Differential Rates of Glycation Following Exposure to Unique Monosaccharides
Abstract
1. Introduction
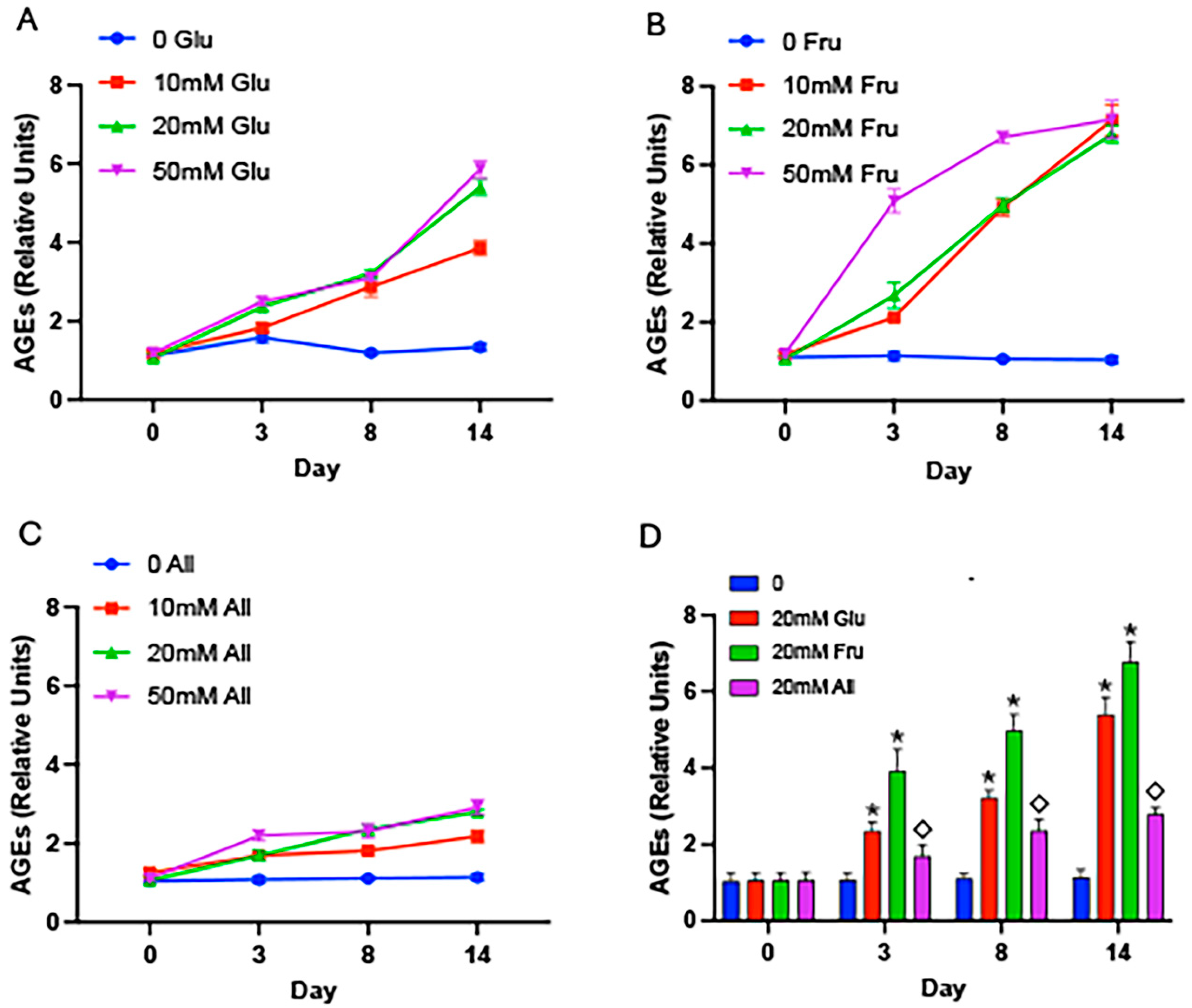
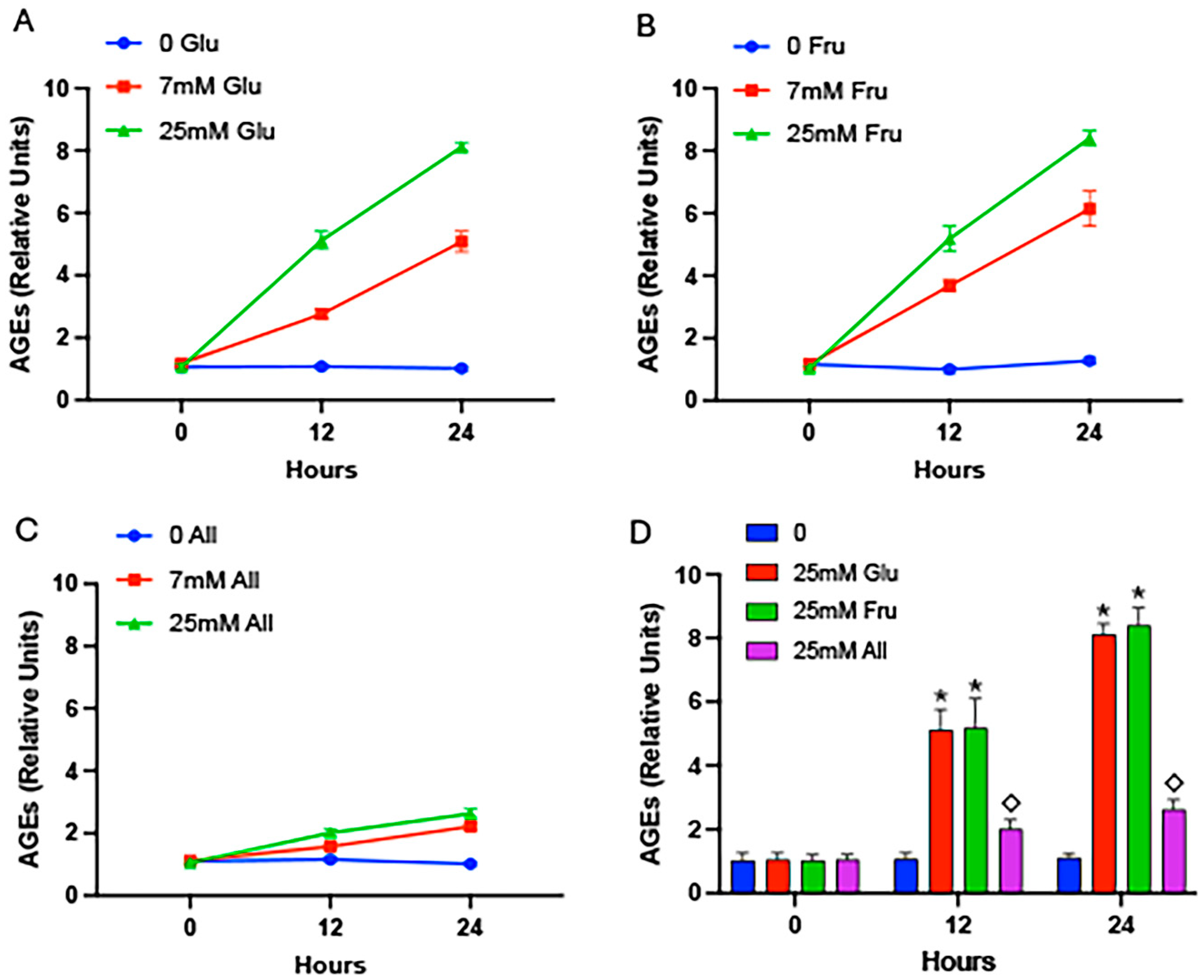
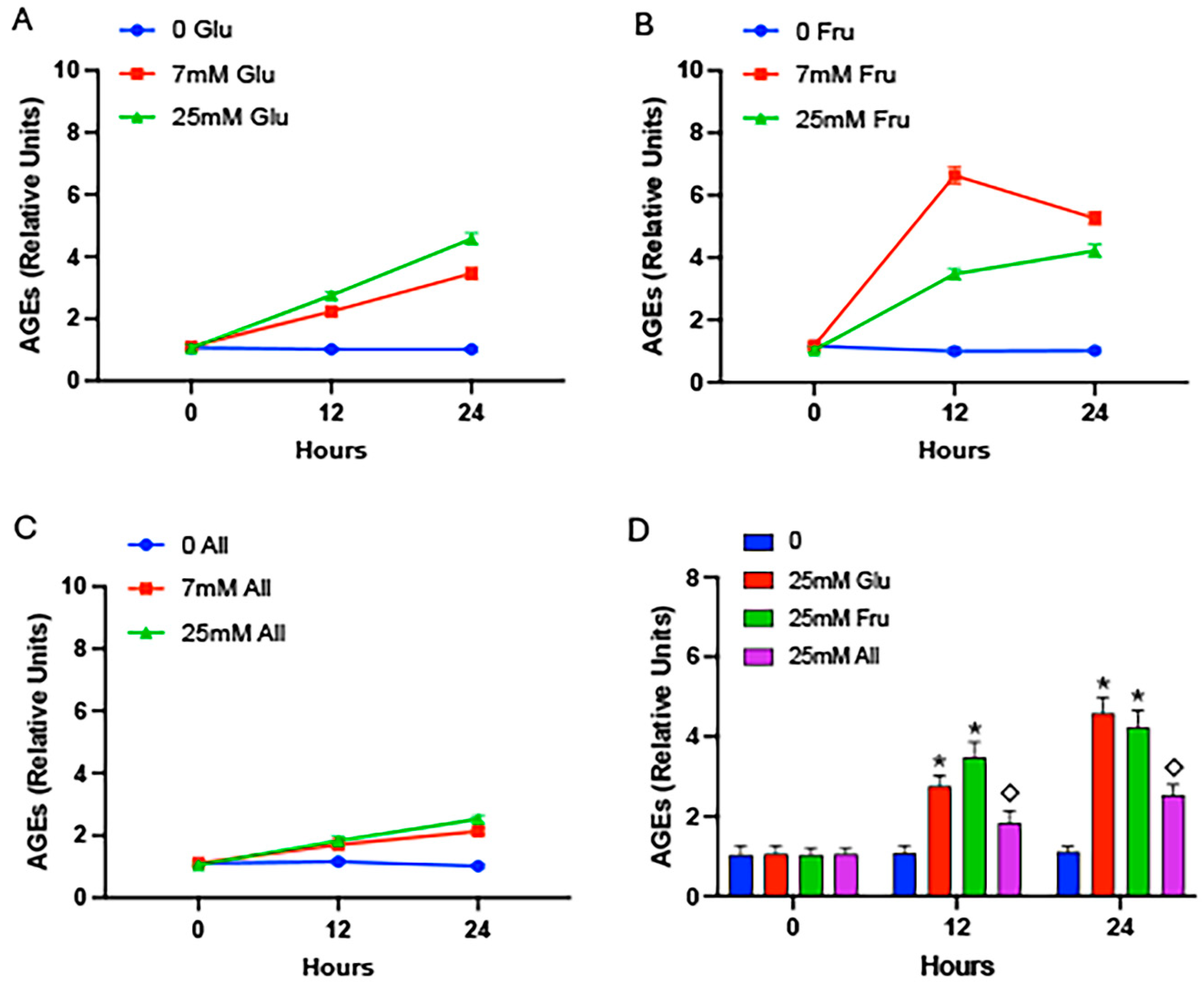
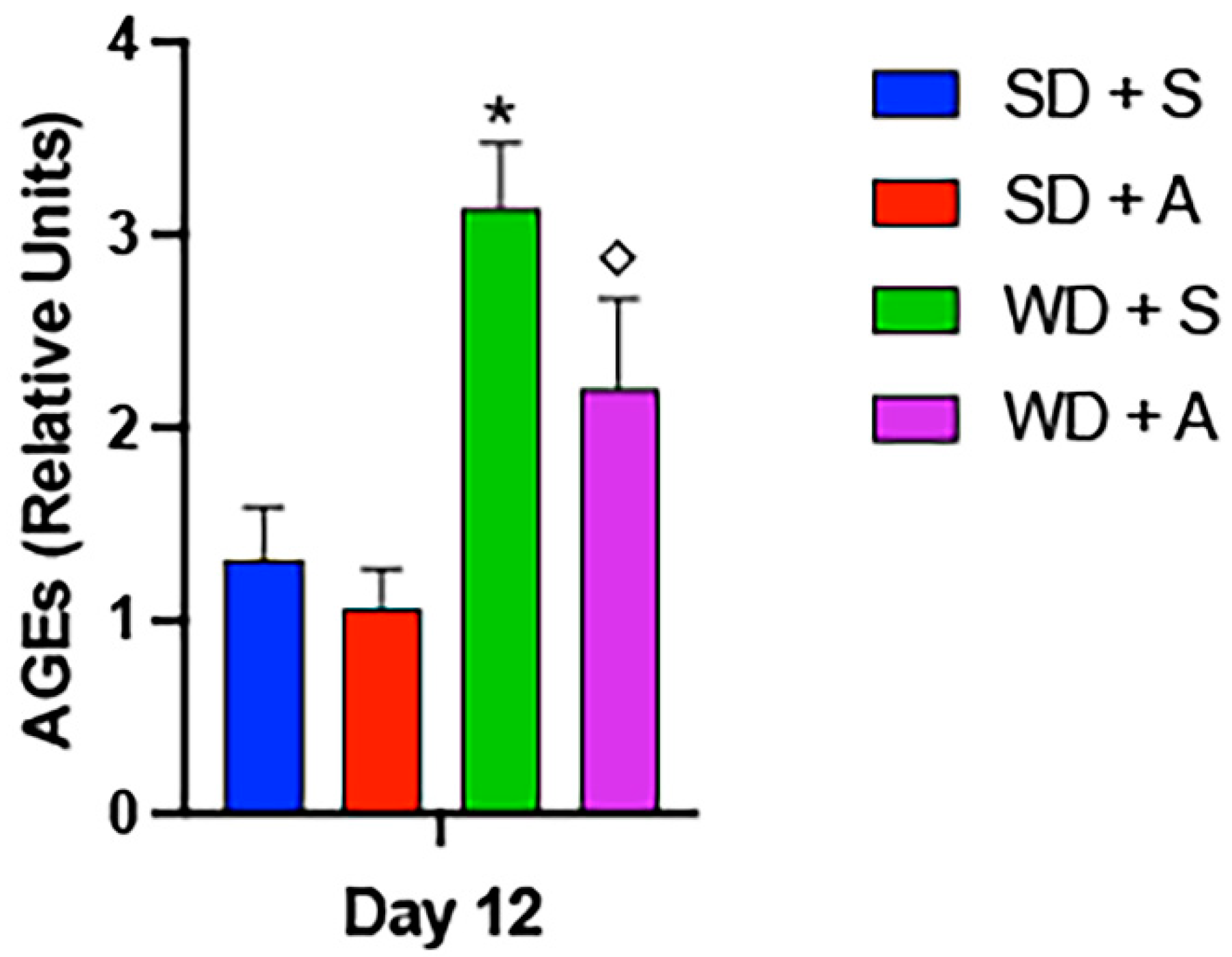
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell-Free Experiments
4.2. Cell Culture Experiments
4.3. Animal Experiments
4.4. Quantification of AGEs
4.5. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, K.E.; Prasad, C.; Vijayagopal, P.; Juma, S.; Imrhan, V. Advanced glycation end products, inflammation, and chronic metabolic diseases: Links in a chain? Crit. Rev. Food Sci. Nutr. 2016, 56, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H.; Hauner, H.; Hornef, M.; Clavel, T. Allulose in human diet: The knowns and the unknowns. Br. J. Nutr. 2021, 128, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang WenLi, Z.W.; Yu ShuHuai, Y.S.; Zhang Tao, Z.T.; Jiang Bo, J.B.; Mu WanMeng, M.W. Recent advances in d-allulose: Physiological functionalities, applications, and biological production. Trends Food Sci. Technol. 2016, 54, 127–137. [Google Scholar] [CrossRef]
- Chen, J.-H.; Lin, X.; Bu, C.; Zhang, X. Role of advanced glycation end products in mobility and considerations in possible dietary and nutritional intervention strategies. Nutr. Metab. 2018, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ding, L.; Wang, P.; Wu, Y.; Areeprasert, C.; Wang, M.; Chen, X.; Wang, F.; Yu, G. Formation of melanoidins and development of characterization techniques during thermal pretreatment of organic solid waste: A critical review. Fuel 2023, 334, 126790. [Google Scholar] [CrossRef]
- Grillo, M.A.; Colombatto, S. Advanced glycation end-products (AGEs): Involvement in aging and in neurodegenerative diseases. Amino Acids 2007, 35, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Trivelli, L.A.; Ranney, H.M.; Lai, H.-T. Hemoglobin Components in Patients with Diabetes Mellitus. N. Engl. J. Med. 1971, 284, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Saudek, C.D.; Derr, R.L.; Kalyani, R.R. Assessing Glycemia in Diabetes Using Self-monitoring Blood Glucose and Hemoglobin A1c. JAMA 2006, 295, 1688–1697. [Google Scholar] [CrossRef]
- Pinkas, A.; Aschner, M. Advanced Glycation End-Products and Their Receptors: Related Pathologies, Recent Therapeutic Strategies, and a Potential Model for Future Neurodegeneration Studies. Chem. Res. Toxicol. 2016, 29, 707–714. [Google Scholar] [CrossRef]
- Prasad, C.; Davis, K.E.; Imrhan, V.; Juma, S.; Vijayagopal, P. Advanced Glycation End Products and Risks for Chronic Diseases: Intervening Through Lifestyle Modification. Am. J. Lifestyle Med. 2019, 13, 384–404. [Google Scholar] [CrossRef]
- Yan, S.D.; Chen, X.; Fu, J.; Chen, M.; Zhu, H.; Roher, A.; Slattery, T.; Zhao, L.; Nagashima, M.; Morser, J. RAGE and amyloid-β peptide neurotoxicity in Alzheimer’s disease. Nature 1996, 382, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M.; Du Yan, S.; Yan, S.F.; Stern, D.M. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J. Clin. Investig. 2001, 108, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Tobon-Velasco, J.C.; Cuevas, E.; Torres-Ramos, M.A. Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinflammation and oxidative stress. CNS Neurol. Disord. -Drug Targets 2014, 13, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Cooper, M.E. Mechanisms of Diabetic Complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Control, D.; Group, C.T.R. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar]
- Turner, R.; Holman, R.R.; Cull, C.A.; Stratton, I.M.; Matthews, D.R.; Frighi, V.; Manley, S.E.; Neil, A.; Mcelroy, K.; Wright, D.H.; et al. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Palace, M. Diabetes and advanced glycation endproducts. J. Intern. Med. 2002, 251, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Yaylayan, V.A.; Huyghues-Despointes, A.; Feather, M. Chemistry of Amadori rearrangement products: Analysis, synthesis, kinetics, reactions, and spectroscopic properties. Crit. Rev. Food Sci. Nutr. 1994, 34, 321–369. [Google Scholar] [CrossRef]
- Giardino, I.; Edelstein, D.; Brownlee, M. BCL-2 expression or antioxidants prevent hyperglycemia-induced formation of intracellular advanced glycation endproducts in bovine endothelial cells. J. Clin. Investig. 1996, 97, 1422–1428. [Google Scholar] [CrossRef]
- Bunn, H.F.; Higgins, P.J. Reaction of Monosaccharides with Proteins: Possible Evolutionary Significance. Science 1981, 213, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Williamson, J.R.; Brownlee, M. Glucose and diabetic vascular disease 1. FASEB J. 1992, 6, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Hayase, F.; Shin, D.B.; Oimomi, M.; Baba, S. 3-Deoxyglucosone, an intermediate product of the Maillard reaction. Prog. Clin. Biol. Res. 1989, 304, 69–84. [Google Scholar] [PubMed]
- Tessier, F.J.; Monnier, V.M.; Sayre, L.M.; Kornfield, J.A. Triosidines: Novel Maillard reaction products and cross-links from the reaction of triose sugars with lysine and arginine residues. Biochem. J. 2003, 369, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Koh, Y.H.; Taniguchi, N. The cytotoxicity of methylglyoxal and 3-deoxyglucosone is decreased in the aldehyde reductase gene-transfected cells. In Enzymology and Molecular Biology of Carbonyl Metabolism; Chapter 7; Springer: Boston, MA, USA, 1999; pp. 509–515. [Google Scholar]
- McPherson, J.D.; Shilton, B.H.; Walton, D.J. Role of fructose in glycation and cross-linking of proteins. Biochemistry 1988, 27, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Dornadula, S.; Elango, B.; Balashanmugam, P.; Palanisamy, R.; Mohanram, R.K. Pathophysiological Insights of Methylglyoxal Induced Type-2 Diabetes. Chem. Res. Toxicol. 2015, 28, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Semchyshyn, H.M.; Miedzobrodzki, J.; Bayliak, M.M.; Lozinska, L.M.; Homza, B.V. Fructose compared with glucose is more a potent glycoxidation agent in vitro, but not under carbohydrate-induced stress in vivo: Potential role of antioxidant and antiglycation enzymes. Carbohydr. Res. 2014, 384, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Mou, L.; Hu, P.; Cao, X.; Chen, Y.; Xu, Y.; He, T.; Wei, Y.; He, R. Comparison of bovine serum albumin glycation by ribose and fructose in vitro and in vivo. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2022, 1868, 166283. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Yamaguchi, F.; Matsunaga, T.; Hirata, Y.; Kamitori, K.; Dong, Y.; Sui, L.; Tsukamoto, I.; Ueno, M.; Tokuda, M. Rare sugar d-psicose protects pancreas β-islets and thus improves insulin resistance in OLETF rats. Biochem. Biophys. Res. Commun. 2012, 425, 717–723. [Google Scholar] [CrossRef]
- Hossain, M.A.; Kitagaki, S.; Nakano, D.; Nishiyama, A.; Funamoto, Y.; Matsunaga, T.; Tsukamoto, I.; Yamaguchi, F.; Kamitori, K.; Dong, Y.; et al. Rare sugar d-psicose improves insulin sensitivity and glucose tolerance in type 2 diabetes Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biochem. Biophys. Res. Commun. 2011, 405, 7–12. [Google Scholar] [CrossRef]
- Iida, T.; Kishimoto, Y.; Yoshikawa, Y.; Hayashi, N.; Okuma, K.; Tohi, M.; Yagi, K.; Matsuo, T.; Izumori, K. Acute D-Psicose Administration Decreases the Glycemic Responses to an Oral Maltodextrin Tolerance Test in Normal Adults. J. Nutr. Sci. Vitaminol. 2008, 54, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Iida, T.; Yamada, T.; Okuma, K.; Takehara, I.; Yamamoto, T.; Yamada, K.; Tokuda, M. Study on the Postprandial Blood Glucose Suppression Effect ofD-Psicose in Borderline Diabetes and the Safety of Long-Term Ingestion by Normal Human Subjects. Biosci. Biotechnol. Biochem. 2010, 74, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.; Martinez, G.; Iida, T.; Yamada, T.; Ferraris, R.P.; Toyoda, Y. d-Allulose is a substrate of glucose transporter type 5 (GLUT5) in the small intestine. Food Chem. 2018, 277, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Sim, L.; Mohan, S.; Kumarasamy, J.; Liu, H.; Avery, S.; Naim, H.Y.; Quezada-Calvillo, R.; Nichols, B.L.; Pinto, B.M.; et al. Mapping the intestinal alpha-glucogenic enzyme specificities of starch digesting maltase-glucoamylase and sucrase-isomaltase. Bioorganic Med. Chem. 2011, 19, 3929–3934. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Yamaguchi, F.; Hirose, K.; Matsunaga, T.; Sui, L.; Hirata, Y.; Noguchi, C.; Katagi, A.; Kamitori, K.; Dong, Y.; et al. Rare sugar D-psicose prevents progression and development of diabetes in T2DM model Otsuka Long-Evans Tokushima Fatty rats. Drug Des. Dev. Ther. 2015, 9, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, X.; Guan, Y.; Sun, Y. Characteristics and Antioxidant Activity of Maillard Reaction Products from Psicose-Lysine and Fructose-Lysine Model Systems. J. Food Sci. 2011, 76, C398–C403. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.R.; Wasley, K.M.; Allison, C.H. Diesel Particulate Matter Induces Receptor for Advanced Glycation End-Products (RAGE) Expression in Pulmonary Epithelial Cells, and RAGE Signaling Influences NF-κB–Mediated Inflammation. Environ. Health Perspect. 2011, 119, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.B.; Hirschi, K.M.; Arroyo, J.A.; Bikman, B.T.; Kooyman, D.L.; Reynolds, P.R. Plausible Roles for RAGE in Conditions Exacerbated by Direct and Indirect (Secondhand) Smoke Exposure. Int. J. Mol. Sci. 2017, 18, 652. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.B.; Stogsdill, J.A.; Lewis, J.B.; Wood, T.T.; Reynolds, P.R. RAGE and tobacco smoke: Insights into modeling chronic obstructive pulmonary disease. Front. Physiol. 2012, 3, 27769. [Google Scholar] [CrossRef] [PubMed]
- Pratchayasakul, W.; Jinawong, K.; Pongkan, W.; Jaiwongkam, T.; Arunsak, B.; Chunchai, T.; Tokuda, M.; Chattipakorn, N.; Chattipakorn, S.C. Not only metformin, but also D-allulose, alleviates metabolic disturbance and cognitive decline in prediabetic rats. Nutr. Neurosci. 2022, 25, 1115–1127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarke, D.M.; Koutnik, A.P.; Johnson, R.J.; DeBlasi, J.M.; Bikman, B.T.; Arroyo, J.A.; Reynolds, P.R. Differential Rates of Glycation Following Exposure to Unique Monosaccharides. Int. J. Mol. Sci. 2024, 25, 6921. https://doi.org/10.3390/ijms25136921
Clarke DM, Koutnik AP, Johnson RJ, DeBlasi JM, Bikman BT, Arroyo JA, Reynolds PR. Differential Rates of Glycation Following Exposure to Unique Monosaccharides. International Journal of Molecular Sciences. 2024; 25(13):6921. https://doi.org/10.3390/ijms25136921
Chicago/Turabian StyleClarke, Derek M, Andrew P Koutnik, Richard J Johnson, Janine M DeBlasi, Benjamin T Bikman, Juan A Arroyo, and Paul R Reynolds. 2024. "Differential Rates of Glycation Following Exposure to Unique Monosaccharides" International Journal of Molecular Sciences 25, no. 13: 6921. https://doi.org/10.3390/ijms25136921
APA StyleClarke, D. M., Koutnik, A. P., Johnson, R. J., DeBlasi, J. M., Bikman, B. T., Arroyo, J. A., & Reynolds, P. R. (2024). Differential Rates of Glycation Following Exposure to Unique Monosaccharides. International Journal of Molecular Sciences, 25(13), 6921. https://doi.org/10.3390/ijms25136921








