E.C.; Bryce, N.S.; Gunning, P.W. Impact of the Actin Cytoskeleton on Cell Development and Function Mediated via Tropomyosin Isoforms. Semin. Cell Dev. Biol. 2020, 102, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Khaitlina, S.Y. Tropomyosin as a Regulator of Actin Dynamics. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 318, pp. 255–291. ISBN 978-0-12-802279-5. [Google Scholar]
- Vindin, H.; Gunning, P. Cytoskeletal Tropomyosins: Choreographers of Actin Filament Functional Diversity. J. Muscle Res. Cell Motil. 2013, 34, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Meiring, J.C.M.; Bryce, N.S.; Wang, Y.; Taft, M.H.; Manstein, D.J.; Liu Lau, S.; Stear, J.; Hardeman, E.C.; Gunning, P.W. Co-Polymers of Actin and Tropomyosin Account for a Major Fraction of the Human Actin Cytoskeleton. Curr. Biol. 2018, 28, 2331–2337.e5. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.M.; Arndt, K.M. Coiled Coil Domains: Stability, Specificity, and Biological Implications. ChemBioChem 2004, 5, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Nevzorov, I.A.; Levitsky, D.I. Tropomyosin: Double Helix from the Protein World. Biochemistry 2011, 76, 1507–1527. [Google Scholar] [CrossRef] [PubMed]
- Woolfson, D.N. Coiled-Coil Design: Updated and Upgraded. In Fibrous Proteins: Structures and Mechanisms; Parry, D.A.D., Squire, J.M., Eds.; Subcellular Biochemistry; Springer International Publishing: Cham, Switzerland, 2017; Volume 82, pp. 35–61. ISBN 978-3-319-49672-6. [Google Scholar]
- Lau, S.Y.; Taneja, A.K.; Hodges, R.S. Synthesis of a Model Protein of Defined Secondary and Quaternary Structure. Effect of Chain Length on the Stabilization and Formation of Two-Stranded Alpha-Helical Coiled-Coils. J. Biol. Chem. 1984, 259, 13253–13261. [Google Scholar] [CrossRef]
- Woolfson, D.N. The Design of Coiled-Coil Structures and Assemblies. In Advances in Protein Chemistry; Elsevier: Amsterdam, The Netherlands, 2005; Volume 70, pp. 79–112. ISBN 978-0-12-034270-9. [Google Scholar]
- Weber, A.; Pennise, C.R.; Fowler, V.M. Tropomodulin Increases the Critical Concentration of Barbed End-Capped Actin Filaments by Converting ADP·Pi-Actin to ADP-Actin at All Pointed Filament Ends. J. Biol. Chem. 1999, 274, 34637–34645. [Google Scholar] [CrossRef]
- Khaitlina, S.; Fitz, H.; Hinssen, H. The Interaction of Gelsolin with Tropomyosin Modulates Actin Dynamics. FEBS J. 2013, 280, 4600–4611. [Google Scholar] [CrossRef]
- Ostrowska, Z.; Robaszkiewicz, K.; Moraczewska, J. Regulation of Actin Filament Turnover by Cofilin-1 and Cytoplasmic Tropomyosin Isoforms. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2017, 1865, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Ono, K. Tropomyosin Inhibits ADF/Cofilin-Dependent Actin Filament Dynamics. J. Cell Biol. 2002, 156, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Brayford, S.; Bryce, N.S.; Schevzov, G.; Haynes, E.M.; Bear, J.E.; Hardeman, E.C.; Gunning, P.W. Tropomyosin Promotes Lamellipodial Persistence by Collaborating with Arp2/3 at the Leading Edge. Curr. Biol. 2016, 26, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Blanchoin, L.; Pollard, T.D.; Hitchcock-DeGregori, S.E. Inhibition of the Arp2/3 Complex-Nucleated Actin Polymerization and Branch Formation by Tropomyosin. Curr. Biol. 2001, 11, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Manstein, D.J.; Meiring, J.C.M.; Hardeman, E.C.; Gunning, P.W. Actin–Tropomyosin Distribution in Non-Muscle Cells. J. Muscle Res. Cell Motil. 2020, 41, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Gunning, P.; O’Neill, G.; Hardeman, E. Tropomyosin-Based Regulation of the Actin Cytoskeleton in Time and Space. Physiol. Rev. 2008, 88, 1–35. [Google Scholar] [CrossRef]
- Geeves, M.A.; Hitchcock-DeGregori, S.E.; Gunning, P.W. A Systematic Nomenclature for Mammalian Tropomyosin Isoforms. J. Muscle Res. Cell Motil. 2015, 36, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Moraczewska, J.; Nicholson-Flynn, K.; Hitchcock-DeGregori, S.E. The Ends of Tropomyosin Are Major Determinants of Actin Affinity and Myosin Subfragment 1-Induced Binding to F-Actin in the Open State. Biochemistry 1999, 38, 15885–15892. [Google Scholar] [CrossRef] [PubMed]
- Schevzov, G.; Whittaker, S.P.; Fath, T.; Lin, J.J.-C.; Gunning, P.W. Tropomyosin Isoforms and Reagents. BioArchitecture 2011, 1, 135–164. [Google Scholar] [CrossRef]
- Sui, Z.; Gokhin, D.S.; Nowak, R.B.; Guo, X.; An, X.; Fowler, V.M. Stabilization of F-Actin by Tropomyosin Isoforms Regulates the Morphology and Mechanical Behavior of Red Blood Cells. Mol. Biol. Cell 2017, 28, 2531–2542. [Google Scholar] [CrossRef]
- Sung, L.A.; Gao, K.M.; Yee, L.J.; Temm-Grove, C.J.; Helfman, D.M.; Lin, J.J.; Mehrpouryan, M. Tropomyosin Isoform 5b Is Expressed in Human Erythrocytes: Implications of Tropomodulin-TM5 or Tropomodulin-TM5b Complexes in the Protofilament and Hexagonal Organization of Membrane Skeletons. Blood 2000, 95, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Gokhin, D.S.; Fowler, V.M. Feisty Filaments: Actin Dynamics in the Red Blood Cell Membrane Skeleton. Curr. Opin. Hematol. 2016, 23, 206–214. [Google Scholar] [CrossRef]
- Fowler, V.M. The Human Erythrocyte Plasma Membrane. In Current Topics in Membranes; Elsevier: Amsterdam, The Netherlands, 2013; Volume 72, pp. 39–88. ISBN 978-0-12-417027-8. [Google Scholar]
- Lees, J.G.; Ching, Y.W.; Adams, D.H.; Bach, C.T.T.; Samuel, M.S.; Kee, A.J.; Hardeman, E.C.; Gunning, P.; Cowin, A.J.; O’Neill, G.M. Tropomyosin Regulates Cell Migration during Skin Wound Healing. J. Investig. Dermatol. 2013, 133, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Hook, J.; Lemckert, F.; Schevzov, G.; Fath, T.; Gunning, P. Functional Identity of the Gamma Tropomyosin Gene: Implications for Embryonic Development, Reproduction and Cell Viability. BioArchitecture 2011, 1, 49–59. [Google Scholar] [CrossRef]
- Yamashiro, S.; Gokhin, D.S.; Sui, Z.; Bergeron, S.E.; Rubenstein, P.A.; Fowler, V.M. Differential Actin-Regulatory Activities of Tropomodulin1 and Tropomodulin3 with Diverse Tropomyosin and Actin Isoforms. J. Biol. Chem. 2014, 289, 11616–11629. [Google Scholar] [CrossRef]
- Bareja, I.; Wioland, H.; Janco, M.; Nicovich, P.R.; Jégou, A.; Romet-Lemonne, G.; Walsh, J.; Böcking, T. Dynamics of Tpm1.8 Domains on Actin Filaments with Single-Molecule Resolution. Mol. Biol. Cell 2020, 31, 2452–2462. [Google Scholar] [CrossRef] [PubMed]
- Marchenko, M.A.; Nefedova, V.V.; Yampolskaya, D.S.; Borzova, V.A.; Kleymenov, S.Y.; Nabiev, S.R.; Nikitina, L.V.; Matyushenko, A.M.; Levitsky, D.I. Comparative Structural and Functional Studies of Low Molecular Weight Tropomyosin Isoforms, the TPM3 Gene Products. Arch. Biochem. Biophys. 2021, 710, 108999. [Google Scholar] [CrossRef]
- Nefedova, V.V.; Marchenko, M.A.; Kleymenov, S.Y.; Datskevich, P.N.; Levitsky, D.I.; Matyushenko, A.M. Thermal Unfolding of Various Human Non-Muscle Isoforms of Tropomyosin. Biochem. Biophys. Res. Commun. 2019, 514, 613–617. [Google Scholar] [CrossRef]
- Marchenko, M.; Nefedova, V.; Artemova, N.; Kleymenov, S.; Levitsky, D.; Matyushenko, A. Structural and Functional Peculiarities of Cytoplasmic Tropomyosin Isoforms, the Products of TPM1 and TPM4 Genes. Int. J. Mol. Sci. 2021, 22, 5141. [Google Scholar] [CrossRef]
- Temm-Grove, C.J.; Guo, W.; Helfman, D.M. Low Molecular Weight Rat Fibroblast Tropomyosin 5 (TM-5): cDNA Cloning, Actin-Binding, Localization, and Coiled-Coil Interactions. Cell Motil. Cytoskelet. 1996, 33, 223–240. [Google Scholar] [CrossRef]
- Greenfield, N.J.; Huang, Y.J.; Palm, T.; Swapna, G.V.T.; Monleon, D.; Montelione, G.T.; Hitchcock-DeGregori, S.E. Solution NMR Structure and Folding Dynamics of the N Terminus of a Rat Non-Muscle α-Tropomyosin in an Engineered Chimeric Protein. J. Mol. Biol. 2001, 312, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Palm, T.; Greenfield, N.J.; Hitchcock-DeGregori, S.E. Tropomyosin Ends Determine the Stability and Functionality of Overlap and Troponin T Complexes. Biophys. J. 2003, 84, 3181–3189. [Google Scholar] [CrossRef] [PubMed]
- Nevzorov, I.A.; Nikolaeva, O.P.; Kainov, Y.A.; Redwood, C.S.; Levitsky, D.I. Conserved Noncanonical Residue Gly-126 Confers Instability to the Middle Part of the Tropomyosin Molecule. J. Biol. Chem. 2011, 286, 15766–15772. [Google Scholar] [CrossRef] [PubMed]
- Sumida, J.P.; Wu, E.; Lehrer, S.S. Conserved Asp-137 Imparts Flexibility to Tropomyosin and Affects Function. J. Biol. Chem. 2008, 283, 6728–6734. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H.; Kim, K.-H.; Jun, G.; Greenfield, N.J.; Dominguez, R.; Volkmann, N.; Hitchcock-DeGregori, S.E.; Cohen, C. Deciphering the Design of the Tropomyosin Molecule. Proc. Natl. Acad. Sci. USA 2001, 98, 8496–8501. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H. Local Structural Changes in Tropomyosin Detected by a Trypsin-Probe Method. Biochemistry 1984, 23, 4791–4798. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H.; Zhou, Z.; Reshetnikova, L.; Robinson, H.; Yammani, R.D.; Tobacman, L.S.; Cohen, C. Structure of the Mid-Region of Tropomyosin: Bending and Binding Sites for Actin. Proc. Natl. Acad. Sci. USA 2005, 102, 18878–18883. [Google Scholar] [CrossRef] [PubMed]
- Kremneva, E.; Nikolaeva, O.; Maytum, R.; Arutyunyan, A.M.; Kleimenov, S.Y.; Geeves, M.A.; Levitsky, D.I. Thermal Unfolding of Smooth Muscle and Nonmuscle Tropomyosin A-homodimers with Alternatively Spliced Exons. FEBS J. 2006, 273, 588–600. [Google Scholar] [CrossRef]
- Matyushenko, A.M.; Koubassova, N.A.; Shchepkin, D.V.; Kopylova, G.V.; Nabiev, S.R.; Nikitina, L.V.; Bershitsky, S.Y.; Levitsky, D.I.; Tsaturyan, A.K. The Effects of Cardiomyopathy-Associated Mutations in the Head-to-Tail Overlap Junction of α-Tropomyosin on Its Properties and Interaction with Actin. Int. J. Biol. Macromol. 2019, 125, 1266–1274. [Google Scholar] [CrossRef]
- Moraczewska, J.; Robaszkiewicz, K.; Śliwinska, M.; Czajkowska, M.; Ly, T.; Kostyukova, A.; Wen, H.; Zheng, W. Congenital Myopathy-related Mutations in Tropomyosin Disrupt Regulatory Function through Altered Actin Affinity and Tropomodulin Binding. FEBS J. 2019, 286, 1877–1893. [Google Scholar] [CrossRef]
- Zheng, W.; Hitchcock-DeGregori, S.E.; Barua, B. Investigating the Effects of Tropomyosin Mutations on Its Flexibility and Interactions with Filamentous Actin Using Molecular Dynamics Simulation. J. Muscle Res. Cell Motil. 2016, 37, 131–147. [Google Scholar] [CrossRef]
- Ly, S.; Lehrer, S.S. Long-Range Effects of Familial Hypertrophic Cardiomyopathy Mutations E180G and D175N on the Properties of Tropomyosin. Biochemistry 2012, 51, 6413–6420. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.A.; Brooker, H.R.; Gyamfi, I.; O’Brien, J.; Ashley, B.; Brazier, J.E.; Dean, A.; Embling, J.; Grimsey, E.; Tomlinson, A.C.; et al. Temperature Sensitive Point Mutations in Fission Yeast Tropomyosin Have Long Range Effects on the Stability and Function of the Actin-Tropomyosin Copolymer. Biochem. Biophys. Res. Commun. 2018, 506, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Janco, M.; Bonello, T.T.; Byun, A.; Coster, A.C.F.; Lebhar, H.; Dedova, I.; Gunning, P.W.; Böcking, T. The Impact of Tropomyosins on Actin Filament Assembly Is Isoform Specific. BioArchitecture 2016, 6, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock-DeGregori, S.E.; Heald, R.W. Altered Actin and Troponin Binding of Amino-Terminal Variants of Chicken Striated Muscle Alpha-Tropomyosin Expressed in Escherichia Coli. J. Biol. Chem. 1987, 262, 9730–9735. [Google Scholar] [CrossRef]
- Monteiro, P.B.; Lataro, R.C.; Ferro, J.A.; Reinach, F.d.C. Functional Alpha-Tropomyosin Produced in Escherichia Coli. A Dipeptide Extension Can Substitute the Amino-Terminal Acetyl Group. J. Biol. Chem. 1994, 269, 10461–10466. [Google Scholar] [CrossRef]
- Carman, P.J.; Barrie, K.R.; Dominguez, R. Novel Human Cell Expression Method Reveals the Role and Prevalence of Posttranslational Modification in Nonmuscle Tropomyosins. J. Biol. Chem. 2021, 297, 101154. [Google Scholar] [CrossRef]
- Coulton, A.T.; East, D.A.; Galinska-Rakoczy, A.; Lehman, W.; Mulvihill, D.P. The Recruitment of Acetylated and Unacetylated Tropomyosin to Distinct Actin Polymers Permits the Discrete Regulation of Specific Myosins in Fission Yeast. J. Cell Sci. 2010, 123, 3235–3243. [Google Scholar] [CrossRef]
- Kostyukova, A.S.; Hitchcock-DeGregori, S.E. Effect of the Structure of the N Terminus of Tropomyosin on Tropomodulin Function. J. Biol. Chem. 2004, 279, 5066–5071. [Google Scholar] [CrossRef]
- Reindl, T.; Giese, S.; Greve, J.N.; Reinke, P.Y.; Chizhov, I.; Latham, S.L.; Mulvihill, D.P.; Taft, M.H.; Manstein, D.J. Distinct Actin–Tropomyosin Cofilament Populations Drive the Functional Diversification of Cytoskeletal Myosin Motor Complexes. iScience 2022, 25, 104484. [Google Scholar] [CrossRef]
- Gateva, G.; Kremneva, E.; Reindl, T.; Kotila, T.; Kogan, K.; Gressin, L.; Gunning, P.W.; Manstein, D.J.; Michelot, A.; Lappalainen, P. Tropomyosin Isoforms Specify Functionally Distinct Actin Filament Populations In Vitro. Curr. Biol. 2017, 27, 705–713. [Google Scholar] [CrossRef]
- Cagigas, M.L.; Bryce, N.S.; Ariotti, N.; Brayford, S.; Gunning, P.W.; Hardeman, E.C. Correlative Cryo-ET Identifies Actin/Tropomyosin Filaments That Mediate Cell–Substrate Adhesion in Cancer Cells and Mechanosensitivity of Cell Proliferation. Nat. Mater. 2022, 21, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Verhagen, M.P.; Teeuwssen, M.; Sun, W.; Joosten, R.; Sacchetti, A.; Ewing-Graham, P.C.; Jansen, M.P.H.M.; Boere, I.A.; Bryce, N.S.; et al. Tropomyosin1 Isoforms Underlie Epithelial to Mesenchymal Plasticity, Metastatic Dissemination, and Resistance to Chemotherapy in High-Grade Serous Ovarian Cancer. Cell Death Differ. 2024, 31, 360–377. [Google Scholar] [CrossRef] [PubMed]
- Matyushenko, A.M.; Artemova, N.V.; Shchepkin, D.V.; Kopylova, G.V.; Bershitsky, S.Y.; Tsaturyan, A.K.; Sluchanko, N.N.; Levitsky, D.I. Structural and Functional Effects of Two Stabilizing Substitutions, D137L and G126R, in the Middle Part of A-tropomyosin Molecule. FEBS J. 2014, 281, 2004–2016. [Google Scholar] [CrossRef] [PubMed]
- Pardee, J.D.; Aspudich, J. [18] Purification of Muscle Actin. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1982; Volume 85, pp. 164–181. ISBN 978-0-12-181985-9. [Google Scholar]
- Freire, E.; Biltonen, R.L. Statistical Mechanical Deconvolution of Thermal Transitions in Macromolecules. I. Theory and Application to Homogeneous Systems. Biopolymers 1978, 17, 463–479. [Google Scholar] [CrossRef]
- Haeberle, J.R.; Trybus, K.M.; Hemric, M.E.; Warshaw, D.M. The Effects of Smooth Muscle Caldesmon on Actin Filament Motility. J. Biol. Chem. 1992, 267, 23001–23006. [Google Scholar] [CrossRef] [PubMed]
- Shchepkin, D.V.; Nabiev, S.R.; Kopylova, G.V.; Matyushenko, A.M.; Levitsky, D.I.; Bershitsky, S.Y.; Tsaturyan, A.K. Cooperativity of Myosin Interaction with Thin Filaments Is Enhanced by Stabilizing Substitutions in Tropomyosin. J. Muscle Res. Cell Motil. 2017, 38, 183–191. [Google Scholar] [CrossRef]
- Nabiev, S.R.; Ovsyannikov, D.A.; Kopylova, G.V.; Shchepkin, D.V.; Matyushenko, A.M.; Koubassova, N.A.; Levitsky, D.I.; Tsaturyan, A.K.; Bershitsky, S.Y. Stabilizing the Central Part of Tropomyosin Increases the Bending Stiffness of the Thin Filament. Biophys. J. 2015, 109, 373–379. [Google Scholar] [CrossRef]

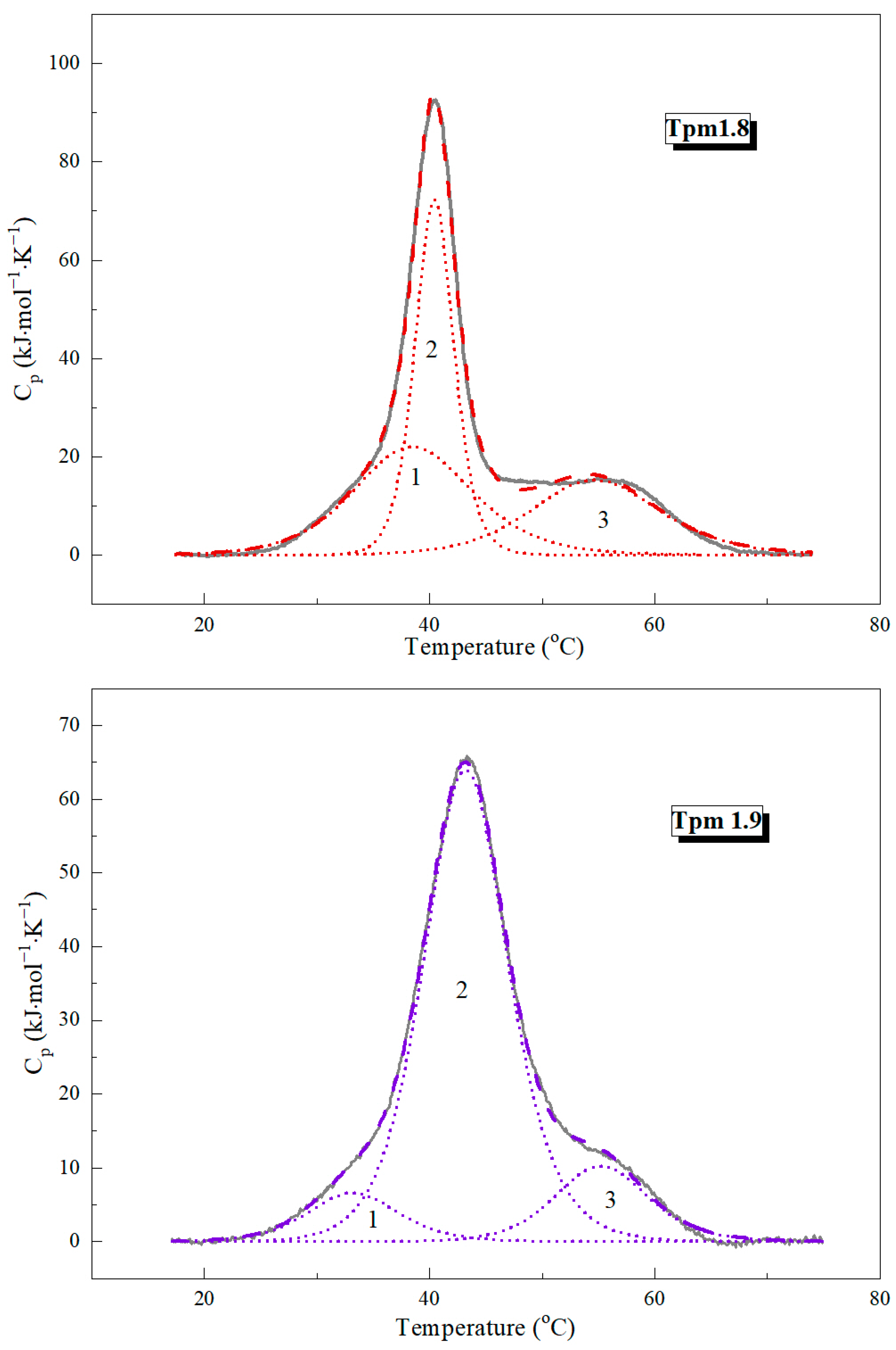

| Tpm Isoforms | Tm (°C) | ΔHcal * (kJ∙mol−1) | ΔHcal (% of Total) | Total ΔHcal (kJ∙mol−1) |
|---|---|---|---|---|
| Tpm1.8 | 874 | |||
| Domain 1 | 38.6 ± 0.09 | 310 | 35 | |
| Domain 2 | 40.4 ± 0.01 | 332 | 38 | |
| Domain 3 | 54.8 ± 0.08 | 232 | 27 | |
| Tpm1.9 | 825 | |||
| Domain 1 | 33.2 ± 0.10 | 70 | 8 | |
| Domain 2 | 43.3 ± 0.02 | 640 | 78 | |
| Domain 3 | 55.3 ± 0.05 | 115 | 14 |
| Sample Name | Bending Stiffness, K∙1026N∙m2 (IQT, N) |
|---|---|
| F-actin | 2.35 (1.6–5.3, 18) |
| F-actin + Tpm1.8 | 3.75 (2.7–5.55, 22) *# |
| F-actin + AS-Tpm1.8 | 8.1 (6.2–10.25, 17) * |
| F-actin + Tpm1.9 | 4.8 (3.87–6.95, 23) *# |
| F-actin + AS-Tpm1.9 | 4.7 (4–7.4, 23) *# |
| Primer Name | Primer Sequence |
|---|---|
| Tpm1.12 fw | 5′ATATATACATATGGCTGGAAGTAGCTCACTTGAAGC 3′ |
| AS-Tpm1.12 fw | 5′ATATATACATATGGCTAGCATGGCTGGAAGTAGCTCACTTGAAGC 3′ |
| Tpm1.8/1.9 mid fw | 5′ GAGAGAGGCATGAAAGTCATTGAGAGTCG 3′ |
| Tpm1.8/1.9 mid rev | 5′ CGACTCTCAATGACTTTCATGCCTCTCTC 3′ |
| Tpm1.9 rev | 5′ATATATAGAATTCTTACATGTTGTTCAACTCCAGTAAAGTC 3′ |
| Tpm1.8 rev | 5′ ATATATAGAATTCTTACATGTTGTTTAACTCCAGTAAAGTCTG 3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapshina, K.K.; Nefedova, V.V.; Nabiev, S.R.; Roman, S.G.; Shchepkin, D.V.; Kopylova, G.V.; Kochurova, A.M.; Beldiia, E.A.; Kleymenov, S.Y.; Levitsky, D.I.; et al. Functional and Structural Properties of Cytoplasmic Tropomyosin Isoforms Tpm1.8 and Tpm1.9. Int. J. Mol. Sci. 2024, 25, 6873. https://doi.org/10.3390/ijms25136873
Lapshina KK, Nefedova VV, Nabiev SR, Roman SG, Shchepkin DV, Kopylova GV, Kochurova AM, Beldiia EA, Kleymenov SY, Levitsky DI, et al. Functional and Structural Properties of Cytoplasmic Tropomyosin Isoforms Tpm1.8 and Tpm1.9. International Journal of Molecular Sciences. 2024; 25(13):6873. https://doi.org/10.3390/ijms25136873
Chicago/Turabian StyleLapshina, Ksenia K., Victoria V. Nefedova, Salavat R. Nabiev, Svetlana G. Roman, Daniil V. Shchepkin, Galina V. Kopylova, Anastasia M. Kochurova, Evgenia A. Beldiia, Sergey Y. Kleymenov, Dmitrii I. Levitsky, and et al. 2024. "Functional and Structural Properties of Cytoplasmic Tropomyosin Isoforms Tpm1.8 and Tpm1.9" International Journal of Molecular Sciences 25, no. 13: 6873. https://doi.org/10.3390/ijms25136873
APA StyleLapshina, K. K., Nefedova, V. V., Nabiev, S. R., Roman, S. G., Shchepkin, D. V., Kopylova, G. V., Kochurova, A. M., Beldiia, E. A., Kleymenov, S. Y., Levitsky, D. I., & Matyushenko, A. M. (2024). Functional and Structural Properties of Cytoplasmic Tropomyosin Isoforms Tpm1.8 and Tpm1.9. International Journal of Molecular Sciences, 25(13), 6873. https://doi.org/10.3390/ijms25136873






