Basidiomycetes Polysaccharides Regulate Growth and Antioxidant Defense System in Wheat
Abstract
:1. Introduction
2. Results
2.1. Fungal EPS Features
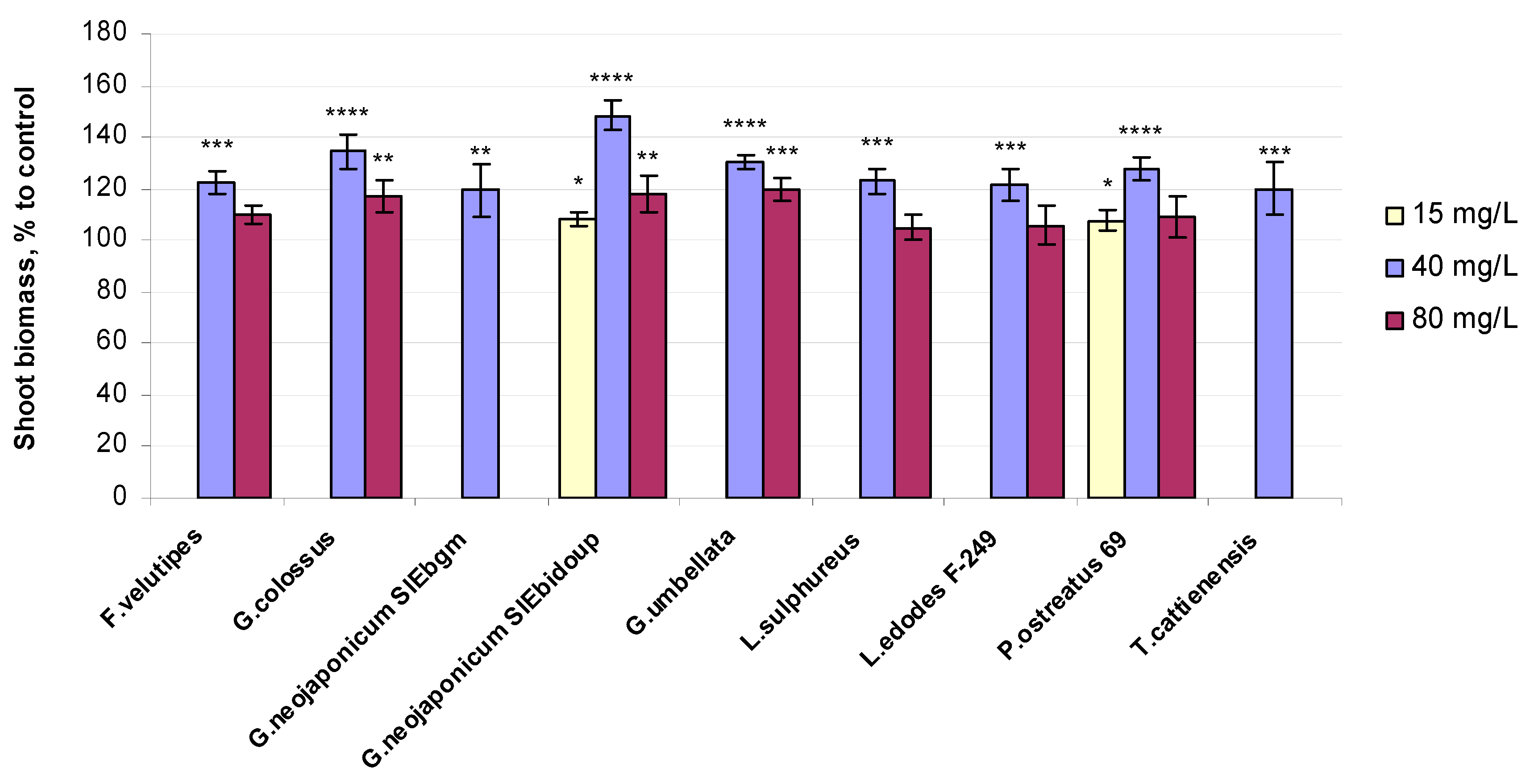
2.2. Morphological and Physiological Variables of Wheat Seedlings Exposed to Fungal EPS
2.3. Biochemical Variables of Wheat Seedlings Exposed to Fungal EPS
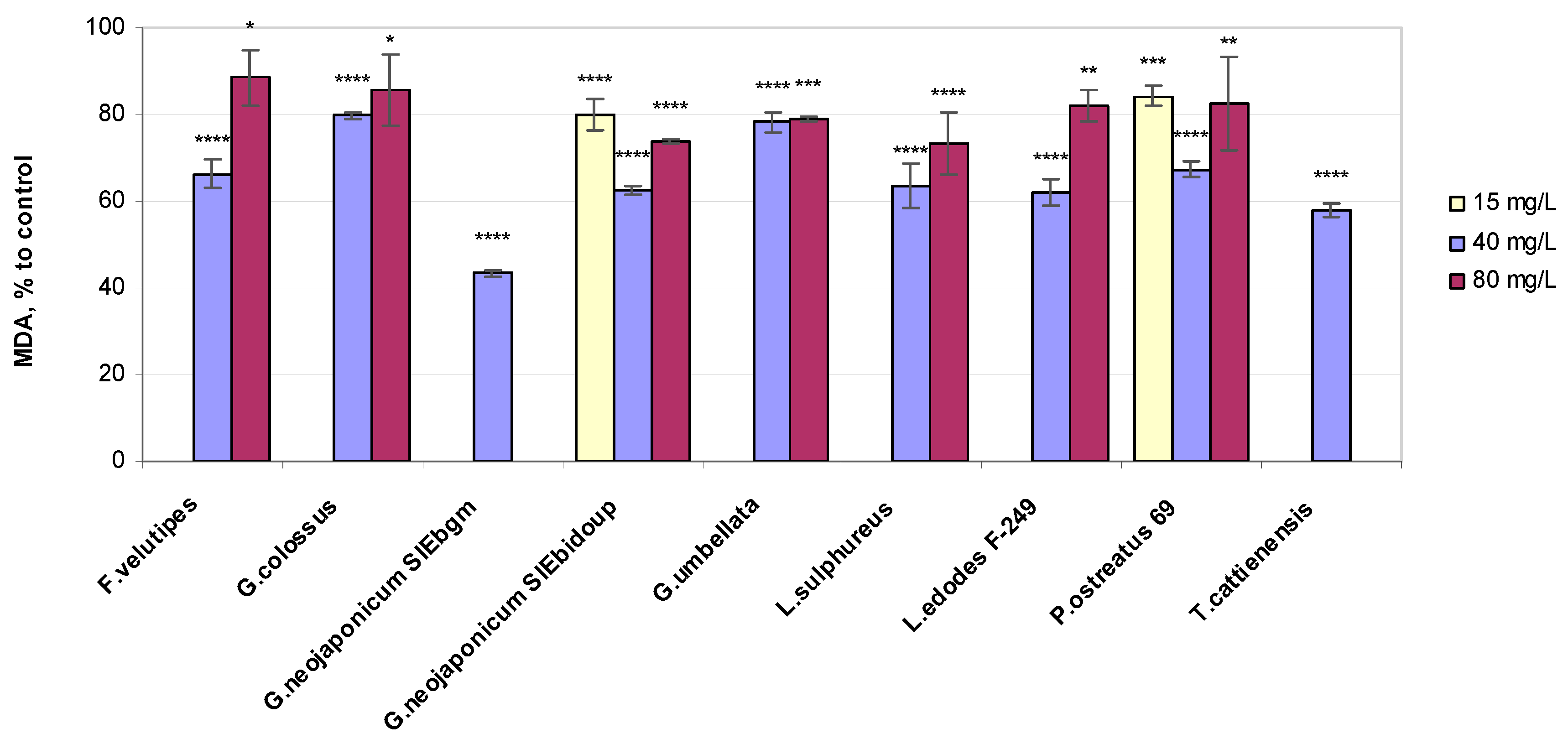
2.3.1. Lipid Peroxidation Status
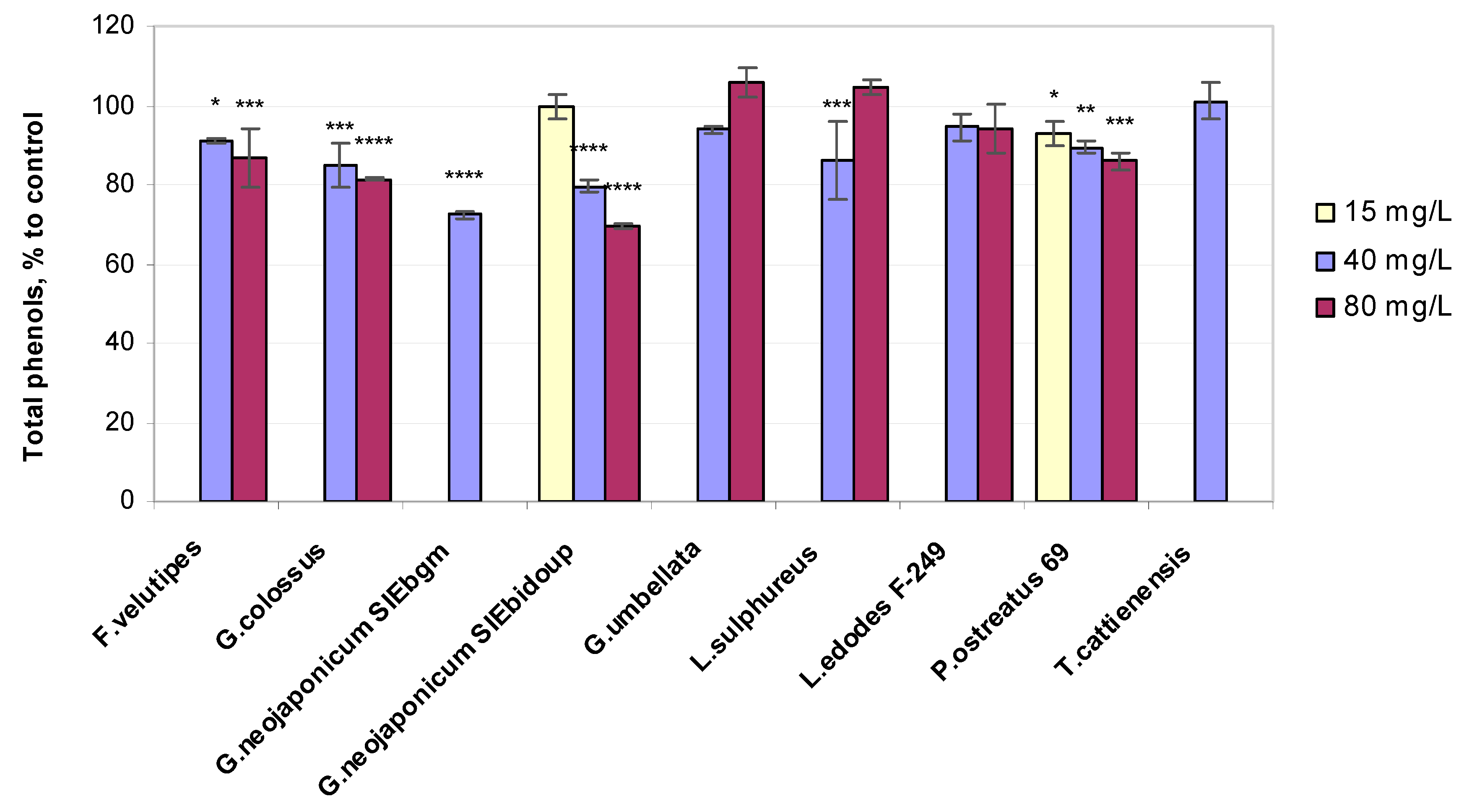
2.3.2. Total Phenols Content
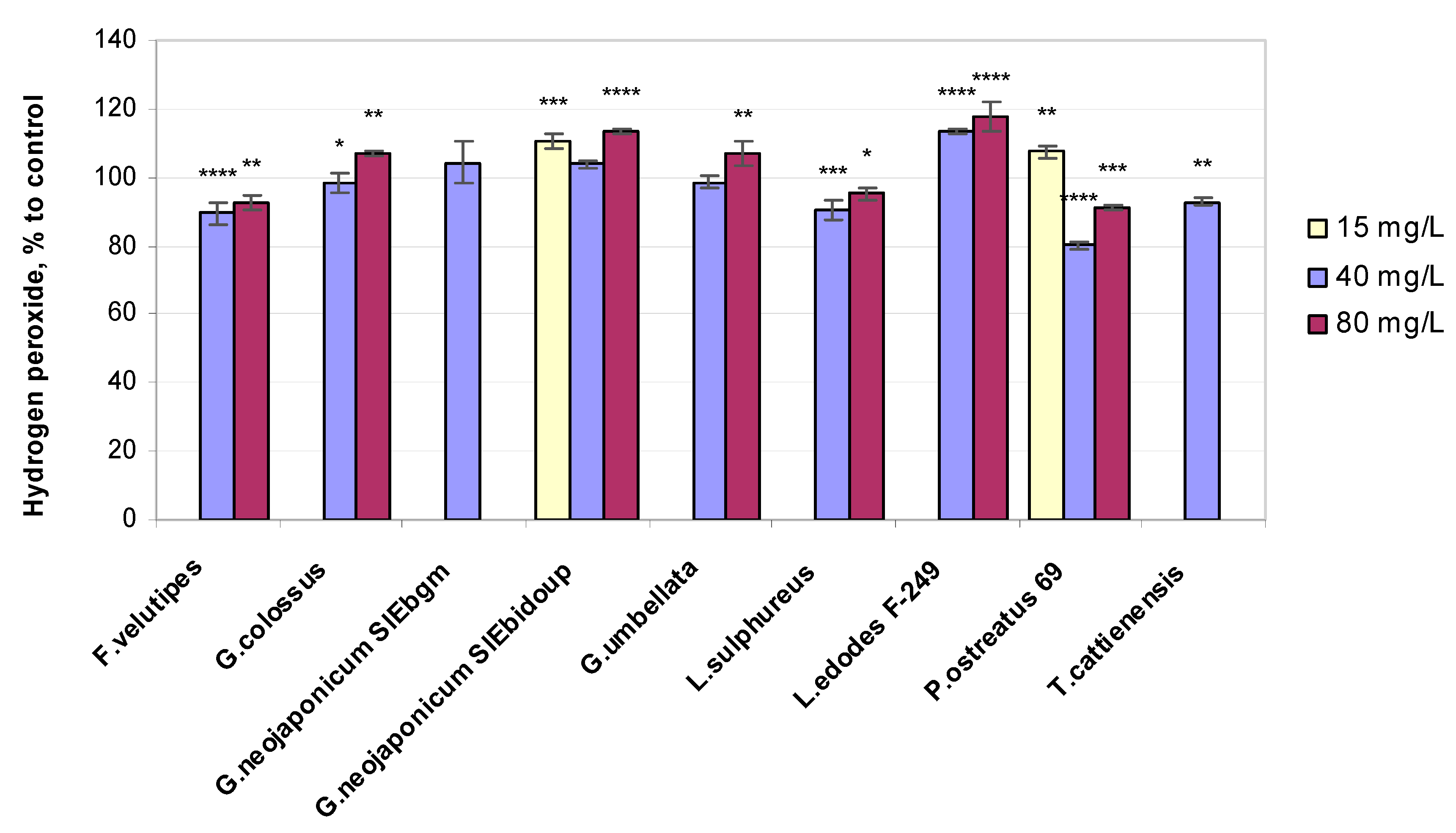
2.3.3. Hydrogen Peroxide Level
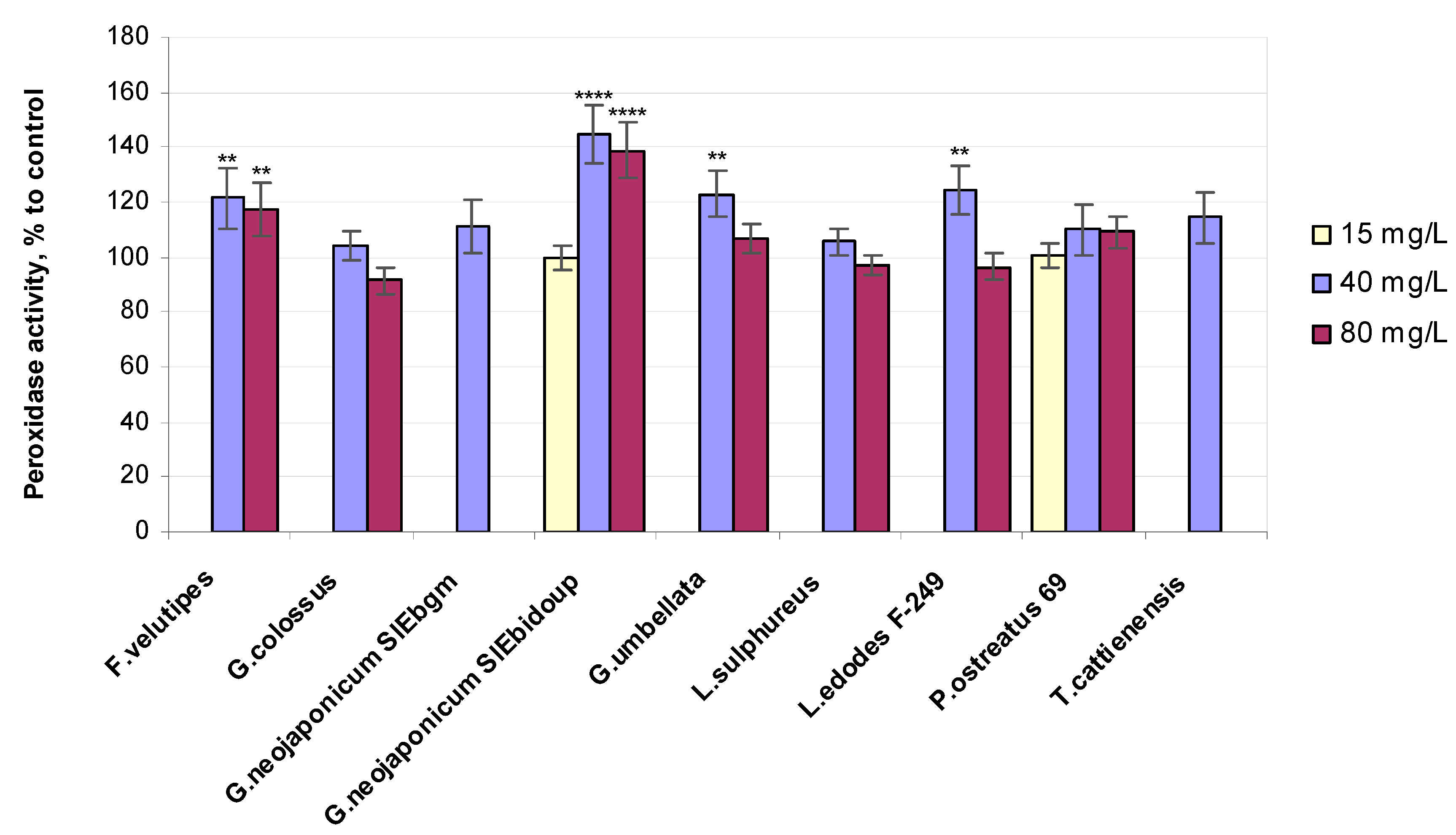
2.3.4. Enzyme-Related Bioassays
3. Discussion
3.1. Morphology-Related Response in Wheat Plants Exposed to Fungal EPS
3.2. Biomass-Related Response in Wheat Plants Exposed to Fungal EPS
3.3. Non-Enzymatic Antioxidant-Related Response in Wheat Plants Exposed to Fungal EPS
3.4. Enzymatic Antioxidant-Related Response in Wheat Plants Exposed to Fungal EPS
4. Materials and Methods
4.1. Fungal Material and Preparations
4.2. Plant Material and Treatment
4.3. Malondialdehyde Measurement
4.4. Total Phenol Content Measurement
4.5. Hydrogen Peroxide Measurement
4.6. Enzymatic Activity Determinations
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive Oxygen Species in Plants: From Source to Sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, X.; Gong, B.; Lü, G.; Li, J.; Gao, H. Review of the mechanisms by which transcription factors and exogenous substances regulate ROS metabolism under abiotic stress. Antioxidants 2022, 11, 2106. [Google Scholar] [CrossRef] [PubMed]
- Vega-Muñoz, I.; Herrera-Estrella, A.; Martínez-de la Vega, O.; Heil, M. ATM and ATR, two central players of the DNA damage response, are involved in the induction of systemic acquired resistance by extracellular DNA, but not the plant wound response. Front. Immunol. 2023, 14, 1175786. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Gao, Q.; Liu, J.; Tang, J.; Hua, Z.; Sun, X. Categories of exogenous substances and their effect on alleviation of plant salt stress. Eur. J. Agron. 2023, 142, 126656. [Google Scholar] [CrossRef]
- Huang, C.; Tian, Y.; Zhang, B.; Hassan, M.J.; Li, Z.; Zhu, Y. Chitosan (CTS) Alleviates Heat-Induced Leaf Senescence in Creeping Bentgrass by Regulating Chlorophyll Metabolism, Antioxidant Defense, and the Heat Shock Pathway. Molecules 2021, 26, 5337. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Hossain, M.A.; Fujita, M.; Tran, L.-S.P. Physiological and biochemical mechanisms associated with trehalose-induced copper-stress tolerance in rice. Sci. Rep. 2015, 5, 11433. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-Q.; Li, T.-T.; Hao, Z.-J.; Cheng, M.-L.; Tao, J. Exogenous trehalose confers high temperature stress tolerance to herbaceous peony by enhancing antioxidant systems, activating photosynthesis, and protecting cell structure. Cell Stress Chaperon 2019, 24, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Rohman, M.; Islam, R.; Monsur, M.B.; Amiruzzaman, M.; Fujita, M.; Hasanuzzaman, M. Trehalose Protects Maize Plants from Salt Stress and Phosphorus Deficiency. Plants 2019, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R.V. Chitosan as a coating material for nanoparticles intended for biomedical applications. React. Funct. Polym. 2020, 147, 104459. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Yang, S.; Yue, L.; Jiang, Q.; Xia, W. Synthesis and antioxidant properties of chitosan and carboxymethyl chitosan-stabilized selenium nanoparticles. Carbohydr. Polym. 2015, 132, 574–581. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Al-Huqail, A.A.; Al-Harbi, M.S.; Ali, E.F.; Wang, J.; Ding, Z.; Rekaby, S.A.; Ghoneim, A.M.; Eissa, M.A. Mechanisms of Chitosan Nanoparticles in the Regulation of Cold Stress Resistance in Banana Plants. Nanomaterials 2021, 11, 2670. [Google Scholar] [CrossRef] [PubMed]
- Lesnichaya, M.V.; Aleksandrova, G.P.; Feoktistova, L.P.; Sapozhnikov, A.N.; Fadeeva, T.V.; Sukhov, B.G.; Trofimov, B.A. Silver-containing nanocomposites based on galactomannan and carrageenan: Synthesis, structure, and antimicrobial properties. Russ. Chem. Bull. 2010, 59, 2323–2328. [Google Scholar] [CrossRef]
- Lesnichaya, M.V.; Aleksandrova, G.P.; Sukhov, B.G.; Rokhin, A.V. Molecular-weight characteristics of galactomannan and carrageenan. Chem. Nat. Compd. 2013, 49, 405–410. [Google Scholar] [CrossRef]
- Lesnichaya, M.V.; Sukhov, B.G.; Aleksandrova, G.P.; Gasilova, E.R.; Vakul’skaya, T.I.; Khutsishvili, S.S.; Sapozhnikov, A.N.; Klimenkov, I.V.; Trofimov, B.A. Chiroplasmonic magnetic gold nanocomposites produced by one-step aqueous method using κ-carrageenan. Carbohydr. Polym. 2017, 175, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Sultana, F.; Khan, S.; Nayeema, J.; Mostafa, M.; Ferdus, H.; Tran, L.-S.P.; Mostofa, M.G. Carrageenans as biostimulants and bio-elicitors: Plant growth and defense responses. Stress Biol. 2024, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Hijri, M. Microbial-Based Plant Biostimulants. Microorganisms 2023, 11, 686. [Google Scholar] [CrossRef] [PubMed]
- Lehman, A.P.; Long, S.R. Exopolysaccharides from Sinorhizobium meliloti can protect against H2O2-dependent damage. J. Bacteriol. 2013, 195, 5362–5369. [Google Scholar] [CrossRef] [PubMed]
- Kawaharada, Y.; Kelly, S.; Wibroe Nielsen, M.; Hjuler, C.T.; Gysel, K.; Muszynski, A.; Carlson, R.W.; Thygesen, M.B.; Sandal, N.; Asmussen, M.H.; et al. Receptor-mediated exopolysaccharide perception controls bacterial infection. Nature 2015, 523, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Yegorenkova, I.V.; Tregubova, K.V.; Ignatov, V.V. Paenibacillus polymyxa rhizobacteria and their synthesized exoglycans in interaction with wheat roots: Colonization and root hair deformation. Curr. Microbiol. 2013, 66, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Yegorenkova, I.V.; Tregubova, K.V.; Krasov, A.I.; Evseeva, N.V.; Matora, L.Y. Effect of exopolysaccharides of Paenibacillus polymyxa rhizobacteria on physiological and morphological variables of wheat seedlings. J. Microbiol. 2021, 59, 729–735. [Google Scholar] [CrossRef]
- Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front. Microbiol. 2018, 8, 2580. [Google Scholar] [CrossRef]
- Isfahani, F.M.; Tahmourespour, A.; Hoodaji, M.; Ataabadi, M.; Mohammadi, A. Infuence of exopolysaccharide-producing bacteria and SiO2 nanoparticles on proline content and antioxidant enzyme activities of tomato seedlings (Solanum lycopersicum L.) under salinity stress. Pol. J. Environ. Stud. 2019, 28, 153–163. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A. Exopolysaccharide producing rhizobacteria and their impact on growth and drought tolerance of wheat grown under rainfed conditions. PLoS ONE 2019, 14, e0222302. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Mishra, S.; Dames, J.F. Arbuscular mycorrhizal inoculation improves growth and antioxidative response of Jatropha curcas (L.) under Na2SO4 salt stress. Plant Biosyst.–Int. J. Deal. All Asp. Plant Biol. 2015, 149, 260–269. [Google Scholar]
- Slimani, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Boutasknit, A.; Anli, M.; Abouraicha, E.F.; Oufdou, K.; Meddich, A.; Baslam, M. Signals and Machinery for Mycorrhizae and Cereal and Oilseed Interactions towards Improved Tolerance to Environmental Stresses. Plants 2024, 13, 826. [Google Scholar] [CrossRef]
- Bidabadi, S.S.; Masoumian, M. Arbuscular mycorrhizal symbiosis improves growth and antioxidative response of Stevia rebaudiana (Bert.) under salt stress. Trends Hortic. 2018, 1, 549. [Google Scholar]
- Basiru, S.; Hijri, M. The Potential Applications of Commercial Arbuscular Mycorrhizal Fungal Inoculants and Their Ecological Consequences. Microorganisms 2022, 10, 1897. [Google Scholar] [CrossRef]
- Basiru, S.; Hijri, M. Does commercial inoculation promote arbuscular mycorrhizal fungi invasion? Microorganisms 2022, 10, 404. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. The application of arbuscular mycorrhizal fungi as microbial biostimulant, sustainable approaches in modern agriculture. Plants 2023, 12, 3101. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Butnariu, M.; Ezzat, S.M.; Adetunji, C.O.; Imran, M.; Sobhani, S.R.; Tufail, T.; Hosseinabadi, T.; Ramírez-Alarcón, K.; Martorell, M.; et al. Mushrooms-Rich Preparations on Wound Healing: From Nutritional to Medicinal Attributes. Front. Pharmacol. 2020, 11, 567518. [Google Scholar] [CrossRef]
- Anusiya, G.; Gowthama Prabu, U.; Yamini, N.V.; Sivarajasekar, N.; Rambabu, K.; Bharath, G.; Banat, F. A review of the therapeutic and biological effects of edible and wild mushrooms. Bioengineered 2021, 12, 11239–11268. [Google Scholar] [CrossRef]
- Sivanesan, I.; Muthu, M.; Gopal, J.; Oh, J.W. Mushroom polysaccharide-assisted anticarcinogenic mycotherapy: Reviewing its clinical trials. Molecules 2022, 27, 4090. [Google Scholar] [CrossRef]
- Fernandes, A.; Nair, A.; Kulkarni, N.; Todewale, N.; Jobby, R. Exploring Mushroom Polysaccharides for the Development of Novel Prebiotics: A Review. Int. J. Med. Mushrooms 2023, 25, 1–10. [Google Scholar] [CrossRef]
- Pinar, O.; Rodríguez-Couto, S. Biologically active secondary metabolites from white-rot fungi. Front. Chem. 2024, 12, 1363354. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Ren, L.; Dai, X.; Zhao, J.; Gao, C.; Zhang, S.; Dong, J.; Zhao, Z.; Li, Y.; et al. Extraction, purification, structural characteristics and biological properties of the polysaccharides from Armillaria mellea (Vahl) P. Kumm.: A review. Int. J. Biol. Macromol. 2024, 259, 129175. [Google Scholar] [CrossRef]
- Su, Y.; Chen, L.; Yang, F.; Cheung, P.C.K. Beta-D-glucan-based drug delivery system and its potential application in targeting tumor associated macrophages. Carbohydr. Polym. 2021, 253, 117258. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural features, modification, and functionalities of beta-glucan. Fibers 2020, 8, 1. [Google Scholar] [CrossRef]
- Legentil, L.; Paris, F.; Ballet, C.; Trouvelot, S.; Daire, X.; Vetvicka, V.; Ferrières, V. Molecular interactions of β-(1→3)-glucans with their receptors. Molecules 2015, 20, 9745–9766. [Google Scholar] [CrossRef]
- Chen, L.; Ge, M.D.; Zhu, Y.J.; Song, Y.; Cheung, P.C.; Zhang, B.B.; Liu, L.M. Structure, bioactivity and applications of natural hyperbranched polysaccharides. Carbohydr. Polym. 2019, 223, 115076. [Google Scholar] [CrossRef]
- Tsivileva, O.M.; Perfileva, A.I. Mushroom-derived novel selenium nanocomposites’ effects on potato plant growth and tuber germination. Molecules 2022, 27, 4438. [Google Scholar] [CrossRef]
- Nowak, A.; Tyśkiewicz, R.; Wiater, A.; Jaroszuk-Ściseł, J. (1→3)-α-D-glucooligosaccharides as elicitors influencing the activity of plant resistance pathways in wheat tissues. Agronomy 2022, 12, 1170. [Google Scholar] [CrossRef]
- Tsivileva, O.M.; Pozdnyakov, A.S.; Ivanova, A.A. Polymer nanocomposites of selenium biofabricated using fungi. Molecules 2021, 26, 3657. [Google Scholar] [CrossRef] [PubMed]
- Tsivileva, O.M.; Koftin, O.V.; Ibragimova, D.N.; Fedotova, O.V.; Nikitina, V.E. Production of biometal complexes based on the systems incorporating 4-hydroxycoumarin fragnment and their effect on microorganisms. Proc. RAS Ufa Sci. Cent. 2019, 2019, 89–98. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, Y.; Ma, X.; Ouyang, Z.; Deng, L.; Shen, S.; Dong, X.; Du, N.; Dong, H.; Guo, Z.; et al. Melatonin alleviates copper toxicity via improving ROS metabolism and antioxidant defense response in tomato seedlings. Antioxidants 2022, 11, 758. [Google Scholar] [CrossRef] [PubMed]
- Bouremani, N.; Cherif-Silini, H.; Silini, A.; Bouket, A.C.; Luptakova, L.; Alenezi, F.N.; Baranov, O.; Belbahri, L. Plant growth-promoting rhizobacteria (PGPR): A rampart against the adverse effects of drought stress. Water 2023, 15, 418. [Google Scholar] [CrossRef]
- Sami, H.; Karim, N.; Angelo, S. Alleviation of heat stress by ACC Deaminase and Exopolysaccharide Producing Plant-Growth-Promoting Rhizobacteria in Solanum melongena L. Adv. Life Sci. 2024, 11, 513–524. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, W.; Jing, B.; Liu, L. Modulation of soil aeration and antioxidant defenses with hydrogen peroxide improves the growth of winter wheat (Triticum aestivum L.) plants. J. Clean. Prod. 2024, 435, 140565. [Google Scholar] [CrossRef]
- Nadimi, M.; Hawley, E.; Liu, J.; Hildebrand, K.; Sopiwnyk, E.; Paliwal, J. Enhancing traceability of wheat quality through the supply chain. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2495–2522. [Google Scholar] [CrossRef]
- Sun, Y.; Xie, X.; Jiang, C.-J. Antioxidant Agriculture for Stress-Resilient Crop Production: Field Practice. Antioxidants 2024, 13, 164. [Google Scholar] [CrossRef]
- Pomortsev, A.V.; Dorofeev, N.V.; Adamovich, S.N.; Oborina, E.N. Effect of protatranes on the physiological parameters of spring wheat under chloride salinity conditions. Izv. Vuzov. Prikl. Khimiya I Biotekhnologiya = Proc. Universities. Appl. Chem. Biotechnol. 2022, 12, 485–490. [Google Scholar] [CrossRef]
- Jochum, M.D.; McWilliams, K.L.; Borrego, E.J.; Kolomiets, M.V.; Niu, G.; Pierson, E.A.; Jo, Y.K. Bioprospecting plant growth-promoting rhizobacteria that mitigate drought stress in grasses. Front. Microbiol. 2019, 10, 466447. [Google Scholar] [CrossRef]
- Bekkaye, M.; Baha, N.; Behairi, S.; MariaPerez-Clemente, R.; Kaci, Y. Impact of bio-inoculation with halotolerant rhizobacteria on growth, physiological, and hormonal responses of durum wheat under salt stress. J. Plant Growth Regul. 2023, 42, 6549–6564. [Google Scholar] [CrossRef]
- Sesiz, U.; Alsaleh, A.; Bektas, H.; Topu, M.; Özkan, H. Genome-wide association analysis of coleoptile length and interaction with plant height in durum wheat. Agron. J. 2024, 116, 1–17. [Google Scholar] [CrossRef]
- Cetinel, A.H.S.; Yalcinkaya, T.; Akyol, T.Y.; Gokce, A.; Turkan, I. Pretreatment of seeds with hydrogen peroxide improves deep-sowing tolerance of wheat seedlings. Plant Physiol. Biochem. 2021, 167, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Gholami, H.; Abdolshahi, R.; Mohayeji, M.; Esmaeilizadeh-Moghadam, M. Investigation of coleoptile and mesocotyl as the most important factors for the establishment of bread wheat seed under rain-fed conditions. Iran. J. Seed Res. 2023, 9, 63–76. [Google Scholar] [CrossRef]
- Licaj, I.; Germinario, C.; Di Meo, M.C.; Varricchio, E.; Rocco, M. The physiology and anatomy study in leaves of Saragolla and Svevo wheat cultivars under polyethylene glycol-simulated drought stress. Funct. Plant Biol. 2024, 51, FP23151. [Google Scholar] [CrossRef]
- Jahan, E.; Sharwood, R.E.; Tissue, D.T. Effects of leaf age during drought and recovery on photosynthesis, mesophyll conductance and leaf anatomy in wheat leaves. Front. Plant Sci. 2023, 14, 1091418. [Google Scholar] [CrossRef]
- Wu, A.; Brider, J.; Busch, F.A.; Chen, M.; Chenu, K.; Clarke, V.C.; Collins, B.; Ermakova, M.; Evans, J.R.; Farquhar, G.D.; et al. A cross-scale analysis to understand and quantify the effects of photosynthetic enhancement on crop growth and yield across environments. Plant Cell Environ. 2023, 46, 23–44. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- Ngumbi, E.; Kloepper, J. Bacterial-mediated drought tolerance: Current and future prospects. Appl. Soil Ecol. 2016, 105, 109–125. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plant. 2017, 161, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Birouste, M.; Zamora-Ledezma, E.; Bossard, C.; Perez-Ramos, I.M.; Roumet, C. Measurement of fine root tissue density: A comparison of three methods reveals the potential of root dry matter content. Plant Soil 2014, 374, 299–313. [Google Scholar] [CrossRef]
- Mundim, G.d.S.M.; Maciel, G.M.; de Oliveira Mendes, G. Aspergillus niger as a Biological Input for Improving Vegetable Seedling Production. Microorganisms 2022, 10, 674. [Google Scholar] [CrossRef] [PubMed]
- Noureen, S.; Iqbal, A.; Muqeet, H.A. Potential of Drought Tolerant Rhizobacteria Amended with Biochar on Growth Promotion in Wheat. Plants 2024, 13, 1183. [Google Scholar] [CrossRef] [PubMed]
- Naseem, H.; Bano, A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J. Plant Interact. 2014, 9, 689–701. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, Y.; Shi, Z. Arbuscular Mycorrhiza Enhances Biomass Production and Salt Tolerance of Sweet Sorghum. Microorganisms 2019, 7, 289. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Ali, D.F.I.; Xiong, Y.-C.; Brestic, M.; Skalicky, M.; Hamoud, Y.A.; Ulhassan, Z.; Shaghaleh, H.; AbdElgawad, H.; Farooq, M.; et al. Physiological and Biochemical Responses of Soybean Plants Inoculated with Arbuscular Mycorrhizal Fungi and Bradyrhizobium under Drought Stress. BMC Plant Biol. 2021, 21, 195. [Google Scholar] [CrossRef]
- Wierzchowski, K.; Kawka, M.; Wrzecionek, M.; Urbanek, J.; Pietrosiuk, A.; Sykłowska-Baranek, K.; Gadomska-Gajadhur, A.; Pilarek, M. Stress-induced intensification of deoxyshikonin production in Rindera graeca hairy root cultures with ester-based scaffolds. Plants 2022, 11, 3462. [Google Scholar] [CrossRef]
- Salwan, R.; Sharma, A.; Sharma, V. Microbes mediated plant stress tolerance in saline agricultural ecosystem. Plant Soil 2019, 442, 1–22. [Google Scholar] [CrossRef]
- Ahmed, M.; Tóth, Z.; Decsi, K. The Impact of Salinity on Crop Yields and the Confrontational Behavior of Transcriptional Regulators, Nanoparticles, and Antioxidant Defensive Mechanisms under Stressful Conditions: A Review. Int. J. Mol. Sci. 2024, 25, 2654. [Google Scholar] [CrossRef] [PubMed]
- Kolupaev, Y.E.; Shakhov, I.V.; Kokorev, A.I.; Dyachenko, A.I.; Dmitriev, A.P. The Role of Reactive Oxygen Species and Calcium Ions in Implementing the Stress-Protective Effect of γ-Aminobutyric Acid on Wheat Seedlings Under Heat Stress Conditions. Cytol. Genet. 2024, 58, 81–91. [Google Scholar] [CrossRef]
- Lastochkina, O.; Aliniaeifard, S.; Garshina, D.; Garipova, S.; Pusenkova, L.; Allagulova, C.; Fedorova, K.; Baymiev, A.; Koryakov, I.; Sobhani, M. Seed priming with endophytic Bacillus subtilis strain-specifically improves growth of Phaseolus vulgaris plants under normal and salinity conditions and exerts anti-stress effect through induced lignin deposition in roots and decreased oxidative and osmotic damages. J. Plant Phys. 2021, 263, 153462. [Google Scholar]
- Tkachenko, O.V.; Evseeva, N.V.; Kargapolova, K.Y.; Denisova, A.Y.; Pozdnyakova, N.N.; Kulikov, A.A.; Burygin, G.L. Rhizobacteria Increase the Adaptation Potential of Potato Microclones under Aeroponic Conditions. Microorganisms 2023, 11, 1866. [Google Scholar] [CrossRef]
- Rashid, U.; Yasmin, H.; Hassan, M.N.; Naz, R.; Nosheen, A.; Sajjad, M.; Ilyas, N.; Keyani, R.; Jabeen, Z.; Mumtaz, S.; et al. Drought-tolerant Bacillus megaterium isolated from semi-arid conditions induces systemic tolerance of wheat under drought conditions. Plant Cell Rep. 2022, 41, 549–569. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.N.; Wu, Q.S.; Kuča, K. Unravelling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biol. 2021, 23, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Salami, M.; Heidari, B.; Batley, J.; Wang, J.; Tan, X.L.; Richards, C.; Tan, H. Integration of genome-wide association studies, metabolomics, and transcriptomics reveals phenolic acid-and flavonoid-associated genes and their regulatory elements under drought stress in rapeseed flowers. Front. Plant Sci. 2024, 14, 1249142. [Google Scholar] [CrossRef]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant Flavonoids—Biosynthesis, Transport and Involvement in Stress Responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef] [PubMed]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Bhakthavatchalu, S.; Shivakumar, S.; Sullia, S.B. Characterization of multiple plant growth promotion traits of Pseudomonas aeruginosa FP6, a potential stress tolerant biocontrol agent. Ann. Biol. Res. 2013, 4, 214–223. [Google Scholar]
- Jha, Y.; Mohamed, H.I. Enhancement of disease resistance, growth potential, and biochemical markers in maize plants by inoculation with plant growth-promoting bacteria under biotic stress. J. Plant Pathol. 2023, 105, 729–748. [Google Scholar] [CrossRef]
- Ghosh, R.; Bryant, D.L.; Farone, A.L. Panax quinquefolius (North American Ginseng) Polysaccharides as Immunomodulators: Current Research Status and Future Directions. Molecules 2020, 25, 5854. [Google Scholar] [CrossRef] [PubMed]
- Dehghanian, Z.; Habibi, K.; Dehghanian, M.; Aliyar, S.; Lajayer, B.A.; Astatkie, T.; Minkina, T.; Keswani, C. Reinforcing the bulwark: Unravelling the efficient applications of plant phenolics and tannins against environmental stresses. Heliyon 2022, 8, e09094. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Chauhan, P.S.; Jha, P.N.; Verma, R.K.; Singh, S.; Yadav, V.K.; Sahoo, D.K.; Patel, A. Secretory molecules from secretion systems fine-tune the host-beneficial bacteria (PGPRs) interaction. Front. Microbiol. 2024, 15, 1355750. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Peng, M.; Wang, Y.; Chen, Z.; Zhu, S. Preharvest hydrogen peroxide treatment delays leaf senescence of Chinese flowering cabbage during storage by reducing water loss and activating antioxidant defense system. Front. Plant Sci. 2022, 13, 856646. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Qureshi, M.K.; Gawroński, P.; Munir, S.; Jindal, S.; Kerchev, P. Hydrogen peroxide-induced stress acclimation in plants. Cell. Mol. Life Sci. 2022, 79, 129. [Google Scholar] [CrossRef]
- Bitterlich, M.; Franken, P.; Graefe, J. Atmospheric drought and low light impede mycorrhizal effects on leaf photosynthesis—A glasshouse study on tomato under naturally fluctuating environmental conditions. Mycorrhiza 2019, 29, 13–28. [Google Scholar] [CrossRef]
- Pan, J.; Huang, C.; Peng, F.; Zhang, W.; Luo, J.; Ma, S.; Xue, X. Effect of arbuscular mycorrhizal fungi (AMF) and plant growth-promoting bacteria (PGPR) inoculations on Elaeagnus angustifolia L. in saline soil. Appl. Sci. 2020, 10, 945. [Google Scholar] [CrossRef]
- Yang, J.; Luo, J.; Tian, X.; Zhao, Y.; Li, Y.; Wu, X. Progress in Understanding Oxidative Stress, Aging, and Aging-Related Diseases. Antioxidants 2024, 13, 394. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. The key roles of ROS and RNS as a signaling molecule in plant–microbe interactions. Antioxidants 2023, 12, 268. [Google Scholar] [CrossRef]
- Chasov, A.V.; Minibayeva, F.V. Effect of exogenous phenols on superoxide production by extracellular peroxidase from wheat seedling roots. Biochemistry 2009, 74, 766–774. [Google Scholar] [CrossRef]
- Aksenova, M.A.; Nechaeva, T.L.; Goncharuk, E.A.; Zubova, M.Y.; Kazantseva, V.V.; Lapshin, P.V.; Frolov, A.; Zagoskina, N.V. Changes in the Antioxidant Potential of Camellia sinensis Cultures under the Influence of Phenolic Precursors. Molecules 2024, 29, 474. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Goncharuk, E.A.; Zagoskina, N.V. Heavy metals, their phytotoxicity, and the role of phenolic antioxidants in plant stress responses with focus on cadmium. Molecules 2023, 28, 3921. [Google Scholar] [CrossRef]
- Tsivileva, O.M.; Perfileva, A.I. Selenium compounds biotransformed by mushrooms: Not only dietary sources, but also toxicity mediators. Curr. Nutr. Food Sci. 2017, 13, 82–96. [Google Scholar] [CrossRef]
- Pankratov, A.N.; Tsymbal, O.A.; Tsivileva, O.M.; Yurasov, N.A. Fragmentation Canals of Molecular Ions of GC-MS-Registered Components of Media of Shiitake Basidiomycete Submerged Cultivation in the Presence of Diacetophenonylselenide. Isomerization of Dihydrofurans and Their Cation Radicals. Izv. Saratov Univ. Chem. Biol. Ecol. 2015, 15, 16–25. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Tsivileva, O.M.; Koftin, O.V.; Anis’Kov, A.A.; Ibragimova, D.N. Selenium-Containing Nanobiocomposites of Fungal Origin Reduce the Viability and Biofilm Formation of the Bacterial Phytopathogen Clavibacter michiganensis subsp. sepedonicus. Nanotechnol. Russ. 2018, 13, 268–276. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Asakawa, T.; Matsushita, S. Coloring conditions of thiobarbituric acid test for detecting lipid hydroperoxides. Lipids 1980, 15, 137–140. [Google Scholar] [CrossRef]
- Tortora, M.L.; Díaz-Ricci, J.C.; Pedraza, R.O. Protection of strawberry plants (Fragaria ananassa Duch.) against anthracnose disease induced by Azospirillum brasilense. Plant Soil 2012, 356, 279–290. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.; Jeong, S.C.; Lee, S.; Khoo, C.; Koyyalamudi, S.R. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chin. Med. 2012, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Wu, Y.X.; von Tiedemann, A. Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ. Pollut. 2002, 116, 37–47. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, K.; Fu, Y.; Ma, H.; Zhu, F. Toxic action and baseline sensitivity of boscalid against Penicillium digitatum. Crop Prot. 2020, 137, 105272. [Google Scholar] [CrossRef]
- Mirshekari, A.; Madani, B.; Golding, J.B. Aloe vera gel treatment delays postharvest browning of white button mushroom (Agaricus bisporus). J. Food Meas. Charact. 2019, 13, 1250–1256. [Google Scholar] [CrossRef]
- García-Limones, C.; Hervás, A.; Navas-Cortés, J.A.; Jiménez-Díaz, R.M.; Tena, M. Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp. ciceris. Physiol. Mol. Plant Pathol. 2002, 61, 325–337. [Google Scholar] [CrossRef]
- Hanaka, A.; Nowak, A.; Plak, A.; Dresler, S.; Ozimek, E.; Jaroszuk-Ściseł, J.; Wójciak-Kosior, M.; Sowa, I. Bacterial isolate inhabiting Spitsbergen soil modifies the physiological response of Phaseolus coccineus in control conditions and under exogenous application of methyl jasmonate and copper excess. Int. J. Mol. Sci. 2019, 20, 1909. [Google Scholar] [CrossRef] [PubMed]
- Jaroszuk-Sciseł, J.; Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Majewska, M.; Hanaka, A.; Tyśkiewicz, K.; Pawlik, A.; Janusz, G. Phytohormones (auxin, gibberellin) and ACC deaminase in vitro synthesized by the mycoparasitic Trichoderma DEMTkZ3A0 strain and changes in the level of auxin and plant resistance markers in wheat seedlings inoculated with this strain conidia. Int. J. Mol. Sci. 2019, 20, 4923. [Google Scholar] [CrossRef] [PubMed]
- Sentchouk, V.V.; Grintsevich, E.E. Oxidation of benzidine and its derivatives by thyroid peroxidase. Biochemistry 2004, 69, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: II. Purification and quantitative relationship with water-soluble protein in seedlings. Plant Physiol. 1977, 59, 315–318. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| Assay Mode | Fungal Producent | Applied Concentration, mg/L | Coleoptile Length, cm | Leaf Length, cm | Root Length, cm | Root Number |
|---|---|---|---|---|---|---|
| Control | Water | 0 | 1.19 b–e | 4.77 b–g | 2.23 ef | 2.20 b–h |
| 1 | Armillaria mellea 0738 | 40 | 1.09 a–d | 4.71 b–f | 2.45 e–g | 2.20 b–h |
| 2 | Armillaria mellea 1346 | 40 | 0.98 ab | 4.17 b–e | 1.90 de | 2.30 d–h |
| 3 | Flammulina velutipes 0535 | 40 | 0.95 a | 6.39 h–j | 3.51 hi | 2.29 c–h |
| 4 | Flammulina velutipes 0535 | 80 | 1.07 a–c | 5.30 e–i | 1.88 de | 1.77 a–d |
| 5 | Ganoderma applanatum 0154 | 40 | 1.17 b–e | 4.90 c–g | 1.73 c–e | 1.72 a–c |
| 6 | Ganoderma applanatum SIE1304 | 40 | 0.92 a | 3.53 ab | 0.99 ab | 1.77 a–d |
| 7 | Ganoderma colossus SIE1301 | 40 | 1.10 a–d | 5.61 f–i | 3.41 hi | 2.24 c–h |
| 8 | Ganoderma colossus SIE1301 | 80 | 1.64 jk | 5.27 d–i | 1.38 b–d | 3.00 ij |
| 9 | Ganoderma lucidum 1315 | 40 | 0.93 a | 3.93 a–d | 0.97 ab | 1.94 a–e |
| 10 | Ganoderma lucidum SIE1303 | 40 | 1.18 b–e | 4.46 b–f | 1.45 b–d | 2.56 f–i |
| 11 | Ganoderma neojaponicum SIEbgm | 40 | 1.29 d–g | 6.36 h–j | 4.28 j | 1.48 a |
| 12 | Ganoderma neojaponicum SIEbidoup | 15 | 1.19 bcde | 5.66 fghi | 1.81 cde | 1.87 abcd |
| 13 | Ganoderma neojaponicum SIEbidoup | 40 | 1.69 jk | 7.63 j | 3.98 ij | 3.20 jk |
| 14 | Ganoderma neojaponicum SIEbidoup | 80 | 1.61 i–k | 6.46 ij | 3.20 h | 3.20 jk |
| 15 | Ganoderma valesiacum 120702 | 40 | 1.21 c–e | 5.61 f–i | 2.24 ef | 1.58 a |
| 16 | Grifola frondosa 0917 | 40 | 0.91 a | 4.56 b–f | 2.26 ef | 2.01 a–f |
| 17 | Grifola umbellata 1622 | 40 | 1.54 h–j | 6.55 ij | 2.91 f–h | 3.20 jk |
| 18 | Grifola umbellata 1622 | 80 | 1.21 c–e | 6.12 g–i | 2.36 ef | 1.82 a–d |
| 19 | Laetiporus sulphureus 120707 | 40 | 1.49 g–j | 5.05 c–h | 1.86 de | 3.00 ij |
| 20 | Laetiporus sulphureus 120707 | 80 | 1.35 e–h | 3.82 a–c | 0.99 ab | 2.73 h–j |
| 21 | Lentinula edodes 198 | 40 | 1.37 e–h | 4.37 b–f | 1.11 a–c | 3.13 j |
| 22 | Lentinula edodes F-249 | 40 | 0.91 a | 6.52 ij | 3.16 h | 2.16 b–g |
| 23 | Lentinula edodes F-249 | 80 | 1.16 b–e | 5.34 e–i | 3.06 gh | 2.44 e–h |
| 24 | Pleurotus ostreatus 69 | 15 | 1.04 a–c | 4.58 b–f | 1.33 b–d | 1.67 ab |
| 25 | Pleurotus ostreatus 69 | 40 | 1.79 k | 6.57 ij | 3.12 gh | 3.27 jk |
| 26 | Pleurotus ostreatus 69 | 80 | 1.43 f–i | 5.55 f–i | 3.38 hi | 3.00 ij |
| 27 | Pleurotus ostreatus BK1702 | 40 | 0.90 a | 4.11 b–e | 0.53 a | 2.59 g–i |
| 28 | Pleurotus ostreatus HK352 | 40 | 1.03 a–c | 4.81 b–g | 1.20 a–d | 1.87 a–d |
| 29 | Tomophagus cattienensis SIE1302 | 40 | 1.25 c–f | 2.80 a | 0.95 ab | 3.67 k |
| Fungal Producent | Applied Concent- Ration, mg/L | Sample Group | Biochemical Variable | Coleoptile Length R (p-Value) | Leaf Length R (p-value) | Root Length R (p-Value) | Root Number R (p-Value) |
|---|---|---|---|---|---|---|---|
| Water | 0 | I | MDA level | ns * | ns | −0.2635 (0.2310) | ns |
| Flammulina velutipes 0535 | 40 | ||||||
| Ganoderma colossus SIE1301 | 40 | Phenol content | −0.2619 (0.2324) | −0.5485 (0.0503) | −0.7406 (0.0071) | +0.4087 (0.1205) | |
| Ganoderma neojaponicum SIEbgm | 40 | ||||||
| Ganoderma neojaponicum SIEbidoup | 40 | H2O2 concent- ration | −0.4129 (0.1178) | +0.3527 (0.1588) | +0.2330 (0.2586) | −0.4308 (0.1069) | |
| Grifola umbellata 1622 | 40 | ||||||
| Laetiporus sulphureus 120707 | 40 | Pox activity | ns | +0.4567 (0.0923) | +0.5058 (0.0679) | +0.2818 (0.2151) | |
| Lentinula edodes F-249 | 40 | ||||||
| Pleurotus ostreatus 69 | 40 | SOD activity | +0.3671 (0.1483) | ns | ns | +0.4520 (0.0948) | |
| Tomophagus cattienensis SIE1302 | 40 | ||||||
| Water | 0 | II | MDA level | −0.5403 (0.0834) | ns | −0.3540 (0.1948) | −0.7244 (0.0211) |
| Flammulina velutipes 0535 | 80 | ||||||
| Ganoderma colossus SIE1301 | 80 | Phenol content | ns | −0.6455 (0.0419) | −0.8190 (0.0064) | ns | |
| Ganoderma neojaponicum SIEbidoup | 80 | ||||||
| H2O2 concent- ration | ns | +0.4840 (0.1121) | +0.3505 (0.1973) | ns | |||
| Grifola umbellata 1622 | 80 | ||||||
| Laetiporus sulphureus 120707 | 80 | Pox activity | +0.4173 (0.1518) | +0.5751 (0.0679) | +0.7543 (0.0153) | +0.4810 (0.1138) | |
| Lentinula edodes F-249 | 80 | SOD activity | +0.4592 (0.1262) | +0.4099 (0.1566) | +0.7135 (0.0234) | +0.4194 (0.1505) | |
| Pleurotus ostreatus 69 | 80 | ||||||
| Water | 0 | III | MDA level | −0.9406 (0.1103) | −0.9454 (0.1056) | −0.9712 (0.0766) | −0.9933 (0.0368) |
| Ganoderma neojaponicum SIEbidoup | 15 | Phenol content | −0.6287 (0.2836) | ns | ns | −0.4343 (0.3570) | |
| H2O2 concent- ration | +0.8660 (0.1667) | +0.9881 (0.0492) | +0.9980 (0.0403) | +0.9574 (0.0933) | |||
| Pleurotus ostreatus 69 | 15 | Pox activity | +0.6287 (0.2836) | ns | ns | +0.4343 (0.3570) | |
| SOD activity | +0.9962 (0.0277) | +0.8303 (0.1882) | +0.8776 (0.1591) | +0.9897 (0.0457) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsivileva, O.; Shaternikov, A.; Evseeva, N. Basidiomycetes Polysaccharides Regulate Growth and Antioxidant Defense System in Wheat. Int. J. Mol. Sci. 2024, 25, 6877. https://doi.org/10.3390/ijms25136877
Tsivileva O, Shaternikov A, Evseeva N. Basidiomycetes Polysaccharides Regulate Growth and Antioxidant Defense System in Wheat. International Journal of Molecular Sciences. 2024; 25(13):6877. https://doi.org/10.3390/ijms25136877
Chicago/Turabian StyleTsivileva, Olga, Andrei Shaternikov, and Nina Evseeva. 2024. "Basidiomycetes Polysaccharides Regulate Growth and Antioxidant Defense System in Wheat" International Journal of Molecular Sciences 25, no. 13: 6877. https://doi.org/10.3390/ijms25136877
APA StyleTsivileva, O., Shaternikov, A., & Evseeva, N. (2024). Basidiomycetes Polysaccharides Regulate Growth and Antioxidant Defense System in Wheat. International Journal of Molecular Sciences, 25(13), 6877. https://doi.org/10.3390/ijms25136877






