The Extremely-Low-Frequency Electromagnetic Field Affects Apoptosis and Oxidative-Stress-Related Genes and Proteins in the Porcine Endometrium—An In Vitro Study
Abstract
:1. Introduction
2. Results
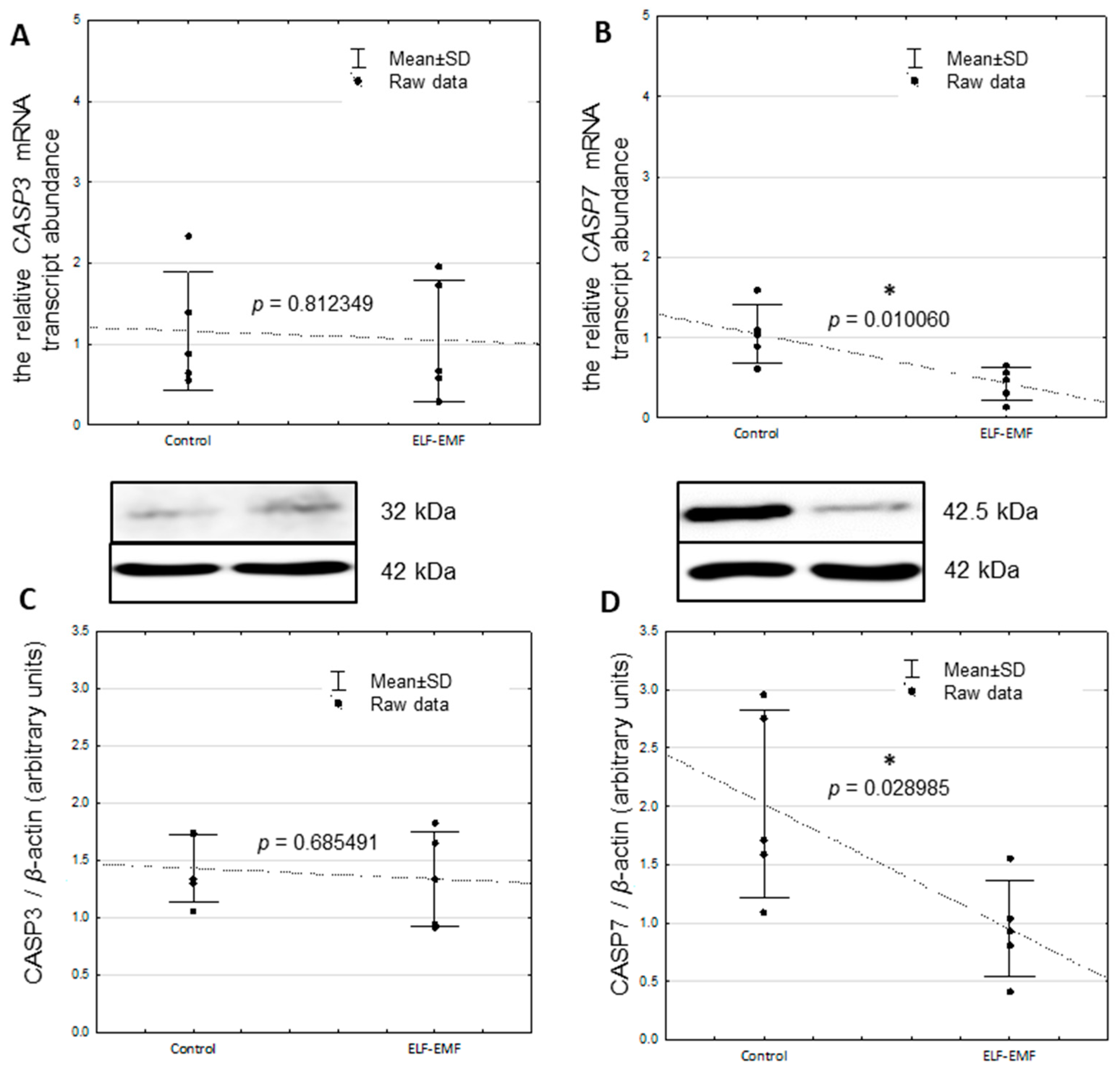
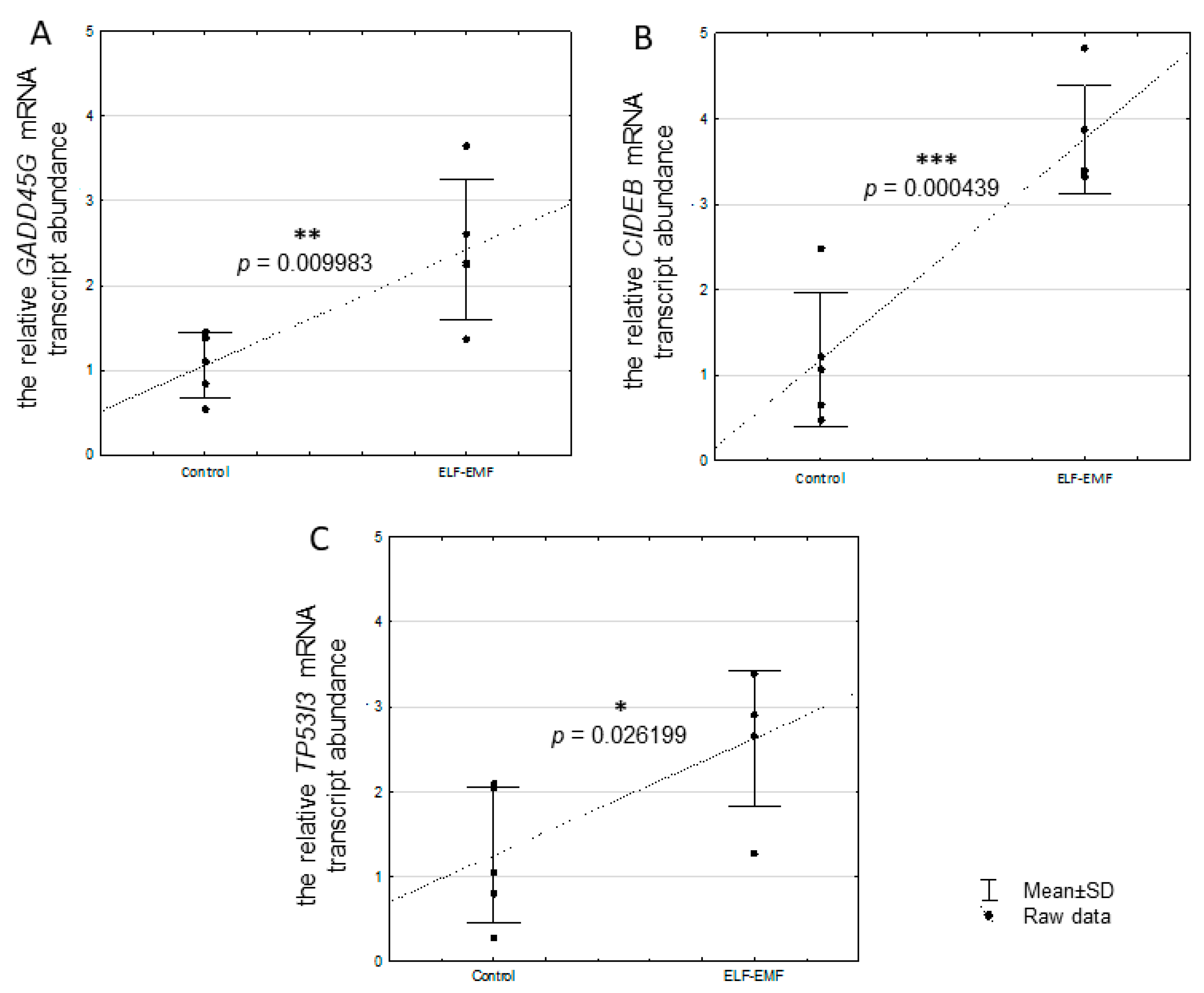
2.1. The Relative mRNA Transcript Abundance of CASP3, CASP7, CIDEB, GADD45G, and TP53I3 in the Endometrium Exposed to ELF-EMF In Vitro
2.2. The Relative Protein Abundance of CASP3 and CASP7 in the Endometrium Exposed to the ELF-EMF In Vitro
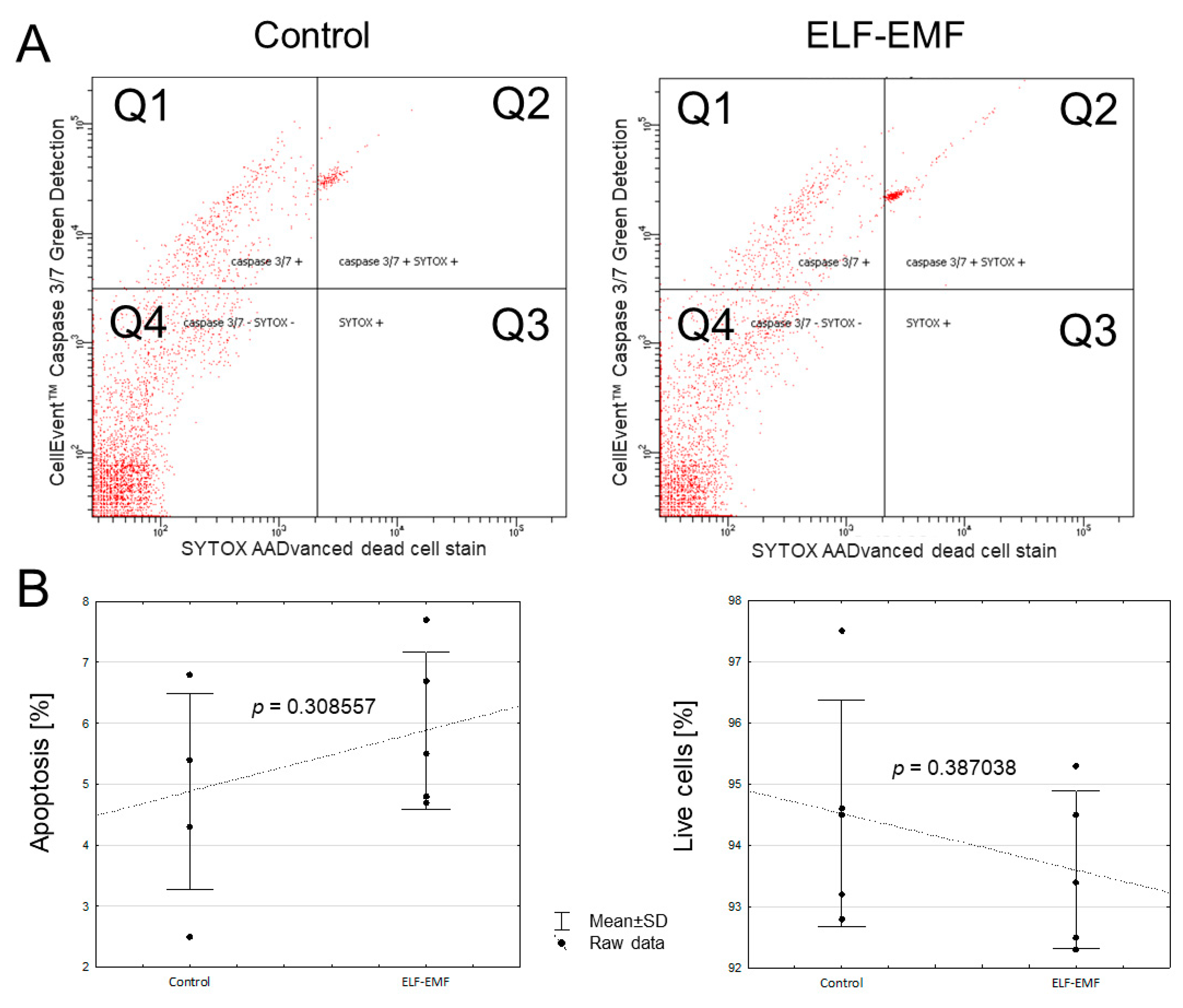
2.3. The In Vitro Effect of ELF-EMF on the Apoptosis Process in the Endometrium
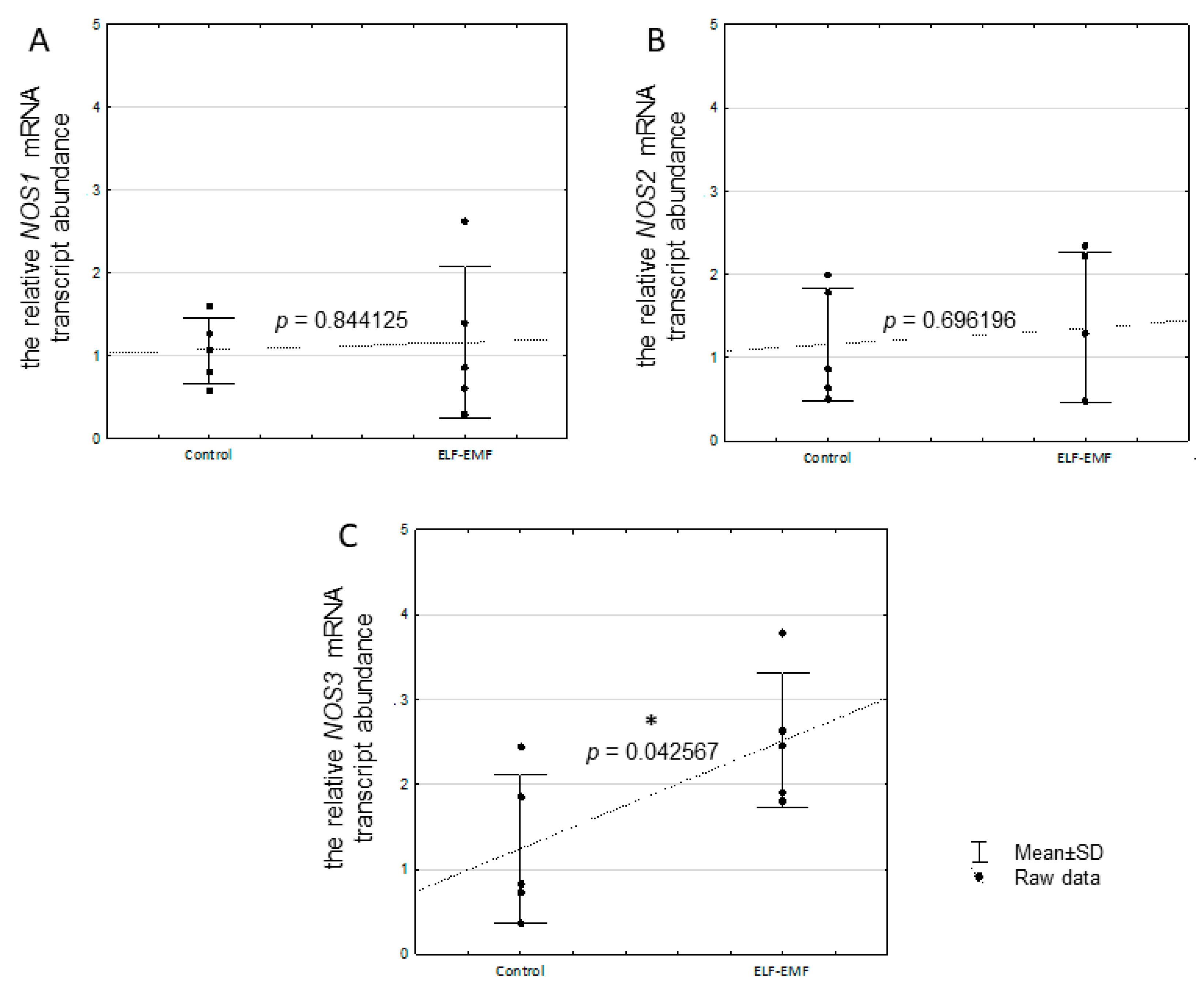
2.4. The Relative mRNA Transcript Abundance of NOS1, NOS2, and NOS3 in the Endometrium Exposed to the ELF-EMF In Vitro
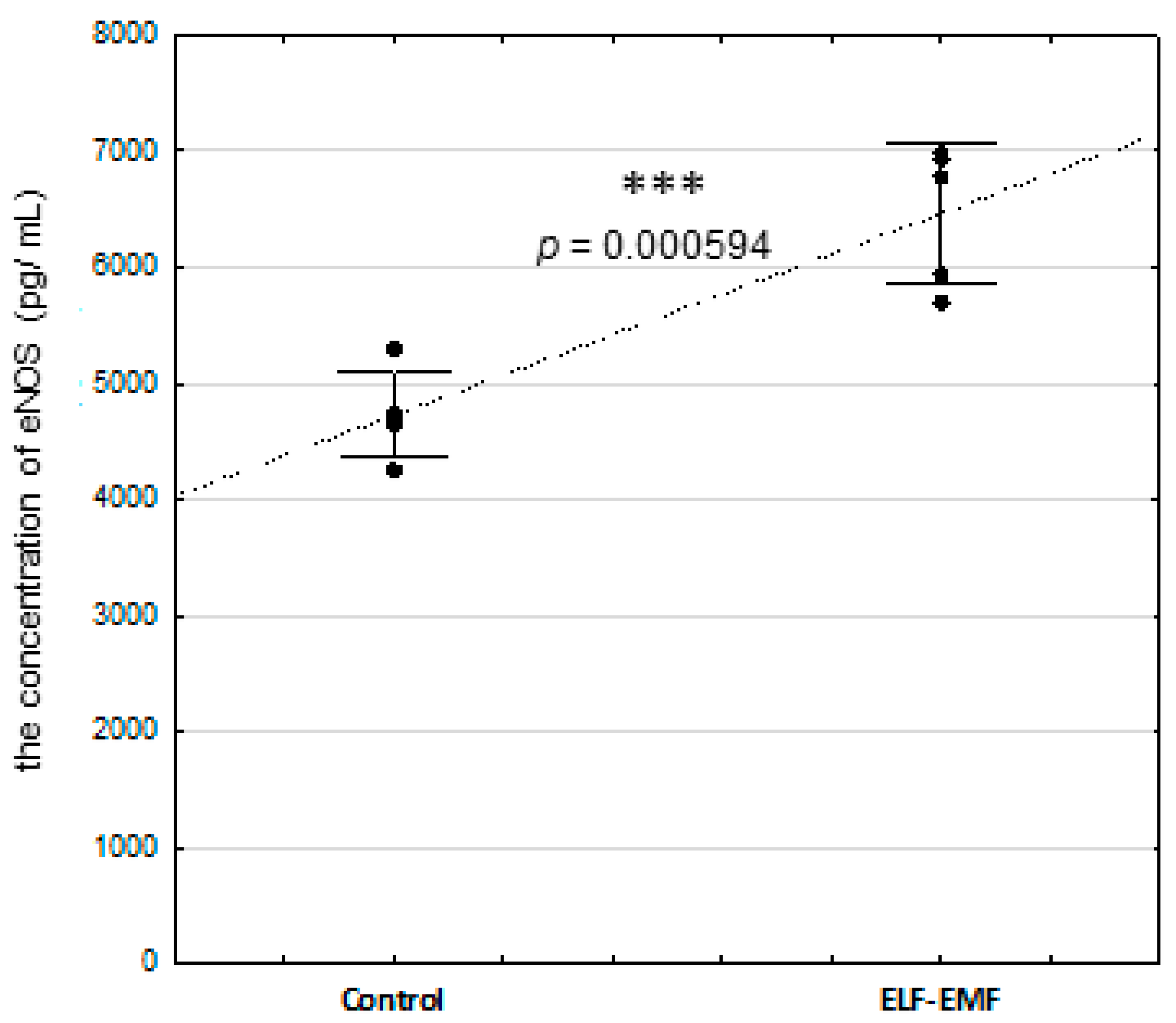
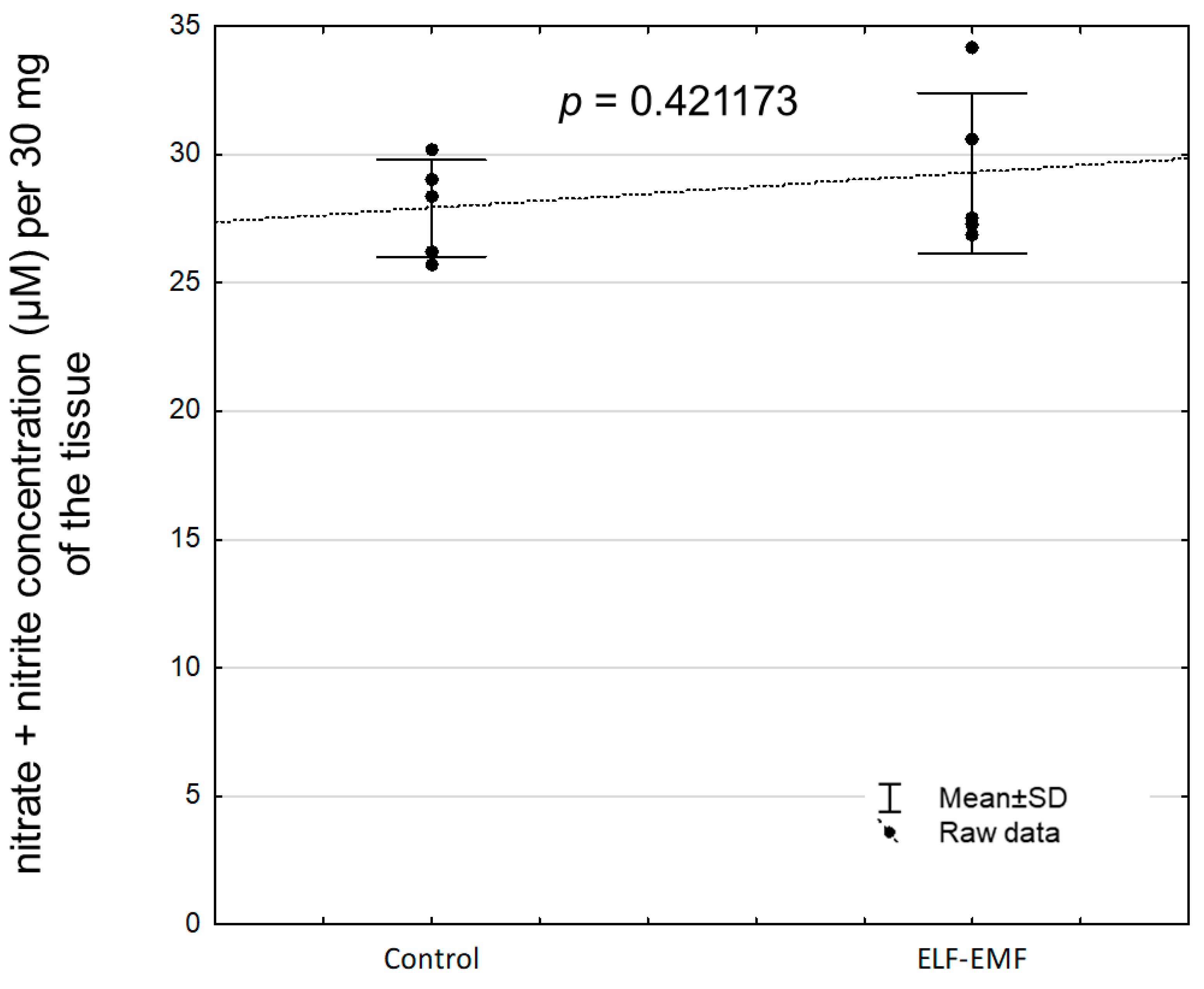
2.5. The Abundance of eNOS Protein and NOS Activity (Nitrate + Nitrite Concentration) in the Endometrium Exposed to the ELF-EMF In Vitro
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Animals, Endometrial Slice Collection, and ELF-EMF Exposure
4.3. RNA Isolation
4.4. Determination of Relative mRNA Transcript Abundance of CASP3, CASP7, CIDEB, GADD45G, NOS1, NOS2, NOS3, and TP53I3
4.5. Protein Isolation
4.6. Determination of Pro-CASP3 and Pro-CASP7 Protein Abundance
4.7. Endometrial Cell Isolation and Flow Cytometer Analysis of CASP3/7 Activity
4.8. Measurement of NOS Activity In Vitro—Nitrate/Nitrite Colorimetric Assay (LDH Method)
4.9. The eNOS Concentration
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lai, H.; Levitt, B.B. Cellular and Molecular Effects of Non-Ionizing Electromagnetic Fields. Rev. Environ. Health 2023. [Google Scholar] [CrossRef]
- Samaila, B.; Sagagi, Y.M.; Tampul, H.M. Exposure and Biological Impacts Assessment of Non-Ionizing Electromagnetic Radiation. Sci. Set. J. Phys. 2023, 2, 11. [Google Scholar]
- Karimi, A.; Ghadiri Moghaddam, F.; Valipour, M. Insights in the Biology of Extremely Low-Frequency Magnetic Fields Exposure on Human Health. Mol. Biol. Rep. 2020, 47, 5621–5633. [Google Scholar] [CrossRef]
- Gajšek, P.; Ravazzani, P.; Grellier, J.; Samaras, T.; Bakos, J.; Thuróczy, G. Review of Studies Concerning Electromagnetic Field (EMF) Exposure Assessment in Europe: Low Frequency Fields (50 Hz–100 KHz). Int. J. Environ. Res. Public Health 2016, 13, 875. [Google Scholar] [CrossRef]
- Grandolfo, M. Protection of Workers from Power Frequency Electric and Magnetic Fields: A Practical Guide; Occupational Safety and Health Series, 69; International Radiation Protection Association. International Non-Ionising Radiation Committee: Munich, Germany, 1994. [Google Scholar]
- Funk, R.H.W.; Monsees, T.K. Effects of Electromagnetic Fields on Cells: Physiological and Therapeutical Approaches and Molecular Mechanisms of Interaction. A Review. Cells Tissues Organs 2006, 182, 59–78. [Google Scholar] [CrossRef]
- Drzewiecka, E.M.; Kozlowska, W.; Paukszto, L.; Zmijewska, A.; Wydorski, P.J.; Jastrzebski, J.P.; Franczak, F. Effect of the Electromagnetic Field (EMF) Radiation on Transcriptomic Profile of Pig Myometrium during the Peri-implantation Period—An In Vitro Study. Int. J. Mol. Sci. 2021, 22, 7322. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, W.; Drzewiecka, E.M.; Paukszto, L.; Zmijewska, A.; Wydorski, P.J.; Jastrzebski, J.P.; Franczak, A. Exposure to the Electromagnetic Field Alters the Transcriptomic Profile in the Porcine Endometrium During the Peri-Implantation Period. J. Physiol. Pharmacol. 2021, 72, 1–15. [Google Scholar] [CrossRef]
- Wydorski, P.J.; Kozlowska, W.; Drzewiecka, E.M.; Zmijewska, A.; Franczak, A. Extremely Low-Frequency Electromagnetic Field Exposure Alters DNA Methylation Levels in the Endometrium of Pigs during the Peri-Implantation Period. Reprod. Fertil. Dev. 2023, 35, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Franczak, A.; Drzewiecka, E.M.; Kozlowska, W.; Zmijewska, A.; Wydorski, P.J. Extremely Low-Frequency Electromagnetic Field (ELF-EMF) Induces Alterations in Epigenetic Regulation in the Myometrium—An In Vitro Study. Theriogenology 2023, 200, 136–146. [Google Scholar] [CrossRef]
- Wydorski, P.J.; Kozlowska, W.; Zmijewska, A.; Franczak, A. Exposure to the Extremely Low-Frequency Electromagnetic Field Induces Changes in the Epigenetic Regulation of Gene Expression in the Endometrium. Theriogenology 2024, 217, 72–82. [Google Scholar] [CrossRef]
- Franczak, A.; Waszkiewicz, E.M.; Kozlowska, W.; Zmijewska, A.; Koziorowska, A. Consequences of Electromagnetic Field (EMF) Radiation during Early Pregnancy—Androgen Synthesis and Release from the Myometrium of Pigs in Vitro. Anim. Reprod. Sci. 2020, 218, 106465. [Google Scholar] [CrossRef] [PubMed]
- Drzewiecka, E.M.; Kozlowska, W.; Zmijewska, A.; Wydorski, P.J.; Franczak, A. Electromagnetic Field (Emf) Radiation Alters Estrogen Release from the Pig Myometrium during the Peri-Implantation Period. Int. J. Mol. Sci. 2021, 22, 2920. [Google Scholar] [CrossRef]
- Kozlowska, W.; Drzewiecka, E.; Zmijewska, A.; Koziorowska, A.; Franczak, A. Effects of Electromagnetic Field (EMF) Radiation on Androgen Synthesis and Release from the Pig Endometrium during the Fetal Peri-Implantation Period. Anim. Reprod. Sci. 2021, 226, 106694. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, W.; Drzewiecka, E.M.; Zmijewska, A.; Franczak, A. Electromagnetic Field Exposure Alters In Vitro Estrogen Biosynthesis and Its Release by the Porcine Endometrium in the Peri-Implantation Period. Reprod. Biol. 2022, 22, 100642. [Google Scholar] [CrossRef]
- Garip, A.; Akan, Z. Effect of ELF-EMF on Number of Apoptotic Cells; Correlation with Reactive Oxygen Species and HSP. Acta Biol. Hung. 2010, 61, 158–167. [Google Scholar] [CrossRef]
- Barati, M.; Javidi, M.A.; Darvishi, B.; Shariatpanahi, S.P.; Mesbah Moosavi, Z.S.; Ghadirian, R.; Khani, T.; Sanati, H.; Simaee, H.; Shokrollahi Barough, M.; et al. Necroptosis Triggered by ROS Accumulation and Ca2+ Overload, Partly Explains the Inflammatory Responses and Anti-Cancer Effects Associated with 1Hz, 100 MT ELF-MF In Vivo. Free Radic. Biol. Med. 2021, 169, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, G.; Del Re, B. Epigenetic Dysregulation in Various Types of Cells Exposed to Extremely Low-Frequency Magnetic Fields. Cell Tissue Res. 2021, 386, 1–15. [Google Scholar] [CrossRef]
- Shayeghan, M.; Forouzesh, F.; Madjid Ansari, A.; Javidi, M.A. DNMT1 and MiRNAs: Possible Epigenetics Footprints in Electromagnetic Fields Utilization in Oncology. Med. Oncol. 2021, 38, 125. [Google Scholar] [CrossRef]
- Mumtaz, S.; Rana, J.N.; Choi, E.H.; Han, I. Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects. Int. J. Mol. Sci. 2022, 23, 9288. [Google Scholar] [CrossRef]
- Okano, A.; Ogawa, H.; Takahashi, H.; Geshi, M. Apoptosis in the Porcine Uterine Endometrium during the Estrous Cycle, Early Pregnancy and Post Partum. J. Reprod. Dev. 2007, 53, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Pampfer, S.; Donnay, I. Apoptosis at the Time of Embryo Implantation in Mouse and Rat. Cell Death Differ. 1999, 6, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Von Rango, U.; Classen-Linke, I.; Krusche, C.A.; Beier, H.M. The Receptive Endometrium Is Characterized by Apoptosis in the Glands. Hum. Reprod. 1998, 13, 3177–3189. [Google Scholar] [CrossRef]
- Ellis, H.M.; Horvitz, R. Genetic Control of Programmed Cell Death in the Nematode C. elegans. Cell 1986, 44, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, New and Emerging Functions of Caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, B.W.; Hille, R.; Raile, K.; Kiess, W. Apoptosis: Live or Die—Hard Work Either Way! Horm. Metab. Res. 2001, 33, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.M.; Cohen, G.M.; MacFarlane, M. Different Subcellular Distribution of Caspase-3 and Caspase-7 Following Fas-Induced Apoptosis in Mouse Liver. J. Biol. Chem. 1998, 273, 10815–10818. [Google Scholar] [CrossRef]
- Salvesen, G.S.; Dixit, V.M. Caspase Activation: The Induced-Proximity Model. Proc. Natl. Acad. Sci. USA 1999, 96, 10964–10967. [Google Scholar] [CrossRef] [PubMed]
- Cristofolini, A.; Sanchis, G.; Moliva, M.; Alonso, L.; Chanique, A.; Koncurat, M.; Merkis, C. Cellular Remodelling by Apoptosis during Porcine Placentation. Reprod. Domest. Anim. 2013, 48, 584–590. [Google Scholar] [CrossRef]
- McLendon, B.A.; Seo, H.; Kramer, A.C.; Burghardt, R.C.; Bazer, F.W.; Johnson, G.A. Pig Conceptuses Secrete Interferon Gamma to Recruit T Cells to the Endometrium during the Peri-Implantation Period. Biol. Reprod. 2020, 103, 1018–1029. [Google Scholar] [CrossRef]
- Thorburn, A. Apoptosis Signaling. In Encyclopedia of Cancer; Springer: Berlin/Heidelberg, Germany, 2011; pp. 244–249. [Google Scholar] [CrossRef]
- Mirzaei, M.R.; Najafi, A.; Arababadi, M.K.; Asadi, M.H.; Mowla, S.J. Altered Expression of Apoptotic Genes in Response to OCT4B1 Suppression in Human Tumor Cell Lines. Tumor Biol. 2014, 35, 9999–10009. [Google Scholar] [CrossRef]
- Yu, M.; Wang, H.; Zhao, J.; Yuan, Y.; Wang, C.; Li, J.; Zhang, L.; Zhang, L.; Li, Q.; Ye, J. Expression of CIDE Proteins in Clear Cell Renal Cell Carcinoma and Their Prognostic Significance. Mol. Cell. Biochem. 2013, 378, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Srivastava, G.; Hsieh, W.-S.; Gao, Z.; Murray, P.; Liao, S.-K.; Ambinder, R.; Tao, Q. The Stress-Responsive Gene GADD45G Is a Functional Tumor Suppressor, with Its Response to Environmental Stresses Frequently Disrupted Epigenetically in MultipleTumors. Clin. Cancer Res. 2005, 11, 6442–6449. [Google Scholar] [CrossRef] [PubMed]
- Bruemmer, D.; Yin, F.; Liu, J.; Berger, J.P.; Sakai, T.; Blaschke, F.; Fleck, E.; Van Herle, A.J.; Forman, B.M.; Law, R.E. Regulation of the Growth Arrest and DNA Damage-Inducible Gene 45 (GADD45) by Peroxisome Proliferator-Activated Receptor Gamma in Vascular Smooth Muscle Cells. Circ. Res. 2003, 93, e38–e47. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Jain, S.K. Oxidative Stress and Apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Liu, Y. GADD45 Proteins: Roles in Cellular Senescence and Tumor Development. Exp. Biol. Med. 2014, 239, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, T.; Czyz, J.; Rolletschek, A.; Blyszczuk, P.; Fuchs, J.; Jovtchev, G.; Schulderer, J.; Kuster, N.; Wobus, A.M. Electromagnetic Fields Affect Transcript Levels of Apoptosis-related Genes in Embryonic Stem Cell-derived Neural Progenitor Cells. FASEB J. 2005, 19, 1686–1688. [Google Scholar] [CrossRef]
- Simko, M. Cell Type Specific Redox Status Is Responsible for Diverse Electromagnetic Field Effects. Curr. Med. Chem. 2007, 14, 1141–1152. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Tavárez-Alonso, S.; Murria-Estal, R.; Megías-Vericat, J.; Tortajada-Girbés, M.; Alonso-Iglesias, E. Nitric Oxide Production Is Increased in Severely Obese Children and Related to Markers of Oxidative Stress and Inflammation. Atherosclerosis 2011, 215, 475–480. [Google Scholar] [CrossRef]
- Wang, Y.; Marsden, P.A. Nitric Oxide Synthases: Gene Structure and Regulation. Adv. Pharmacol. 1995, 34, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Cameron, I.T.; Campbell, S. Nitric Oxide in the Endometrium. Hum. Reprod. Update 1998, 4, 565–569. [Google Scholar] [CrossRef]
- Andronowska, A.; Wąsowska, B.; Całka, J.; Doboszyńska, T. Localization and Correlation between NADPH-Diaphorase and Nitric Oxide Synthase Isoforms in the Porcine Uterus during the Estrous Cycle. Cell Tissue Res. 2005, 321, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Roberto da Costa, R.P.; Ferreira-Dias, G.; Mateus, L.; Korzekwa, A.; Andronowska, A.; Platek, R.; Skarzynski, D.J. Endometrial Nitric Oxide Production and Nitric Oxide Synthases in the Equine Endometrium: Relationship with Microvascular Density during the Estrous Cycle. Domest. Anim. Endocrinol. 2007, 32, 287–302. [Google Scholar] [CrossRef]
- Telfer, J.F.; Lyall, F.; Norman, J.E.; Cameron, I.T. Identification of Nitric Oxide Synthase in Human Uterus. Hum. Reprod. 1995, 10, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Van Langendonckt, A.; Casanas-Roux, F.; Donnez, J. Oxidative Stress and Peritoneal Endometriosis. Fertil. Steril. 2002, 77, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Najafi, T.; Novin, M.G.; Ghazi, R.; Khorram, O. Altered Endometrial Expression of Endothelial Nitric Oxide Synthase in Women with Unexplained Recurrent Miscarriage and Infertility. Reprod. Biomed. Online 2012, 25, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Patruno, A.; Tabrez, S.; Pesce, M.; Shakil, S.; Kamal, M.A.; Reale, M. Effects of Extremely Low Frequency Electromagnetic Field (ELF-EMF) on Catalase, Cytochrome P450 and Nitric Oxide Synthase in Erythro-Leukemic Cells. Life Sci. 2015, 121, 117–123. [Google Scholar] [CrossRef]
- Barzilai, A.; Yamamoto, K.I. DNA Damage Responses to Oxidative Stress. DNA Repair. 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Contente, A.; Dittmer, A.; Koch, M.C.; Roth, J.; Dobbelstein, M. A Polymorphic Microsatellite That Mediates Induction of PIG3 by P53. Nat. Genet. 2002, 30, 315–320. [Google Scholar] [CrossRef]
- Kotsinas, A.; Aggarwal, V.; Tan, E.J.; Levy, B.; Gorgoulis, V.G. PIG3: A Novel Link between Oxidative Stress and DNA Damage Response in Cancer. Cancer Lett. 2012, 327, 97–102. [Google Scholar] [CrossRef]
- Flatt, P.M.; Polyak, K.; Tang, L.J.; Scatena, C.D.; Westfall, M.D.; Rubinstein, L.A.; Yu, J.; Kinzler, K.W.; Vogelstein, B.; Hill, D.E.; et al. P53-Dependent Expression of PIG3 during Proliferation, Genotoxic Stress, and Reversible Growth Arrest. Cancer Lett. 2000, 156, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.R.; Lopes, J.; Levin, N.K.; Kalpage, H.; Tainsky, M.A. Germline Mutations in Apoptosis Pathway Genes in Ovarian Cancer; the Functional Role of a TP53I3 (PIG3) Variant in ROS Production and DNA Repair. Cell Death Discov. 2021, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Yokus, B.; Cakir, D.U.; Akdag, M.Z.; Sert, C.; Mete, N. Oxidative DNA Damage in Rats Exposed to Extremely Low Frequency Electro Magnetic Fields. Free Radic. Res. 2005, 39, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K. Advances in Swine Biomedical Model Genomics. Int. J. Biol. Sci. 2007, 3, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Mordhorst, B.R.; Prather, R.S. Pig Models of Reproduction. In Animal Models and Human Reproduction; Constantinescu, G., Schatten, H., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2017; Chapter 9; pp. 213–234. [Google Scholar] [CrossRef]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the Pig as a Human Biomedical Model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; DeMayo, F.J. Animal Models of Implantation. Reproduction 2004, 128, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Spencer, T.E.; Johnson, G.A.; Burghardt, R.C. Uterine Receptivity to Implantation of Blastocysts in Mammals. Front. Biosci. 2011, S3, 745–767. [Google Scholar] [CrossRef]
- Geisert, R.D.; Renegar, R.H.; Thatcher, W.W.; Roberts, R.M.; Bazer, F.W. Establishment of Pregnancy in the Pig: I. Interrelationships between Preimplantation Development of the Pig Blastocyst and Uterine Endometrial Secretions. Biol. Reprod. 1982, 27, 925–939. [Google Scholar] [CrossRef]
- Bazer, F.W.; Johnson, G.A. Pig Blastocyst-Uterine Interactions. Differentiation 2014, 87, 52–65. [Google Scholar] [CrossRef]
- Idelevich, A.; Vilella, F. Mother and Embryo Cross-Communication. Genes 2020, 11, 376. [Google Scholar] [CrossRef]
- Jarvis, G.E. Early Embryo Mortality in Natural Human Reproduction: What the Data Say. F1000Research 2016, 5, 2765. [Google Scholar] [CrossRef]
- Salker, M.; Teklenburg, G.; Molokhia, M.; Lavery, S.; Trew, G.; Aojanepong, T.; Mardon, H.J.; Lokugamage, A.U.; Rai, R.; Landles, C.; et al. Natural Selection of Human Embryos: Impaired Decidualization of Endometrium Disables Embryo-Maternal Interactions and Causes Recurrent Pregnancy Loss. PLoS ONE 2010, 5, 0010287. [Google Scholar] [CrossRef] [PubMed]
- Kokawa, K.; Shikone, T.; Nakano, R. Apoptosis in the Human Uterine Endometrium during the Menstrual Cycle. J. Clin. Endrocrinol. Metab. 1996, 81, 4144–4147. [Google Scholar]
- Gebel, M.H.; Braun, D.P.; Tambur, A.; Frame, D.; Rana, N.; Dmowski, W.P. Spontaneous Apoptosis of Endometrial Tissue Is Impaired in Women with Endometriosis. Fertil. Steril. 1998, 69, 1042–1047. [Google Scholar] [CrossRef]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA Damage and Disease: Induction, Repair and Significance. Mutat Res. 2004, 567, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A Novel and Compact Review on the Role of Oxidative Stress in Female Reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menoca, L.; Ladron De Guevara, R.; Cepero, E.; Boise, L.H. Caspase-9, Caspase-3 and Caspase-7 Have Distinct Roles during Intrinsic Apoptosis. Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef]
- Walsh, J.G.; Cullen, S.P.; Sheridan, C.; Lüthi, A.U.; Gerner, C.; Martin, S.J. Executioner Caspase-3 and Caspase-7 Are Functionally Distinct Proteases. Proc. Natl. Acad. Sci. USA 2008, 105, 12815–12819. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Moreira, L.O.; Makena, P.; Spierings, D.C.J.; Boyd, K.; Murray, P.J.; Green, D.R.; Kanneganti, T.D. Caspase-7 Deficiency Protects from Endotoxin-Induced Lymphocyte Apoptosis and Improves Survival. Blood 2009, 113, 2742–2745. [Google Scholar] [CrossRef]
- Nakatsumi, H.; Yonehara, S. Identification of Functional Regions Defining Different Activity in Caspase-3 and Caspase-7 within Cells. J. Biol. Chem. 2010, 285, 25418–25425. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yamauchi, L.; Hunter, T.; Kikkawa, U.; Kamada, S. Possible Involvement of Caspase-7 in Cell Cycle Progression at Mitosis. Genes Cells 2008, 13, 609–621. [Google Scholar] [CrossRef]
- Su, Y.; Shi, H. High Androgen Level Causes Recurrent Miscarriage and Impairs Endometrial Receptivity. Trop. J. Pharm. Res. 2019, 18, 1547–1552. [Google Scholar] [CrossRef]
- Salilew-Wondim, D.; Hölker, M.; Rings, F.; Ghanem, N.; Ulas-Cinar, M.; Peippo, J.; Tholen, E.; Looft, C.; Schellander, K.; Tesfaye, D. Bovine Pretransfer Endometrium and Embryo Transcriptome Fingerprints as Predictors of Pregnancy Success after Embryo Transfer. Physiol. Genom. 2010, 42, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Z.; Ye, J.; Xue, B.; Qi, J.; Zhang, J.; Zhou, Z.; Li, Q. Cideb Regulates Diet-Induced Obesity, Liver Steatosis, and Insulin Sensitivity by Controlling Lipogenesis and Fatty Acid Oxidation. Diabetes 2007, 56, 2523–2532. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, L.; Li, P. CIDE Proteins and Lipid Metabolism. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1094–1098. [Google Scholar] [CrossRef]
- Li, J.Z.; Lei, Y.; Wang, Y.; Zhang, Y.; Ye, J.; Xia, X.; Pan, X.; Li, P. Control of Cholesterol Biosynthesis, Uptake and Storage in Hepatocytes by Cideb. Biochim. Biophys. Acta 2010, 1801, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Christenson, L.K.; Strauss, J.F. Steroidogenic Acute Regulatory Protein (StAR) and the Intramitochondrial Translocation of Cholesterol. Biochim. Biophys. Acta 2000, 1529, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.H.; Hales, D.B. Overview of Steroidogenic Enzymes in the Pathway from Cholesterol to Active Steroid Hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef]
- Liebermann, D.A.; Hoffman, B. Gadd45 in Stress Signaling. J. Mol. Signal. 2008, 3, 15. [Google Scholar] [CrossRef]
- Salvador, J.M.; Brown-Clay, J.D.; Fornace, A.J. Gadd45 in Stress Signaling, Cell Cycle Control, and Apoptosis. In Gadd45 Stress Sensor Genes; Liebermann, D.A., Hoffman, B., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 793, pp. 1–19. [Google Scholar] [CrossRef]
- Wang, X.W.; Zhan, Q.; Coursen, J.D.; Khan, M.A.; Kontny, H.U.; Yu, L.; Hollander, M.C.; O’Connor, P.M.; Fornace, A.J.; Harris, C.C. GADD45 Induction of a G2/M Cell Cycle Checkpoint. Proc. Natl. Acad. Sci. USA 1999, 96, 3706–3711. [Google Scholar] [CrossRef]
- Font, L.P.; Cardonne, M.M.; Kemps, H.; Meesen, R.; Salmon, O.F.; González, F.G.; Lambrichts, I.; Rigo, J.M.; Brône, B.; Bronckaers, A. Non-Pulsed Sinusoidal Electromagnetic Field Rescues Animals from Severe Ischemic Stroke via NO Activation. Front. Neurosci. 2019, 13, 561. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kum, C.; Choi, H.J.; Park, E.S.; Sohn, U.D. Extremely Low Frequency Magnetic Field Induces Hyperalgesia in Mice Modulated by Nitric Oxide Synthesis. Life Sci. 2006, 78, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Patruno, A.; Amerio, P.; Pesce, M.; Vianale, G.; Di Luzio, S.; Tulli, A.; Franceschelli, S.; Grilli, A.; Muraro, R.; Reale, M. Extremely Low Frequency Electromagnetic Fields Modulate Expression of Inducible Nitric Oxide Synthase, Endothelial Nitric Oxide Synthase and Cyclooxygenase-2 in the Human Keratinocyte Cell Line HaCat: Potential Therapeutic Effects in Wound Healing. Br. J. Dermatol. 2010, 162, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.I.; Torsello, A.; Tedesco, B.; Fasanella, S.; Boninsegna, A.; D’Ascenzo, M.; Grassi, C.; Azzena, G.B.; Cittadini, A. 50-Hz Extremely Low Frequency Electromagnetic Fields Enhance Cell Proliferation and DNA Damage: Possible Involvement of a Redox Mechanism. Biochim. Biophys. Acta Mol. Cell Res. 2005, 1743, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Murohara, T.; Asahara, T.; Silver, M.; Bauters, C.; Masuda, H.; Kalka, C.; Kearney, M.; Chen, D.; Chen, D.; Symes, J.F.; et al. Nitric Oxide Synthase Modulates Angiogenesis in Response to Tissue Ischemia. J. Clin. Investig. 1998, 101, 2567–2578. [Google Scholar] [CrossRef]
- Monache, S.D.; Alessandro, R.; Iorio, R.; Gualtieri, G.; Colonna, R. Extremely Low Frequency Electromagnetic Fields (ELF-EMFs) Induce Invitro Angiogenesis Process in Human Endothelial Cells. Bioelectromagnetics 2008, 29, 640–648. [Google Scholar] [CrossRef]
- Bryan, N.S.; Grisham, M.B. Methods to Detect Nitric Oxide and Its Metabolites in Biological Samples. Free Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L.; Cowan, N.J. Measurement of Endothelial Cytosolic Calcium Concentration and Nitric Oxide Production Reveals Discrete Mechanisms of Endothelium-Dependent Pulmonary Vasodilatation. Circ. Res. 1991, 68, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Pohóczky, K.; Kun, J.; Szalontai, B.; Szöke, É.; Sághy, É.; Payrits, M.; Kajtár, B.; Kovács, K.; Környei, J.L.; Garai, J.; et al. Estrogen-Dependent up-Regulation of TRPA1 and TRPV1 Receptor Proteins in the Rat Endometrium. J. Mol. Endocrinol. 2016, 56, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Persoons, E.; Hennes, A.; De Clercq, K.; Van Bree, R.; Vriens, G.; Dorien, F.O.; Peterse, D.; Vanhie, A.; Meuleman, C.; Voets, T.; et al. Functional Expression of TRP Ion Channels in Endometrial Stromal Cells of Endometriosis Patients. Int. J. Mol. Sci. 2018, 19, 2467. [Google Scholar] [CrossRef]
- Kong, X.; Li, M.; Shao, K.; Yang, Y.; Wang, Q.; Cai, M. Progesterone Induces Cell Apoptosis via the CACNA2D3/Ca2+/P38 MAPK Pathway in Endometrial Cancer. Oncol. Rep. 2020, 43, 121–132. [Google Scholar] [CrossRef]
- Vangeel, L.; Voets, T. Transient Receptor Potential Channels and Calcium Signaling. Cold Spring Harb. Perspect. Biol. 2019, 11, a035048. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Ding, Q.; Liu, C.; Wu, H. Electromagnetic Fields Regulate Calcium-Mediated Cell Fate of Stem Cells: Osteogenesis, Chondrogenesis and Apoptosis. Stem Cell Res. Ther. 2023, 14, 133. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, K.; Van Den Eynde, C.; Hennes, A.; Van Bree, R.; Voets, T.; Vriens, J. The Functional Expression of Transient Receptor Potential Channels in the Mouse Endometrium. Hum. Reprod. 2017, 32, 615–630. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Biological Origin of Free Radicals, and Mechanisms of Antioxidant Protection. Chem.-Biol. Interact. 1994, 91, 133–140. [Google Scholar] [CrossRef]
- Raggi, F.; Vallesi, G.; Rufini, S.; Gizzi, S.; Ercolani, E.; Rossi, R. ELF Magnetic Therapy and Oxidative Balance. Electromagn. Biol. Med. 2008, 27, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Dizdaroglu, M.; Jaruga, P. Mechanisms of Free Radical-Induced Damage to DNA. Free Radic. Res. 2012, 46, 382–419. [Google Scholar] [CrossRef] [PubMed]
- Akpolat, V.; Celik, M.S.; Işık, B.; Yavaş, M.C. The Effect of Low-Frequency Electromagnetic Field (ELF-EMF) on Serum Total Antioxidant Capacity (TAOC) and Total Oxidant Stres (TOS). Int. Arch. Med. Res. 2014, 6, 21–25. [Google Scholar]
- Koziorowska, A.; Pasiud, E.; Fila, M.; Romerowicz-Misielak, M. The Impact of Electromagnetic Field at a Frequency of 50 Hz and a Magnetic Induction of 2.5 MT on Viability of Pineal Cells In Vitro. J. Biol. Regul. Homeost. Agents 2016, 30, 1067–1072. [Google Scholar]
- Koziorowska, A.; Waszkiewicz, E.M.; Romerowicz-Misielak, M.; Zglejc-Waszak, K.; Franczak, A. Extremely Low-Frequency Electromagnetic Field (EMF) Generates Alterations in the Synthesis and Secretion of Oestradiol-17β (E2) in Uterine Tissues: An In Vitro Study. Theriogenology 2018, 110, 86–95. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The Nitrate-Nitrite-Nitric Oxide Pathway in Physiology and Therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
| TaqMan Probes | Target Gene Name | Assay ID |
|---|---|---|
| CASP3 | Caspase 3 | Ss03382792_u1 |
| CASP7 | Caspase 7 | Ss06867774_m1 |
| CIDEB | Cell-death-inducing DFFA-like effector B | Ss03389752_g1 |
| GADD45G | Growth arrest, and DNA damage inducible 45γ | Ss04246840_g1 |
| NOS1 | Nitric oxide synthase 1 | Ss06838170_m1 |
| NOS2 | Nitric oxide synthase 2 | Ss03374608_u1 |
| NOS3 | Nitric oxide synthase 3 | Ss03383840_u1 |
| TP53I3 | Tumor protein P53 inducible protein 3 | Ss06911275_g1 |
| Reference genes | ||
| ACTB | β-actin | Ss03376081_u1 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Ss03374854_g1 |
| Primary Antibody | Catalog Number | Dilution | Company | Host |
|---|---|---|---|---|
| CASP3 | MA1-91637 | 1:1000 | Invitrogen | Mouse |
| CASP7 | NBP1-19229 | 1:1000 | Biotechne | Mouse |
| Secondary antibody | ||||
| Anti-rabbit HRP | 314460 | 1:5000 | Invitrogen | Goat |
| Anti-mouse HRP | 31430 | 1:5000 | Invitrogen | Goat |
| Reference protein | ||||
| ACTB | A2066 | 1:200 | Sigma Aldrich | Rabbit |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wydorski, P.J.; Zmijewska, A.; Franczak, A. The Extremely-Low-Frequency Electromagnetic Field Affects Apoptosis and Oxidative-Stress-Related Genes and Proteins in the Porcine Endometrium—An In Vitro Study. Int. J. Mol. Sci. 2024, 25, 6931. https://doi.org/10.3390/ijms25136931
Wydorski PJ, Zmijewska A, Franczak A. The Extremely-Low-Frequency Electromagnetic Field Affects Apoptosis and Oxidative-Stress-Related Genes and Proteins in the Porcine Endometrium—An In Vitro Study. International Journal of Molecular Sciences. 2024; 25(13):6931. https://doi.org/10.3390/ijms25136931
Chicago/Turabian StyleWydorski, Pawel Jozef, Agata Zmijewska, and Anita Franczak. 2024. "The Extremely-Low-Frequency Electromagnetic Field Affects Apoptosis and Oxidative-Stress-Related Genes and Proteins in the Porcine Endometrium—An In Vitro Study" International Journal of Molecular Sciences 25, no. 13: 6931. https://doi.org/10.3390/ijms25136931





