Transporter Proteins as Therapeutic Drug Targets—With a Focus on SGLT2 Inhibitors
Abstract
1. Introduction
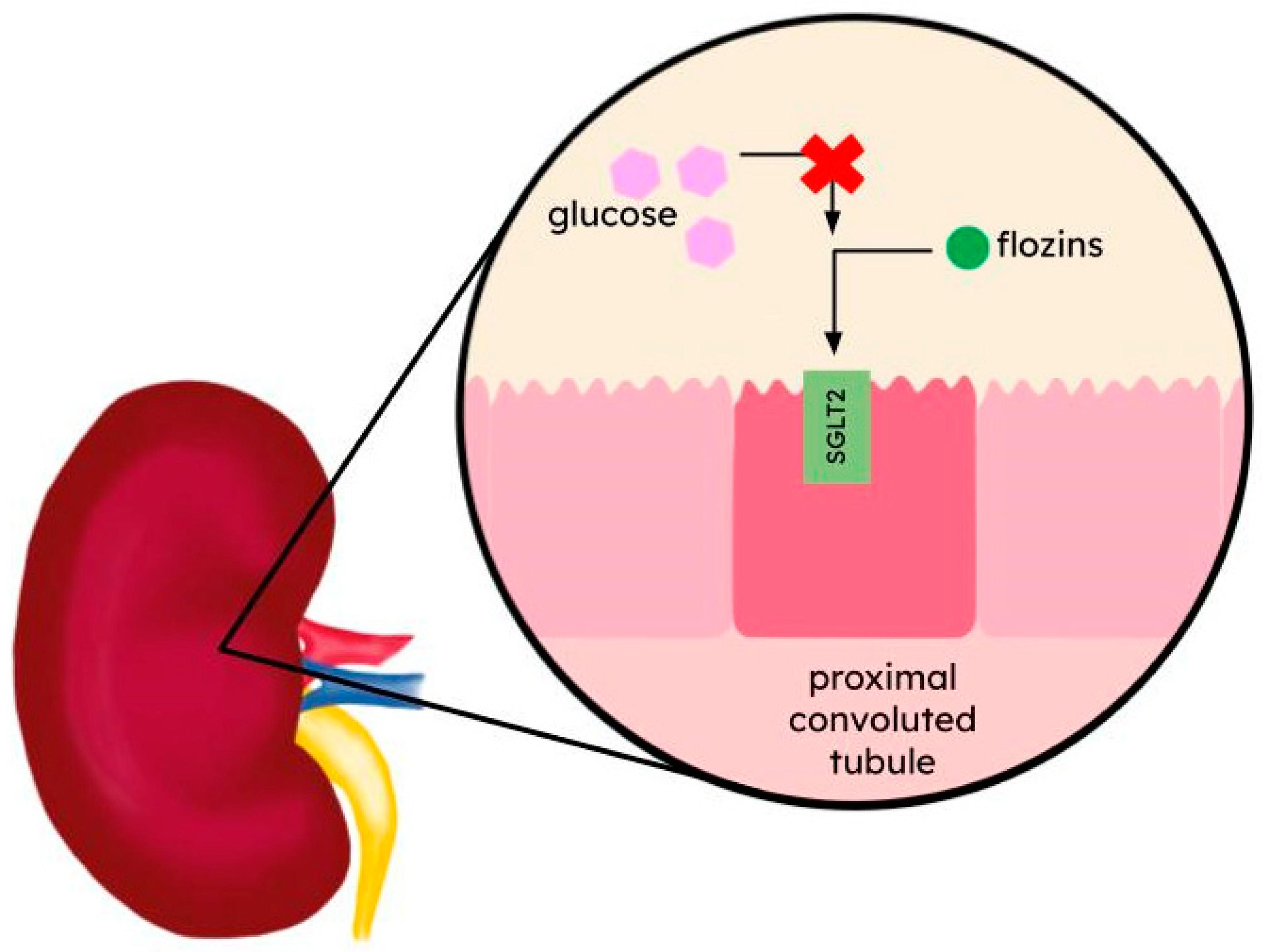
2. Flozins—SGLT2 Inhibitors
2.1. Dapagliflozin
2.2. Canagliflozin
2.3. Empagliflozin
2.4. Ertugliflozin
2.5. Sotagliflozin
3. Myrcludex B
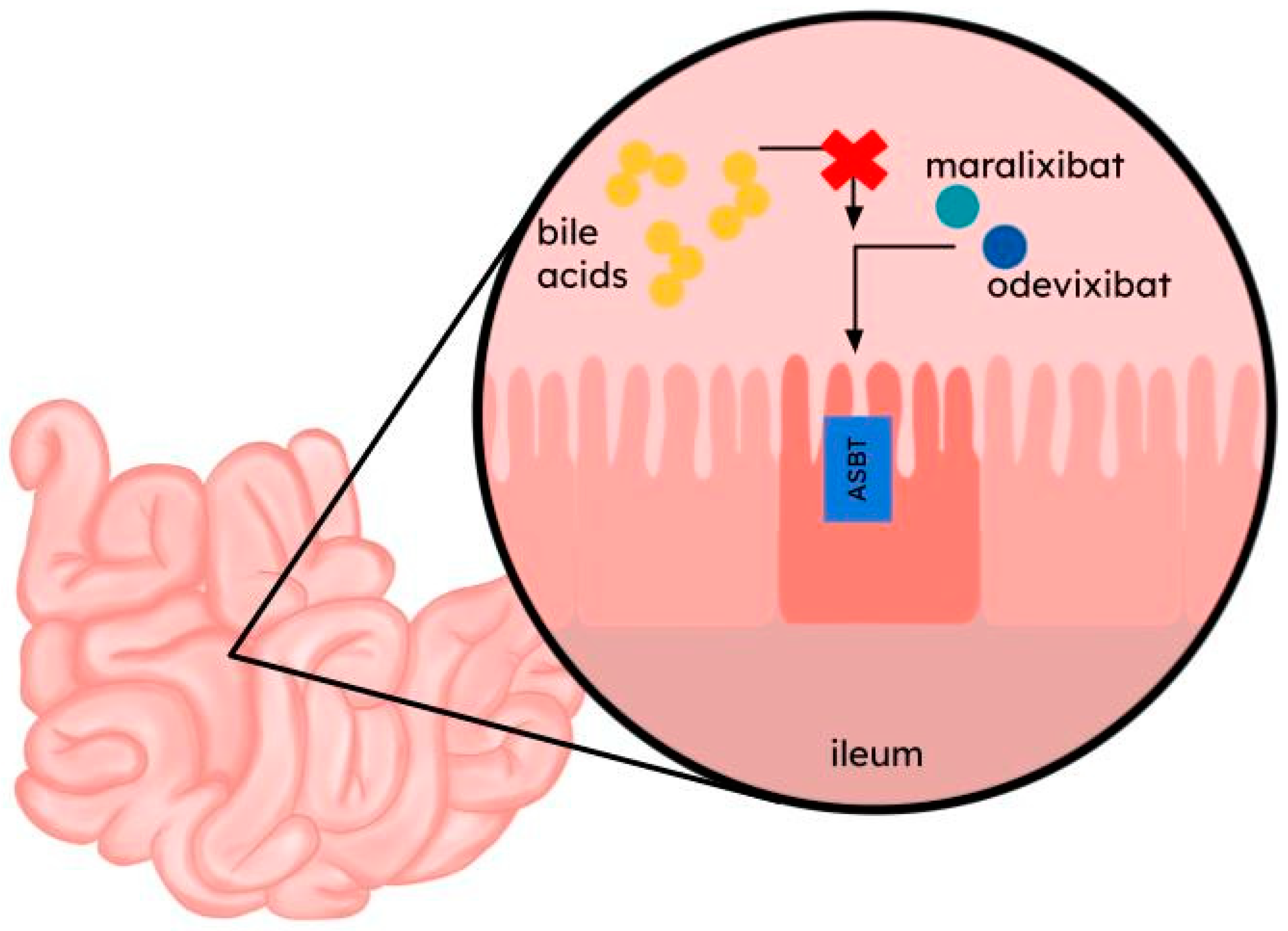
4. Odevixibat and Maralixibat
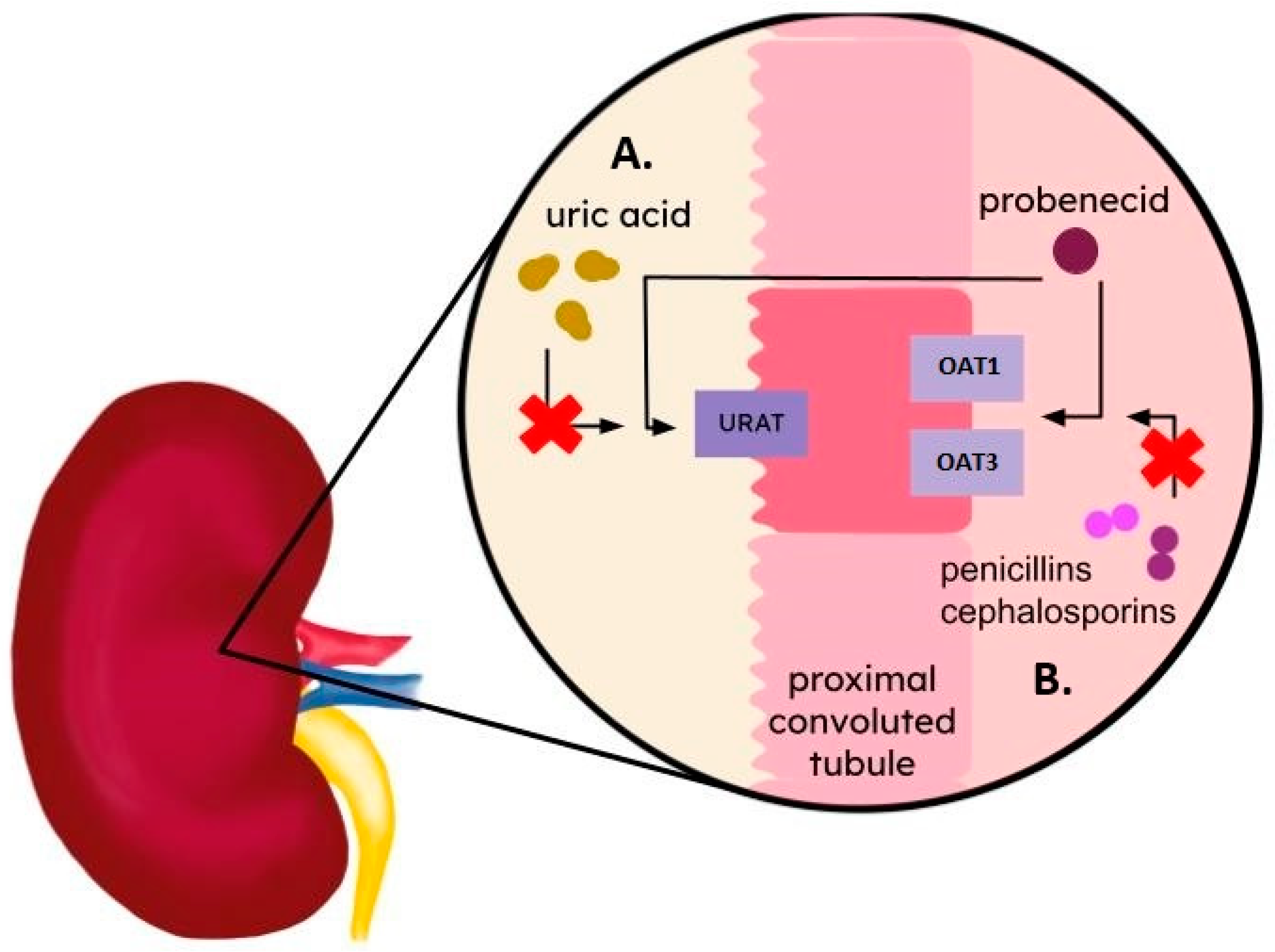
5. Probenecid and Benzbromarone
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drozdzik, M.; Czekawy, I.; Oswald, S.; Drozdzik, A. Intestinal Drug Transporters in Pathological States: An Overview. Pharmacol. Rep. 2020, 72, 1173–1194. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, S.; Chen, L. The Physiological Role of Drug Transporters. Protein Cell 2015, 6, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, C.Y.-T.; Kong, A.-N.T. Induction of Phase I, II and III Drug Metabolism/Transport by Xenobiotics. Arch. Pharm. Res. 2005, 28, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Austin Doyle, L.; Ross, D.D. Multidrug Resistance Mediated by the Breast Cancer Resistance Protein BCRP (ABCG2). Oncogene 2003, 22, 7340–7358. [Google Scholar] [CrossRef]
- Ferrada, E.; Superti-Furga, G. A Structure and Evolutionary-Based Classification of Solute Carriers. iScience 2022, 25, 105096. [Google Scholar] [CrossRef] [PubMed]
- You, G.; Morris, M.E. Overview of Drug Transporter Families. In Drug Transporters: Molecular Characterization and Role in Drug Disposition; You, G., Morris, M.E., Binghe, W., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 1–10. [Google Scholar]
- Zamek-Gliszczynski, M.J.; Taub, M.E.; Chothe, P.P.; Chu, X.; Giacomini, K.M.; Kim, R.B.; Ray, A.S.; Stocker, S.L.; Unadkat, J.D.; Wittwer, M.B.; et al. Transporters in Drug Development: 2018 ITC Recommendations for Transporters of Emerging Clinical Importance. Clin. Pharmacol. Ther. 2018, 104, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Bahadduri, P.M.; Polli, J.E.; Swaan, P.W.; Ekins, S. Targeting Drug Transporters–Combining in Silico and in Vitro Approaches to Predict in Vivo. In Membrane Transporters in Drug Discovery and Development: Methods and Protocols; Yan, Q., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; pp. 65–103. [Google Scholar]
- Ravna, A.W.; Sager, G.; Dahl, S.G.; Sylte, I. Membrane Transporters: Structure, Function and Targets for Drug Design. In Transporters as Targets for Drugs; Napier, S., Bingham, M., Eds.; Topics in Medicinal Chemistry; Springer: Berlin/Heidelberg, Germany, 2009; pp. 15–51. [Google Scholar]
- Mizuno, N.; Niwa, T.; Yotsumoto, Y.; Sugiyama, Y. Impact of Drug Transporter Studies on Drug Discovery and Development. Pharmacol. Rev. 2003, 55, 425–461. [Google Scholar] [CrossRef] [PubMed]
- Rieg, T.; Vallon, V. Development of SGLT1 and SGLT2 Inhibitors. Diabetologia 2018, 61, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Platt, K.A.; Cunard, R.; Schroth, J.; Whaley, J.; Thomson, S.C.; Koepsell, H.; Rieg, T. SGLT2 Mediates Glucose Reabsorption in the Early Proximal Tubule. J. Am. Soc. Nephrol. 2011, 22, 104–112. [Google Scholar] [CrossRef]
- Ferrannini, E.; Ramos, S.J.; Salsali, A.; Tang, W.; List, J.F. Dapagliflozin Monotherapy in Type 2 Diabetic Patients With Inadequate Glycemic Control by Diet and Exercise: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial. Diabetes Care 2010, 33, 2217–2224. [Google Scholar] [CrossRef]
- Stenlöf, K.; Cefalu, W.T.; Kim, K.-A.; Alba, M.; Usiskin, K.; Tong, C.; Canovatchel, W.; Meininger, G. Efficacy and Safety of Canagliflozin Monotherapy in Subjects with Type 2 Diabetes Mellitus Inadequately Controlled with Diet and Exercise. Diabetes Obes. Metab. 2013, 15, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.; Weng, J.; Eilbracht, J.; Delafont, B.; Kim, G.; Woerle, H.J.; Broedl, U.C.; EMPA-REG MONO Trial Investigators. Empagliflozin Monotherapy with Sitagliptin as an Active Comparator in Patients with Type 2 Diabetes: A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Diabetes Endocrinol. 2013, 1, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Terra, S.G.; Focht, K.; Davies, M.; Frias, J.; Derosa, G.; Darekar, A.; Golm, G.; Johnson, J.; Saur, D.; Lauring, B.; et al. Phase III, Efficacy and Safety Study of Ertugliflozin Monotherapy in People with Type 2 Diabetes Mellitus Inadequately Controlled with Diet and Exercise Alone. Diabetes Obes. Metab. 2017, 19, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Zeeuw, D.d.; Mahaffey, K.W.; Fulcher, G.; Erondu, N.; Shaw, W.; Barrett, T.D.; Weidner-Wells, M.; Deng, H.; Matthews, D.R.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes: Results from the CANVAS Program Randomised Clinical Trials. Lancet Diabetes Endocrinol. 2018, 6, 691–704. [Google Scholar] [CrossRef]
- Levin, A.; Perkovic, V.; Wheeler, D.C.; Hantel, S.; George, J.T.; von Eynatten, M.; Koitka-Weber, A.; Wanner, C.; EMPA-REG OUTCOME Investigators. Empagliflozin and Cardiovascular and Kidney Outcomes across KDIGO Risk Categories: Post Hoc Analysis of a Randomized, Double-Blind, Placebo-Controlled, Multinational Trial. Clin. J. Am. Soc. Nephrol. 2020; 15, 1433–1444. [Google Scholar]
- The EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar]
- Barnett, A.H.; Mithal, A.; Manassie, J.; Jones, R.; Rattunde, H.; Woerle, H.J.; Broedl, U.C. Efficacy and Safety of Empagliflozin Added to Existing Antidiabetes Treatment in Patients with Type 2 Diabetes and Chronic Kidney Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Diabetes Endocrinol. 2014, 2, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Grunberger, G.; Camp, S.; Johnson, J.; Huyck, S.; Terra, S.G.; Mancuso, J.P.; Jiang, Z.W.; Golm, G.; Engel, S.S.; Lauring, B. Ertugliflozin in Patients with Stage 3 Chronic Kidney Disease and Type 2 Diabetes Mellitus: The VERTIS RENAL Randomized Study. Diabetes Ther. 2018, 9, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Cannon, C.P.; Cherney, D.Z.I.; Masiukiewicz, U.; Pratley, R.; Dagogo-Jack, S.; Frederich, R.; Charbonnel, B.; Mancuso, J.; Shih, W.J.; et al. Efficacy of Ertugliflozin on Heart Failure–Related Events in Patients with Type 2 Diabetes Mellitus and Established Atherosclerotic Cardiovascular Disease. Circulation 2020, 142, 2205–2215. [Google Scholar] [CrossRef]
- Qi, X.; Li, W. Unlocking the Secrets to Human NTCP Structure. Innovation 2022, 3, 100294. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Li, T. Regulation of the HBV Entry Receptor NTCP and Its Potential in Hepatitis B Treatment. Front. Mol. Biosci. 2022, 9, 879817. [Google Scholar] [CrossRef] [PubMed]
- Sagnelli, C.; Sagnelli, E.; Russo, A.; Pisaturo, M.; Occhiello, L.; Coppola, N. HBV/HDV Co-Infection: Epidemiological and Clinical Changes, Recent Knowledge and Future Challenges. Life 2021, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F.; Hagey, L.R. Key Discoveries in Bile Acid Chemistry and Biology and Their Clinical Applications: History of the Last Eight Decades. J. Lipid Res. 2014, 55, 1553–1595. [Google Scholar] [CrossRef] [PubMed]
- Kamath, B.M.; Stein, P.; Houwen, R.H.J.; Verkade, H.J. Potential of Ileal Bile Acid Transporter Inhibition as a Therapeutic Target in Alagille Syndrome and Progressive Familial Intrahepatic Cholestasis. Liver Int. 2020, 40, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Craddock, A.L.; Love, M.W.; Daniel, R.W.; Kirby, L.C.; Walters, H.C.; Wong, M.H.; Dawson, P.A. Expression and Transport Properties of the Human Ileal and Renal Sodium-Dependent Bile Acid Transporter. Am. J. Physiol. 1998, 274, G157–G169. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Polli, J.E. Apical Sodium Dependent Bile Acid Transporter (ASBT, SLC10A2): A Potential Prodrug Target. Mol. Pharm. 2006, 3, 223–230. [Google Scholar] [CrossRef]
- Mitchell, E.; Gilbert, M.; Loomes, K.M. Alagille Syndrome. Clin. Liver Dis. 2018, 22, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Amirneni, S.; Haep, N.; Gad, M.A.; Soto-Gutierrez, A.; Squires, J.E.; Florentino, R.M. Molecular Overview of Progressive Familial Intrahepatic Cholestasis. World J. Gastroenterol. 2020, 26, 7470–7484. [Google Scholar] [CrossRef] [PubMed]
- Scheffel, A.R. Use of Benemid in Enhancement of Serum Blood Levels of Pencillin. Ph.D. Thesis, University of Nebraska Medical Center, Omaha, NE, USA, 1952. [Google Scholar]
- Koepsell, H. The SLC22 Family with Transporters of Organic Cations, Anions and Zwitterions. Mol. Asp. Med. 2013, 34, 413–435. [Google Scholar] [CrossRef]
- Eraly, S.A.; Hamilton, B.A.; Nigam, S.K. Organic Anion and Cation Transporters Occur in Pairs of Similar and Similarly Expressed Genes. Biochem. Biophys. Res. Commun. 2003, 300, 333–342. [Google Scholar] [CrossRef]
- Hilgendorf, C.; Ahlin, G.; Seithel, A.; Artursson, P.; Ungell, A.-L.; Karlsson, J. Expression of Thirty-Six Drug Transporter Genes in Human Intestine, Liver, Kidney, and Organotypic Cell Lines. Drug Metab. Dispos. 2007, 35, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Alebouyeh, M.; Takeda, M.; Onozato, M.L.; Tojo, A.; Noshiro, R.; Hasannejad, H.; Inatomi, J.; Narikawa, S.; Huang, X.-L.; Khamdang, S.; et al. Expression of Human Organic Anion Transporters in the Choroid Plexus and Their Interactions with Neurotransmitter Metabolites. J. Pharmacol. Sci. 2003, 93, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Gutman, A.B.; Yu, T.F. Benemid (p-Di-n-Propylsulfamyl)-Benzoic Acid) as Uricosuric Agent in Chronic Gouty Arthritis. Trans. Assoc. Am. Physicians 1951, 64, 279–288. [Google Scholar] [PubMed]
- Bartels, E.C.; Matossian, G.S. Gout: Six-Year Follow-up on Probenecid (Benemid) Therapy. Arthritis Rheum. 1959, 2, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular Identification of a Renal Urate Anion Exchanger That Regulates Blood Urate Levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Bleasby, K.; Castle, J.C.; Roberts, C.J.; Cheng, C.; Bailey, W.J.; Sina, J.F.; Kulkarni, A.V.; Hafey, M.J.; Evers, R.; Johnson, J.M.; et al. Expression Profiles of 50 Xenobiotic Transporter Genes in Humans and Pre-Clinical Species: A Resource for Investigations into Drug Disposition. Xenobiotica 2006, 36, 963–988. [Google Scholar] [CrossRef] [PubMed]
- EMA Summary of Product Characteristics. ZURAMPIC, INN-Lesinurad 2017. Available online: https://www.ema.europa.eu/en/documents/product-information/zurampic-epar-product-information_en.pdf (accessed on 27 February 2024).
- Lee, M.-H.H.; Graham, G.G.; Williams, K.M.; Day, R.O. A Benefit-Risk Assessment of Benzbromarone in the Treatment of Gout. Was Its Withdrawal from the Market in the Best Interest of Patients? Drug Saf. 2008, 31, 643–665. [Google Scholar] [CrossRef]
- Moriwaki, Y.; Yamamoto, T.; Takahashi, S.; Hada, T.; Higashino, K. Analysis of Uric Acid Transport in Renal Tubules Using Benzbromarone and Pyrazinamide. Int. J. Clin. Pharmacol. Ther. Toxicol. 1990, 28, 84–88. [Google Scholar]
- Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process (accessed on 27 February 2024).
- BfArM-Arzneimittel Recherchieren. Available online: https://www.bfarm.de/DE/Arzneimittel/Arzneimittelinformationen/Arzneimittel-recherchieren/_node.html (accessed on 27 February 2024).
- SAI-Detailed View. Available online: https://sai.refdata.ch/detail/37885 (accessed on 27 February 2024).
- Wright, E.M.; Loo, D.D.F.; Hirayama, B.A. Biology of Human Sodium Glucose Transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef]
- Rieg, T.; Masuda, T.; Gerasimova, M.; Mayoux, E.; Platt, K.; Powell, D.R.; Thomson, S.C.; Koepsell, H.; Vallon, V. Increase in SGLT1-Mediated Transport Explains Renal Glucose Reabsorption during Genetic and Pharmacological SGLT2 Inhibition in Euglycemia. Am. J. Physiol. Ren. Physiol. 2014, 306, F188–F193. [Google Scholar] [CrossRef]
- Santer, R.; Calado, J. Familial Renal Glucosuria and SGLT2: From a Mendelian Trait to a Therapeutic Target. Clin. J. Am. Soc. Nephrol. 2010, 5, 133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lu, Y.; Hajifathalian, K.; Bentham, J.; Cesare, M.D.; Danaei, G.; Bixby, H.; Cowan, M.J.; Ali, M.K.; Taddei, C.; et al. Worldwide Trends in Diabetes since 1980: A Pooled Analysis of 751 Population-Based Studies with 4.4 Million Participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Wright, E. Diseases of Renal Glucose Handling. In Genetic Diseases of the Kidney; Lifton, R.P., Somlo, S., Giebisch, G.H., Seldin, D.W., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 131–140. [Google Scholar]
- Laakso, M. Cardiovascular Disease in Type 2 Diabetes From Population to Man to Mechanisms: The Kelly West Award Lecture 2008. Diabetes Care 2010, 33, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.C.; Deng, A.; Bao, D.; Satriano, J.; Blantz, R.C.; Vallon, V. Ornithine Decarboxylase, Kidney Size, and the Tubular Hypothesis of Glomerular Hyperfiltration in Experimental Diabetes. J. Clin. Investig. 2001, 107, 217–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vallon, V.; Rose, M.; Gerasimova, M.; Satriano, J.; Platt, K.A.; Koepsell, H.; Cunard, R.; Sharma, K.; Thomson, S.C.; Rieg, T. Knockout of Na-Glucose Transporter SGLT2 Attenuates Hyperglycemia and Glomerular Hyperfiltration but Not Kidney Growth or Injury in Diabetes Mellitus. Am. J. Physiol. Ren. Physiol. 2013, 304, F156–F167. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Gerasimova, M.; Rose, M.A.; Masuda, T.; Satriano, J.; Mayoux, E.; Koepsell, H.; Thomson, S.C.; Rieg, T. SGLT2 Inhibitor Empagliflozin Reduces Renal Growth and Albuminuria in Proportion to Hyperglycemia and Prevents Glomerular Hyperfiltration in Diabetic Akita Mice. Am. J. Physiol. Ren. Physiol. 2014, 306, F194–F204. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Luo, Y.; Wang, D.; Adorini, L.; Pruzanski, M.; Dobrinskikh, E.; Levi, M. A Dual Agonist of Farnesoid X Receptor (FXR) and the G Protein–Coupled Receptor TGR5, INT-767, Reverses Age-Related Kidney Disease in Mice. J. Biol. Chem. 2017, 292, 12018–12024. [Google Scholar] [CrossRef] [PubMed]
- Khunti, K.; Davies, M.; Majeed, A.; Thorsted, B.L.; Wolden, M.L.; Paul, S.K. Hypoglycemia and Risk of Cardiovascular Disease and All-Cause Mortality in Insulin-Treated People with Type 1 and Type 2 Diabetes: A Cohort Study. Diabetes Care 2015, 38, 316–322. [Google Scholar] [CrossRef]
- Vallon, V.; Thomson, S.C. Targeting Renal Glucose Reabsorption to Treat Hyperglycaemia: The Pleiotropic Effects of SGLT2 Inhibition. Diabetologia 2017, 60, 215–225. [Google Scholar] [CrossRef]
- Grempler, R.; Thomas, L.; Eckhardt, M.; Himmelsbach, F.; Sauer, A.; Sharp, D.E.; Bakker, R.A.; Mark, M.; Klein, T.; Eickelmann, P. Empagliflozin, a Novel Selective Sodium Glucose Cotransporter-2 (SGLT-2) Inhibitor: Characterisation and Comparison with Other SGLT-2 Inhibitors. Diabetes Obes. Metab. 2012, 14, 83–90. [Google Scholar] [CrossRef]
- Choi, C.-I. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors from Natural Products: Discovery of Next-Generation Antihyperglycemic Agents. Molecules 2016, 21, 1136. [Google Scholar] [CrossRef] [PubMed]
- Isaji, M. SGLT2 Inhibitors: Molecular Design and Potential Differences in Effect. Kidney Int. 2011, 79, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Richter, K.; Blantz, R.C.; Thomson, S.; Osswald, H. Glomerular Hyperfiltration in Experimental Diabetes Mellitus: Potential Role of Tubular Reabsorption. J. Am. Soc. Nephrol. 1999, 10, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.C.; Rieg, T.; Miracle, C.; Mansoury, H.; Whaley, J.; Vallon, V.; Singh, P. Acute and Chronic Effects of SGLT2 Blockade on Glomerular and Tubular Function in the Early Diabetic Rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R75–R83. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Devineni, D.; Ghosh, A.; Polidori, D.; Chien, S.; Wexler, D.; Shalayda, K.; Demarest, K.; Rothenberg, P. Canagliflozin, a Novel Inhibitor of Sodium Glucose Co-transporter 2, Dose Dependently Reduces Calculated Renal Threshold for Glucose Excretion and Increases Urinary Glucose Excretion in Healthy Subjects. Diabetes Obes. Metab. 2011, 13, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Komoroski, B.; Vachharajani, N.; Boulton, D.; Kornhauser, D.; Geraldes, M.; Li, L.; Pfister, M. Dapagliflozin, a Novel SGLT2 Inhibitor, Induces Dose-Dependent Glucosuria in Healthy Subjects. Clin. Pharmacol. Ther. 2009, 85, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; Seewaldt-Becker, E.; Macha, S.; Hantel, S.; Pinnetti, S.; Seman, L.; Woerle, H.J. Safety, Tolerability, Pharmacokinetics and Pharmacodynamics Following 4 Weeks’ Treatment with Empagliflozin Once Daily in Patients with Type 2 Diabetes. Diabetes Obes. Metab. 2013, 15, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Gorboulev, V.; Schürmann, A.; Vallon, V.; Kipp, H.; Jaschke, A.; Klessen, D.; Friedrich, A.; Scherneck, S.; Rieg, T.; Cunard, R.; et al. Na+-d-Glucose Cotransporter SGLT1 Is Pivotal for Intestinal Glucose Absorption and Glucose-Dependent Incretin Secretion. Diabetes 2011, 61, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.R.; DaCosta, C.M.; Gay, J.; Ding, Z.-M.; Smith, M.; Greer, J.; Doree, D.; Jeter-Jones, S.; Mseeh, F.; Rodriguez, L.A.; et al. Improved Glycemic Control in Mice Lacking Sglt1 and Sglt2. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E117–E130. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- EMA Summary of Product Characteristics. FORXIGA, INN-Canagliflozin 2020. Available online: https://www.ema.europa.eu/en/documents/product-information/forxiga-epar-product-information_en.pdf (accessed on 6 March 2024).
- Forxiga|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/forxiga (accessed on 6 March 2024).
- Edistride|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/edistride (accessed on 6 March 2024).
- FDA Medication Guide Farxiga 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202293s020lbl.pdf (accessed on 6 March 2024).
- Dapagliflozin Viatris|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/dapagliflozin-viatris (accessed on 7 March 2024).
- Ebymect|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ebymect (accessed on 7 March 2024).
- Xigduo|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/xigduo (accessed on 7 March 2024).
- Qtern|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/qtern (accessed on 7 March 2024).
- Bailey, C.J.; Morales Villegas, E.C.; Woo, V.; Tang, W.; Ptaszynska, A.; List, J.F. Efficacy and Safety of Dapagliflozin Monotherapy in People with Type 2 Diabetes: A Randomized Double-Blind Placebo-Controlled 102-Week Trial. Diabet. Med. 2015, 32, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Del Prato, S.; Meier, J.J.; Durán-García, S.; Rohwedder, K.; Elze, M.; Parikh, S.J. Dapagliflozin versus Glipizide as Add-on Therapy in Patients with Type 2 Diabetes Who Have Inadequate Glycemic Control with Metformin: A Randomized, 52-Week, Double-Blind, Active-Controlled Noninferiority Trial. Diabetes Care 2011, 34, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, S.A.; Hardy, E.; Sugg, J.; Parikh, S.; Study 10 Group. Dapagliflozin Is Effective as Add-on Therapy to Sitagliptin with or without Metformin: A 24-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study. Diabetes Care 2014, 37, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Matthaei, S.; Bowering, K.; Rohwedder, K.; Grohl, A.; Parikh, S.; Study 05 Group. Dapagliflozin Improves Glycemic Control and Reduces Body Weight as Add-on Therapy to Metformin plus Sulfonylurea: A 24-Week Randomized, Double-Blind Clinical Trial. Diabetes Care 2015, 38, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Woo, V.; Soler, N.G.; Pahor, A.; Sugg, J.; Rohwedder, K.; Parikh, S. Long-Term Efficacy of Dapagliflozin in Patients with Type 2 Diabetes Mellitus Receiving High Doses of Insulin. Ann. Intern. Med. 2012, 156, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Frías, J.P.; Guja, C.; Hardy, E.; Ahmed, A.; Dong, F.; Öhman, P.; Jabbour, S.A. Exenatide Once Weekly plus Dapagliflozin Once Daily versus Exenatide or Dapagliflozin Alone in Patients with Type 2 Diabetes Inadequately Controlled with Metformin Monotherapy (DURATION-8): A 28 Week, Multicentre, Double-Blind, Phase 3, Randomised Controlled Trial. Lancet Diabetes Endocrinol. 2016, 4, 1004–1016. [Google Scholar] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Polidori, D.; Sha, S.; Mudaliar, S.; Ciaraldi, T.P.; Ghosh, A.; Vaccaro, N.; Farrell, K.; Rothenberg, P.; Henry, R.R. Canagliflozin Lowers Postprandial Glucose and Insulin by Delaying Intestinal Glucose Absorption in Addition to Increasing Urinary Glucose Excretion: Results of a Randomized, Placebo-Controlled Study. Diabetes Care 2013, 36, 2154–2161. [Google Scholar] [CrossRef]
- EMA Summary of Product Characteristics. INVOKANA, INN-Canagliflozin 2023. Available online: https://www.ema.europa.eu/en/documents/product-information/invokana-epar-product-information_en.pdf (accessed on 8 March 2024).
- FDA Medication Guide Invokana 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/204042s026lbl.pdf (accessed on 8 March 2024).
- Vokanamet|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/vokanamet (accessed on 8 March 2024).
- Fulcher, G.; Matthews, D.R.; Perkovic, V.; de Zeeuw, D.; Mahaffey, K.W.; Weiss, R.; Rosenstock, J.; Capuano, G.; Desai, M.; Shaw, W.; et al. Efficacy and Safety of Canagliflozin Used in Conjunction with Sulfonylurea in Patients with Type 2 Diabetes Mellitus: A Randomized, Controlled Trial. Diabetes Ther. 2015, 6, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; de Zeeuw, D.; Mahaffey, K.W.; Fulcher, G.; Ways, K.; Desai, M.; Shaw, W.; Capuano, G.; Alba, M.; et al. Efficacy and Safety of Canagliflozin, an Inhibitor of Sodium–Glucose Cotransporter 2, When Used in Conjunction with Insulin Therapy in Patients with Type 2 Diabetes. Diabetes Care 2014, 38, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Rodbard, H.W.; Seufert, J.; Aggarwal, N.; Cao, A.; Fung, A.; Pfeifer, M.; Alba, M. Efficacy and Safety of Titrated Canagliflozin in Patients with Type 2 Diabetes Mellitus Inadequately Controlled on Metformin and Sitagliptin. Diabetes Obes. Metab. 2016, 18, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Charpentier, G.; Hollander, P.; González-Gálvez, G.; Mathieu, C.; Vercruysse, F.; Usiskin, K.; Law, G.; Black, S.; Canovatchel, W.; et al. Efficacy and Safety of Canagliflozin in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Metformin and Sulphonylurea: A Randomised Trial. Int. J. Clin. Pract. 2013, 67, 1267–1282. [Google Scholar] [CrossRef] [PubMed]
- Forst, T.; Guthrie, R.; Goldenberg, R.; Yee, J.; Vijapurkar, U.; Meininger, G.; Stein, P. Efficacy and Safety of Canagliflozin over 52 Weeks in Patients with Type 2 Diabetes on Background Metformin and Pioglitazone. Diabetes Obes. Metab. 2014, 16, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T.; Leiter, L.A.; Yoon, K.-H.; Arias, P.; Niskanen, L.; Xie, J.; Balis, D.A.; Canovatchel, W.; Meininger, G. Efficacy and Safety of Canagliflozin versus Glimepiride in Patients with Type 2 Diabetes Inadequately Controlled with Metformin (CANTATA-SU): 52 Week Results from a Randomised, Double-Blind, Phase 3 Non-Inferiority Trial. Lancet 2013, 382, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Schernthaner, G.; Gross, J.L.; Rosenstock, J.; Guarisco, M.; Fu, M.; Yee, J.; Kawaguchi, M.; Canovatchel, W.; Meininger, G. Canagliflozin Compared with Sitagliptin for Patients with Type 2 Diabetes Who Do Not Have Adequate Glycemic Control with Metformin Plus Sulfonylurea: A 52-Week Randomized Trial. Diabetes Care 2013, 36, 2508–2515. [Google Scholar] [CrossRef] [PubMed]
- Jardine, M.J.; Zhou, Z.; Mahaffey, K.W.; Oshima, M.; Agarwal, R.; Bakris, G.; Bajaj, H.S.; Bull, S.; Cannon, C.P.; Charytan, D.M.; et al. Renal, Cardiovascular, and Safety Outcomes of Canagliflozin by Baseline Kidney Function: A Secondary Analysis of the CREDENCE Randomized Trial. J. Am. Soc. Nephrol. 2020, 31, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- EMA Summary of Product Characteristics. JARDIANCE, INN-Empagliflozin 2024. Available online: https://www.ema.europa.eu/en/documents/product-information/jardiance-epar-product-information_en.pdf (accessed on 8 March 2024).
- FDA Medication Guide Jardiance 2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/204629s033lbl.pdf (accessed on 8 March 2024).
- Glyxambi|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/glyxambi (accessed on 8 March 2024).
- Synjardy|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/synjardy (accessed on 8 March 2024).
- Häring, H.-U.; Merker, L.; Seewaldt-Becker, E.; Weimer, M.; Meinicke, T.; Broedl, U.C.; Woerle, H.J.; EMPA-REG MET Trial Investigators. Empagliflozin as Add-on to Metformin in Patients with Type 2 Diabetes: A 24-Week, Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Care 2014, 37, 1650–1659. [Google Scholar] [CrossRef]
- Raedler, L.A. Glyxambi (Empagliflozin/Linagliptin): A Dual-Acting Oral Medication Approved for the Treatment of Patients with Type 2 Diabetes. Am. Health Drug Benefits 2015, 8, 171–175. [Google Scholar]
- Laffel, L.M.; Danne, T.; Klingensmith, G.J.; Tamborlane, W.V.; Willi, S.; Zeitler, P.; Neubacher, D.; Marquard, J.; DINAMO Study Group. Efficacy and Safety of the SGLT2 Inhibitor Empagliflozin versus Placebo and the DPP-4 Inhibitor Linagliptin versus Placebo in Young People with Type 2 Diabetes (DINAMO): A Multicentre, Randomised, Double-Blind, Parallel Group, Phase 3 Trial. Lancet Diabetes Endocrinol. 2023, 11, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Lewin, A.; DeFronzo, R.A.; Patel, S.; Liu, D.; Kaste, R.; Woerle, H.J.; Broedl, U.C. Initial Combination of Empagliflozin and Linagliptin in Subjects with Type 2 Diabetes. Diabetes Care 2015, 38, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Ridderstråle, M.; Andersen, K.R.; Zeller, C.; Kim, G.; Woerle, H.J.; Broedl, U.C.; EMPA-REG H2H-SU Trial Investigators. Comparison of Empagliflozin and Glimepiride as Add-on to Metformin in Patients with Type 2 Diabetes: A 104-Week Randomised, Active-Controlled, Double-Blind, Phase 3 Trial. Lancet Diabetes Endocrinol. 2014, 2, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, S.; Rosenstock, J.; Meinicke, T.; Woerle, H.J.; Broedl, U.C. Initial Combination of Empagliflozin and Metformin in Patients with Type 2 Diabetes. Diabetes Care 2016, 39, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Jelaska, A.; Zeller, C.; Kim, G.; Broedl, U.C.; Woerle, H.J.; EMPA-REG BASALTM Trial Investigators. Impact of Empagliflozin Added on to Basal Insulin in Type 2 Diabetes Inadequately Controlled on Basal Insulin: A 78-Week Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Obes. Metab. 2015, 17, 936–948. [Google Scholar] [CrossRef]
- Rosenstock, J.; Jelaska, A.; Frappin, G.; Salsali, A.; Kim, G.; Woerle, H.J.; Broedl, U.C.; EMPA-REG MDI Trial Investigators. Improved Glucose Control with Weight Loss, Lower Insulin Doses, and No Increased Hypoglycemia with Empagliflozin Added to Titrated Multiple Daily Injections of Insulin in Obese Inadequately Controlled Type 2 Diabetes. Diabetes Care 2014, 37, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- EMA Summary of Product Characteristics. STEGLATRO, INN-Ertugliflozin 2018. Available online: https://www.ema.europa.eu/en/documents/product-information/steglatro-epar-product-information_en.pdf (accessed on 8 March 2024).
- FDA Medication Guide Steglatro 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209803s000lbl.pdf (accessed on 8 March 2024).
- Segluromet|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/segluromet (accessed on 8 March 2024).
- Steglujan|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/steglujan (accessed on 8 March 2024).
- Markham, A. Ertugliflozin: First Global Approval. Drugs 2018, 78, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Aronson, R.; Frias, J.; Goldman, A.; Darekar, A.; Lauring, B.; Terra, S.G. Long-Term Efficacy and Safety of Ertugliflozin Monotherapy in Patients with Inadequately Controlled T2DM despite Diet and Exercise: VERTIS MONO Extension Study. Diabetes Obes. Metab. 2018, 20, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Frias, J.; Páll, D.; Charbonnel, B.; Pascu, R.; Saur, D.; Darekar, A.; Huyck, S.; Shi, H.; Lauring, B.; et al. Effect of Ertugliflozin on Glucose Control, Body Weight, Blood Pressure and Bone Density in Type 2 Diabetes Mellitus Inadequately Controlled on Metformin Monotherapy (VERTIS MET). Diabetes Obes. Metab. 2018, 20, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Hollander, P.; Liu, J.; Hill, J.; Johnson, J.; Jiang, Z.W.; Golm, G.; Huyck, S.; Terra, S.G.; Mancuso, J.P.; Engel, S.S.; et al. Ertugliflozin Compared with Glimepiride in Patients with Type 2 Diabetes Mellitus Inadequately Controlled on Metformin: The VERTIS SU Randomized Study. Diabetes Ther. 2018, 9, 193–207. [Google Scholar] [CrossRef]
- Pratley, R.E.; Eldor, R.; Raji, A.; Golm, G.; Huyck, S.B.; Qiu, Y.; Sunga, S.; Johnson, J.; Terra, S.G.; Mancuso, J.P.; et al. Ertugliflozin plus Sitagliptin versus Either Individual Agent over 52 Weeks in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Metformin: The VERTIS FACTORIAL Randomized Trial. Diabetes Obes. Metab. 2018, 20, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Krumins, T.; Zhou, H.; Huyck, S.; Johnson, J.; Golm, G.; Terra, S.G.; Mancuso, J.P.; Engel, S.S.; Lauring, B. Ertugliflozin and Sitagliptin Co-Initiation in Patients with Type 2 Diabetes: The VERTIS SITA Randomized Study. Diabetes Ther. 2018, 9, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, S.; Liu, J.; Eldor, R.; Amorin, G.; Johnson, J.; Hille, D.; Liao, Y.; Huyck, S.; Golm, G.; Terra, S.G.; et al. Efficacy and Safety of the Addition of Ertugliflozin in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Metformin and Sitagliptin: The VERTIS SITA2 Placebo-controlled Randomized Study. Diabetes Obes. Metab. 2018, 20, 530–540. [Google Scholar] [CrossRef] [PubMed]
- FDA Medication Guide Inpefa 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216203s000lbl.pdf (accessed on 7 March 2024).
- Zynquista|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zynquista (accessed on 7 March 2024).
- Asami, J.; Kimura, K.T.; Fujita-Fujiharu, Y.; Ishida, H.; Zhang, Z.; Nomura, Y.; Liu, K.; Uemura, T.; Sato, Y.; Ono, M.; et al. Structure of the Bile Acid Transporter and HBV Receptor NTCP. Nature 2022, 606, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Irobalieva, R.N.; Bang-Sørensen, R.; Nosol, K.; Mukherjee, S.; Agrawal, P.; Stieger, B.; Kossiakoff, A.A.; Locher, K.P. Structure of Human NTCP Reveals the Basis of Recognition and Sodium-Driven Transport of Bile Salts into the Liver. Cell Res. 2022, 32, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Wedemeyer, H.; Aleman, S.; Brunetto, M.R.; Blank, A.; Andreone, P.; Bogomolov, P.; Chulanov, V.; Mamonova, N.; Geyvandova, N.; Morozov, V.; et al. A Phase 3, Randomized Trial of Bulevirtide in Chronic Hepatitis D. N. Engl. J. Med. 2023, 389, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Han, B.; Zhang, W.; Wu, W. Clinical Effects of NTCP-Inhibitor Myrcludex B. J. Viral Hepat. 2021, 28, 852–858. [Google Scholar] [CrossRef]
- Wedemeyer, H.; Bogomolov, P.; Blank, A.; Allweiss, L.; Dandri-Petersen, M.; Bremer, B.; Voronkova, N.; Schoneweis, K.; Pathil, A.; Burhenne, J.; et al. Final Results of a Multicenter, Open-Label Phase 2b Clinical Trial to Assess Safety and Efficacy of Myrcludex B in Combination with Tenofovir in Patients with Chronic HBV/HDV Co-Infection. J. Hepatol. 2018, 68, S3. [Google Scholar] [CrossRef]
- Yurdaydin, C. New Treatment Options for Delta Virus: Is a Cure in Sight? J. Viral Hepat. 2019, 26, 618–626. [Google Scholar] [CrossRef]
- Wedemeyer, H.; Schoneweis, K.; Bogomolov, P.; Voronkova, N.; Chulanov, V.; Stepanova, T.; Bremer, B.; Allweiss, L.; Dandri, M.; Burhenne, J.; et al. GS-13-Final Results of a Multicenter, Open-Label Phase 2 Clinical Trial (MYR203) to Assess Safety and Efficacy of Myrcludex B in Cwith PEG-Interferon Alpha 2a in Patients with Chronic HBV/HDV Co-Infection. J. Hepatol. 2019, 70, e81. [Google Scholar] [CrossRef]
- Dawson, P.A.; Oelkers, P. Bile Acid Transporters. J. Lipid Res. 2009, 50, 2340–2357. [Google Scholar] [CrossRef] [PubMed]
- Kamath, B.M.; Ye, W.; Goodrich, N.P.; Loomes, K.M.; Romero, R.; Heubi, J.E.; Leung, D.H.; Spinner, N.B.; Piccoli, D.A.; Alonso, E.M.; et al. Outcomes of Childhood Cholestasis in Alagille Syndrome: Results of a Multicenter Observational Study. Hepatol. Commun. 2020, 22, 387–398. [Google Scholar] [CrossRef]
- Wessel, D.; Thompson, R.; Grammatikopoulos, T.; Kadaristiana, A.; Jankowska, I.; Lipiński, P.; Czubkowski, P.; Gonzales, E.; Jacquemin, E.; Spraul, A.; et al. The Natural Course of FIC1 Deficiency and BSEP Deficiency: Initial Results from the NAPPED-Consortium (Natural Course and Prognosis of PFIC and Effect of Biliary Diversion). J. Hepatol. 2018, 68, S626–S627. [Google Scholar] [CrossRef]
- De Vloo, C.; Nevens, F. Cholestatic Pruritus: An Update. Acta Gastroenterol. Belg. 2019, 82, 75–82. [Google Scholar] [PubMed]
- Kremer, A.E.; Oude Elferink, R.P.J.; Beuers, U. Pathophysiology and Current Management of Pruritus in Liver Disease. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 89–97. [Google Scholar] [CrossRef]
- Gonzales, E.; Hardikar, W.; Stormon, M.; Baker, A.; Hierro, L.; Gliwicz, D.; Lacaille, F.; Lachaux, A.; Sturm, E.; Setchell, K.D.R.; et al. Efficacy and Safety of Maralixibat Treatment in Patients with Alagille Syndrome and Cholestatic Pruritus (ICONIC): A Randomised Phase 2 Study. Lancet 2021, 398, 1581–1592. [Google Scholar] [CrossRef]
- Deeks, E.D. Odevixibat: First Approval. Drugs 2021, 81, 1781–1786. [Google Scholar] [CrossRef]
- Thompson, R.J.; Arnell, H.; Artan, R.; Baumann, U.; Calvo, P.L.; Czubkowski, P.; Dalgic, B.; D’Antiga, L.; Durmaz, Ö.; Fischler, B.; et al. Odevixibat Treatment in Progressive Familial Intrahepatic Cholestasis: A Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Gastroenterol. Hepatol. 2022, 7, 830–842. [Google Scholar] [CrossRef]
- Thompson, R.J.; Artan, R.; Baumann, U.; Calvo, P.L.; Czubkowski, P.; Dalgic, B.; D’Antiga, L.; Di Giorgio, A.; Durmaz, Ö.; Gonzalès, E.; et al. Interim Results from an Ongoing, Open-Label, Single-Arm Trial of Odevixibat in Progressive Familial Intrahepatic Cholestasis. JHEP Rep. 2023, 5, 100782. [Google Scholar] [CrossRef]
- Thompson, R.J.; Horn, P.; Houwen, R.H.J.; Lacaille, F.; Ni, Q.; Stein, P.; Tessier, M.E.; Thompson, C.; Vittorio, J.M.; Kjems, L. Substantial Clinical Benefits with Odevixibat Treatment across Progressive Familial Intrahepatic Cholestasis Genetic Deficiencies: Subgroup Analysis of Serum Bile Acids, Pruritus, and Safety Using Pooled Data from the PEDFIC 1 and 2 Studies. In Proceedings of the International Liver Congress, online, 23 June 2021. [Google Scholar]
- Beyer, R.H.; Wiebelhaus, V.D.; Russe, H.F. Benemid: An Anticatabolite; Its Pharmacological Properties. Fed. Proc. 1950, 9, 258. [Google Scholar]
- Cunningham, R.F.; Israili, Z.H.; Dayton, P.G. Clinical Pharmacokinetics of Probenecid. Clin. Pharmacokinet. 1981, 6, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Selen, A.; Amidon, G.L.; Welling, P.G. Pharmacokinetics of Probenecid Following Oral Doses to Human Volunteers. J. Pharm. Sci. 1982, 71, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Boger, W.P.; Pitts, F.W.; Gallagher, M.E. Benemid and Carinamide: Comparison of Effect on Para-Aminosalicylic Acid (PAS) Plasma Concentrations. J. Lab. Clin. Med. 1950, 36, 276–282. [Google Scholar] [PubMed]
- Robbins, N.; Koch, S.E.; Tranter, M.; Rubinstein, J. The History and Future of Probenecid. Cardiovasc. Toxicol. 2012, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.C.; Arkell, P.; Riezk, A.; Gilchrist, M.; Wheeler, G.; Hope, W.; Holmes, A.H.; Rawson, T.M. Addition of Probenecid to Oral β-Lactam Antibiotics: A Systematic Review and Meta-Analysis. J. Antimicrob. Chemother. 2022, 77, 2364–2372. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Anderson, G.D.; Wang, J. Drug-Drug Interactions Involving Membrane Transporters in the Human Kidney. Expert. Opin. Drug Metab. Toxicol. 2006, 2, 505–532. [Google Scholar] [CrossRef] [PubMed]
- Roddy, E. Revisiting the Pathogenesis of Podagra: Why Does Gout Target the Foot? J. Foot Ankle Res. 2011, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Sivera, F.; Andres, M.; Dalbeth, N. A Glance into the Future of Gout. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221114098. [Google Scholar] [CrossRef]
- Pui, K.; Gow, P.J.; Dalbeth, N. Efficacy and Tolerability of Probenecid as Urate-Lowering Therapy in Gout; Clinical Experience in High-Prevalence Population. J. Rheumatol. 2013, 40, 872–876. [Google Scholar] [CrossRef]
- Kim, S.C.; Neogi, T.; Kang, E.H.; Liu, J.; Desai, R.J.; Zhang, M.; Solomon, D.H. Cardiovascular Risks of Probenecid versus Allopurinol in Older Patients with Gout. J. Am. Coll. Cardiol. 2018, 71, 994–1004. [Google Scholar] [CrossRef]
- Anzai, N.; Enomoto, A.; Endou, H. Renal Urate Handling: Clinical Relevance of Recent Advances. Curr. Rheumatol. Rep. 2005, 7, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruiz, F.; Alonso-Ruiz, A.; Calabozo, M.; Herrero-Beites, A.; García-Erauskin, G.; Ruiz-Lucea, E. Efficacy of Allopurinol and Benzbromarone for the Control of Hyperuricaemia. A Pathogenic Approach to the Treatment of Primary Chronic Gout. Ann. Rheum. Dis. 1998, 57, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Reinders, M.K.; Haagsma, C.; Jansen, T.L.T.A.; van Roon, E.N.; Delsing, J.; van de Laar, M.a.F.J.; Brouwers, J.R.B.J. A Randomised Controlled Trial on the Efficacy and Tolerability with Dose Escalation of Allopurinol 300–600 Mg/Day versus Benzbromarone 100–200 Mg/Day in Patients with Gout. Ann. Rheum. Dis. 2009, 68, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruiz, F.; Calabozo, M.; Pijoan, J.I.; Herrero-Beites, A.M.; Ruibal, A. Effect of Urate-Lowering Therapy on the Velocity of Size Reduction of Tophi in Chronic Gout. Arthritis Rheum. 2002, 47, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.-W.; Chiu, H.-T.; Tsai, C.-W.; Ting, I.-W.; Yeh, H.-C.; Huang, H.-C.; Kuo, C.-C.; CMUH Kidney Research Group. Comparative Effectiveness of Allopurinol, Febuxostat and Benzbromarone on Renal Function in Chronic Kidney Disease Patients with Hyperuricemia: A 13-Year Inception Cohort Study. Nephrol. Dial. Transplant. 2018, 33, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Dong, Y.-Q.; Jia, G.-X.; Fan, S.-M.; Li, S.-Z.; Yang, S.-S.; Li, Y.-B. ASBT(SLC10A2): A Promising Target for Treatment of Diseases and Drug Discovery. Biomed. Pharmacother. 2020, 132, 110835. [Google Scholar] [CrossRef]
- Carmichael, N.; Day, P.J.R. Cell Surface Transporters and Novel Drug Developments. Front. Pharmacol. 2022, 13, 852938. [Google Scholar] [CrossRef]

| Transporter Family | Transporter Protein | Predominant Substrates | Localization | Associated Diseases/Disorders | Corresponding Drugs |
|---|---|---|---|---|---|
| SLC5 (solute carrier 5) | SGLT2 (sodium–glucose transport protein 2) | Glucose [11] | Early proximal tubule of the kidney [12] | Type 2 diabetes mellitus (T2DM) [13,14,15,16] chronic kidney disease (CKD) [17,18,19,20,21], heart failure [22,23] | Dapagliflozin; canagliflozin; empagliflozin; ertugliflozin; sotagliflozin |
| SLC10 (solute carrier 10) | NTCP (sodium taurocholate co-transporting polypeptide) | Bile salts [24] | Liver, pancreas [24] | Hepatitis B virus (HBV) and hepatitis delta virus (HDV) coinfection [25,26] | Myrcludex B |
| ASBT (apical sodium-dependent bile salt transporter) | Bile salts [27,28,29] | Terminal ileum, kidney, cholangiocytes [27,30] | Alagille syndrome (ALGS), Progressive Familial Intrahepatic Cholestasis (PFIC) with associated cholestasis and pruritus [28,31,32] | Odevixibat; maralixibat | |
| SLC22 (solute carrier 22) | OAT1 (organic anion transporter 1) | Uric acid; endogenous anions, anionic drugs incl. β-lactam antibiotics, ciprofloxacin, and some non-steroidal anti-inflammatory drugs [33,34,35] | Proximal tubule of the kidney (basolateral membrane), brain, choroid plexus, and spinal cord [34,36,37] | Gout [38,39] | Probenecid; benzbromarone |
| OAT3 (organic anion transporter 3) | Uric acid; endogenous anions, anionic drugs incl. β-lactam antibiotics, ciprofloxacin, and some non-steroidal anti-inflammatory drugs [33,34,35] | Proximal tubule of the kidney (basolateral membrane), brain, choroid plexus, and spinal cord [34,36,37] | Gout [38,39] | Probenecid | |
| URAT1 (urate transporter 1) | Uric acid [34] | Proximal tubule of the kidney (apical membrane), heart, liver, prostate, skeletal muscles, small intestine, colon, and adrenal gland. pituitary gland [40,41] | Gout [38,39,42,43,44,45,46,47] | Probenecid; lesinurad; benzbromarone |
| Drug Name | Conducted Trials | Only Patients with T2DM | Safety with CKD | Safety with Cardiovascular Diseases | Other Drugs Used in Treatment | Observed Adverse Effects |
|---|---|---|---|---|---|---|
| Dapagliflozin | Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise [13] | Yes | Not tested | Not tested | Not tested | Most common AEs: nasopharyngitis, diarrhea, and headache—other significant observed AEs: nocturia, increased incidence in signs, symptoms, and other reports suggestive of UTIs and genital infections, hypotensive events, hypoglycemia events |
| Efficacy and safety of dapagliflozin monotherapy in people with type 2 diabetes [81] | Yes | Not tested | Not tested | Dapagliflozin/metformin | Most common AE: nasopharyngitis, headache, and influenza—other significant AEs events of hypoglycemia, benign breast neoplasm, events of renal impairment, events suggestive of genital infections and UTIs, pyelonephritis | |
| Dapagliflozin Versus Glipizide as Add-on Therapy in Patients With Type 2 Diabetes Who Have Inadequate Glycemic Control With Metformin [82] | Yes | Not tested | Not tested | Metformin/dapagliflozin or metformin/glipizide | Most common Aes: nasopharyngitis, hypertension, and influenza—other significant AEs: complex ventricular arrhythmia, decreased calculated creatinine clearance, epigastric pain, prostate cancer, pulmonary embolism, and worsening of coronary artery disease; drug-induced acute hepatitis; hypoglycemic events; renal failure; hypotension; dehydration; hypovolemia; signs and symptoms suggestive of genital infections or UTIs | |
| Dapagliflozin Is Effective as Add-on Therapy to Sitagliptin With or Without Metformin [83] | Yes | Not tested | Not tested | Dapagliflozin or dapagliflozin/sitagliptin or dapagliflozin/metformin/sitagliptin | Few events of hypoglycemia, one event of major hypoglycemia, symptoms, and events suggestive of genital infection, vulvovaginal mycotic infection, hypotension, dehydration, hypovolemia, renal impairment, prostate neoplasm, thyroid neoplasm | |
| Dapagliflozin Improves Glycemic Control and Reduces Body Weight as Add-on Therapy to Metformin Plus Sulfonylurea [84] | Yes | Not tested | Not tested | Metformin/sulfonylurea or dapagliflozin/metformin/sulfonylurea | Most common AEs: hypoglycemia, UTIs and genital infections, bronchitis—other significant AEs: two events of renal impairment/failure; orthostatic hypotension; elevated bilirubin | |
| Long-Term Efficacy of Dapagliflozin in Patients With Type 2 Diabetes Mellitus Receiving High Doses of Insulin [85] | Yes | Not tested | Not tested | Insulin/oral antidiabetic drugs or dapagliflozin/insulin/oral antidiabetic drugs | Most common AEs: nasopharyngitis, UTIs and genital infections, headaches, back pain, hypertension—other significant AEs: hypoglycemia, renal impairment/failure, hypotension, dehydration or hypovolemia | |
| Safety and Efficacy of Exenatide Once Weekly Plus Dapagliflozin Once Daily Versus Exenatide or Dapagliflozin Alone in Patients With Type 2 Diabetes Inadequately Controlled With Metformin Monotherapy [86] | Yes | Not tested | Not tested | Exenatide/dapagliflozin or exenatide or dapagliflozin | Most common AEs: nausea, UTIs and genital infections, headache, diarrhea—other significant AEs: gastrointestinal AEs, pancreatitis, hypoglycemia | |
| Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes [87] | Yes | Not tested | Yes | Not tested | UTIs and genital infections, symptoms of volume depletion, acute kidney injury, amputations, neoplasms, hepatic events, major hypoglycemic events, diabetic ketoacidosis | |
| The Dapagliflozin And Prevention of Adverse-outcomes in Heart Failure (DAPA-HF) trial [70] | No | Not tested | Yes | All patients received standard drug and device therapy for heart failure | Only SAEs, AEs leading to treatment discontinuation/interruption/dose reduction, and AEs of special interest were collected: volume depletion, renal dysfunction, major hypoglycemic episodes, fractures, and diabetic ketoacidosis | |
| Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction [71] | No | Not tested | Yes | Dapagliflozin, in addition to the patient’s usual therapy | Only SAEs, AEs leading to treatment discontinuation/interruption/dose reduction, and AEs of special interest were collected: UTIs, renal impairment, cardiac failure, COVID-19, pneumonia, ischemic stroke | |
| Dapagliflozin in Patients with Chronic Kidney Disease [72] | No | Yes | Not tested | Dapagliflozin and ACE inhibitor or ARB for at least 4 weeks before screening | Only SAEs, AEs leading to treatment discontinuation/interruption/dose reduction, and AEs of special interest were collected: amputations, fracture, renal-related AEs, major hypoglycemia, volume depletion | |
| Canagliflozin | Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise [14] | Yes | Not tested | Not tested | Not tested | Selected AEs: UTIs, genital mycotic infection, osmotic diuresis-related AEs, postural dizziness, orthostatic hypotension |
| Efficacy and Safety of Canagliflozin Used in Conjunction with Sulfonylurea in Patients with Type 2 Diabetes Mellitus [92] | Yes | Not tested | Not tested | Canagliflozin/sulfonylurea | Selected AEs: UTIs, genital mycotic infections, osmotic diuresis-related events, postural dizziness, orthostatic hypotension, hypoglycemia events | |
| Efficacy and safety of canagliflozin, an inhibitor of sodium–glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes [93] | Yes | Not tested | Not tested | Canagliflozin/stable background glucose-lowering therapy | Selected AEs: genital mycotic infections, UTIs, hypoglycemia events, osmotic diuresis-related events, volume-related AEs, renal-related AEs, photosensitivity events, fractures | |
| Efficacy and safety of titrated canagliflozin in patients with type 2 diabetes mellitus inadequately controlled on metformin and sitagliptin [94] | Yes | Not tested | Not tested | Metformin/sitagliptin or metformin/sitagliptin/canagliflozin | Selected AEs: UTIs, genital mycotic infections, osmotic diuresis-related events, volume depletion-related events, fracture, ketone-related events, hypoglycemia events | |
| Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea [95] | Yes | Not tested | Not tested | Metformin/sulfonylurea or metformin/sulfonylurea/canagliflozin | Selected AEs: UTIs, genital mycotic infection, osmotic diuresis-related AEs, volume-related AEs, hypoglycemia events | |
| Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone [96] | Yes | Not tested | Not tested | Metformin/pioglitazone or metformin/pioglitazone/canagliflozin | Selected AEs: UTIs, genital mycotic infection, osmotic diuresis-related events, volume depletion events | |
| Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU) [97] | Yes | Not tested | Not tested | Canagliflozin/metformin or glimepiride/metformin | Selected AEs: genital mycotic infections, UTIs, osmotic diuresis-related events | |
| Canagliflozin Compared With Sitagliptin for Patients With Type 2 Diabetes Who Do Not Have Adequate Glycemic Control With Metformin Plus Sulfonylurea [98] | Yes | Not tested | Not tested | Sulfonylurea/metformin/canagliflozin or sulfonylurea/metformin/sitagliptin | Selected AEs: genital mycotic infections, UTIs, pollakiuria, polyuria | |
| Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomized clinical trials [17] | No | Yes | Not tested | Not tested | Renal AEs: acute kidney injury events, hyperkalemia events | |
| Kidney, Cardiovascular, and Safety Outcomes of Canagliflozin according to Baseline Albuminuria: A CREDENCE Secondary Analysis [99] | Yes | Yes | Yes | Not tested | Renal AEs: acute kidney injury events, volume depletion events, hyperkalemia events, UTIs, hypoglycemia | |
| Empagliflozin | Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes [15] | Yes | Not tested | Not tested | Empagliflozin or sitagliptin | Most common AEs: nasopharyngitis, UTIs, hyperglycemia, dyslipidemia—other significant AEs: hypoglycemia, genital infections |
| Empagliflozin as Add-On to Metformin in Patients With Type 2 Diabetes [104] | Yes | Not tested | Not tested | Metformin or metformin/empagliflozin | Nasopharyngitis, hyperglycemia, UTIs, genital infections | |
| Glyxambi (Empagliflozin/Linagliptin): A Dual-Acting Oral Medication Approved for the Treatment of Patients with Type 2 Diabetes [105] | Yes | Not tested | Not tested | Linagliptin or empagliflozin or linagliptin/empagliflozin | Most common AEs: UTIs, genital mycotic infections, upper respiratory tract infections, increased urination, dyslipidemia, arthralgia, nausea—other significant AEs: nasopharyngitis, diarrhea, cough, hypoglycemic events | |
| Efficacy and safety of the SGLT2 inhibitor empagliflozin versus placebo and the DPP-4 inhibitor linagliptin versus placebo in young people with type 2 diabetes (DINAMO) [106] | Yes | Not tested | Not tested | Empagliflozin or linagliptin | Hypoglycemia | |
| Initial Combination of Empagliflozin and Linagliptin in Subjects With Type 2 Diabetes [107] | Yes | Not tested | Not tested | Linagliptin/empagliflozin or empagliflozin or linagliptin | Most common AEs: UTIs, upper respiratory tract infections, nasopharyngitis, influenza, hyperglycemia—other significant AEs: hypoglycemic events, volume depletion events, hypersensitivity reactions, pancreatitis | |
| Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes [108] | Yes | Not tested | Not tested | Glimepiride/metformin or empagliflozin/metformin | Most common AEs: hyperglycemia, UTIs, nasopharyngitis, upper respiratory tract infections—other significant AEs: genital infections, volume depletion events, bone fracture events | |
| Initial Combination of Empagliflozin and Metformin in Patients With Type 2 Diabetes [109] | Yes | Not tested | Not tested | Empagliflozin, metformin, or empagliflozin/metformin | Most common AEs: UTIs, upper respiratory tract infections, dyslipidemia, dizziness, diarrhea—other significant AEs: hypoglycemia, genital infections, increased urination events, volume depletion events, dehydration, hypotension | |
| Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin [110] | Yes | Not tested | Not tested | Empagliflozin as an add-on to insulin | Most common AEs: hypoglycemia, nasopharyngitis, UTIs, hyperglycemia, dizziness—other significant AEs: genital infections, severe hypoglycemic events | |
| Empagliflozin and Cardiovascular and Kidney Outcomes across KDIGO Risk Categories [18] | Yes | Yes | Yes | Empagliflozin as an add-on to the standard of care for T2DM and cardiovascular risk management | Most common AEs: UTIs, genital infections—other significant AEs: bone fracture, hyperkalemia, hypoglycemia | |
| Empagliflozin in Patients with Chronic Kidney Disease [19] | No | Yes | Yes | Empagliflozin as an add-on to single-agent RAS inhibitor | Selected AEs: UTIs, genital infections, hyperkalemia, acute kidney injury, dehydration, liver injury, ketoacidosis, lower limb amputation, bone fracture, hypoglycemia | |
| Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease [20] | Yes | Yes | Not tested | Empagliflozin as an add-on to antidiabetic drugs (excluding other SGLT2 inhibitors) | Selected AEs: hypoglycemia, UTIs, upper respiratory tract infections, nasopharyngitis, hyperglycemia, back pain, nausea, volume depletion | |
| Cardiovascular and Renal Outcomes of Empagliflozin in Heart Failure [22] | No | Not tested | Yes | Empagliflozin as an add-on to treatment for heart failure (diuretics, inhibitors of the renin–angiotensin system, and neprilysin, beta-blockers, mineralocorticoid receptor antagonists, and cardiac devices) | Selected AEs: hypotension, volume depletion, hypoglycemia, UTIs, genital infections, bone fractures, lower limb amputation | |
| Ertugliflozin | Phase III, efficacy and safety study of ertugliflozin monotherapy in people with type 2 diabetes mellitus inadequately controlled with diet and exercise alone [16] | Yes | Not tested | Not tested | Not tested | Selected AEs: UTIs, genital mycotic infections, hypoglycemia, hypovolemia, pollakiuria, polyuria, constipation |
| Long-term efficacy and safety of ertugliflozin monotherapy in patients with inadequately controlled T2DM despite diet and exercise [117] | Yes | Not tested | Not tested | Metformin in phase B of the study in participants from the placebo group | Selected AEs: genital mycotic infections, UTIs, hypoglycemia, hypovolemia, pollakiuria, polyuria, one event of hepatic adjudication | |
| Effect of ertugliflozin on glucose control, body weight, blood pressure, and bone density in type 2 diabetes mellitus inadequately controlled on metformin monotherapy (VERTIS MET) [118] | Yes | Not tested | Not tested | Ertugliflozin as an add-on to metformin | Selected AEs: genital mycotic infections, UTIs, hypoglycemia, hypovolemia, pollakiuria, dizziness, orthostatic blood pressure decrease events | |
| Ertugliflozin Compared with Glimepiride in Patients with Type 2 Diabetes Mellitus Inadequately Controlled on Metformin (VERTIS SU) [119] | Yes | Not tested | Not tested | Ertugliflozin or glimepiride | Selected AEs: hypoglycemia, genital mycotic infections, UTIs, hypovolemia, acute kidney injury event, diabetic ketoacidosis event, toe amputation events | |
| Ertugliflozin plus sitagliptin versus either individual agent over 52 weeks in patients with type 2 diabetes mellitus inadequately controlled with metformin (VERTIS FACTORIAL) [120] | Yes | Not tested | Not tested | Ertugliflozin or sitagliptin | Selected AEs: genital mycotic infections, UTIs, hypoglycemia, hypovolemia, diabetic ketoacidosis event | |
| Ertugliflozin and Sitagliptin Co-initiation in Patients with Type 2 Diabetes (VERTIS SITA) [121] | Yes | Not tested | Not tested | Ertugliflozin as an add-on to sitagliptin | Selected AEs: genital mycotic infections, UTIs, hypoglycemia, hypovolemia, a decrease from baseline of >30% in eGFR events | |
| Efficacy and safety of the addition of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sitagliptin (VERTIS SITA2) [122] | Yes | Not tested | Not tested | Ertugliflozin as an add-on to sitagliptin and metformin | Selected AEs: genital mycotic infections, UTIs, hypoglycemia, hypovolemia | |
| Efficacy of Ertugliflozin on Heart Failure–Related Events in Patients With Type 2 Diabetes Mellitus and Established Atherosclerotic Cardiovascular Disease [23] | Yes | Not tested | Yes | Not tested | --- | |
| Ertugliflozin in Patients with Stage 3 Chronic Kidney Disease and Type 2 Diabetes Mellitus (VERTIS RENAL) [21] | Yes | Yes | Not tested | Not tested | Selected AEs: UTIs, genital mycotic infections, hypoglycemia, hypovolemia, albuminuria events |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komaniecka, N.; Maroszek, S.; Drozdzik, M.; Oswald, S.; Drozdzik, M. Transporter Proteins as Therapeutic Drug Targets—With a Focus on SGLT2 Inhibitors. Int. J. Mol. Sci. 2024, 25, 6926. https://doi.org/10.3390/ijms25136926
Komaniecka N, Maroszek S, Drozdzik M, Oswald S, Drozdzik M. Transporter Proteins as Therapeutic Drug Targets—With a Focus on SGLT2 Inhibitors. International Journal of Molecular Sciences. 2024; 25(13):6926. https://doi.org/10.3390/ijms25136926
Chicago/Turabian StyleKomaniecka, Nina, Sonia Maroszek, Maria Drozdzik, Stefan Oswald, and Marek Drozdzik. 2024. "Transporter Proteins as Therapeutic Drug Targets—With a Focus on SGLT2 Inhibitors" International Journal of Molecular Sciences 25, no. 13: 6926. https://doi.org/10.3390/ijms25136926
APA StyleKomaniecka, N., Maroszek, S., Drozdzik, M., Oswald, S., & Drozdzik, M. (2024). Transporter Proteins as Therapeutic Drug Targets—With a Focus on SGLT2 Inhibitors. International Journal of Molecular Sciences, 25(13), 6926. https://doi.org/10.3390/ijms25136926







