Biofungicides Based on Plant Extracts: On the Road to Organic Farming
Abstract
1. Introduction
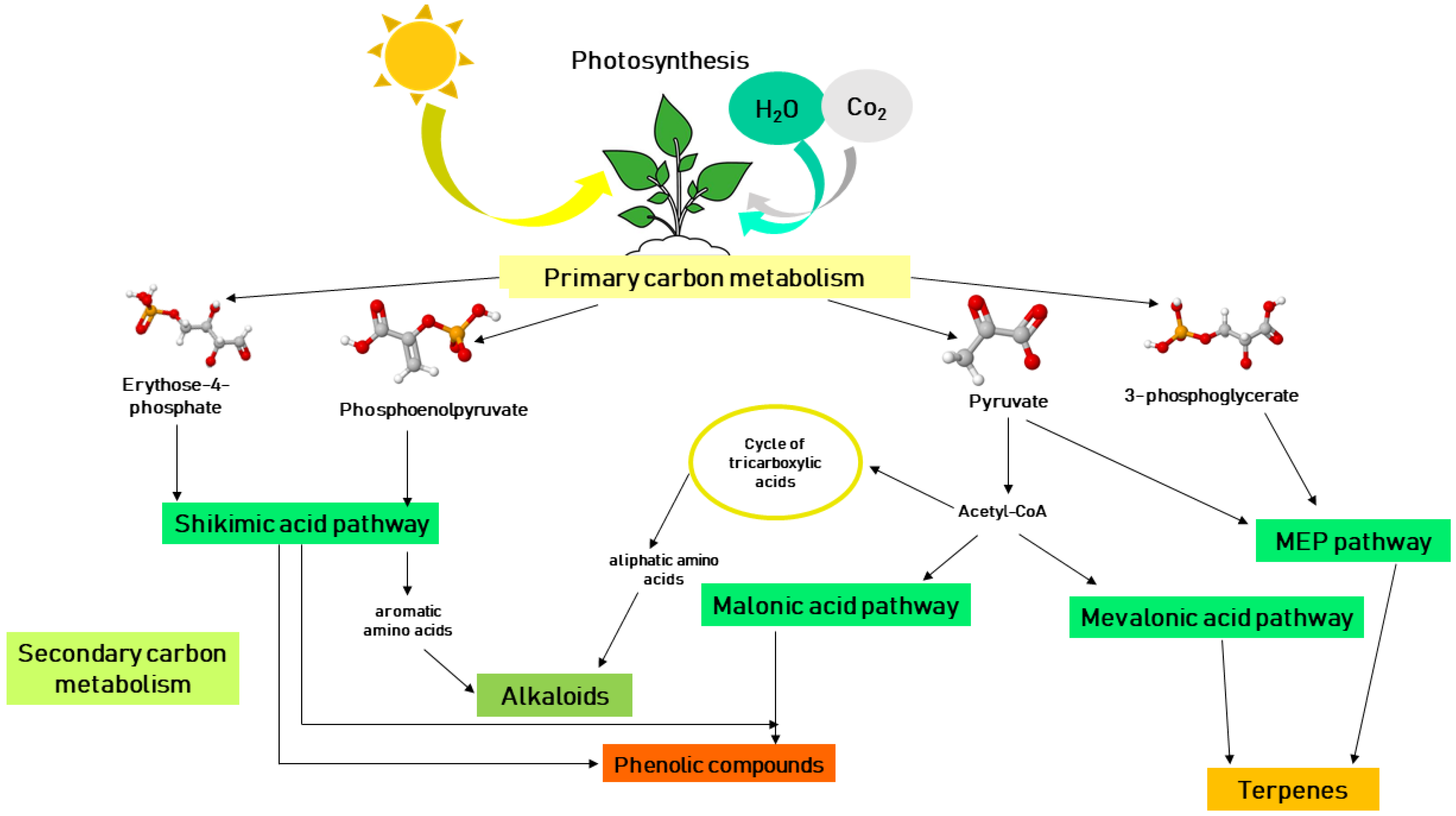
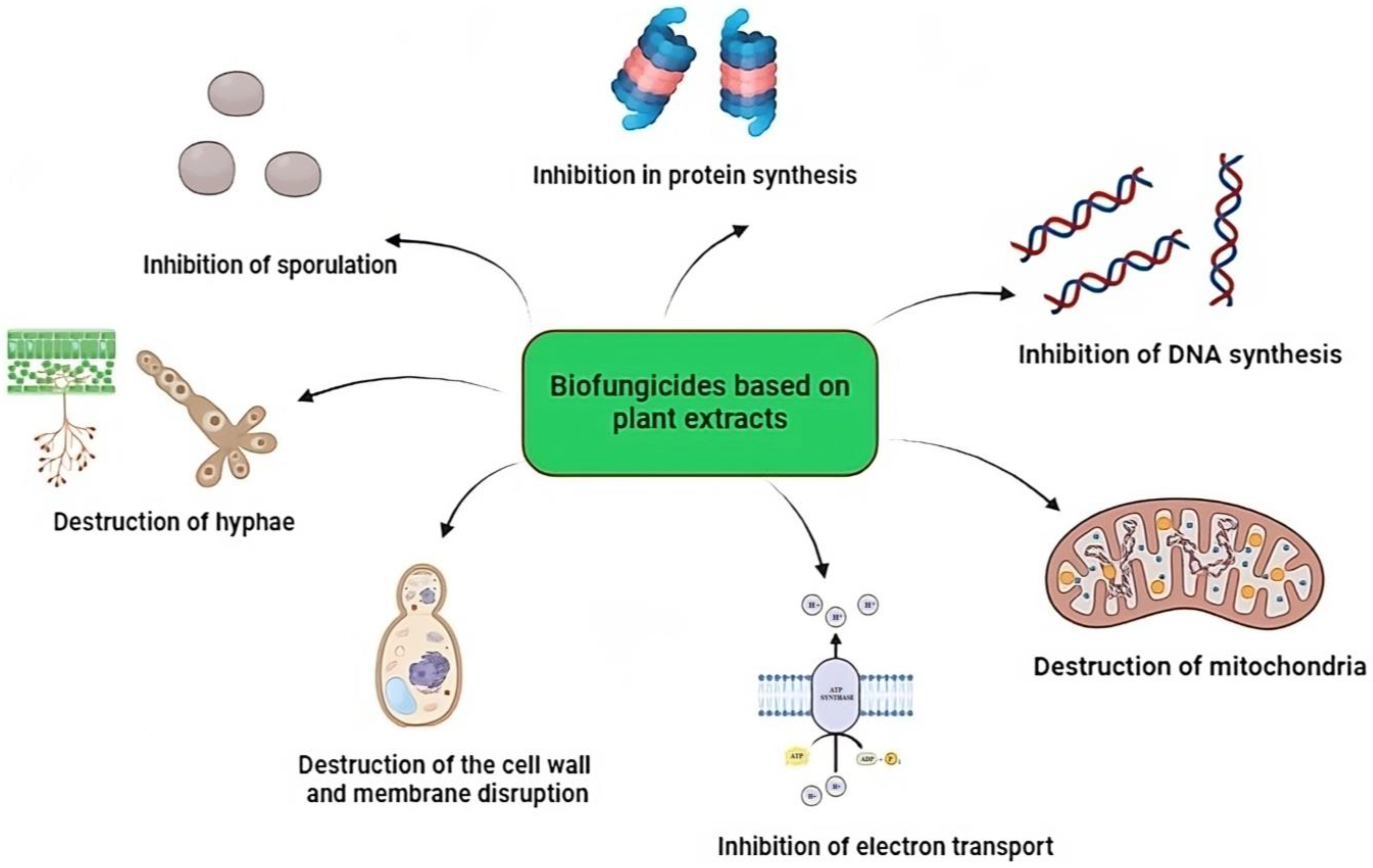
2. Biosynthesis of Secondary Metabolites in Plants and Their Mechanisms of Action against Phytopathogenic Fungi
3. Terpenes
4. Alkaloids
5. Phenolic Compounds
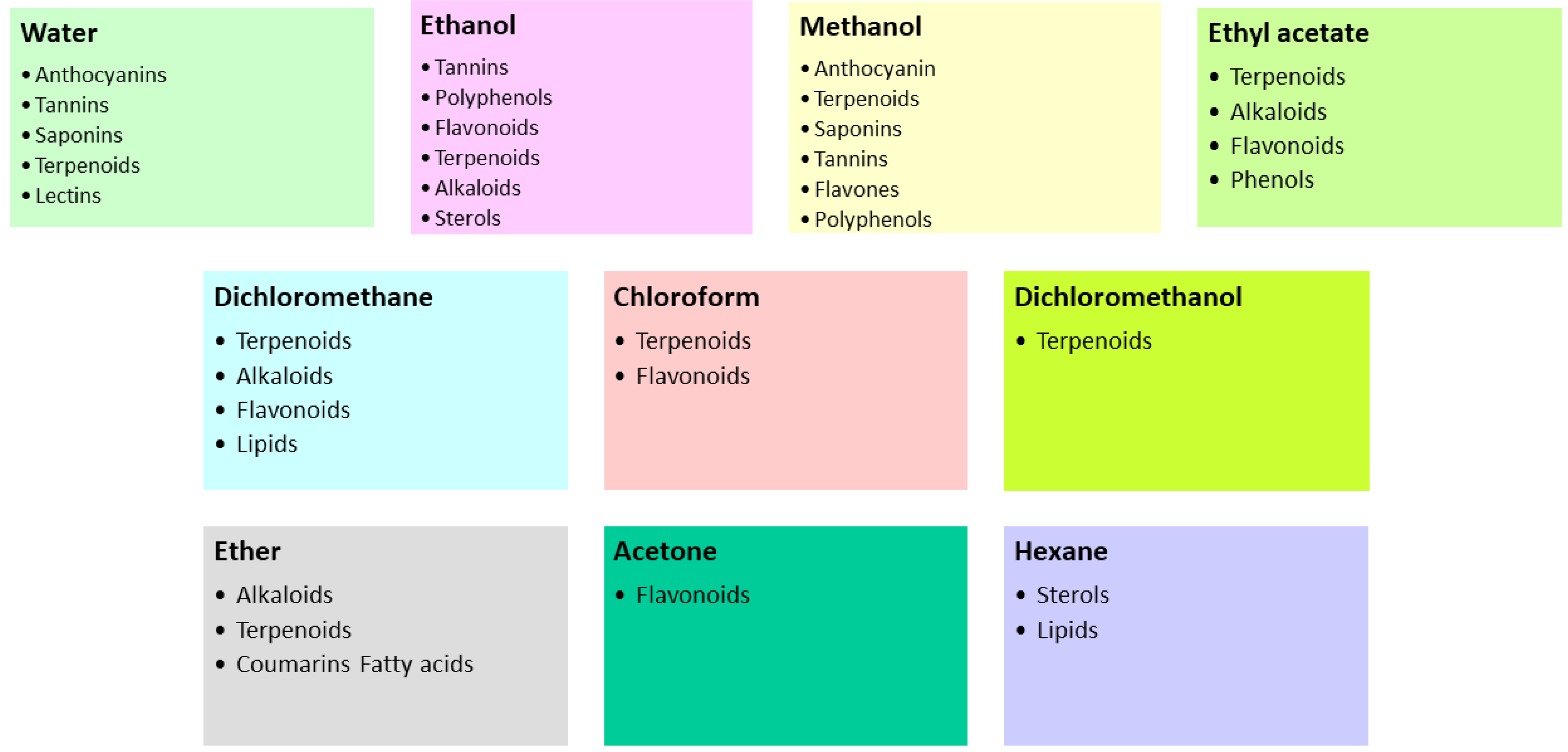
6. Methods for Extraction of Secondary Metabolites and Formulation of Biofungicides
7. Conventional Techniques
7.1. Soxhlet Extraction
7.2. Maceration
7.3. Hydrodistillation
8. Unconventional Techniques
8.1. Ultrasound-Assisted Extraction (UAE)
8.2. Pulsed Electric Field Extraction (PEF)
8.3. Enzyme-Assisted Extraction (EAE)
8.4. Microwave-Assisted Extraction (MAE)
8.5. Pressurized Liquid Extraction (PLE)
8.6. Supercritical Fluid Extraction (SFE)
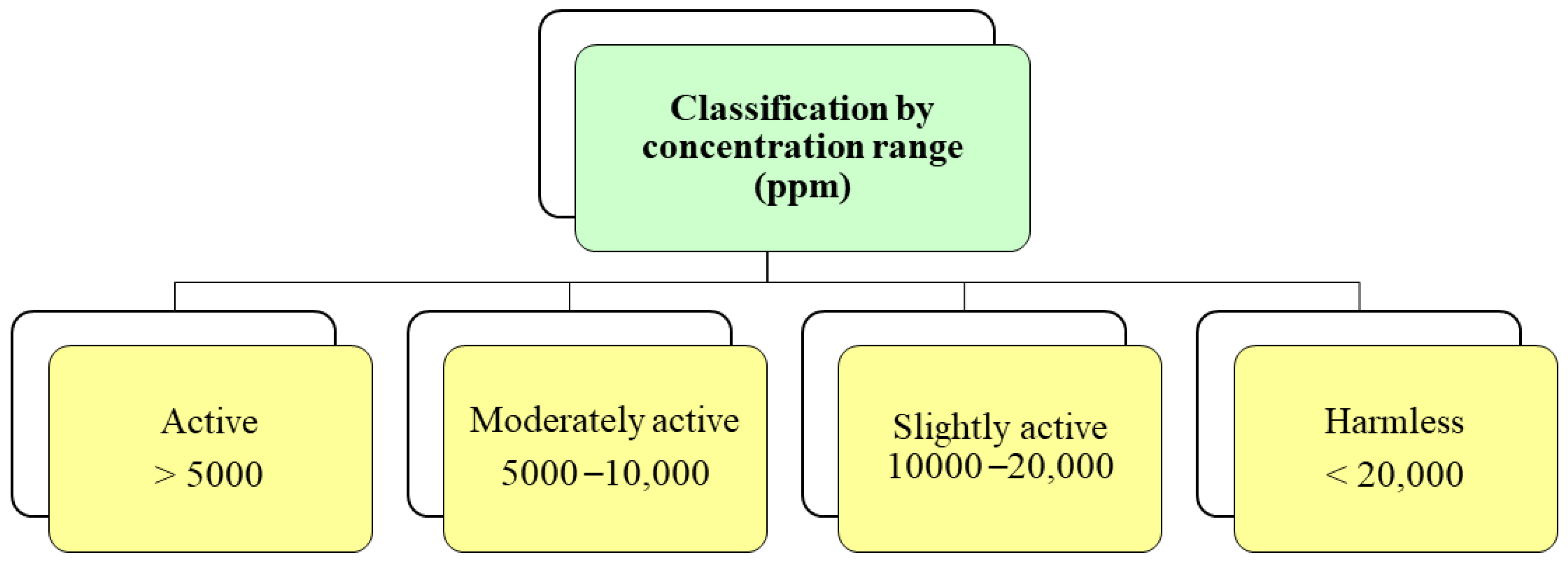
9. Biofungicides in the Control of Phytopathogenic Fungi
10. Regulation, Advantages, Disadvantages, and Current Panorama of Biofungicides in Agriculture
11. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Ray, M.K.; Mishra, A.K.; Mohanta, Y.K.; Mahanta, S.; Chakrabartty, I.; Kungwani, N.A.; Pudake, R.N. Nanotechnology as a promising tool against phytopathogens: A futuristic approach to agriculture. Agriculture 2023, 13, 1856. [Google Scholar] [CrossRef]
- Košćak, L.; Lamovšek, J.; Đermić, E.; Prgomet, I.; Godena, S. Microbial and plant-based compounds as alternatives for the control of phytopathogenic bacteria. Horticulturae 2023, 9, 1124. [Google Scholar] [CrossRef]
- De Mello-Sampayo, C.; Viana, P.; Lopes, A.; Carvalho da Silva, R.; de Jesus, R.; Sarmento, G.; Meisel, L. Survey of Antifungal in Surface-and Groundwater: A Portuguese Environmental Case Study. Sustainability 2024, 16, 594. [Google Scholar] [CrossRef]
- Lengai, G.M.; Fulano, A.M.; Muthomi, J.W. Improving access to export market for fresh vegetables through reduction of phytosanitary and pesticide residue constraints. Sustainability 2022, 14, 8183. [Google Scholar] [CrossRef]
- Boonupara, T.; Udomkun, P.; Khan, E.; Kajitvichyanukul, P. Airborne pesticides from agricultural practices: A critical review of pathways, influencing factors, and human health implications. Toxics 2023, 11, 858. [Google Scholar] [CrossRef] [PubMed]
- Pânzaru, R.L.; Firoiu, D.; Ionescu, G.H.; Ciobanu, A.; Medelete, D.M.; Pîrvu, R. Organic Agriculture in the Context of 2030 Agenda Implementation in European Union Countries. Sustainability 2023, 15, 10582. [Google Scholar] [CrossRef]
- Joshi, N.; Bhattarai, K.; Sinha, S.; Rawat, B.; Rai, N.; Anand, J.; Rawat, J.M. Production of secondary metabolites from medicinal plants through tissue culture. In Secondary Metabolites and Biotherapeutics, 1st ed.; Kumar, A., Kumar, S., Eds.; Academic Press: Kolkata, India, 2024; Volume 1, pp. 63–77. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S.B. From Nature to lab: A review of secondary metabolite biosynthetic pathways, environmental influences, and in vitro approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, K.; Chakravarthi, G.; Rai, S.; Singh, P.; Mishra, S.; Mishra, D.; Tiwari, N. Biochemical characterization of plant secondary metabolites. In Secondary Metabolites and Biotherapeutics, 1st ed.; Kumar, A., Kumar, S., Eds.; Academic Press: Kolkata, India, 2024; Volume 1, pp. 39–61. [Google Scholar] [CrossRef]
- Barthwal, R.; Mahar, R. Exploring the Significance, Extraction, and Characterization of Plant-Derived Secondary Metabolites in Complex Mixtures. Metabolites 2024, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Verma, S.K.; Shyam, P. Identification and purification of plant secondary metabolite as medicinal raw materials. In Secondary Metabolites and Biotherapeutics, 1st ed.; Kumar, A., Kumar, S., Eds.; Academic Press: Kolkata, India, 2024; Volume 1, pp. 9–38. [Google Scholar] [CrossRef]
- Hernandez-Tenorio, F.; Miranda, A.M.; Rodríguez, C.A.; Giraldo-Estrada, C.; Sáez, A.A. Potential strategies in the biopesticide formulations: A bibliometric analysis. Agronomy 2022, 12, 2665. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of botanical pesticides in agriculture as an alternative to synthetic pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Šunjka, D.; Mechora, Š. An alternative source of biopesticides and improvement in their formulation—Recent advances. Plants 2022, 11, 3172. [Google Scholar] [CrossRef] [PubMed]
- Almeida, N.A.; Freire, L.; Carnielli-Queiroz, L.; Bragotto, A.P.; Silva, N.C.; Rocha, L.O. Essential oils: An eco-friendly alternative for controlling toxigenic fungi in cereal grains. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13251. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhang, M.; Tang, S.; Li, M.; Wu, R.; Wan, S.; Li, F. Research Progress on Benzimidazole Fungicides: A Review. Molecules 2024, 29, 1218. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.; Singh, R.; Muthusamy, S.; Sharma, M.; Grewal, K.; Singh, H.P.; Batish, D.R. Plant essential oils as biopesticides: Applications, mechanisms, innovations, and constraints. Plants 2023, 12, 2916. [Google Scholar] [CrossRef] [PubMed]
- Twaij, B.M.; Hasan, M.N. Bioactive secondary metabolites from plant sources: Types, synthesis, and their therapeutic uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Kumari, S.; Nazir, F.; Maheshwari, C.; Kaur, H.; Gupta, R.; Siddique, K.H.; Khan, M.I.R. Plant hormones and secondary metabolites under environmental stresses: Shedding light on defense molecules. Plant Physiol. Biochem. 2024, 206, 108238. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy. 2021, 11, 968. [Google Scholar] [CrossRef]
- Chen, R.; Wang, M.; Keasling, J.D.; Hu, T.; Yin, X. Expanding the structural diversity of terpenes by synthetic biology approaches. Trends Biotechnol. 2024, 42, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, J.D.; Chang, C.Y. Terpene synthases in disguise: Enzymology, structure, and opportunities of non-canonical terpene synthases. Nat Prod Rep. 2020, 37, 425–463. [Google Scholar] [CrossRef]
- Li, R.L.; Zhang, Q.; Liu, J.; He, L.Y.; Huang, Q.W.; Peng, W.; Wu, C.J. Processing methods and mechanisms for alkaloid-rich Chinese herbal medicines: A review. J. Integr. Med. 2021, 19, 89–103. [Google Scholar] [CrossRef]
- Manna, K.; Debnath, B.; Singh, W.S. Major metabolites of certain marketed plant alkaloids. Front. Nat. Prod. Chem. 2020, 6, 124–150. [Google Scholar] [CrossRef]
- Samira, O.; Laila, B.; Moussa, N.A.; Mohamed, I.; Devkota, K.; Abdelhakim, B.; Said, G. Recent advances in the extraction of bioactive compounds from plant matrices and their use as potential antioxidants for vegetable oils enrichment. J. Food Compos. Anal. 2024, 128, 105995. [Google Scholar] [CrossRef]
- Jiang, Y.; Fang, Z.; Leonard, W.; Zhang, P. Phenolic compounds in Lycium berry: Composition, health benefits and industrial applications. J. Funct. Foods 2021, 77, 104340. [Google Scholar] [CrossRef]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Hasanuzzaman, M. Diverse physiological roles of flavonoids in plant environmental stress responses and tolerance. Plants 2022, 11, 3158. [Google Scholar] [CrossRef] [PubMed]
- Contreras Martínez, O.I.; Angulo Ortíz, A.; Santafé Patiño, G.; Peñata-Taborda, A.; Berrio Soto, R. Isoespintanol Antifungal Activity Involves Mitochondrial Dysfunction, Inhibition of Biofilm Formation, and Damage to Cell Wall Integrity in Candida tropicalis. Int. J. Mol. Sci. 2023, 24, 10187. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, L.; Du, F.; Sun, L.; Shi, J.; Long, M.; Chen, Z. Activity and mechanism of action of antifungal peptides from microorganisms: A review. Molecules 2021, 26, 3438. [Google Scholar] [CrossRef]
- Hang, S.; Lu, H.; Jiang, Y. Marine-Derived Metabolites Act as Promising Antifungal Agents. Marine Drugs 2024, 22, 180. [Google Scholar] [CrossRef] [PubMed]
- da Silva, W.P.; Lopes, G.V.; Ramires, T.; Kleinubing, N.R. May phenolics mitigate the antimicrobial resistance in foodborne pathogens? Curr. Opin. Food Sci. 2023, 25, 101107. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, S.; Liu, J. Recent advances in chitin biosynthesis associated with the morphology and secondary metabolite synthesis of filamentous fungi in submerged fermentation. J. Fungi 2023, 9, 205. [Google Scholar] [CrossRef]
- Sulaiman, M.; Jannat, K.; Nissapatorn, V.; Rahmatullah, M.; Paul, A.K.; de Lourdes Pereira, M.; Wiart, C. Antibacterial and antifungal alkaloids from Asian angiosperms: Distribution, mechanisms of action, structure-activity, and clinical potentials. Antibiotics 2022, 11, 1146. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Wong, K.S.; Fong, W.P.; Tsang, P.W.K. Metergoline-induced cell death in Candida krusei. Fungal Biol. 2011, 115, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Chen, Y.; Wang, Y. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS ONE 2012, 7, e30147. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Z.; Chang, W.Q.; Cheng, A.X.; Sun, L.M.; Lou, H.X. Plagiochin E, an antifungal active macrocyclic bis (bibenzyl), induced apoptosis in Candida albicans through a metacaspase-dependent apoptotic pathway. Biochim. Biophys. Acta 2010, 1800, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Bagiu, R.V.; Vlaicu, B.; Butnariu, M. Chemical composition and in vitro antifungal activity screening of the Allium ursinum L. (Liliaceae). Int. J. Mol. Sci. 2012, 13, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Henao Castañeda, I.; Pereañez, J.A.; Preciado, L.M.; Jios, J. Sulfur compounds as inhibitors of enzymatic activity of a snake venom phospholipase A2: Benzyl 4-nitrobenzenecarbodithioate as a case of study. Molecules 2020, 25, 1373. [Google Scholar] [CrossRef] [PubMed]
- Kubec, R.; Dadáková, E. Chromatographic methods for determination of S-substituted cysteine derivatives—A comparative study. J. Chromatogr. A 2009, 1216, 6957–6963. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.; Whiteman, M.; Moore, P.K.; Zhu, Y.Z. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: The chemistry of potential therapeutic agents. Nat. Prod. Rep. 2005, 22, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Jikah, A.N.; Edo, G.I. Mechanisms of Action by Sulphur Compounds in Allium sativum. A Review. Pharmacol. Res.-Mod. Chin. Med. 2023, 9, 100323. [Google Scholar] [CrossRef]
- Massi, F.; Torriani, S.; Borghi, L.; Toffolatti, S.L. Fungicide resistance evolution and detection in plant pathogens: Plasmopara viticola as a Case Study. Microorganisms 2021, 9, 119. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Akinola, S.A.; Adeleke, B.S.; Gbadegesin, L.A.; Adejumo, G.D.; Glick, B.R.; Babalola, O.O. Biopesticides versus synthetic pesticides usage in Africa. Microbiome-Based Decontam. Environ. Pollut. 2024, 1, 417–450. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Baroi, A.M.; Ortan, A. Selected aspects related to medicinal and aromatic plants as alternative sources of bioactive compounds. Int. J. Mol. Sci. 2021, 22, 1521. [Google Scholar] [CrossRef]
- Selvamuthukumaran, M.; Shi, J. Recent advances in extraction of antioxidants from plant by-products processing industries. Food Qual. Saf. 2017, 1, 61–81. [Google Scholar] [CrossRef]
- Gopalasatheeskumar, K. Significant role of soxhlet extraction process in phytochemical research. MJPMS 2018, 7, 43–47. [Google Scholar]
- Dhawan, D.; Gupta, J. Research article comparison of different solvents for phytochemical extraction potential from datura metel plant leaves. Int. J. Biol. Chem. 2017, 11, 17–22. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, J.; Dai, X.; Li, X. Extraction and Analysis of Chemical Compositions of Natural Products and Plants. Separations 2023, 10, 598. [Google Scholar] [CrossRef]
- Verep, D.; Ateş, S.; Karaoğul, E. A Review of Extraction Methods for Obtaining Bioactive Compounds in Plant-Based Raw Materials. Bartın Orman Fakültesi Derg. 2023, 25, 492–513. [Google Scholar] [CrossRef]
- Keyes, C.A.; Giltrow, K.R.; Mahon, T.J. A comparison of maceration methods for the preparation of infant skeletal remains for forensic anthropological analysis. Int. J. Leg. 2024, 138, 1085–1092. [Google Scholar] [CrossRef]
- Naviglio, D.; Trifuoggi, M.; Varchetta, F.; Nebbioso, V.; Perrone, A.; Avolio, L.; Gallo, M. Efficiency of Recovery of the Bioactive Principles of Plants by Comparison between Solid–Liquid Extraction in Mixture and Single-Vegetable Matrices via Maceration and RSLDE. Plants 2023, 12, 2900. [Google Scholar] [CrossRef]
- Dunkić, V.; Nazlić, M.; Ruščić, M.; Vuko, E.; Akrap, K.; Topić, S.; Kremer, D. Hydrodistillation and Microwave Extraction of Volatile Compounds: Comparing Data for Twenty-One Veronica Species from Different Habitats. Plants 2022, 11, 902. [Google Scholar] [CrossRef]
- Katekar, V.P.; Rao, A.B.; Sardeshpande, V.R. A hydrodistillation-based essential oils extraction: A quest for the most effective and cleaner technology. Sustain. Chem. Pharm. 2023, 36, 101270. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem 2021, 70, 105325. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Ren, X. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 35, 100547. [Google Scholar] [CrossRef]
- Polachini, T.C.; Norwood, E.A.; Le-Bail, P.; Le-Bail, A.; Cárcel, J.A. Pulsed electric field (PEF) application on wheat malting process: Effect on hydration kinetics, germination and amylase expression. IFSET 2023, 86, 103375. [Google Scholar] [CrossRef]
- Ghoshal, G. Comprehensive review in pulsed electric field (PEF) in food preservation: Gaps in current studies for potential future research. Heliyon 2023, 9, e17532. [Google Scholar] [CrossRef]
- Sanjeewa, K.A.; Herath, K.H.I.N.M.; Kim, Y.S.; Jeon, Y.J.; Kim, S.K. Enzyme-assisted extraction of bioactive compounds from seaweeds and microalgae. TrAC Trends Anal. Chem. 2023, 167, 117266. [Google Scholar] [CrossRef]
- Choulot, M.; Michalak, I.; Jing, L.; Szymczycha-Madeja, A.; Wełna, M.; Bourgougnon, N.; Le Guillard, C. The Enzyme-Assisted Extraction of compounds of interest in agriculture: Case study of the red seaweed Solieria chordalis (C. Agardh) J. Agardh. Algal Res. 2023, 75, 103239. [Google Scholar] [CrossRef]
- Mabate, B.; Pletschke, B.I. Sequential and enzyme-assisted extraction of algal bioproducts from Ecklonia maxima. Enzyme Mcrob. Technol. 2024, 173, 110364. [Google Scholar] [CrossRef] [PubMed]
- Giap, V.D.; Nhan, N.T. Study on enzyme-assisted extraction of the total phenolic content, vitamin C and antioxidant activity from Beta vulgaris var. rubra. Vietnam J. Chem. 2023, 61, 134–139. [Google Scholar] [CrossRef]
- López-Salazar, H.; Camacho-Díaz, B.H.; Ocampo, M.L.A.; Jiménez-Aparicio, A.R. Microwave-assisted extraction of functional compounds from plants: A Review. Bioresources 2023, 18, 6614. [Google Scholar] [CrossRef]
- Adeel, S.; Azeem, M.; Habib, N.; Hussaan, M.; Kiran, A.; Haji, A.; Haddar, W. Sustainable application of microwave assisted extracted tea based tannin natural dye for chemical and bio-mordanted wool fabric. J. Nat. Fibers 2023, 20, 2136322. [Google Scholar] [CrossRef]
- Vo, T.P.; Pham, T.V.; Tran, T.N.H.; Vo, L.T.V.; Vu, T.T.; Pham, N.D.; Nguyen, D.Q. Ultrasonic-assisted and microwave-assisted extraction of phenolics and terpenoids from abelmoschus sagittifolius (kurz) merr roots using natural deep eutectic solvents. ACS Omega 2023, 8, 29704–29716. [Google Scholar] [CrossRef] [PubMed]
- Barp, L.; Višnjevec, A.M.; Moret, S. Pressurized Liquid Extraction: A powerful tool to implement extraction and purification of food contaminants. Foods 2023, 12, 2017. [Google Scholar] [CrossRef] [PubMed]
- Leonarski, E.; Kuasnei, M.; Moraes, P.A.D.; Cesca, K.; de Oliveira, D.; Zielinski, A.A.F. Pressurized liquid extraction as an eco-friendly approach to recover anthocyanin from black rice bran. IFSET 2023, 86, 103372. [Google Scholar] [CrossRef]
- Banafi, A.; Wee, S.K.; Tiong, A.N.T.; Kong, Z.Y.; Saptoro, A.; Sunarso, J. Modeling of supercritical fluid extraction bed: A critical review. Chem. Eng. Res. Des. 2023, 193, 685–712. [Google Scholar] [CrossRef]
- Atwi-Ghaddar, S.; Destandau, E.; Lesellier, E. Optimization of supercritical fluid extraction of polar flavonoids from Robinia pseudoacacia L. heartwood. J. CO2 Util. 2023, 70, 102440. [Google Scholar] [CrossRef]
- Chañi-Paucar, L.O.; dos Santos, L.C.; Scopel, E.; Torres-Mayanga, P.C.; Hatami, T.; Martínez, J. Supercritical fluid extraction of bioactive compounds from quinilla (Manilkara bidentata) seed. J. Supercrit. Fluids 2023, 193, 105831. [Google Scholar] [CrossRef]
- Larocca, V.; Martino, M.; Trupo, M.; Magarelli, R.A.; Spagnoletta, A.; Ambrico, A. Evaluation of carbon dioxide supercritical fluid extraction (CO2-SFE) on carotenoids recovery from red yeast cells. Biomass Convers. Biorefinery 2023, 1, 1–10. [Google Scholar] [CrossRef]
- Toffolatti, S.L.; Davillerd, Y.; D’Isita, I.; Facchinelli, C.; Germinara, G.S.; Ippolito, A.; Romanazzi, G. Are Basic Substances a Key to Sustainable Pest and Disease Management in Agriculture? An Open Field Perspective. Plants 2023, 12, 3152. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, D.; Ianiri, G.; Del Grosso, C.; Barone, G.; De Curtis, F.; Castoria, R.; Lima, G. Advances and perspectives in the use of biocontrol agents against fungal plant diseases. Horticulturae 2022, 8, 577. [Google Scholar] [CrossRef]
- Tripathi, Y.N.; Divyanshu, K.; Kumar, S.; Jaiswal, L.K.; Khan, A.; Birla, H.; Upadhyay, R.S. Biopesticides: Current Status and Future Prospects in India. Bioeconomy Sustain. Dev. 2020, 1, 79–109. [Google Scholar] [CrossRef]
- Al-Mekhlafi, F.A.; Abutaha, N.; Farooq, M.; Wadaan, M.A.; Al-Khalifa, M.S. Laboratory evaluation of the effects of Portunus pelagicus extracts against Culex pipiens larvae and aquatic non-target organisms. J. King Saud. Univ. Sci. 2023, 35, 102924. [Google Scholar] [CrossRef]
- Rana, A.K.; Kaur, K.; Vyas, P. Biofungicides and plant growth promoters: Advantages and opportunities in entrepreneurship. Entrep. Microorg. 2024, 1, 259–277. [Google Scholar] [CrossRef]
- Teixeira, A.; Sánchez-Hernández, E.; Noversa, J.; Cunha, A.; Cortez, I.; Marques, G.; Oliveira, R. Antifungal activity of plant waste extracts against phytopathogenic fungi: Allium sativum peels extract as a promising product targeting the fungal plasma membrane and cell wall. Horticulturae 2023, 9, 136. [Google Scholar] [CrossRef]
- Pane, C.; Manganiello, G.; Vitti, A.; Celano, R.; Piccinelli, A.L.; De Falco, E. Phytochemical Extracts of Dittrichia viscosa (L.) Greuter from Agroecological Systems: Seed Antigerminative Properties and Effectiveness in Counteracting Alternaria Leaf Spot Disease on Baby-Leaf Spinach. Biology 2023, 12, 790. [Google Scholar] [CrossRef]
- Cruz, A.; Sánchez-Hernández, E.; Teixeira, A.; Oliveira, R.; Cunha, A.; Martín-Ramos, P. Phytoconstituents and Ergosterol Biosynthesis-Targeting Antimicrobial Activity of Nutmeg (Myristica fragans Houtt.) against Phytopathogens. Molecules 2024, 29, 471. [Google Scholar] [CrossRef]
- Cruz, A.; Sánchez-Hernández, E.; Teixeira, A.; Martín-Ramos, P.; Cunha, A.; Oliveira, R. Antifungal and Antioomycete Activities of a Curcuma longa L. Hydroethanolic Extract Rich in Bisabolene Sesquiterpenoids. Horticulturae 2024, 10, 124. [Google Scholar] [CrossRef]
- Sobhy, S.; Al-Askar, A.A.; Bakhiet, E.K.; Elsharkawy, M.M.; Arishi, A.A.; Behiry, S.I.; Abdelkhalek, A. Phytochemical Characterization and Antifungal Efficacy of Camphor (Cinnamomum camphora L.) Extract against Phytopathogenic Fungi. Separations 2023, 10, 189. [Google Scholar] [CrossRef]
- Salas-Gómez, A.L.; Espinoza Ahumada, C.A.; Castillo Godina, R.G.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Segura Martínez, M.T.D.J.; Osorio-Hernández, E. Antifungal In Vitro Activity of Phoradendron sp. Extracts on Fungal Isolates from Tomato Crop. Plants 2023, 12, 672. [Google Scholar] [CrossRef] [PubMed]
- Al-Askar, A.A.; Bashir, S.; Mohamed, A.E.; Sharaf, O.A.; Nabil, R.; Su, Y.; Behiry, S.I. Antimicrobial Efficacy and HPLC Analysis of Polyphenolic Compounds in a Whole-Plant Extract of Eryngium campestre. Separations 2023, 10, 362. [Google Scholar] [CrossRef]
- García-Ramírez, E.; Contreras-Oliva, A.; Salinas-Ruiz, J.; Hernández-Ramírez, G.; Spinoso-Castillo, J.L.; Colmenares Cuevas, S.I. Plant Extracts Control In Vitro Growth of Disease-Causing Fungi in Chayote. Plants 2023, 12, 1800. [Google Scholar] [CrossRef]
- Hernández-Ceja, A.; Loeza-Lara, P.D.; Espinosa-García, F.J.; García-Rodríguez, Y.M.; Medina-Medrano, J.R.; Gutiérrez-Hernández, G.F.; Ceja-Torres, L.F. In vitro antifungal activity of plant extracts on pathogenic fungi of blueberry (Vaccinium sp.). Plants 2021, 10, 852. [Google Scholar] [CrossRef]
- Ordóñez, Y.F.; Ruano, J.; Avila, P.; Berutti, L.; Guerrero, P.C.; Ordóñez, P.E. In Vitro Antimicrobial Activity of Plant Species against the Phytopathogens Ralstonia solanacearum, Phytophthora infestans, and Neopestalotiopsis javaensis. Agriculture 2023, 13, 2029. [Google Scholar] [CrossRef]
- Hernández, A.; Ruiz-Moyano, S.; Galván, A.I.; Merchán, A.V.; Nevado, F.P.; Aranda, E.; Martín, A. Anti-fungal activity of phenolic sweet orange peel extract for controlling fungi responsible for post-harvest fruit decay. Fungal Biol. 2020, 2, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, P.G.; Ramírez, D.G.; Mejía, E.Z.; Ocampo, S.A.; Díaz, C.N.; Martínez, R.I.R. Extracts of Stevia rebaudiana against Fusarium oxysporum associated with tomato cultivation. Sci. Hortic. 2020, 259, 108683. [Google Scholar] [CrossRef]
- Gasca, C.A.; Dassoler, M.; Brand, G.D.; de Medeiros Nóbrega, Y.K.; Gomes, S.M.; Jamal, C.M.; Silveira, D. Chemical composition and antifungal effect of ethanol extract from Sapindus saponaria L. fruit against banana anthracnose. Sci. Hortic. 2020, 259, 108842. [Google Scholar] [CrossRef]
- Dudoit, A.; Mertz, C.; Chillet, M.; Cardinault, N.; Brat, P. Antifungal activity of Brazilian red propolis extract and isolation of bioactive fractions by thin-layer chromatography-bioautography. Food Chem. 2020, 1, 127060. [Google Scholar] [CrossRef] [PubMed]
- Elkhetabi, A.; Lahlali, R.; Askarne, L.; Ezrari, S.; El Ghadaroui, L.; Tahiri, A.; Amiri, S. Efficacy assessment of pomegranate peel aqueous extract for brown rot (Monilinia spp.) disease control. Physiol. Mol. Plant Pathol. 2020, 110, 101482. [Google Scholar] [CrossRef]
- Liang, C.; Yang, L.; Shao, Y.; Zhu, X.; Zhao, H.; Chen, B.; Sun, R. Broad-spectrum antifungal activity of dichloromethane extract of Waltheria indica stems and isolated compounds. Ind. Crops Prod. 2019, 142, 111855. [Google Scholar] [CrossRef]
- Hernández- Soto, I.; Prieto-Méndez, J.; Aquino Torres, E.; Madariaga Navarrete, A.; Reyes Santamaría, M.I.; Pacheco Trejo, J. Evaluation of the effect of the methanolic extract of Argemone ochroleuca for environmentally friendly control of Colletotrichum gloeosporioides, Fusarium oxysporum and Rhizoctonia solani. Cienc. Tec. Vitivinic. 2018, 33, 65–74. [Google Scholar]
- Pazolini, K.; dos Santos, I.; Giaretta, R.D.; Marcondes, M.M.; Reiner, D.A.; Citadin, I. The use of brassica extracts and thermotherapy for the postharvest control of brown rot in peach. Sci. Hortic. 2016, 209, 41–46. [Google Scholar] [CrossRef]
- De Rodríguez, D.J.; Trejo-González, F.A.; Rodríguez-García, R.; Díaz-Jimenez, M.L.V.; Sáenz-Galindo, A.; Hernández-Castillo, F.D.; Peña-Ramos, F.M. Antifungal activity in vitro of Rhus muelleri against Fusarium oxysporum f. sp. lycopersici. Ind. Crops Prod. 2015, 75, 150–158. [Google Scholar] [CrossRef]
- Al-Rahmah, A.N.; Mostafa, A.A.; Abdel-Megeed, A.; Yakout, S.M.; Hussein, S.A. Fungicidal activities of certain methanolic plant extracts against tomato phytopathogenic fungi. Afr. J. Microbiol. Res. 2013, 7, 517–524. [Google Scholar]
- De Rodríguez, D.J.; García, R.R.; Castillo, F.H.; González, C.A.; Galindo, A.S.; Quintanilla, J.V.; Zuccolotto, L.M. In vitro antifungal activity of extracts of Mexican Chihuahuan Desert plants against postharvest fruit fungi. Ind. Crops Prod. 2011, 34, 960–966. [Google Scholar] [CrossRef]
- Tabanca, N.; Demirci, B.; Crockett, S.L.; Başer, K.H.C.; Wedge, D.E. Chemical composition and antifungal activity of Arnica longifolia, Aster hesperius, and Chrysothamnus nauseosus essential oils. J. Agric. Food Chem. 2007, 55, 8430–8435. [Google Scholar] [CrossRef]
- Cáceres Rueda de León, I.; Colorado Vargas, R.; Salas Muñoz, E.; Muñoz Castellanos, L.N.; Hernández Ochoa, L. Actividad Antifúngica in vitro de Extractos Acuosos de Especias contra Fusarium oxysporum, Alternaría alternata, Geotrichum candidum, Trichoderma spp., Penicillum digitatum y Aspergillus niger. Rev. Mex. Fitopatol. 2013, 31, 105–112. [Google Scholar]
- Hernández, T.; Canales, M.; García, A.M.; Duran, Á.; Meráz, S.; Dávila, P.; Ávila, J.G. Antifungal activity of the essential oils of two verbenaceae: Lantana achyranthifolia and Lippia graveolens of Zapotitlán de las Salinas, Puebla (México). Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas. 2008, 7, 202–206. [Google Scholar]
- Jaramillo, B.E.; Duarte, E.; Delgado, W. Bioactividad del aceite esencial de Chenopodium ambrosioides colombiano. Rev. Cubana Plant Med. 2012, 17, 54–64. [Google Scholar]
- De Rodríguez, D.J.; Salas-Méndez, E.D.J.; Rodríguez-García, R.; Hernández-Castillo, F.D.; Díaz-Jiménez, M.L.V.; Sáenz-Galindo, A.; Carrillo-Lomelí, D.A. Antifungal activity in vitro of ethanol and aqueous extracts of leaves and branches of Flourensia spp. against postharvest fungi. Ind. Crops Prod. 2017, 107, 499–508. [Google Scholar] [CrossRef]
- Vogt, V.; Cifuente, D.; Tonn, C.; Sabini, L.; Rosas, S. Antifungal activity in vitro and in vivo of extracts and lignans isolated from Larrea divaricata Cav. against phytopathogenic Fungi. Ind. Crops Prod. 2013, 42, 583–586. [Google Scholar] [CrossRef]
- Al-Askar, A.A. In vitro antifungal activity of three Saudi plant extracts against some phytopathogenic fungi. JACB 2012, 3, 277–284. [Google Scholar] [CrossRef]
- Bruni, R.; Medici, A.; Andreotti, E.; Fantin, C.; Muzzoli, M.; Dehesa, M.; Sacchetti, G. Chemical composition and biological activities of Ishpingo essential oil, a traditional Ecuadorian spice from Ocotea quixos (Lam.) Kosterm.(Lauraceae) flower calices. Food Chem. 2004, 85, 415–421. [Google Scholar] [CrossRef]
- Toledo, E.; Félix, C.; Vicente, T.F.; Augusto, A.; Félix, R.; Toledo, B.; Lemos, M.F. Seaweed extracts to control postharvest phytopathogenic fungi in Rocha Pear. J. Fungi 2023, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Astacio, J.D.; Espeso, E.A.; Melgarejo, P.; De Cal, A. Monilinia fructicola Response to White Light. J. Fungi 2023, 9, 988. [Google Scholar] [CrossRef]
- Baltazar, E.; Rodrigues, S.; Ares, A.; Camelo, A.; Brandão, I.; Espirito Santo, C.; Costa, J. Morphological, Molecular and Genomic Identification and Characterisation of Monilinia fructicola in Prunus persica from Portugal. Agronomy 2023, 13, 1493. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, W.; Han, X.; Wu, B.; Song, Z.; Shi, J. Plant glycerol suppresses brown rot of peach fruit by enhancing disease resistance. Physiol. Mol. Plant Pathol. 2024, 129, 102204. [Google Scholar] [CrossRef]
- Elsherbiny, A.S.; Galal, A.; Ghoneem, K.M.; Salahuddin, N.A. Graphene oxide-based nanocomposites for outstanding eco-friendly antifungal potential against tomato phytopathogens. Biomater. Adv. 2024, 160, 213863. [Google Scholar] [CrossRef] [PubMed]
- Lieu, M.D.; Phuong, T.V.; Nguyen, T.T.B.; Dang, T.K.T.; Nguyen, T.H. A review of preservation approaches for extending avocado fruit shelf-life. J. Agric. Food Res. 2024, 16, 101102. [Google Scholar] [CrossRef]
- Peralta-Ruiz, Y.; Rossi, C.; Grande-Tovar, C.D.; Chaves-López, C. Green management of postharvest anthracnose caused by Colletotrichum gloeosporioides. J. Fungi 2023, 9, 623. [Google Scholar] [CrossRef]
- Hsieh, T.F.; Shen, Y.M.; Huang, J.H.; Tsai, J.N.; Lu, M.T.; Lin, C.P. Insights into Grape Ripe Rot: A Focus on the Colletotrichum gloeosporioides Species Complex and Its Management Strategies. Plants 2023, 12, 2873. [Google Scholar] [CrossRef] [PubMed]
- Nasimi, Z.; Barriuso, J.; Keshavarz, T.; Zheng, A. Molecular, physiological, and biochemical properties of sclerotia metamorphosis in Rhizoctonia solani. Fungal Biol. Rev. 2024, 48, 100351. [Google Scholar] [CrossRef]
- Abbas, A.; Ali, A.; Hussain, A.; Ali, A.; Alrefaei, A.F.; Naqvi, S.A.H.; Baloch, F.S. Assessment of Genetic Variability and Evolutionary Relationships of Rhizoctonia solani Inherent in Legume Crops. Plants 2023, 12, 2515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Zheng, X.R.; Li, H.; Chen, F.M. Alternaria alternata, the causal agent of a new needle blight disease on Pinus bungeana. J. Fungi 2024, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, S.; Jo, M.; An, S.; Kim, Y.; Yoon, J.; Park, S.Y. Isolation and Identification of Alternaria alternata from Potato Plants Affected by Leaf Spot Disease in Korea: Selection of Effective Fungicides. J. Fungi 2024, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Mesa, A.M.; Marin, P.A.; Ocampo, O.; Calle, J.; Monsalve Fonnegra, Z.I. Fungicidas a partir de extractos vegetales: Una alternativa en el manejo integrado de hongos fitopatógenos. Rev. Investig. Agropecu. 2019, 45, 23–30. [Google Scholar]
- Hernández-Soto, I.; González-García, Y.; Juárez-Maldonado, A.; Hernández-Fuentes, A.D. Impact of Argemone mexicana L. on tomato plants infected with Phytophthora infestans. PeerJ 2024, 12, e16666. [Google Scholar] [CrossRef]
- El Khetabi, A.; Lahlali, R.; Ezrari, S.; Radouane, N.; Lyousfi, N.; Banani, H.; Barka, E.A. Role of plant extracts and essential oils in fighting against postharvest fruit pathogens and extending fruit shelf life: A review. Trends Food Sci. 2022, 120, 402–417. [Google Scholar] [CrossRef]
- Dos Santos Gomes, A.C.; da Silva, R.R.; Moreira, S.I.; Vicentini, S.N.; Ceresini, P.C. Biofungicides: An Eco-Friendly Approach for Plant Disease Management. Encycl. Mycol. 2021, 2, 641–649. [Google Scholar] [CrossRef]
- Ahmed, H.F.; Seleiman, M.F.; Mohamed, I.A.; Taha, R.S.; Wasonga, D.O.; Battaglia, M.L. Activity of essential oils and plant extracts as biofungicides for suppression of soil-borne fungi associated with root rot and wilt of marigold (Calendula officinalis L.). Horticulturae 2023, 9, 222. [Google Scholar] [CrossRef]
- McLaughlin, M.S.; Roy, M.; Abbasi, P.A.; Carisse, O.; Yurgel, S.N.; Ali, S. Why Do We Need Alternative Methods for Fungal Disease Management in Plants? Plants 2023, 12, 3822. [Google Scholar] [CrossRef]
- Matrose, N.A.; Obikeze, K.; Belay, Z.A.; Caleb, O.J. Plant extracts and other natural compounds as alternatives for post-harvest management of fruit fungal pathogens: A review. Food Biosci. 2021, 41, 100840. [Google Scholar] [CrossRef]
- Khadiri, M.; Boubaker, H.; Lahmamsi, H.; Taoussi, M.; Ezzouggari, R.; Askarne, L.; Lahlali, R. Challenges in apple preservation: Fungicide resistance and emerging biocontrols. Physiol. Mol. Plant Pathol. 2023, 129, 102205. [Google Scholar] [CrossRef]
- Daraban, G.M.; Hlihor, R.M.; Suteu, D. Pesticides vs. Biopesticides: From Pest Management to Toxicity and Impacts on the Environment and Human Health. Toxics 2023, 11, 983. [Google Scholar] [CrossRef]
- Meena, R.K.; Mishra, P. Bio-pesticides for agriculture and environment sustainability. In Resources Use Efficiency in Agriculture; Springer: Singapore, 2020; pp. 85–107. [Google Scholar] [CrossRef]
- Liu, H.W.; Begley, T. Comprehensive Natural Products III; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–633. [Google Scholar]
- Villaverde, J.J.; Sandín-España, P.; Sevilla-Morán, B.; López-Goti, C.; Alonso-Prados, J.L. Biopesticides from Natural Products: Current Development, Legislative Framework, and Future Trends. BioResources 2016, 11, 5618–5640. [Google Scholar] [CrossRef]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Ntougias, S. Status and prospects of botanical biopesticides in Europe and Mediterranean countries. Biomolecules 2022, 12, 311. [Google Scholar] [CrossRef]
- DAFF (Department of Agriculture, Fisheries and Forestry). Guidelines on the Data Required for Registration of Biological/Biopesticides Remedies in South Africa. 2015. Available online: https://www.nda.agric.za/doaDev/sideMenu/ActNo36_1947/AIC/Guidelines%20for%20Registration%20of%20Biological%20Remedies%202015%20Registrar%20of%20Act%2036%20of%201947.pdf (accessed on 5 June 2024).
- Pest Control Products Act. Available online: http://kenyalaw.org/kl/fileadmin/pdfdownloads/Acts/PestControlProductsAct__Cap346_.pdf (accessed on 5 June 2024).
- NAFDAC (National Agency for Food and Drug Administration and Control). Biopesticide Registration Regulations. 2019. Available online: https://www.nafdac.gov.ng/wp-content/uploads/Files/Resources/Regulations/New_Draft_Regulations/Bio-Pesticides-Registraton-Regulations.pdf (accessed on 5 June 2024).
- Panday, D.; Bhusal, N.; Das, S.; Ghalehgolabbehbahani, A. Rooted in Nature: The Rise, Challenges, and Potential of Organic Farming and Fertilizers in Agroecosystems. Sustainability 2024, 16, 1530. [Google Scholar] [CrossRef]
- Ashaolu, C.A.; Okonkwo, C.O.; Njuguna, E.; Ndolo, D. Recommendations for effective and sustainable regulation of biopesticides in Nigeria. Sustainability 2022, 14, 2846. [Google Scholar] [CrossRef]
- Benbrook, C.M.; Benbrook, R. A minimum data set for tracking changes in pesticide use. In Herbicides; Elsevier: Amsterdam, The Netherlands, 2021; pp. 21–39. [Google Scholar] [CrossRef]
- Priya, A.K.; Alagumalai, A.; Balaji, D.; Song, H. Bio-based agricultural products: A sustainable alternative to agrochemicals for promoting a circular economy. RSC Sustain. 2023, 1, 746–762. [Google Scholar] [CrossRef]
- Mesnage, R.; Benbrook, C. Use of the concept ‘environmentally relevant level’ in linking the results of pesticide toxicity studies to public health outcomes. All Life 2023, 16, 2167872. [Google Scholar] [CrossRef]
| Techniques | |
|---|---|
| Conventional Extraction | Non-Conventional Extraction |
| Soxhlet extraction | Ultrasound-assisted extraction (UAE) |
| Maceration | Pulsed electric field extraction (PEF) |
| Hydrodistillation | Enzyme-assisted extraction (EAE) |
| Microwave-assisted extraction (MAE) | |
| Pressurized liquid extraction (PLE) | |
| Supercritical fluid extraction (SFE) | |
| Phytopathogenic Fungus | Disease | Vegetable Extract | Bioactive Compound with Biological Activity | Reference |
|---|---|---|---|---|
| Colletotrichum acutatum | Anthracnose | Ethanolic Garlic peel extract | Allyl trisulfide; allyl methyl trisulfide; allyl disulfide; allyl trans-1-propenyl disulfide; allyl methyl sulfide; and 2-vinyl-4H-1,3-dithiine | [81] |
| Alternaria alternata | Leaf-spot | Aqueous Dittrichia viscosa L. extract | Caffeoyl quinic acids; methoxylated flavonoids; sesquiterpene | [82] |
| Botrytis cinerea; Colletotrichum acutatum; Diplodia corticola; Phytophthora cinnamomic; Fusarium culmorum | Gray mold; anthracnose; trunk canker; chestnut ink; vascular wilting | Hydroethanolic extract Myristica fragans Houtt | Tetradecanoic acid, 9-octadecenoic acid; n-hexadecanoic acid; dodecanoic acid; octadecanoic acid; veratone; gelsevirine; and montanine | [83] |
| Botrytis cinerea; Colletotrichum acutatum; Diplodia corticola; Phytophthora cinnamomic; Fusarium culmorum | Gray mold; anthracnose; trunk canker; chestnut ink; vascular wilting | Hydroethanolic Curcuma longa L. extract | β-turmerone; α-turmerone; -ar-turmerone; α-atlantone; γ-curcumene; zingiberene; isoelemicin; and gibberellin | [84] |
| Alternaria alternata; Fusarium solani; Fusarium oxysporum | Black mold; vascular wilting | Methanolic Cinnamomum camphora (L.) J. Presl extract | Mono(2-ethylhexyl) ester of 1,2-benzene dicarboxylic acid | [85] |
| Alternaria alternata; Fusarium oxysporum; Rhizoctonia solani | Black mold; vascular wilting; damping-off | Polyphenol extracts: Mesquite (Prosopis glandulosa Torr), Cedar (Cedrus Trew), and Oak (Quercus L.) | Anthocyanins; flavonols and flavones | [86] |
| Fusarium oxysporum; Rhizoctonia solani | Vascular wilting; damping-off | Methanolic Eryngium campestre L. extract | Benzoic acid; catechol; quercetin; vanillic acid; resveratrol; naringenin; and quinol | [87] |
| Fusarium oxysporum, Fusarium solani Phytophthora capsici | Vascular wilting; pepper blight | Essential oil: Cinnamon (Cinnamomum zeylanicum J. Presl), Neem (Azadirachta indica A.Juss ) oil, Sapote (Diospyros digyna (J.F.Gmel.) Perr) extract | Cinnamaldehyde; kaempferol; cinnamic alcohol; alkaloids; essential oils; polyphenols; tannins; and saponins | [88] |
| Pestalotiopsis clavispora; Colletotrichum gloeosporioides; Lasiodiplodia pseudotheobromae | Crown rot; antracnosis; gummosis | Mixture of ethanol and ethyl acetate: Lantana hirta L., Argemone ochroleuca L. and Adenophyllum porophyllum (Cav.) extract | L. hirta extract: phytol and α-Sitosterol. In A. ochroleuca: toluene and benzene, 1,3-bis(3-phenoxyphenoxy)-. In A. porophyllum: hexanedioic acid, bis(2-ethylhexyl) ester | [89] |
| Ralstonia solanacearu; Phytophthora infestans; Neopestalotiopsis javaensis | Banana bacterial wilt; late blight; scab diseases | Methanol Pernettya prostrata (Cav.) and Rubus roseus Schott | Phenolic compounds | [90] |
| Monilinia fructicola; Botrytis cinerea; Alternaria alternata | Brown rot; gray mold; black mold | Orange (Citrus × sinensis L.) peel polyphenolic extract | Ferulic acid and p-coumaric acid | [91] |
| Fusarium oxysporum | Vascular wilting | Hexane extract of Stevia rebaudiana Bertoni leaves | Austroinulin | [92] |
| Colletotrichum spp. | Anthracnose | Ethanolic extract of the fruit of Sapindus saponaria L. | Saponin 3-O-(β-d-xylopyranosyl)-(1→3)-α-l-rhamnopyranosyl- (1→2)-α-l-;arabinopyranosyl hederagenin and acyclic sesquiterpene oligoglycoside | [93] |
| Colletotrichum musae | Anthracnose | Propolis ethanolic extract of Brazilian Red (Sesuvium portulacastrum L.) | Medicarpin, (3S)-vestitol, and (3S)-neovestitol | [94] |
| Monilinia laxa; M. fructigena | Brown rot | Pomegranate (Punica granatum L.) peel aqueous extract | Phenolic and flavonoid compounds | [95] |
| Botryosphaeria dothidea; Colletotrichum musae; Pestalotipsis guepinii; Colletotrichum orbiculare; Phylophthora nicotianae; Pestalotiopsis longiseta; Sclerotinia sclerotiorum | Canker; anthracnose; leaf spots; anthracnose; late blight; peduncular rots; white mold | The dichloromethane extract of Waltheria Indica L. | Alkaloids; antidesmone; and waltherione C | [96] |
| Colletotrichum gloesoporioides, Fusarium oxysporum, Rhizoctonia solani | Anthracnose; vascular wilting; damping-off | Methanol extract of leaves of Argemone ochroleuca L. | Berberine; Isoquinoline; Ehydrocorydalmine; and Oxyberberine | [97] |
| Monilinia fructicola | Brown rot | Aqueous extracts of Brassica napus L. and Brassica juncea L. | Glucosinolates: nitriles, thiocyanates, epinitriles; but, mainly, isothiocyanates | [98] |
| Fusarium oxysporum | Vascular wilting | Ethanolic extract of Rhus muelleri Müller | Ethyl iso-allocholate (steroid); 7,8-epoxylanostan-11-ol,3-actoxy (alcoholic compound); and 3-trifluoro acetoxy pentadecane (flourcompound) | [99] |
| Fusarium oxysporum; Pythium aphanidermatum; Rhizoctonia solani | Vascular wilting; mildew; damping-off | Methanolic extracts of the leaves of Thymus vulgaris L. and Zingiber officinale (Rosc., shengjiang) | Timol; carvacrol; β cimeno; α-terpinoleno; gingerol; cedreno; zingibereno; and αcurcumeno | [100] |
| Rhizopus stolonifer; Colletotricum gloesporoides; Penicillium digitatum | Soft rot; anthracnose; green rot | Ethanolic and hexane extracts of Lippia graveolens Kunth, Agave lechuguilla Torr, Yucca carnerosana (Trel.) Mc Kelvey, and Yucca filifera Chaub | Saponins, tannins, and flavonoids | [101] |
| Colletotrichum acutatum; C. fragariae; C. gloeosporioides | Anthracnose | Essential oil of Arnica longifolia L., Aster hesparius Michx, and Chrysothamnus nauseous L. | R-bisabolol and carvacrol | [102] |
| Fusarium oxysporum; Alternaria alternata; Geotrichum candidum; Penicillum digitatum; Aspergillus niger | Vascular wilting; black mold; rot; green mold; black mold | Aqueous extracts of Lippia berlandieri L. | Eugenol | [103] |
| Fusarium monoliforme; Rhizoctonia solani | Panicle blight; damping-off | Essential oil of Lantana achyranthifolia L. and Lippia graveolens L. | Carvacrol; α-bisabolol, isocaryophyllene, and thymol | [104] |
| Fusarium oxysporum | Vascular wilting | Essential oil of Chenopodium ambrosioides L. | α-terpinene; P-cimene; 4-carene; and Trans-ascaridol | [105] |
| Alternaria sp.; Rhizoctonia solani; Fusarium oxysporum | Leaf spots; damping-off; vascular wilting | Ethanol extracts of Flourensia microphylla (A.Gray), Flourensia cernua S.F. Blake, Flourensia retinophylla S.F. Blake | Benzofurans, benzopyrans, dehydrofluorenic acid, flourensadiol, methyl orselinate | [106] |
| Fusarium graminearum; Fusarium solani; Fusarium verticillioides; Macrophomina phaseolina | Head blight; stem base rot in vegetables; ear rot; collar rot in soybeans | Chloroform Extract of Larrea divaricata | Apigenine-7-methylether; nordihydroguaiaretic acid; 3,4’-dihydroxy-3’,4-dimethoxy-6,7’-cyclolignan | [107] |
| Alternaria alternata, Fusarium oxysporum, Phoma destructive, Rhizoctonia solani Sclerotium rolfsii | Black mold; vascular wilting; leaf spot; damping-off; root and stem rot | The ethanolic extract of seeds of Alhagi maurorum Medik | Flavonoids; glycosides; alkaloids; saponins; tannins; steroids; and anthraquinone | [108] |
| Phytium ultimum | Root rot | Essential oil of Ocotea quixos Lauraceae Lam | trans-Cinnamaldehyde | [109] |
| Alternaria alternata; Botrytis cinerea; Fusarium oxysporum; Penicillium expansum | Black mold; gray mold; vascular wilting; blue mold | Five different extracts of each seaweed (n-hexane, ethyl acetate, aqueous, ethanolic, and hydroethanolic) | Polysaccharides commonly present in seaweeds, such as laminarin fucoidans or alginates and phlorotannins | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cenobio-Galindo, A.d.J.; Hernández-Fuentes, A.D.; González-Lemus, U.; Zaldívar-Ortega, A.K.; González-Montiel, L.; Madariaga-Navarrete, A.; Hernández-Soto, I. Biofungicides Based on Plant Extracts: On the Road to Organic Farming. Int. J. Mol. Sci. 2024, 25, 6879. https://doi.org/10.3390/ijms25136879
Cenobio-Galindo AdJ, Hernández-Fuentes AD, González-Lemus U, Zaldívar-Ortega AK, González-Montiel L, Madariaga-Navarrete A, Hernández-Soto I. Biofungicides Based on Plant Extracts: On the Road to Organic Farming. International Journal of Molecular Sciences. 2024; 25(13):6879. https://doi.org/10.3390/ijms25136879
Chicago/Turabian StyleCenobio-Galindo, Antonio de Jesús, Alma Delia Hernández-Fuentes, Uriel González-Lemus, Ana Karen Zaldívar-Ortega, Lucio González-Montiel, Alfredo Madariaga-Navarrete, and Iridiam Hernández-Soto. 2024. "Biofungicides Based on Plant Extracts: On the Road to Organic Farming" International Journal of Molecular Sciences 25, no. 13: 6879. https://doi.org/10.3390/ijms25136879
APA StyleCenobio-Galindo, A. d. J., Hernández-Fuentes, A. D., González-Lemus, U., Zaldívar-Ortega, A. K., González-Montiel, L., Madariaga-Navarrete, A., & Hernández-Soto, I. (2024). Biofungicides Based on Plant Extracts: On the Road to Organic Farming. International Journal of Molecular Sciences, 25(13), 6879. https://doi.org/10.3390/ijms25136879







