The Significance of Genetically Determined Methylation and Folate Metabolism Disorders in the Pathogenesis of Coronary Artery Disease: A Target for New Therapies?
Abstract
1. Introduction
2. Overview of the Methylation Pathway
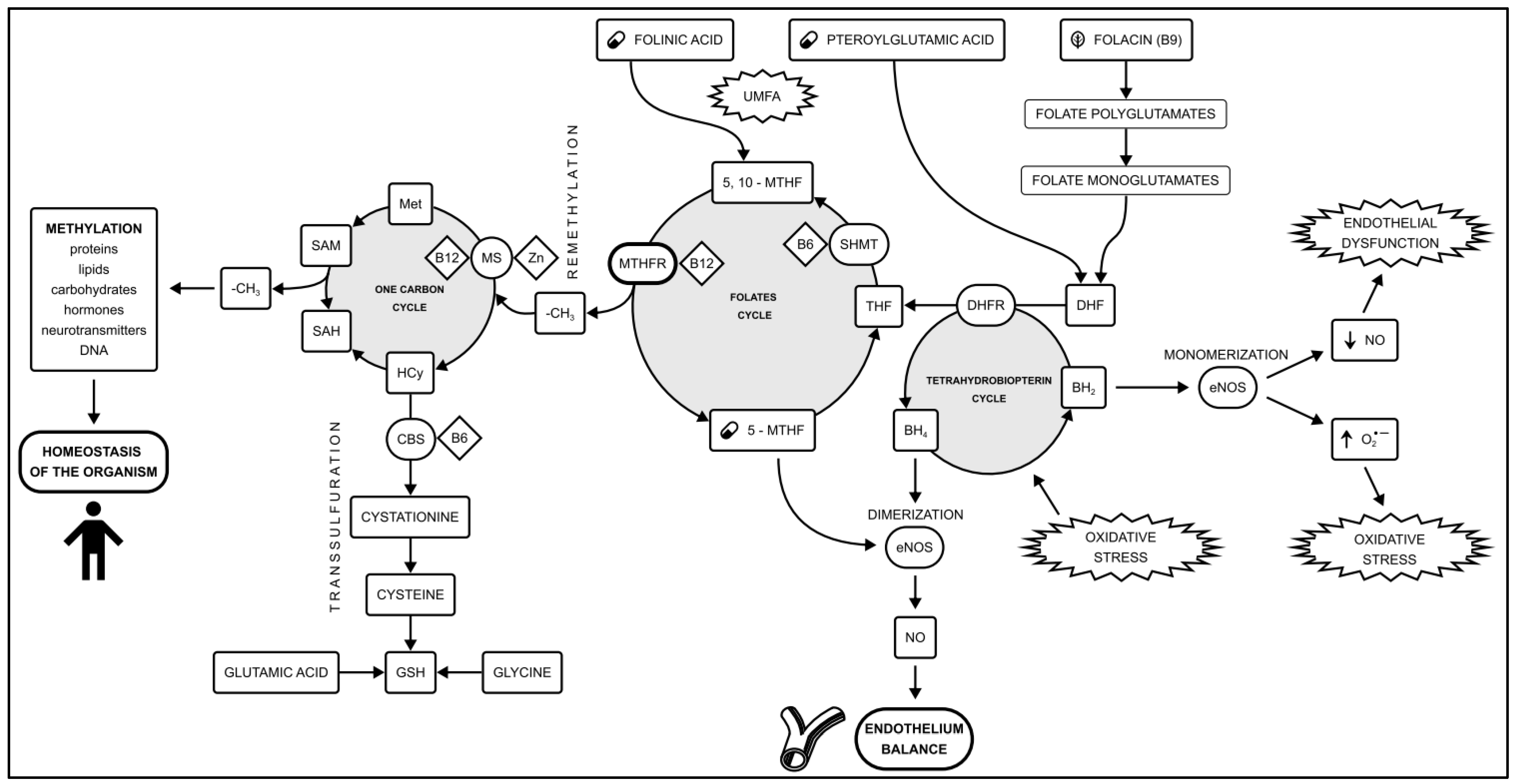
3. Folate Metabolism
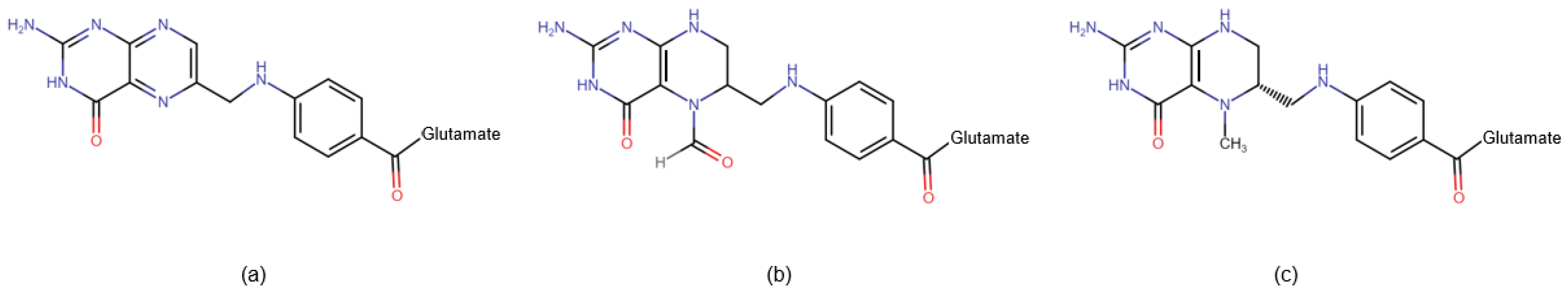
- Folic acid (also known as folacin or vitamin B9): This water-soluble molecule is predominantly found in green leafy vegetables such as asparagus, spinach, lettuce, and broccoli. In food, folic acid exists as a complex compound known as polyglutamine conjugates. These compounds are broken down in the small intestine into monoglutamates, which are absorbed into enterocytes. Within the cell, folic acid is converted to dihydrofolate (DHF) and then to tetrahydrofolate (THF) by the enzyme dihydrofolate reductase (DHFR). Subsequently, the enzyme serine hydroxymethyltransferase (SHMT) transfers the methylene group from the serine side chain to THF, resulting in the production of 5,10-methylenetetrahydrofolate (5,10-MTHF) and glycine. Methylenetetrahydrofolate reductase (MTHFR) then facilitates the formation of 5-methyltetrahydrofolate (5-MTHF), the biologically active form of folate.
- Pteroylglutamic acid (synthetic oxidized folate): This synthetically produced molecule consists of a pteroyl residue and 2 to 7 glutamine residues. It is used as a dietary supplement and for food fortification. Before entering the folate cycle, it must be reduced by DHFR to DHF and then to THF, ultimately being converted to biologically active 5-MTHF. However, synthetic folic acid is very hard for DHFR to recycle. Supplementation with synthetic folic acid may lead to a syndrome known as unmetabolized folic acid (UMFA) syndrome.
- Folinic acid (leucovorin): This synthetic molecule is a 5-formyl derivative of THF, which is converted into 5,10-MTHF without the need for DHFR. MTHFR is required for its conversion to 5-MTHF. Folinic acid is used to mitigate the toxic effects of chemotherapy agents that disrupt folate metabolism by inhibiting DHFR (e.g., methotrexate).
- 5-methyltetrahydrofolate (levomefolic acid, L-methylfolate, 5-MTHF): This represents the predominant physiological form of folate in the blood. The availability of 5-MTHF facilitates the conversion of methionine to S-adenosylmethionine (SAM), a universal methylation effector. After the release of the methyl group, S-adenosylhomocysteine (SAH) and homocysteine are produced, exerting feedback that inhibits methylation. Compared to other folates, which require conversion by enzymes, 5-MTHF is directly involved in nitric oxide production, protecting the vascular endothelium. Moreover, the novel concept of identifying CAD patients with 5-MTHF deficits may translate into the use of 5-MTHF in pharmacotherapy to restore the proper function of the vascular endothelium, but further research is needed on this topic.
Differences between Folates—Diagnostic Insights
4. Pathogenesis of CAD and Its Risk Factors
4.1. Genetics, Genomics, and Epigenetics—A Novel Approach to Understanding the Pathogenesis of CAD
Large-Scale Research or Candidate Genes Approach?
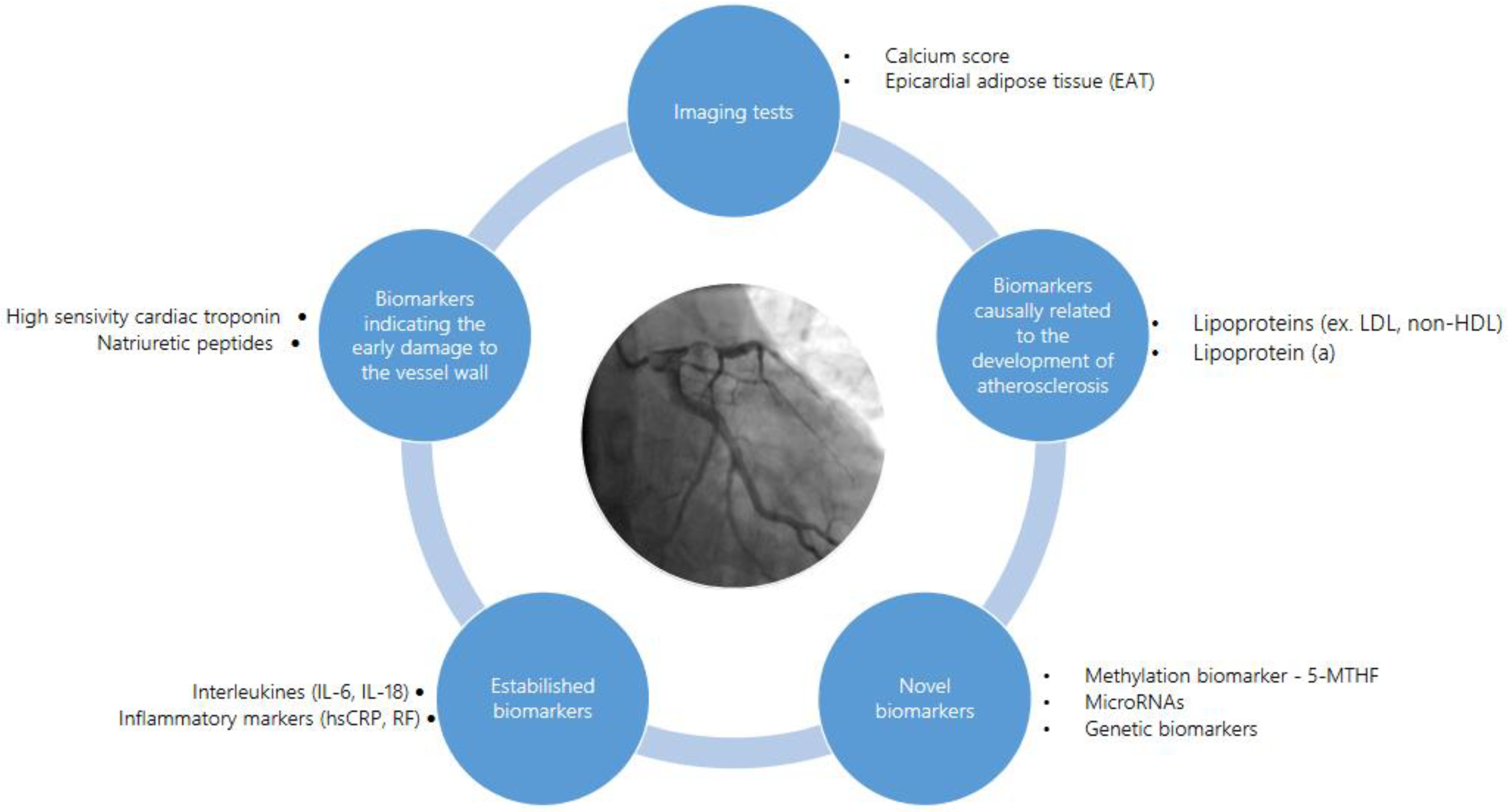
5. CAD Biomarkers
5.1. Patomechanisms at the Early Stage of Atherosclerosis and Related Biomarkers
5.2. Public Health Perspective
6. Methylation Disorders
6.1. Genetically Determined Methylation Disorders
- absorption of folate from food: enzyme encoding glutamine carboxypeptidase II—polymorphism 475H>Y (rs61886492)
- folate transport—polymorphism (rs1051266) of the RFC1 gene
- activity of folate receptors—genes encoding FOLR1, FOLR2
- folate metabolism—polymorphisms encoding the genes dihydrofolate reductase (DHFR), methylenetetrahydrofolate reductase (MTHFR), and methionine synthase (rs1805087).
Methylenetetrahydrofolate Reductase Gene Polymorphisms
| Current Nomenclature | Alleles | Past Nomenclature | Genetic “Raw Data” | |
|---|---|---|---|---|
| c.665C>T MTHFR polymorphism | C677T MTHFR polymorphism | |||
| Genotypes | c.[665C=]c;[665C=] | Both “wild type” alleles | C677C | G/G |
| c.[665C>T];[665C=] | One polymorphic allele: c.665 C>T heterozygote | C677T | A/G | |
| c.[665C>T];[665C>T] | Both polymorphic alleles: c.665 C>T homozygote | T677T | A/A | |
| c.1286A>C MTHFR polymorphism | A1298C MTHFR polymorphism | |||
| Genotypes | c.[1286A=];[1286A=] | Both “wild type” alleles | A1298A | T/T |
| c.[1286A>C];[1286A=] | One polymorphic allele: c.1286 A>C heterozygote | A1298C | G/T | |
| c.[1286A>C];[1286A>C] | Both polymorphic alleles: c.1286 A>C homozygote | C1298C | G/G | |
7. The Role of Methylation Pathway in Endothelial Dysfunction
8. Practical Aspects of Genetics and Pharmacogenomics in CAD Patients
9. Translating the Pathomechanism into Clinical Practice—New CAD Therapies
Folic Acid versus 5-MTHF in CAD Therapy
10. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Irzmański, R.; Glowczynska, R.; Banach, M.; Szalewska, D.; Piotrowicz, R.; Kowalik, I.; Pencina, M.J.; Zareba, W.; Orzechowski, P.; Pluta, S.; et al. Prognostic Impact of Hybrid Comprehensive Telerehabilitation Regarding Diastolic Dysfunction in Patients with Heart Failure with Reduced Ejection Fraction-Subanalysis of the TELEREH-HF Randomized Clinical Trial. J. Clin. Med. 2022, 11, 1844. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Estimates: Life Expectancy and Leading Causes of Death and Disability 2019. Available online: www.who.int/data/gho/data/themes/mortality-and-global-health-estimates (accessed on 17 April 2024).
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, R. Methylation. Brittanica 2018. Available online: https://www.britannica.com/science/methylation (accessed on 17 April 2024).
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Menezo, Y.; Clement, P.; Clement, A.; Elder, K. Methylation: An Ineluctable Biochemical and Physiological Process Essential to the Transmission of Life. Int. J. Mol. Sci. 2020, 21, 9311. [Google Scholar] [CrossRef] [PubMed]
- James, S.J.; Melnyk, S.; Pogribna, M.; Pogribny, I.P.; Caudill, M.A. Elevation in S-adenosylhomocysteine and DNA hypomethylation: Potential epigenetic mechanism for homocysteine-related pathology. J. Nutr. 2002, 132 (Suppl. S8), 2361s–2366s. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liao, R.; Dai, X.; Guo, H.; Wang, D.; Xia, M.; Ling, W.; Xiao, Y. Association between plasma S-adenosylmethionine and risk of mortality in patients with coronary artery disease: A cohort study. Am. J. Clin. Nutr. 2021, 114, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Karthika, C.L.; Ahalya, S.; Radhakrishnan, N.; Kartha, C.C.; Sumi, S. Hemodynamics mediated epigenetic regulators in the pathogenesis of vascular diseases. Mol. Cell Biochem. 2021, 476, 125–143. [Google Scholar] [CrossRef]
- Fardous, A.M.; Heydari, A.R. Uncovering the Hidden Dangers and Molecular Mechanisms of Excess Folate: A Narrative Review. Nutrients 2023, 15, 4699. [Google Scholar] [CrossRef] [PubMed]
- Menezo, Y.; Elder, K.; Clement, A.; Clement, P. Folic Acid, Folinic Acid, 5 Methyl TetraHydroFolate Supplementation for Mutations That Affect Epigenesis through the Folate and One-Carbon Cycles. Biomolecules 2022, 12, 197. [Google Scholar] [CrossRef]
- Costello, J.F.; Plass, C. Methylation matters. J. Med. Genet. 2001, 38, 285–303. [Google Scholar] [CrossRef]
- Cosar, A.; Ipcioglu, O.M.; Ozcan, O.; Gultepe, M. Folate and homocysteine metabolisms and their roles in the biochemical basis of neuropsychiatry. Turk. J. Med. Sci. 2014, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ziolkowska, K.; Tobola-Wrobel, K.; Pietryga, M.; Kasprzak, G.; Jamsheer, A.; Wysocka, E. An Assessment of Selected Molecular and Biochemical Markers of the Folate Pathway as Potential Risk Factors for Fetal Trisomy 21 during the First Trimester of Pregnancy in the Polish Population. J. Clin. Med. 2022, 11, 1190. [Google Scholar] [CrossRef] [PubMed]
- Czechowicz, P.; Malodobra-Mazur, M.; Lebioda, A.; Jonkisz, A.; Dobosz, T.; Smigiel, R. Polymorphisms of the MTHFR gene in mothers of children with trisomy 21 (Down syndrome) in a Polish population. Adv. Clin. Exp. Med. 2020, 29, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Chorąży, M.; Wawrusiewicz-Kurylonek, N.; Gościk, J.; Posmyk, R.; Czarnowska, A.; Więsik, M.; Kapica-Topczewska, K.; Krętowski, A.J.; Kochanowicz, J.; Kułakowska, A. Association between polymorphisms of a folate—Homocysteine—Methionine—SAM metabolising enzyme gene and multiple sclerosis in a Polish population. Neurol. Neurochir. Pol. 2019, 53, 194–198. [Google Scholar] [CrossRef]
- Żur-Wyrozumska, K.; Pera, J.; Dziubek, A.; Sado, M.; Golenia, A.; Słowik, A.; Dziedzic, T. Association between C677T polymorphism of MTHFR gene and risk of amyotrophic lateral sclerosis: Polish population study and a meta-analysis. Neurol. Neurochir. Pol. 2017, 51, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Goracy, I.; Cyryłowski, L.; Kaczmarczyk, M.; Fabian, A.; Koziarska, D.; Goracy, J.; Ciechanowicz, A. C677T polymorphism of the methylenetetrahydrofolate reductase gene and the risk of ischemic stroke in Polish subjects. J. Appl. Genet. 2009, 50, 63–67. [Google Scholar] [CrossRef]
- Wolski, H.; Kurzawinska, G.; Drews, K.; Barlik, M.; Kadziolka, P.; Malewski, Z.; Mikolajska-Ptas, P.; Bylewski, M.; Sere-mak-Mrozikiewicz, A. MTHFR genetic polymorphism and the risk of intrauterine fetal death in Polish women. Ginekol. Pol. 2019, 90, 76–81. [Google Scholar] [CrossRef]
- Nowak, I.; Bylińska, A.; Wilczyńska, K.; Wiśniewski, A.; Malinowski, A.; Wilczyński, J.R.; Radwan, P.; Radwan, M.; Barcz, E.; Płoski, R.; et al. The methylenetetrahydrofolate reductase c.c.677 C>T and c.c.1298 A>C polymorphisms in reproductive failures: Experience from an RSA and RIF study on a Polish population. PLoS ONE 2017, 12, e0186022. [Google Scholar] [CrossRef]
- Stanisławska-Sachadyn, A.; Borzyszkowska, J.; Krzemiński, M.; Janowicz, A.; Dziadziuszko, R.; Jassem, J.; Rzyman, W.; Limon, J. Folate/homocysteine metabolism and lung cancer risk among smokers. PLoS ONE 2019, 14, e0214462. [Google Scholar] [CrossRef]
- Wolski, H.; Barlik, M.; Drews, K.; Klejewski, A.; Kurzawińska, G.; Ożarowski, M.; Łowicki, Z.; Seremak-Mrozikiewicz, A. Contribution of inherited thrombophilia to recurrent miscarriage in the Polish population. Ginekol. Pol. 2017, 88, 385–392. [Google Scholar] [CrossRef]
- Hiraoka, M.; Kagawa, Y. Genetic polymorphisms and folate status. Congenit. Anom. 2017, 57, 142–149. [Google Scholar] [CrossRef]
- Crabtree, M.J.; Hale, A.B.; Channon, K.M. Dihydrofolate reductase protects endothelial nitric oxide synthase from uncoupling in tetrahydrobiopterin deficiency. Free Radic. Biol. Med. 2011, 50, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Moens, A.L.; Vrints, C.J.; Claeys, M.J.; Timmermans, J.-P.; Champion, H.C.; Kass, D.A. Mechanisms and potential therapeutic targets for folic acid in cardiovascular disease. Am. J. Physiol. -Heart Circ. Physiol. 2008, 294, H1971–H1977. [Google Scholar] [CrossRef]
- Duthie, S.J.; Narayanan, S.; Brand, G.M.; Pirie, L.; Grant, G. Impact of Folate Deficiency on DNA Stability. J. Nutr. 2002, 132, 2444S–2449S. [Google Scholar] [CrossRef]
- Magri, V.R.; Rocha, M.A.; de Matos, C.S.; Petersen, P.A.; Leroux, F.; Petrilli, H.M.; Constantino, V.R. Folic acid and sodium folate salts: Thermal behavior and spectroscopic (IR, Raman, and solid-state (13)C NMR) characterization. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 273, 120981. [Google Scholar] [CrossRef] [PubMed]
- Sobczyńska-Malefora, A.; Harrington, D.J. Laboratory assessment of folate (vitamin B(9)) status. J. Clin. Pathol. 2018, 71, 949–956. [Google Scholar] [CrossRef]
- Finer, S.; Saravanan, P.; Hitman, G.; Yajnik, C. The role of the one-carbon cycle in the developmental origins of Type 2 diabetes and obesity. Diabet. Med. 2014, 31, 263–272. [Google Scholar] [CrossRef]
- Marzilli, M.; Merz, C.N.; Boden, W.E.; Bonow, R.O.; Capozza, P.G.; Chilian, W.M.; DeMaria, A.N.; Guarini, G.; Huqi, A.; Morrone, D.; et al. Obstructive coronary atherosclerosis and ischemic heart disease: An elusive link! J. Am. Coll. Cardiol. 2012, 60, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Medina-Leyte, D.J.; Zepeda-Garcia, O.; Dominguez-Perez, M.; Gonzalez-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef]
- Dawson, L.P.; Lum, M.; Nerleker, N.; Nicholls, S.J.; Layland, J. Coronary Atherosclerotic Plaque Regression: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 66–82. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [PubMed]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [PubMed]
- Kotseva, K.; De Backer, G.; De Bacquer, D.; Rydén, L.; Hoes, A.; Grobbee, D.; Maggioni, A.; Marques-Vidal, P.; Jennings, C.; Abreu, A.; et al. Primary prevention efforts are poorly developed in people at high cardiovascular risk: A report from the European Society of Cardiology EURObservational Research Programme EUROASPIRE V survey in 16 European countries. Eur. J. Prev. Cardiol. 2021, 28, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.P.; Inouye, M.; Meikle, P.J.; Nicholls, S.J.; Carrington, M.J.; Marwick, T.H. New Cardiovascular Risk Assessment Techniques for Primary Prevention: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 80, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Chang, C.C.; Hadley, T. Genetic Risk Stratification: A Paradigm Shift in Prevention of Coronary Artery Disease. JACC Basic. Transl. Sci. 2021, 6, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Rizzacasa, B.; Amati, F.; Romeo, F.; Novelli, G.; Mehta, J.L. Epigenetic Modification in Coronary Atherosclerosis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 1352–1365. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, H.; Huang, S.; Yin, L.; Wang, F.; Luo, P.; Huang, H. Epigenetic regulation in cardiovascular disease: Mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 2022, 7, 200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barbalata, T.; Niculescu, L.S.; Stancu, C.S.; Pinet, F.; Sima, A.V. Elevated Levels of Circulating lncRNAs LIPCAR and MALAT1 Predict an Unfavorable Outcome in Acute Coronary Syndrome Patients. Int. J. Mol. Sci. 2023, 24, 12076. [Google Scholar] [CrossRef] [PubMed]
- Thorisson, G.A.; Stein, L.D. The SNP Consortium website: Past, present and future. Nucleic Acids Res. 2003, 31, 124–127. [Google Scholar] [CrossRef]
- Kessler, T.; Schunkert, H. Coronary Artery Disease Genetics Enlightened by Genome-Wide Association Studies. JACC Basic. Transl. Sci. 2021, 6, 610–623. [Google Scholar] [CrossRef]
- Erdmann, J.; Kessler, T.; Munoz Venegas, L.; Schunkert, H. A decade of genome-wide association studies for coronary artery disease: The challenges ahead. Cardiovasc. Res. 2018, 114, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Ito, K.; Terao, C.; Akiyama, M.; Horikoshi, M.; Momozawa, Y.; Matsunaga, H.; Ieki, H.; Ozaki, K.; Onouchi, Y.; et al. Population-specific and trans-ancestry genome-wide analyses identify distinct and shared genetic risk loci for coronary artery disease. Nat. Genet. 2020, 52, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Wiernek, S.; Evans, J.P.; Runge, M.S. Genetics of coronary artery disease and myocardial infarction. World J. Cardiol. 2016, 8, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Schunkert, H.; König, I.R.; Kathiresan, S.; Reilly, M.P.; Assimes, T.L.; Holm, H.; Preuss, M.; Stewart, A.F.; Barbalic, M.; Gieger, C.; et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat. Genet. 2013, 45, 25–33. [Google Scholar]
- Pechlivanis, S.; Lehmann, N.; Hoffmann, P.; Nöthen, M.M.; Jöckel, K.H.; Erbel, R.; Moebus, S. Risk prediction for coronary heart disease by a genetic risk score—Results from the Heinz Nixdorf Recall study. BMC Med. Genet. 2020, 21, 178. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.R. Commentary: What is the case for candidate gene approaches in the era of high-throughput genomics? A response to Border and Keller (2017). J. Child Psychol. Psychiatry 2017, 58, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Scheuner, M.T. Genetic evaluation for coronary artery disease. Genet. Med. 2003, 5, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Timizheva, K.B.; Ahmed, A.A.M.; Ait Aissa, A.; Aghajanyan, A.V.; Tskhovrebova, L.V.; Azova, M.M. Association of the DNA Methyltransferase and Folate Cycle Enzymes’ Gene Polymorphisms with Coronary Restenosis. Life 2022, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Lu, Z.; Muhammad, I.; Chen, Y.; Chen, Q.; Zhang, J.; Song, Y. Associations of the MTHFR rs1801133 polymorphism with coronary artery disease and lipid levels: A systematic review and updated meta-analysis. Lipids Health Dis. 2018, 17, 191. [Google Scholar] [CrossRef]
- Iwanicka, J.; Balcerzyk-Matić, A.; Iwanicki, T.; Mizia-Stec, K.; Bańka, P.; Filipecki, A.; Gawron, K.; Jarosz, A.; Nowak, T.; Krauze, J.; et al. The Association of ADAMTS7 Gene Polymorphisms with the Risk of Coronary Artery Disease Occurrence and Cardiovascular Survival in the Polish Population: A Case-Control and a Prospective Cohort Study. Int. J. Mol. Sci. 2024, 25, 2274. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef]
- Assimes, T.L.; Roberts, R. Genetics: Implications for Prevention and Management of Coronary Artery Disease. J. Am. Coll. Cardiol. 2016, 68, 2797–2818. [Google Scholar] [CrossRef][Green Version]
- Hartmann, D. Biomarker. Encyclopedia Britannica. Available online: https://www.britannica.com/science/biomarker (accessed on 17 April 2024).
- Antoniades, C.; Shirodaria, C.; Warrick, N.; Cai, S.; de Bono, J.; Lee, J.; Leeson, P.; Neubauer, S.; Ratnatunga, C.; Pillai, R.; et al. 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: Effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation 2006, 114, 1193–1201. [Google Scholar] [CrossRef]
- Cundari, G.; Marchitelli, L.; Pambianchi, G.; Catapano, F.; Conia, L.; Stancanelli, G.; Catalano, C.; Galea, N. Imaging biomarkers in cardiac CT: Moving beyond simple coronary anatomical assessment. Radiol. Med. 2024, 129, 380–400. [Google Scholar] [CrossRef] [PubMed]
- Vernon, S.T.; Coffey, S.; Bhindi, R.; Soo Hoo, S.Y.; Nelson, G.I.; Ward, M.R.; Hansen, P.S.; Asrress, K.N.; Chow, C.K.; Celermajer, D.S.; et al. Increasing proportion of ST elevation myocardial infarction patients with coronary atherosclerosis poorly explained by standard modifiable risk factors. Eur. J. Prev. Cardiol. 2017, 24, 1824–1830. [Google Scholar] [CrossRef]
- Pfeiler, S.; Winkels, H.; Kelm, M.; Gerdes, N. IL-1 family cytokines in cardiovascular disease. Cytokine 2019, 122, 154215. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of monosodium salt of l-5-methyltetrahydrofolic acid as a novel food pursuant to Regulation (EU) 2015/2283 and the bioavailability of folate from this source in the context of Directive 2002/46/EC, Regulation (EU) No 609/2013 and Regulation (EC) No 1925/2006. EFSA J. 2023, 21, e8417. [Google Scholar]
- Jiang, Y.; Zhao, Y.; Li, Z.Y.; Chen, S.; Fang, F.; Cai, J.H. Potential roles of microRNAs and long noncoding RNAs as diagnostic, prognostic and therapeutic biomarkers in coronary artery disease. Int. J. Cardiol. 2023, 384, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, V.; Todaro, F.; Cataldi, M.; Tuttolomondo, A. Atherosclerosis and Its Related Laboratory Biomarkers. Int. J. Mol. Sci. 2023, 24, 15546. [Google Scholar] [CrossRef] [PubMed]
- Tudurachi, B.S.; Anghel, L.; Tudurachi, A.; Sascau, R.A.; Statescu, C. Assessment of Inflammatory Hematological Ratios (NLR, PLR, MLR, LMR and Monocyte/HDL-Cholesterol Ratio) in Acute Myocardial Infarction and Particularities in Young Patients. Int. J. Mol. Sci. 2023, 24, 14378. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Chen, Y.; Zhou, X. The roles of microRNAs in epigenetic regulation. Curr. Opin. Chem. Biol. 2019, 51, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Torres-Paz, Y.E.; Gamboa, R.; Fuentevilla-Álvarez, G.; Soto, M.E.; González-Moyotl, N.; Martínez-Alvarado, R.; Torres-Tamayo, M.; Ramírez-Marroquín, E.S.; Vásquez-Jiménez, X.; Sainz-Escarrega, V.; et al. Overexpression of microRNA-21-5p and microRNA-221-5p in Monocytes Increases the Risk of Developing Coronary Artery Disease. Int. J. Mol. Sci. 2023, 24, 8641. [Google Scholar] [CrossRef] [PubMed]
- Figtree, G.A.; Vernon, S.T.; Harmer, J.A.; Gray, M.P.; Arnott, C.; Bachour, E.; Barsha, G.; Brieger, D.; Brown, A.; Celermajer, D.S.; et al. Clinical Pathway for Coronary Atherosclerosis in Patients without Conventional Modifiable Risk Factors: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2023, 82, 1343–1359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karimi Galougahi, K.; Antoniades, C.; Nicholls, S.J.; Channon, K.M.; Figtree, G.A. Redox biomarkers in cardiovascular medicine. Eur. Heart J. 2015, 36, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Ference, B.A.; Staley, J.R.; Freitag, D.F.; Mason, A.M.; Nielsen, S.F.; Willeit, P.; Young, R.; Surendran, P.; Karthikeyan, S.; et al. Association of LPA Variants with Risk of Coronary Disease and the Implications for Lipoprotein(a)-Lowering Therapies: A Mendelian Randomization Analysis. JAMA Cardiol. 2018, 3, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.E.; Besnier, M.; Genetzakis, E.; Tang, O.; Kott, K.A.; Vernon, S.T.; Gray, M.P.; Grieve, S.M.; Kassiou, M.; Figtree, G.A. High-Throughput Measure of Mitochondrial Superoxide Levels as a Marker of Coronary Artery Disease to Accelerate Drug Translation in Patient-Derived Endothelial Cells Using Opera Phenix((R)) Technology. Int. J. Mol. Sci. 2023, 25, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J. New cardiovascular risk factors exist, but are they clinically useful? Eur. Heart J. 2008, 29, 441–444. [Google Scholar] [CrossRef]
- Forstermann, U. Nitric oxide and oxidative stress in vascular disease. Pflug. Arch. 2010, 459, 923–939. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B(12), folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef]
- Doshi, S.N.; McDowell, I.F.; Moat, S.J.; Payne, N.; Durrant, H.J.; Lewis, M.J.; Goodfellow, J. Folic acid improves endothelial function in coronary artery disease via mechanisms largely independent of homocysteine lowering. Circulation 2002, 105, 22–26. [Google Scholar] [CrossRef]
- Bekdash, R.A. Methyl Donors, Epigenetic Alterations, and Brain Health: Understanding the Connection. Int. J. Mol. Sci. 2023, 24, 2346. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Shirodaria, C.; Leeson, P.; Baarholm, O.A.; Van-Assche, T.; Cunnington, C.; Pillai, R.; Ratnatunga, C.; Tousoulis, D.; Stefanadis, C.; et al. MTHFR 677 C>T Polymorphism reveals functional importance for 5-methyltetrahydrofolate, not homocysteine, in regulation of vascular redox state and endothelial function in human atherosclerosis. Circulation 2009, 119, 2507–2515. [Google Scholar] [CrossRef] [PubMed]
- Kadayifci, F.Z.; Zheng, S.; Pan, Y.X. Molecular Mechanisms Underlying the Link between Diet and DNA Methylation. Int. J. Mol. Sci. 2018, 19, 4055. [Google Scholar] [CrossRef]
- Kim, D.J.; Venkataraman, A.; Jain, P.C.; Wiesler, E.P.; DeBlasio, M.; Klein, J.; Tu, S.; Lee, S.; Medzhitov, R.; Iwasaki, A. Vitamin B12 and folic acid alleviate symptoms of nutritional deficiency by antagonizing aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 15837–15845. [Google Scholar] [CrossRef]
- Scaglione, F.; Panzavolta, G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica 2014, 44, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Ryu, C.S.; Lee, J.Y.; Ko, E.J.; Ha, Y.H.; Sung, J.H.; Hwang, T.S.; Kim, I.J.; Kim, N.K. Association of Thymidylate Synthase (TS) Gene Polymorphisms with Incidence and Prognosis of Coronary Artery Disease. Int. J. Mol. Sci. 2023, 24, 12591. [Google Scholar] [CrossRef]
- Liew, S.C.; Gupta, E.D. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: Epidemiology, metabolism and the associated diseases. Eur. J. Med. Genet. 2015, 58, 1–10. [Google Scholar] [CrossRef]
- Undas, A.; Chojnowski, K.; Klukowska, A.; Łętowska, M.; Mital, A.; Młynarski, W.; Musiał, J.; Podolak-Dawidziak, M.; Sąsiadek, M.; Treliński, J.; et al. Determination and interpretation of MTHFR gene mutations in gynecology and internal medicine. Pol. Arch. Intern. Med. 2019, 129, 728–732. [Google Scholar] [CrossRef]
- Moczulska, H.; Pesz, K.; Gach, A.; Borowiec, M.; Sieroszewski, P.; Sąsiadek, M.; Jakubowski, L.; Wielgoś, M. Stanowisko ekspertów Polskiego Towarzystwa Genetyki Człowieka i Polskiego Towarzystwa Ginekologów i Położników w sprawie zlecania i interpretacji wyników badań pod kątem wariantów genetycznych w genie MTHFR. Ginekol. Perinatol. Prakt. 2017, 2, 234–238. [Google Scholar]
- MTHFR Mutation: What Is It? How to Check Your Raw Data. Available online: https://www.geneticlifehacks.com/mthfr/ (accessed on 17 April 2024).
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Wolski, H.; Kocięcka, M.; Mrozikiewicz, A.E.; Barlik, M.; Kurzawińska, G. Coexistence of the 677C>T and 1298A>C MTHFR polymorphisms and its significance in the population of Polish women. Ginekol. Pol. 2015, 86, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Botto, L.D.; Yang, Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: A HuGE review. Am. J. Epidemiol. 2000, 151, 862–877. [Google Scholar] [CrossRef] [PubMed]
- Tsang, B.L.; Devine, O.J.; Cordero, A.M.; Marchetta, C.M.; Mulinare, J.; Mersereau, P.; Guo, J.; Qi, Y.P.; Berry, R.J.; Rosenthal, J.; et al. Assessing the association between the methylenetetrahydrofolate reductase (MTHFR) 677C>T polymorphism and blood folate concentrations: A systematic review and meta-analysis of trials and observational studies. Am. J. Clin. Nutr. 2015, 101, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Janaszak-Jasiecka, A.; Siekierzycka, A.; Płoska, A.; Dobrucki, I.T.; Kalinowski, L. Endothelial Dysfunction Driven by Hypoxia-The Influence of Oxygen Deficiency on NO Bioavailability. Biomolecules 2021, 11, 982. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.C.; Pollock, J.S. Coupled and uncoupled NOS: Separate but equal? Uncoupled NOS in endothelial cells is a critical pathway for intracellular signaling. Circ. Res. 2006, 98, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Yuyun, M.F.; Ng, L.L.; Ng, G.A. Endothelial dysfunction, endothelial nitric oxide bioavailability, tetrahydrobiopterin, and 5-methyltetrahydrofolate in cardiovascular disease. Where are we with therapy? Microvasc. Res. 2018, 119, 7–12. [Google Scholar] [CrossRef]
- Irzmański, R.; Serwa-Stepień, E.; Barylski, M.; Banach, M.; Kowalski, J.; Pawlicki, L. Endothelial dysfunction in hypertension. The role of natriuretic peptides and endothelin. Kardiol. Pol. 2005, 63 (Suppl. 2), S457–S461. [Google Scholar] [PubMed]
- Della Roca Domenico, P.C. Czynność sródbłonka i niepomyślne rokowanie chorób układu krążenia. Kardiol. Po Dyplomie 2011, 10, 14–26. [Google Scholar]
- Goch, A.; Banach, M.; Mikhailidis, D.P.; Rysz, J.; Goch, J.H. Endothelial dysfunction in patients with noncomplicated and complicated hypertension. Clin. Exp. Hypertens. 2009, 31, 20–30. [Google Scholar] [CrossRef]
- Lerman, A.; Zeiher, A.M. Endothelial function: Cardiac events. Circulation 2005, 111, 363–368. [Google Scholar] [CrossRef]
- Szymański, F.M.; Barylski, M.; Cybulska, B.; Wożakowska-Kapłon, B.; Krasiński, Z.; Mamcarz, A.; Widecka, K.; Płatek, A.E.; Dudek, D.; Mickiewicz, A.; et al. Recommendation for the management of dyslipidemia in Poland—Third Declaration of Sopot. Interdisciplinary Expert Position Statement endorsed by the Polish Cardiac Society Working Group on Cardiovascular Pharmacotherapy. Cardiol. J. 2018, 25, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Chapman, M.J.; Humphries, S.E.; Ginsberg, H.N.; Masana, L.; Descamps, O.S.; Wiklund, O.; Hegele, R.A.; Raal, F.J.; Defesche, J.C.; et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease: Consensus Statement of the European Atherosclerosis Society. Eur. Heart J. 2013, 34, 3478–3490. [Google Scholar] [CrossRef] [PubMed]
- Navarese, E.P.; Kolodziejczak, M.; Kereiakes, D.J.; Tantry, U.S.; O’Connor, C.; Gurbel, P.A. Proprotein Convertase Subtilisin/Kexin Type 9 Monoclonal Antibodies for Acute Coronary Syndrome: A Narrative Review. Ann. Intern. Med. 2016, 164, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Mak, M.C.E.; Gurung, R.; Foo, R.S.Y. Applications of Genome Editing Technologies in CAD Research and Therapy with a Focus on Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 14057. [Google Scholar] [CrossRef] [PubMed]
- Dobarro, D.; Gómez-Rubín, M.C.; Sanchez-Recalde, A.; Moreno, R.; Galeote, G.; Jimenez-Valero, S.; Calvo, L.; López de Sá, E.; López-Sendón, J.L. Current pharmacological approach to restore endothelial dysfunction. Cardiovasc. Hematol. Agents Med. Chem. 2009, 7, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Janaszak-Jasiecka, A.; Płoska, A.; Wierońska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: Molecular mechanisms as potential therapeutic targets. Cell Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef] [PubMed]
- Paulo, M.; Costa, D.; Bonaventura, D.; Lunardi, C.N.; Bendhack, L.M. Nitric Oxide Donors as Potential Drugs for the Treatment of Vascular Diseases Due to Endothelium Dysfunction. Curr. Pharm. Des. 2020, 26, 3748–3759. [Google Scholar] [CrossRef] [PubMed]
- Centeno Tablante, E.; Pachon, H.; Guetterman, H.M.; Finkelstein, J.L. Fortification of wheat and maize flour with folic acid for population health outcomes. Cochrane Database Syst. Rev. 2019, 7, CD012150. [Google Scholar] [CrossRef]
- Varadharaj, S.; Kelly, O.J.; Khayat, R.N.; Kumar, P.S.; Ahmed, N.; Zweier, J.L. Role of Dietary Antioxidants in the Preservation of Vascular Function and the Modulation of Health and Disease. Front. Cardiovasc. Med. 2017, 4, 64. [Google Scholar] [CrossRef]
- Bailey, S.W.; Ayling, J.E. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc. Natl. Acad. Sci. USA 2009, 106, 15424–15429. [Google Scholar] [CrossRef]
- Settergren, M.; Böhm, F.; Malmström, R.E.; Channon, K.M.; Pernow, J. L-arginine and tetrahydrobiopterin protects against ischemia/reperfusion-induced endothelial dysfunction in patients with type 2 diabetes mellitus and coronary artery disease. Atherosclerosis 2009, 204, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Lange, H.; Suryapranata, H.; De Luca, G.; Borner, C.; Dille, J.; Kallmayer, K.; Pasalary, N.; Scherer, E.; Dambrink, J.H.E. Folate therapy and in-stent restenosis after coronary stenting. N. Engl. J. Med. 2004, 350, 2673–2681. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Xu, P.; Fu, Z.; Gu, X.; Li, H.; Cui, X.; You, L.; Zhu, L.; Ji, C.; Guo, X. Association of maternal folate status in the second trimester of pregnancy with the risk of gestational diabetes mellitus. Food Sci. Nutr. 2019, 7, 3759–3765. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, L.A.; Reynolds, K.; Holder, K.N.; He, J. Effect of folic acid supplementation on risk of cardiovascular diseases: A meta-analysis of randomized controlled trials. JAMA 2006, 296, 2720–2726. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Ge, X.; Huang, K.; Mao, L.; Yan, S.; Xu, Y.; Huang, S.; Hao, J.; Zhu, P.; Niu, Y.; et al. Folic Acid Supplement Intake in Early Pregnancy Increases Risk of Gestational Diabetes Mellitus: Evidence From a Prospective Cohort Study. Diabetes Care 2016, 39, e36–e37. [Google Scholar] [CrossRef]
- Hu, J.; Wang, B.; Sahyoun, N.R. Application of the Key Events Dose-response Framework to Folate Metabolism. Crit. Rev. Food Sci. Nutr. 2016, 56, 1325–1333. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002; ISBN-10: 0-8153-3218-1ISBN-10: 0-8153-4072-9. [Google Scholar]
- Ferrazzi, E.; Tiso, G.; Di Martino, D. Folic acid versus 5-methyl tetrahydrofolate supplementation in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 312–319. [Google Scholar] [CrossRef]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef]
- Golbahar, J.; Rezaian, G.; Fathi, Z.; Aminzadeh, M.A. Association of low red blood cell folate concentrations with coronary artery disease in Iranians: A matched case-control study. J. Vasc. Res. 2005, 42, 325–330. [Google Scholar] [CrossRef]
- Golbahar, J.; Mostafavi, E. Association between low red blood cell 5-methyltetrahydrofolate and hyperhomocysteinaemia with hypertension: A cross-sectional study. High. Blood Press. Cardiovasc. Prev. 2012, 19, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Z.; Zhou, C.; Li, Q.; He, P.; Zhang, Y.; Li, H.; Liu, C.; Liang, M.; Wang, X.; et al. Relationship of several serum folate forms with the risk of mortality: A prospective cohort study. Clin. Nutr. 2021, 40, 4255–4262. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Holzgreve, W.; Pietrzik, K. Is 5-methyltetrahydrofolate an alternative to folic acid for the prevention of neural tube defects? J. Perinat. Med. 2013, 41, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Wijerathne, C.U.B.; Au-Yeung, K.K.W.; Siow, Y.L.; O, K. 5-Methyltetrahydrofolate Attenuates Oxidative Stress and Improves Kidney Function in Acute Kidney Injury through Activation of Nrf2 and Antioxidant Defense. Antioxidants 2022, 11, 1046. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Z.; Wang, Y.; Zhang, T.; Qi, S.; Tang, Y.; Gao, X. Investigation into the Properties of L-5-Methyltetrahydrofolate and Seal Oil as a Potential Atherosclerosis Intervention in Rats. J. Nutr. Sci. Vitaminol. 2022, 68, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Clément, A.; Menezo, Y.; Cohen, M.; Cornet, D.; Clément, P. 5-Methyltetrahydrofolate reduces blood homocysteine level significantly in C677T methyltetrahydrofolate reductase single-nucleotide polymorphism carriers consulting for infertility. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101622. [Google Scholar] [CrossRef]
- Verhaar, M.C.; Wever, R.M.; Kastelein, J.J.; van Dam, T.; Koomans, H.A.; Rabelink, T.J. 5-methyltetrahydrofolate, the active form of folic acid, restores endothelial function in familial hypercholesterolemia. Circulation 1998, 97, 237–241. [Google Scholar] [CrossRef]

| Type of Inheritance | Genes, Enzymes, Receptors, and Ligands | Clinical Implications |
|---|---|---|
| Monogenic inheritance. Among monogenic mutations, most are involved in lipid metabolism. | Genetic causes of elevated LDL cholesterol | |
| LDLR, APOB, PCSK9 | Familial hyperlipidemia | |
| USF1 | Familial combined hyperlipidemia | |
| Genetic causes of reduced HDL cholesterol levels | ||
| APOA1 | Primary hypoalphalipoproteinemia | |
| ABCA1 | Tangier disease | |
| LCAT | Norum disease, Fish eye disease | |
| ABCG5/8 | Sitosterolemia | |
| Genetic causes of hypertriglyceridemia | ||
| LPL, APOC2, APOAV, GPIHBP1, LMF1 | Familial chylomicronemia syndrome | |
| APOA1/C3/A4/A5 | Familial combined hyperlipidemia and familial hypertriglyceridemia | |
| ATHS | Atherogenic lipoprotein phenotype | |
| Polygenic inheritance. Mutations and polymorphisms of genes encoding enzymes, receptors, and ligands play a role in the development of atherosclerosis. | APOE, APOB, LPL, OLR1 (LOX1), SORT1, TRIB1 | Lipid and apolipoprotein metabolism |
| E-selectin, P-selectin, Interleukin 6, Paraoxonase data | Inflammatory response | |
| Connexin 37, eNOS, metalloproteinase 9, stromelysin 1 | Endothelium function | |
| GP Ia/II receptor, GP IIIa receptor | Platelet function | |
| Factor V Leiden, prothrombin 20210A, protein C deficiency, protein S non-deficiency, anti-thrombin deficiency | Thrombosis and fibrinolysis | |
| MTHFR | Folate metabolism | |
| ACE, AGTR1 | Blood pressure regulation | |
| Genotype | MTHFR c. 665 C>T | |||
|---|---|---|---|---|
| c.[665C=];[665C=] | c.[665C>T];[665C=] | c.[665C>T];[665C>T] | ||
| MTHFR c. 1286A>C | c.[1286A=];[1286A=] | 100 | 60–70 | 30–40 |
| c.[1286A>C];[1286A=] | 70–80 | 50–60 | - | |
| c.[1286A>C];[1286A>C] | 50–60 | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietruszyńska-Reszetarska, A.; Pietruszyński, R.; Irzmański, R. The Significance of Genetically Determined Methylation and Folate Metabolism Disorders in the Pathogenesis of Coronary Artery Disease: A Target for New Therapies? Int. J. Mol. Sci. 2024, 25, 6924. https://doi.org/10.3390/ijms25136924
Pietruszyńska-Reszetarska A, Pietruszyński R, Irzmański R. The Significance of Genetically Determined Methylation and Folate Metabolism Disorders in the Pathogenesis of Coronary Artery Disease: A Target for New Therapies? International Journal of Molecular Sciences. 2024; 25(13):6924. https://doi.org/10.3390/ijms25136924
Chicago/Turabian StylePietruszyńska-Reszetarska, Agnieszka, Robert Pietruszyński, and Robert Irzmański. 2024. "The Significance of Genetically Determined Methylation and Folate Metabolism Disorders in the Pathogenesis of Coronary Artery Disease: A Target for New Therapies?" International Journal of Molecular Sciences 25, no. 13: 6924. https://doi.org/10.3390/ijms25136924
APA StylePietruszyńska-Reszetarska, A., Pietruszyński, R., & Irzmański, R. (2024). The Significance of Genetically Determined Methylation and Folate Metabolism Disorders in the Pathogenesis of Coronary Artery Disease: A Target for New Therapies? International Journal of Molecular Sciences, 25(13), 6924. https://doi.org/10.3390/ijms25136924







