Lipid Droplet–Mitochondria Contacts in Health and Disease
Abstract
1. Introduction
2. Basics of LDs
2.1. LDs’ Structural Characteristics
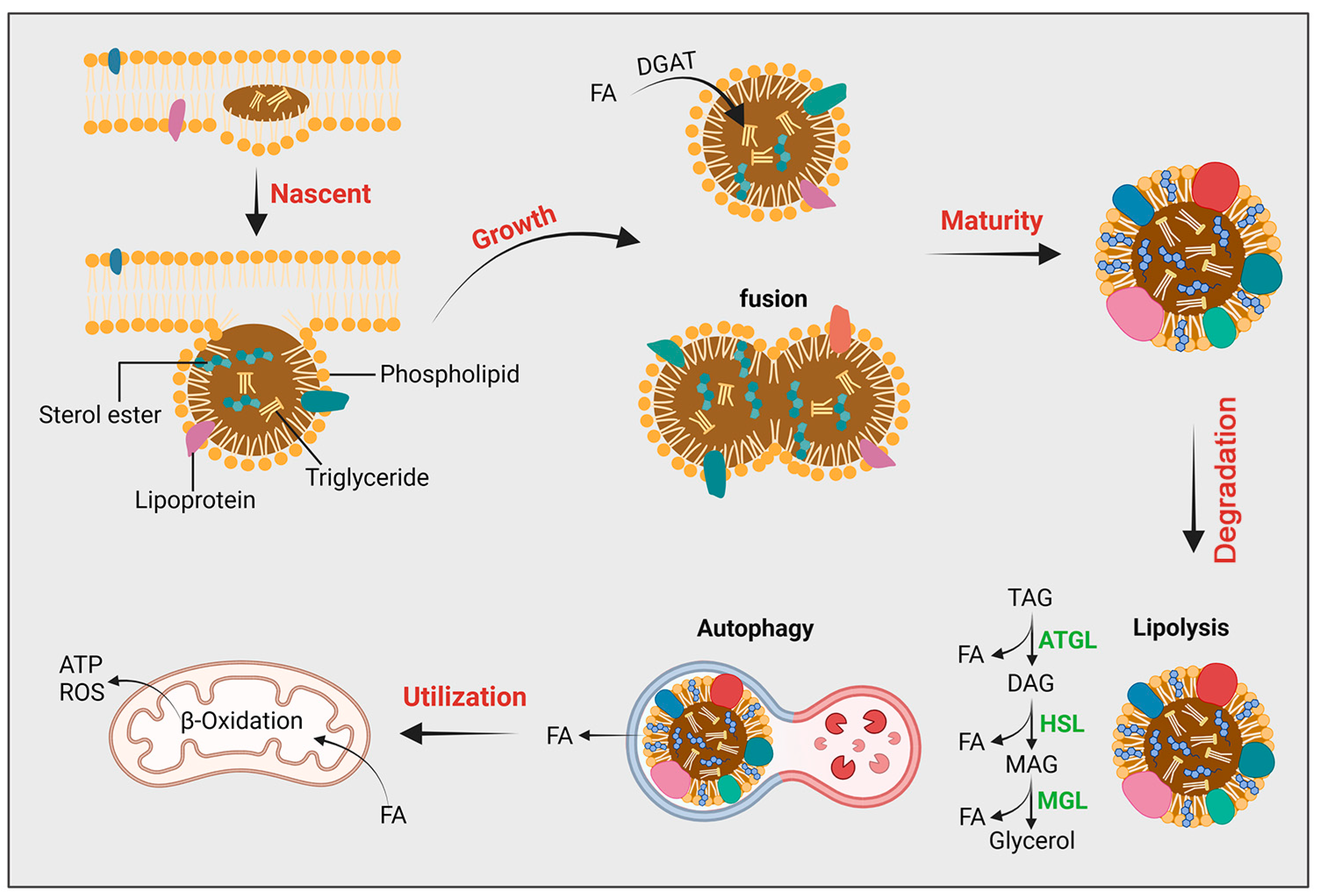
2.2. LDs’ Formation and Growth
2.3. LDs’ Functional Diversity
3. Interplay between LDs and Mitochondria
3.1. Peridroplet Mitochondria
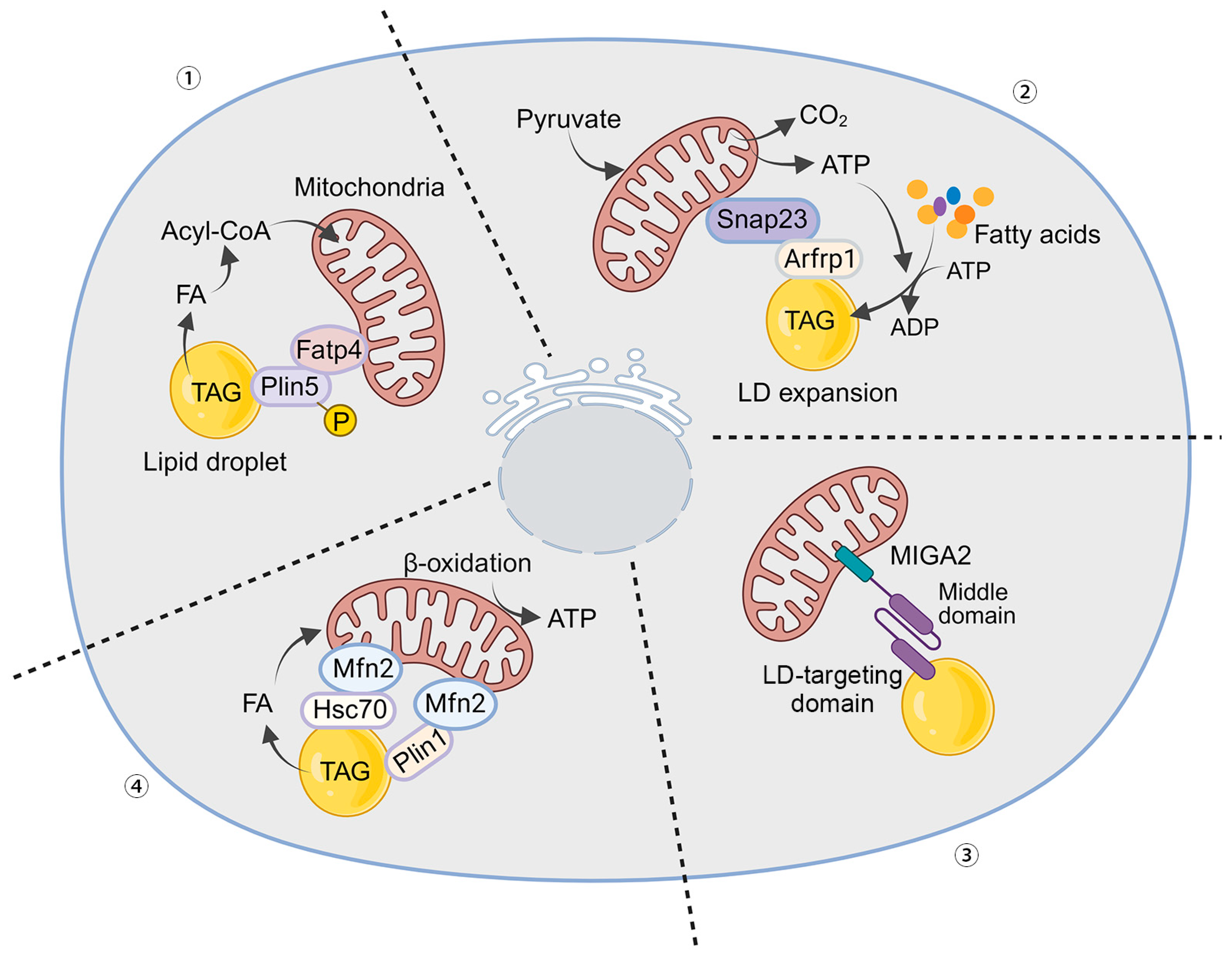
3.2. Mechanisms of LD–Mitochondria Contacts
4. Physiological Relevance of LD–Mitochondria Contacts
4.1. Role in Lipid Transfer and Fatty Acid Oxidation
4.2. Influence on Cellular Energy Homeostasis
4.3. Impact on Oxidative Stress and Redox Signaling
4.4. Role in Promoting Mitophagy
5. Human Diseases Associated with Alterations in LD–Mitochondria Interaction
5.1. Obesity and Type 2 Diabetes
5.2. Hepatic Lipotoxicity
5.3. Skeletal Muscle Exercise Tolerance
5.4. Cardiovascular Diseases (CVDs)
5.5. Virus Replication
5.6. Neurodegenerative Diseases
6. Therapeutic Perspectives
7. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Ouyang, Q.; Chen, Q.; Ke, S.; Ding, L.; Yang, X.; Rong, P.; Feng, W.; Cao, Y.; Wang, Q.; Li, M.; et al. Rab8a as a mitochondrial receptor for lipid droplets in skeletal muscle. Dev. Cell 2023, 58, 289–305.e6. [Google Scholar] [CrossRef] [PubMed]
- Mece, O.; Houbaert, D.; Sassano, M.L.; Durre, T.; Maes, H.; Schaaf, M.; More, S.; Ganne, M.; Garcia-Caballero, M.; Borri, M.; et al. Lipid droplet degradation by autophagy connects mitochondria metabolism to Prox1-driven expression of lymphatic genes and lymphangiogenesis. Nat. Commun. 2022, 13, 2760. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, P.K.; Shivaiah, K.K.; Espinoza-Corral, R. Lipid droplets throughout the evolutionary tree. Prog. Lipid Res. 2020, 78, 101029. [Google Scholar] [CrossRef] [PubMed]
- Guzha, A.; Whitehead, P.; Ischebeck, T.; Chapman, K.D. Lipid Droplets: Packing Hydrophobic Molecules within the Aqueous Cytoplasm. Annu. Rev. Plant Biol. 2023, 74, 195–223. [Google Scholar] [CrossRef] [PubMed]
- Zadoorian, A.; Du, X.; Yang, H. Lipid droplet biogenesis and functions in health and disease. Nat. Rev. Endocrinol. 2023, 19, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Mitrofanova, A.; Merscher, S.; Fornoni, A. Kidney lipid dysmetabolism and lipid droplet accumulation in chronic kidney disease. Nat. Rev. Nephrol. 2023, 19, 629–645. [Google Scholar] [CrossRef] [PubMed]
- Seibert, J.T.; Najt, C.P.; Heden, T.D.; Mashek, D.G.; Chow, L.S. Muscle Lipid Droplets: Cellular Signaling to Exercise Physiology and Beyond. Trends Endocrinol. Metab. 2020, 31, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhao, Z.; Yu, Z.; Chen, L.; Jin, Y.; Wu, J.; Ren, Z. Application of synthetic lipid droplets in metabolic diseases. Clin. Transl. Med. 2023, 13, e1441. [Google Scholar] [CrossRef] [PubMed]
- Monzel, A.S.; Enriquez, J.A.; Picard, M. Multifaceted mitochondria: Moving mitochondrial science beyond function and dysfunction. Nat. Metab. 2023, 5, 546–562. [Google Scholar] [CrossRef]
- Akbari, M.; Kirkwood, T.B.L.; Bohr, V.A. Mitochondria in the signaling pathways that control longevity and health span. Ageing Res. Rev. 2019, 54, 100940. [Google Scholar] [CrossRef]
- Benador, I.Y.; Veliova, M.; Liesa, M.; Shirihai, O.S. Mitochondria Bound to Lipid Droplets: Where Mitochondrial Dynamics Regulate Lipid Storage and Utilization. Cell Metab. 2019, 29, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Talari, N.K.; Mattam, U.; Meher, N.K.; Paripati, A.K.; Mahadev, K.; Krishnamoorthy, T.; Sepuri, N.B.V. Lipid-droplet associated mitochondria promote fatty-acid oxidation through a distinct bioenergetic pattern in male Wistar rats. Nat. Commun. 2023, 14, 766. [Google Scholar] [CrossRef] [PubMed]
- Guyard, V.; Monteiro-Cardoso, V.F.; Omrane, M.; Sauvanet, C.; Houcine, A.; Boulogne, C.; Ben Mbarek, K.; Vitale, N.; Faklaris, O.; El Khallouki, N.; et al. ORP5 and ORP8 orchestrate lipid droplet biogenesis and maintenance at ER-mitochondria contact sites. J. Cell Biol. 2022, 221, e202112107. [Google Scholar] [CrossRef] [PubMed]
- Afonso, M.B.; Islam, T.; Magusto, J.; Amorim, R.; Lenoir, V.; Simoes, R.F.; Teixeira, J.; Silva, L.C.; Wendum, D.; Jeru, I.; et al. RIPK3 dampens mitochondrial bioenergetics and lipid droplet dynamics in metabolic liver disease. Hepatology 2023, 77, 1319–1334. [Google Scholar] [CrossRef] [PubMed]
- Miner, G.E.; So, C.M.; Edwards, W.; Ragusa, J.V.; Wine, J.T.; Wong Gutierrez, D.; Airola, M.V.; Herring, L.E.; Coleman, R.A.; Klett, E.L.; et al. PLIN5 interacts with FATP4 at membrane contact sites to promote lipid droplet-to-mitochondria fatty acid transport. Dev. Cell 2023, 58, 1250–1265.e6. [Google Scholar] [CrossRef] [PubMed]
- Freyre, C.A.C.; Rauher, P.C.; Ejsing, C.S.; Klemm, R.W. MIGA2 Links Mitochondria, the ER, and Lipid Droplets and Promotes De Novo Lipogenesis in Adipocytes. Mol. Cell 2019, 76, 811–825.e14. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Qi, G.; Vitali, F.; Shang, Y.; Raikes, A.C.; Wang, T.; Jin, Y.; Brinton, R.D.; Gu, H.; Yin, F. Loss of fatty acid degradation by astrocytic mitochondria triggers neuroinflammation and neurodegeneration. Nat. Metab. 2023, 5, 445–465. [Google Scholar] [CrossRef] [PubMed]
- Markussen, L.K.; Rondini, E.A.; Johansen, O.S.; Madsen, J.G.S.; Sustarsic, E.G.; Marcher, A.B.; Hansen, J.B.; Gerhart-Hines, Z.; Granneman, J.G.; Mandrup, S. Lipolysis regulates major transcriptional programs in brown adipocytes. Nat. Commun. 2022, 13, 3956. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; McWilliams, T.G. Lipid droplets promote efficient mitophagy. Autophagy 2023, 19, 724–725. [Google Scholar] [CrossRef]
- Cheng, X.; Geng, F.; Pan, M.; Wu, X.; Zhong, Y.; Wang, C.; Tian, Z.; Cheng, C.; Zhang, R.; Puduvalli, V.; et al. Targeting DGAT1 Ameliorates Glioblastoma by Increasing Fat Catabolism and Oxidative Stress. Cell Metab. 2020, 32, 229–242.e8. [Google Scholar] [CrossRef]
- Lin, C.; Chen, J.; Hu, M.; Zheng, W.; Song, Z.; Qin, H. Sesamol promotes browning of white adipocytes to ameliorate obesity by inducing mitochondrial biogenesis and inhibition mitophagy via beta3-AR/PKA signaling pathway. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef] [PubMed]
- Valent, A.M.; Choi, H.; Kolahi, K.S.; Thornburg, K.L. Hyperglycemia and gestational diabetes suppress placental glycolysis and mitochondrial function and alter lipid processing. FASEB J. 2021, 35, e21423. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Herker, E.; Vieyres, G.; Beller, M.; Krahmer, N.; Bohnert, M. Lipid Droplet Contact Sites in Health and Disease. Trends Cell Biol. 2021, 31, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Robichaud, S.; Fairman, G.; Vijithakumar, V.; Mak, E.; Cook, D.P.; Pelletier, A.R.; Huard, S.; Vanderhyden, B.C.; Figeys, D.; Lavallee-Adam, M.; et al. Identification of novel lipid droplet factors that regulate lipophagy and cholesterol efflux in macrophage foam cells. Autophagy 2021, 17, 3671–3689. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.A.; Olzmann, J.A. Protein Quality Control and Lipid Droplet Metabolism. Annu. Rev. Cell Dev. Biol. 2020, 36, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Farese, R.V., Jr.; Walther, T.C. Glycerolipid Synthesis and Lipid Droplet Formation in the Endoplasmic Reticulum. Cold Spring Harb. Perspect. Biol. 2023, 15, a041246. [Google Scholar] [CrossRef] [PubMed]
- Thiam, A.R.; Ikonen, E. Lipid Droplet Nucleation. Trends Cell Biol. 2021, 31, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Yue, F.; Oprescu, S.N.; Qiu, J.; Gu, L.; Zhang, L.; Chen, J.; Narayanan, N.; Deng, M.; Kuang, S. Lipid droplet dynamics regulate adult muscle stem cell fate. Cell Rep. 2022, 38, 110267. [Google Scholar] [CrossRef]
- Najt, C.P.; Khan, S.A.; Heden, T.D.; Witthuhn, B.A.; Perez, M.; Heier, J.L.; Mead, L.E.; Franklin, M.P.; Karanja, K.K.; Graham, M.J.; et al. Lipid Droplet-Derived Monounsaturated Fatty Acids Traffic via PLIN5 to Allosterically Activate SIRT1. Mol. Cell 2020, 77, 810–824.e8. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, M.; Qi, B.; Peng, T.; Liu, M.; Li, Z.; Fu, F.; Guo, Y.; Li, C.; Wang, Y.; et al. Lipid overload-induced RTN3 activation leads to cardiac dysfunction by promoting lipid droplet biogenesis. Cell Death Differ. 2023, 31, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Riella, C.V.; Chung, H.; Shah, S.S.; Wang, M.; Magraner, J.M.; Ribas, G.T.; Ribas, H.T.; Zhang, J.Y.; Alper, S.L.; et al. DGAT2 Inhibition Potentiates Lipid Droplet Formation To Reduce Cytotoxicity in APOL1 Kidney Risk Variants. J. Am. Soc. Nephrol. 2022, 33, 889–907. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.L.S.; Barreto, E.A.; Fazolini, N.P.B.; Viola, J.P.B.; Bozza, P.T. Lipid droplets: Platforms with multiple functions in cancer hallmarks. Cell Death Dis. 2020, 11, 105. [Google Scholar] [CrossRef]
- Zhou, L.; Song, Z.; Hu, J.; Liu, L.; Hou, Y.; Zhang, X.; Yang, X.; Chen, K. ACSS3 represses prostate cancer progression through downregulating lipid droplet-associated protein PLIN3. Theranostics 2021, 11, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, F.; Zeigerer, A.; Kory, N.; Krahmer, N. Hepatic lipid droplet homeostasis and fatty liver disease. Semin. Cell Dev. Biol. 2020, 108, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Han, Y.; Rodriguez Sillke, Y.; Deng, H.; Siddiqui, S.; Treese, C.; Schmidt, F.; Friedrich, M.; Keye, J.; Wan, J.; et al. Lipid droplet-dependent fatty acid metabolism controls the immune suppressive phenotype of tumor-associated macrophages. EMBO Mol. Med. 2019, 11, e10698. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Hu, B.; Li, J.; Chen, R.; Yuan, Z.; Xiao, H.; Chang, H.; Jiu, Y.; Cai, K.; Ding, B. Coronaviral ORF6 protein mediates inter-organelle contacts and modulates host cell lipid flux for virus production. EMBO J. 2023, 42, e112542. [Google Scholar] [CrossRef] [PubMed]
- Veliova, M.; Petcherski, A.; Liesa, M.; Shirihai, O.S. The biology of lipid droplet-bound mitochondria. Semin. Cell Dev. Biol. 2020, 108, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Benador, I.Y.; Veliova, M.; Mahdaviani, K.; Petcherski, A.; Wikstrom, J.D.; Assali, E.A.; Acin-Perez, R.; Shum, M.; Oliveira, M.F.; Cinti, S.; et al. Mitochondria Bound to Lipid Droplets Have Unique Bioenergetics, Composition, and Dynamics that Support Lipid Droplet Expansion. Cell Metab. 2018, 27, 869–885.e6. [Google Scholar] [CrossRef]
- Herms, A.; Bosch, M.; Reddy, B.J.; Schieber, N.L.; Fajardo, A.; Ruperez, C.; Fernandez-Vidal, A.; Ferguson, C.; Rentero, C.; Tebar, F.; et al. AMPK activation promotes lipid droplet dispersion on detyrosinated microtubules to increase mitochondrial fatty acid oxidation. Nat. Commun. 2015, 6, 7176. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Louie, S.M.; Daniele, J.R.; Tran, Q.; Dillin, A.; Zoncu, R.; Nomura, D.K.; Olzmann, J.A. DGAT1-Dependent Lipid Droplet Biogenesis Protects Mitochondrial Function during Starvation-Induced Autophagy. Dev. Cell 2017, 42, 9–21.e5. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Mirza, A.H.; Zhang, S.; Liang, B.; Liu, P. Lipid droplets and mitochondria are anchored during brown adipocyte differentiation. Protein Cell 2019, 10, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, S.; Cui, L.; Wang, W.; Na, H.; Zhu, X.; Li, L.; Xu, G.; Yang, F.; Christian, M.; et al. Lipid droplet remodeling and interaction with mitochondria in mouse brown adipose tissue during cold treatment. Biochim. Biophys. Acta 2015, 1853, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Bosma, M. Lipid droplet dynamics in skeletal muscle. Exp. Cell Res. 2016, 340, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Luo, S.; Yang, J.; Chen, W.; He, L.; Liu, D.; Zhao, L.; Wang, X. Lipid droplet—Mitochondria coupling: A novel lipid metabolism regulatory hub in diabetic nephropathy. Front. Endocrinol. 2022, 13, 1017387. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Tang, D.; Qi, B.; Guo, D.; Wang, Y.; Geng, J.; Zhang, X.; Song, L.; Chang, P.; Chen, W.; et al. Mfn2/Hsc70 Complex Mediates the Formation of Mitochondria-Lipid Droplets Membrane Contact and Regulates Myocardial Lipid Metabolism. Adv. Sci. 2024, 11, e2307749. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Montejano, V.I.; Yang, C.; Hahner, L.; McAfee, J.L.; Johnson, J.A.; Holland, W.L.; Fernandez-Valdivia, R.; Bickel, P.E. Perilipin 5 links mitochondrial uncoupled respiration in brown fat to healthy white fat remodeling and systemic glucose tolerance. Nat. Commun. 2021, 12, 3320. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhao, M.; He, X.; Xue, R.; Li, D.; Yu, X.; Wang, S.; Zang, W. Acetylcholine reduces palmitate-induced cardiomyocyte apoptosis by promoting lipid droplet lipolysis and perilipin 5-mediated lipid droplet-mitochondria interaction. Cell Cycle 2021, 20, 1890–1906. [Google Scholar] [CrossRef] [PubMed]
- Kien, B.; Kolleritsch, S.; Kunowska, N.; Heier, C.; Chalhoub, G.; Tilp, A.; Wolinski, H.; Stelzl, U.; Haemmerle, G. Lipid droplet-mitochondria coupling via perilipin 5 augments respiratory capacity but is dispensable for FA oxidation. J. Lipid Res. 2022, 63, 100172. [Google Scholar] [CrossRef]
- Cui, L.; Liu, P. Two Types of Contact Between Lipid Droplets and Mitochondria. Front. Cell Dev. Biol. 2020, 8, 618322. [Google Scholar] [CrossRef]
- Zhao, T.; Tan, X.Y.; Pantopoulos, K.; Xu, J.J.; Zheng, H.; Xu, Y.C.; Song, Y.F.; Luo, Z. miR-20a-5p targeting mfn2-mediated mitochondria-lipid droplet contacts regulated differential changes in hepatic lipid metabolism induced by two Mn sources in yellow catfish. J. Hazard. Mater. 2024, 462, 132749. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.S.; Palovics, R.; Munson, C.N.; Long, C.; Johansson, P.K.; Yip, O.; Dong, W.; Rawat, E.; West, E.; Schlachetzki, J.C.M.; et al. APOE4/4 is linked to damaging lipid droplets in Alzheimer’s disease microglia. Nature 2024, 628, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Liu, Y.; Zhang, W.; Nie, R.; Ling, Y.; Zhang, B.; Zhang, H.; Wu, C. CircDOCK7 facilitates the proliferation and adipogenic differentiation of chicken abdominal preadipocytes through the gga-miR-301b-3p/ACSL1 axis. J. Anim. Sci. Biotechnol. 2023, 14, 91. [Google Scholar] [CrossRef]
- Huh, J.Y.; Reilly, S.M.; Abu-Odeh, M.; Murphy, A.N.; Mahata, S.K.; Zhang, J.; Cho, Y.; Seo, J.B.; Hung, C.W.; Green, C.R.; et al. TANK-Binding Kinase 1 Regulates the Localization of Acyl-CoA Synthetase ACSL1 to Control Hepatic Fatty Acid Oxidation. Cell Metab. 2020, 32, 1012–1027.e7. [Google Scholar] [CrossRef]
- Gentile, G.M.; Gamarra, J.R.; Engels, N.M.; Blue, R.E.; Hoerr, I.; Wiedner, H.J.; Hinkle, E.R.; Cote, J.L.; Leverence, E.; Mills, C.A.; et al. The synaptosome-associated protein 23 (SNAP23) is necessary for proper myogenesis. FASEB J. 2022, 36, e22441. [Google Scholar] [CrossRef]
- Jagerstrom, S.; Polesie, S.; Wickstrom, Y.; Johansson, B.R.; Schroder, H.D.; Hojlund, K.; Bostrom, P. Lipid droplets interact with mitochondria using SNAP23. Cell Biol. Int. 2009, 33, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Huang, S.; Fan, B.; Niu, B.; Guo, R.; Gu, J.; Gao, S.; Li, B. iTRAQ-based proteome analysis of porcine group A rotavirus-infected porcine IPEC-J2 intestinal epithelial cells. J. Proteom. 2021, 248, 104354. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.S.; Ngo, H.T.; Lee, J.; Son, K.; Park, E.M.; Hwang, S.B. ADP-ribosylation Factor-related Protein 1 Interacts with NS5A and Regulates Hepatitis C Virus Propagation. Sci. Rep. 2016, 6, 31211. [Google Scholar] [CrossRef]
- Wang, J.; Fang, N.; Xiong, J.; Du, Y.; Cao, Y.; Ji, W.K. An ESCRT-dependent step in fatty acid transfer from lipid droplets to mitochondria through VPS13D-TSG101 interactions. Nat. Commun. 2021, 12, 1252. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.; Jun, Y.; Lee, C. Structural basis for mitoguardin-2 mediated lipid transport at ER-mitochondrial membrane contact sites. Nat. Commun. 2022, 13, 3702. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, K.; Li, L.; Hao, Z.; Wang, T.; Liu, Y.; Xing, G.; Liu, Z.; Li, H.; Yuan, H.; et al. Plin2-mediated lipid droplet mobilization accelerates exit from pluripotency by lipidomic remodeling and histone acetylation. Cell Death Differ. 2022, 29, 2316–2331. [Google Scholar] [CrossRef]
- Guo, D.; Tong, Y.; Jiang, X.; Meng, Y.; Jiang, H.; Du, L.; Wu, Q.; Li, S.; Luo, S.; Li, M.; et al. Aerobic glycolysis promotes tumor immune evasion by hexokinase2-mediated phosphorylation of IkappaBalpha. Cell Metab. 2022, 34, 1312–1324.e6. [Google Scholar] [CrossRef]
- Rossi, T.; Zamponi, R.; Chirico, M.; Pisanu, M.E.; Iorio, E.; Torricelli, F.; Gugnoni, M.; Ciarrocchi, A.; Pistoni, M. BETi enhance ATGL expression and its lipase activity to exert their antitumoral effects in triple-negative breast cancer (TNBC) cells. J. Exp. Clin. Cancer Res. 2023, 42, 7. [Google Scholar] [CrossRef]
- Ngo, J.; Choi, D.W.; Stanley, I.A.; Stiles, L.; Molina, A.J.A.; Chen, P.H.; Lako, A.; Sung, I.C.H.; Goswami, R.; Kim, M.Y.; et al. Mitochondrial morphology controls fatty acid utilization by changing CPT1 sensitivity to malonyl-CoA. EMBO J. 2023, 42, e111901. [Google Scholar] [CrossRef] [PubMed]
- Misheva, M.; Kotzamanis, K.; Davies, L.C.; Tyrrell, V.J.; Rodrigues, P.R.S.; Benavides, G.A.; Hinz, C.; Murphy, R.C.; Kennedy, P.; Taylor, P.R.; et al. Oxylipin metabolism is controlled by mitochondrial beta-oxidation during bacterial inflammation. Nat. Commun. 2022, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Bushong, E.A.; Segawa, M.; Tiard, A.; Wong, A.; Brady, M.R.; Momcilovic, M.; Wolf, D.M.; Zhang, R.; Petcherski, A.; et al. Spatial mapping of mitochondrial networks and bioenergetics in lung cancer. Nature 2023, 615, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, N.; Park, S.; Jeon, Y.; Lee, J.; Yoo, S.J.; Lee, J.W.; Moon, C.; Yu, S.W.; Kim, E.K. Tanycytic TSPO inhibition induces lipophagy to regulate lipid metabolism and improve energy balance. Autophagy 2020, 16, 1200–1220. [Google Scholar] [CrossRef]
- Sancar, G.; Liu, S.; Gasser, E.; Alvarez, J.G.; Moutos, C.; Kim, K.; van Zutphen, T.; Wang, Y.; Huddy, T.F.; Ross, B.; et al. FGF1 and insulin control lipolysis by convergent pathways. Cell Metab. 2022, 34, 171–183.e6. [Google Scholar] [CrossRef]
- Liu, R.; Lee, J.H.; Li, J.; Yu, R.; Tan, L.; Xia, Y.; Zheng, Y.; Bian, X.L.; Lorenzi, P.L.; Chen, Q.; et al. Choline kinase alpha 2 acts as a protein kinase to promote lipolysis of lipid droplets. Mol. Cell 2021, 81, 2722–2735.e9. [Google Scholar] [CrossRef]
- Ioannou, M.S.; Jackson, J.; Sheu, S.H.; Chang, C.L.; Weigel, A.V.; Liu, H.; Pasolli, H.A.; Xu, C.S.; Pang, S.; Matthies, D.; et al. Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell 2019, 177, 1522–1535.e14. [Google Scholar] [CrossRef]
- Zhao, W.J.; Bian, Y.P.; Wang, Q.H.; Yin, F.; Yin, L.; Zhang, Y.L.; Liu, J.H. Blueberry-derived exosomes-like nanoparticles ameliorate nonalcoholic fatty liver disease by attenuating mitochondrial oxidative stress. Acta Pharmacol. Sin. 2022, 43, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, Y.; Sun, Y.; He, L. CCDC127 regulates lipid droplet homeostasis by enhancing mitochondria-ER contacts. Biochem. Biophys. Res. Commun. 2023, 683, 149116. [Google Scholar] [CrossRef] [PubMed]
- Amen, T.; Kaganovich, D. Stress granules inhibit fatty acid oxidation by modulating mitochondrial permeability. Cell Rep. 2021, 35, 109237. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Adlakha, J.; Wan, N.; Guinn, E.; Giska, F.; Gupta, K.; Melia, T.J.; Reinisch, K.M. Mitoguardin-2-mediated lipid transfer preserves mitochondrial morphology and lipid droplet formation. J. Cell Biol. 2022, 221, e202207022. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.R.; Butler, L.M.; Hoy, A.J. The diversity and breadth of cancer cell fatty acid metabolism. Cancer Metab. 2021, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, J.; Hou, W.; Kang, R.; Tang, D. STING1 Promotes Ferroptosis Through MFN1/2-Dependent Mitochondrial Fusion. Front. Cell Dev. Biol. 2021, 9, 698679. [Google Scholar] [CrossRef] [PubMed]
- Feher, J.; Elo, A.; Istvan, L.; Nagy, Z.Z.; Radak, Z.; Scuderi, G.; Artico, M.; Kovacs, I. Microbiota mitochondria disorders as hubs for early age-related macular degeneration. Geroscience 2022, 44, 2623–2653. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, J.; Zang, W.J. Mitochondrial homeostasis and redox status in cardiovascular diseases: Protective role of the vagal system. Free Radic. Biol. Med. 2022, 178, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Wanders, R.J.A.; Baes, M.; Ribeiro, D.; Ferdinandusse, S.; Waterham, H.R. The physiological functions of human peroxisomes. Physiol. Rev. 2023, 103, 957–1024. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Z.; Zhang, S.; Zhang, T.; Liu, Y.; Zhang, L. Cellular mitophagy: Mechanism, roles in diseases and small molecule pharmacological regulation. Theranostics 2023, 13, 736–766. [Google Scholar] [CrossRef]
- Li, J.J.; Xiang, Y.; Zhang, L.; Qi, X.L.; Zheng, Z.Q.; Zhou, P.; Tang, Z.S.; Jin, Y.; Zhao, Q.L.; Fu, Y.H.; et al. Enhancer-promoter interaction maps provide insights into skeletal muscle-related traits in pig genome. BMC Biol. 2022, 20, 136. [Google Scholar] [CrossRef]
- Long, M.; Sanchez-Martinez, A.; Longo, M.; Suomi, F.; Stenlund, H.; Johansson, A.I.; Ehsan, H.; Salo, V.T.; Montava-Garriga, L.; Naddafi, S.; et al. DGAT1 activity synchronises with mitophagy to protect cells from metabolic rewiring by iron depletion. EMBO J. 2022, 41, e109390. [Google Scholar] [CrossRef]
- Song, H.; Zhang, X.; Wang, J.; Wu, Y.; Xiong, T.; Shen, J.; Lin, R.; Xiao, T.; Lin, W. The regulatory role of adipocyte mitochondrial homeostasis in metabolism-related diseases. Front. Physiol. 2023, 14, 1261204. [Google Scholar] [CrossRef]
- Anastasia, I.; Ilacqua, N.; Raimondi, A.; Lemieux, P.; Ghandehari-Alavijeh, R.; Faure, G.; Mekhedov, S.L.; Williams, K.J.; Caicci, F.; Valle, G.; et al. Mitochondria-rough-ER contacts in the liver regulate systemic lipid homeostasis. Cell Rep. 2021, 34, 108873. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Liu, S.; Stein, R.; Imai, Y. Lipid Droplets’ Role in the Regulation of beta-Cell Function and beta-Cell Demise in Type 2 Diabetes. Endocrinology 2022, 163, bqac007. [Google Scholar] [CrossRef]
- de Almeida, M.E.; Nielsen, J.; Petersen, M.H.; Wentorf, E.K.; Pedersen, N.B.; Jensen, K.; Hojlund, K.; Ortenblad, N. Altered intramuscular network of lipid droplets and mitochondria in type 2 diabetes. Am. J. Physiol. Cell Physiol. 2023, 324, C39–C57. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Zhao, M.; Nie, Y.; Liu, P.; Zhu, Y.; Zhang, X. Skeletal Muscle Lipid Droplets and the Athlete’s Paradox. Cells 2019, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, C.L.; Fealy, C.E.; Erickson, M.L.; Davuluri, G.; Fujioka, H.; Dantas, W.S.; Huang, E.; Pergola, K.; Mey, J.T.; King, W.T.; et al. Lipids activate skeletal muscle mitochondrial fission and quality control networks to induce insulin resistance in humans. Metabolism 2021, 121, 154803. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.K.; Liu, Y.; Xu, Y.; Jin, Y.; Wu, J.; Ren, Z.Q. Comparative transcriptome and Lipidome analyses suggest a lipid droplet-specific response to heat exposure of brown adipose tissue in normal and obese mice. Life Sci. 2022, 299, 120540. [Google Scholar] [CrossRef]
- Che, L.; Huang, J.; Lin, J.X.; Xu, C.Y.; Wu, X.M.; Du, Z.B.; Wu, J.S.; Lin, Z.N.; Lin, Y.C. Aflatoxin B1 exposure triggers hepatic lipotoxicity via p53 and perilipin 2 interaction-mediated mitochondria-lipid droplet contacts: An in vitro and in vivo assessment. J. Hazard. Mater. 2023, 445, 130584. [Google Scholar] [CrossRef]
- Su, W.; Chi, Y.; An, Y.A. Editorial: Lipid droplets and mitochondria in metabolic diseases. Front. Physiol. 2023, 14, 1266356. [Google Scholar] [CrossRef] [PubMed]
- Borquez, J.C.; Diaz-Castro, F.; La Fuente, F.P.; Espinoza, K.; Figueroa, A.M.; Martinez-Ruiz, I.; Hernandez, V.; Lopez-Soldado, I.; Ventura, R.; Domingo, J.C.; et al. Mitofusin-2 induced by exercise modifies lipid droplet-mitochondria communication, promoting fatty acid oxidation in male mice with NAFLD. Metabolism 2024, 152, 155765. [Google Scholar] [CrossRef] [PubMed]
- Sundquist, K.; Sundquist, J.; Wang, X.; Palmer, K.; Memon, A.A. Baseline mitochondrial DNA copy number and heart failure incidence and its role in overall and heart failure mortality in middle-aged women. Front. Cardiovasc. Med. 2022, 9, 1012403. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.Y.; Kim, S.G.; Kang, Y.J.; Park, S.Y.; Choi, H.C. SIRT1-dependent PGC-1alpha deacetylation by SRT1720 rescues progression of atherosclerosis by enhancing mitochondrial function. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2024, 1869, 159453. [Google Scholar]
- Cuello, F.; Knaust, A.E.; Saleem, U.; Loos, M.; Raabe, J.; Mosqueira, D.; Laufer, S.; Schweizer, M.; van der Kraak, P.; Flenner, F.; et al. Impairment of the ER/mitochondria compartment in human cardiomyocytes with PLN p.Arg14del mutation. EMBO Mol. Med. 2021, 13, e13074. [Google Scholar] [CrossRef] [PubMed]
- Combot, Y.; Salo, V.T.; Chadeuf, G.; Holtta, M.; Ven, K.; Pulli, I.; Ducheix, S.; Pecqueur, C.; Renoult, O.; Lak, B.; et al. Seipin localizes at endoplasmic-reticulum-mitochondria contact sites to control mitochondrial calcium import and metabolism in adipocytes. Cell Rep. 2022, 38, 110213. [Google Scholar] [CrossRef] [PubMed]
- Herker, E. Lipid Droplets in Virus Replication. FEBS Lett. 2024, 598, 1299–1300. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Ferri, E.; Calvani, R.; Coelho-Junior, H.J.; Marzetti, E.; Arosio, B. Age-Associated Glia Remodeling and Mitochondrial Dysfunction in Neurodegeneration: Antioxidant Supplementation as a Possible Intervention. Nutrients 2022, 14, 2406. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Vrijsen, S.; Vrancx, C.; Del Vecchio, M.; Swinnen, J.V.; Agostinis, P.; Winderickx, J.; Vangheluwe, P.; Annaert, W. Inter-organellar Communication in Parkinson’s and Alzheimer’s Disease: Looking Beyond Endoplasmic Reticulum-Mitochondria Contact Sites. Front. Neurosci. 2022, 16, 900338. [Google Scholar] [CrossRef]
- Choi, J.; Ravipati, A.; Nimmagadda, V.; Schubert, M.; Castellani, R.J.; Russell, J.W. Potential roles of PINK1 for increased PGC-1alpha-mediated mitochondrial fatty acid oxidation and their associations with Alzheimer disease and diabetes. Mitochondrion 2014, 18, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, F.; Gao, Q.; Lu, Y.; Xu, X.; Hu, R.; Wang, Z.; Peng, M.; Yang, Z.; Tang, B.Z. Visualizing Dynamic Performance of Lipid Droplets in a Parkinson’s Disease Model via a Smart Photostable Aggregation-Induced Emission Probe. iScience 2019, 21, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Barilani, M.; Lovejoy, C.; Dossena, M.; Vigano, M.; Seresini, A.; Piga, D.; Gandhi, S.; Pezzoli, G.; Abramov, A.Y.; et al. Mitochondrial dysfunction in Parkinsonian mesenchymal stem cells impairs differentiation. Redox Biol. 2018, 14, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Moschandrea, C.; Kondylis, V.; Evangelakos, I.; Herholz, M.; Schneider, F.; Schmidt, C.; Yang, M.; Ehret, S.; Heine, M.; Jaeckstein, M.Y.; et al. Mitochondrial dysfunction abrogates dietary lipid processing in enterocytes. Nature 2024, 625, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Bulow, M.H.; Sellin, J. New discoveries in ER-mitochondria communication. Biochem. Soc. Trans. 2023, 51, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Guo, X.; Cui, S.; Wu, Y.; Zhang, Y.; Shen, X.; Xie, C.; Li, J. Dephosphorylation of AMP-activated protein kinase exacerbates ischemia/reperfusion-induced acute kidney injury via mitochondrial dysfunction. Kidney Int. 2022, 101, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, J.; Shen, S.; Tong, Q.; Ma, X.; Lin, L. SIRT3 promotes lipophagy and chaperon-mediated autophagy to protect hepatocytes against lipotoxicity. Cell Death Differ. 2020, 27, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Lundsgaard, A.M.; Fritzen, A.M.; Kiens, B. Molecular Regulation of Fatty Acid Oxidation in Skeletal Muscle during Aerobic Exercise. Trends Endocrinol. Metab. 2018, 29, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, J.; Takahashi, A.; Takabatake, Y.; Sakai, S.; Minami, S.; Yamamoto, T.; Fujimura, R.; Namba-Hamano, T.; Yonishi, H.; Nakamura, J.; et al. Metabolic effects of RUBCN/Rubicon deficiency in kidney proximal tubular epithelial cells. Autophagy 2020, 16, 1889–1904. [Google Scholar] [CrossRef]
- Blumenfeld, N.R.; Kang, H.J.; Fenzl, A.; Song, Z.; Chung, J.J.; Singh, R.; Johnson, R.; Karakecili, A.; Feranil, J.B.; Rossen, N.S.; et al. A direct tissue-grafting approach to increasing endogenous brown fat. Sci. Rep. 2018, 8, 7957. [Google Scholar] [CrossRef]
- Mece, O.; Houbaert, D.; Agostinis, P. Eating your own fat to stay fit: Lipophagy sustains lymphangiogenesis. Autophagy 2023, 19, 1351–1353. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.; Tan, Y. Lipid Droplet–Mitochondria Contacts in Health and Disease. Int. J. Mol. Sci. 2024, 25, 6878. https://doi.org/10.3390/ijms25136878
Fan H, Tan Y. Lipid Droplet–Mitochondria Contacts in Health and Disease. International Journal of Molecular Sciences. 2024; 25(13):6878. https://doi.org/10.3390/ijms25136878
Chicago/Turabian StyleFan, Hongjun, and Yanjie Tan. 2024. "Lipid Droplet–Mitochondria Contacts in Health and Disease" International Journal of Molecular Sciences 25, no. 13: 6878. https://doi.org/10.3390/ijms25136878
APA StyleFan, H., & Tan, Y. (2024). Lipid Droplet–Mitochondria Contacts in Health and Disease. International Journal of Molecular Sciences, 25(13), 6878. https://doi.org/10.3390/ijms25136878




