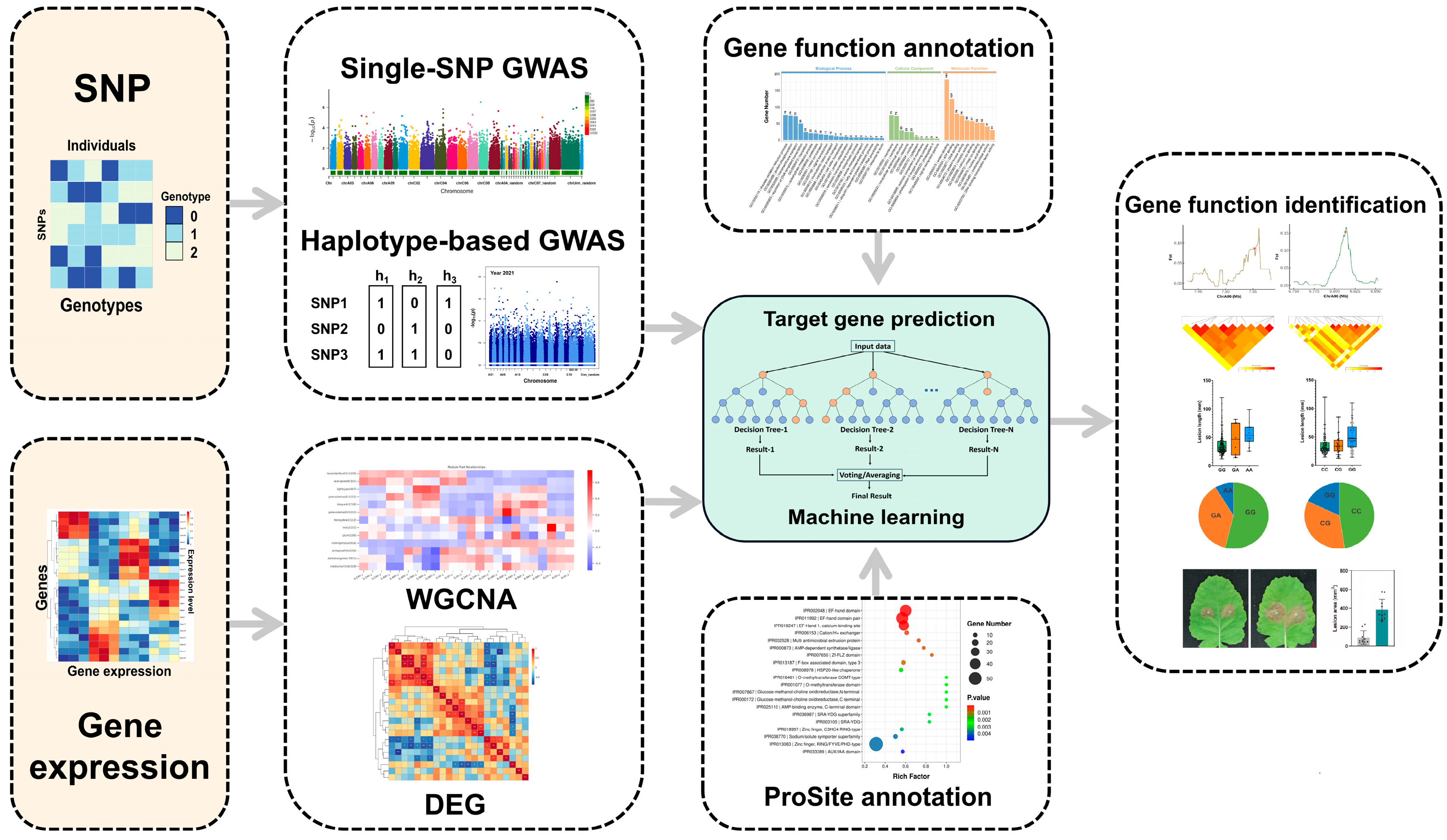
Integrated Assays of Genome-Wide Association Study, Multi-Omics Co-Localization, and Machine Learning Associated Calcium Signaling Genes with Oilseed Rape Resistance to Sclerotinia sclerotiorum
Abstract
1. Introduction
2. Results
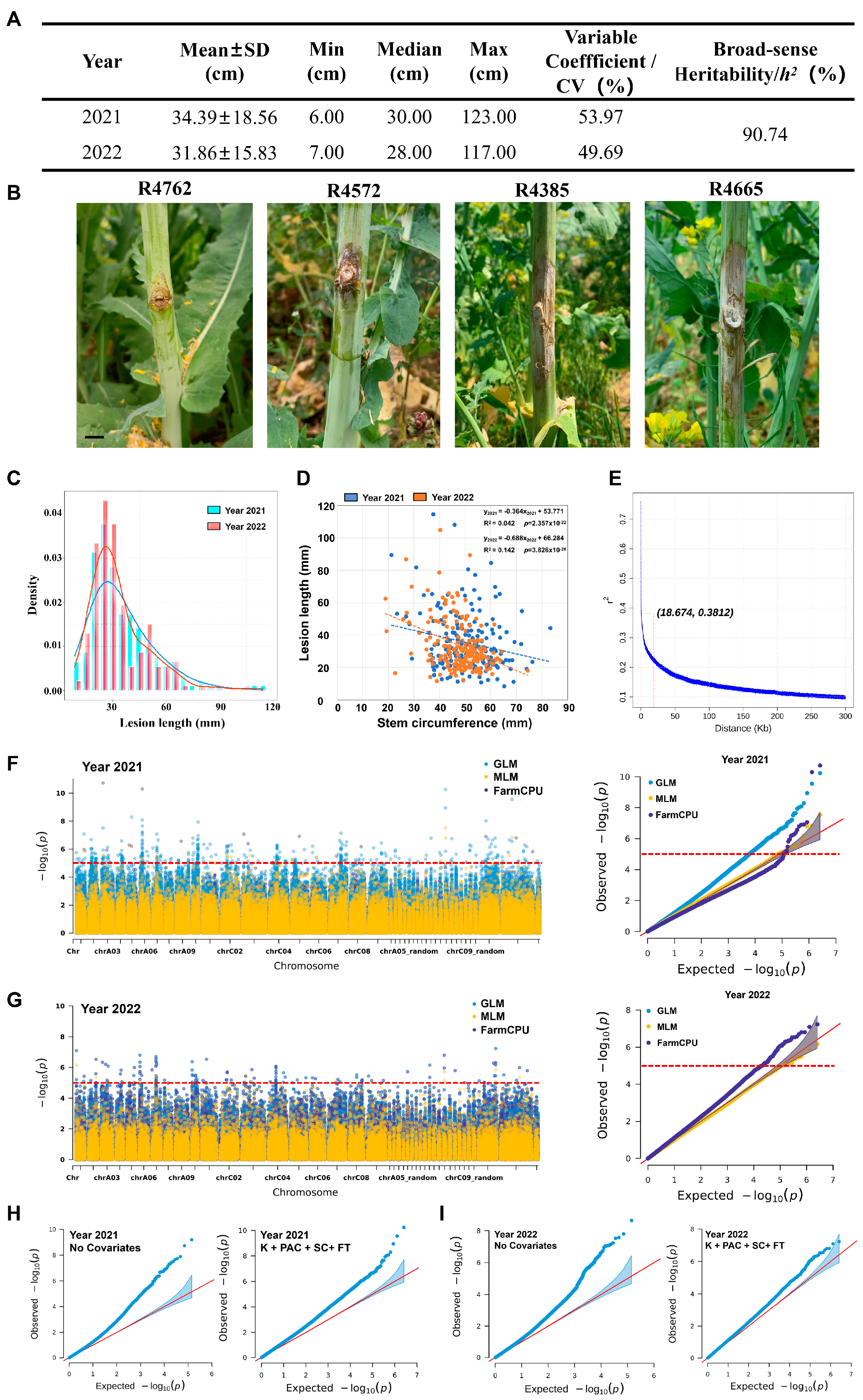
2.1. Optimization for Improved Single-SNP GWAS for SSR Resistance in Oilseed Rape
2.2. Identification of Resistance-Associated Loci and Candidate Genes by Optimized Single-SNP GWAS
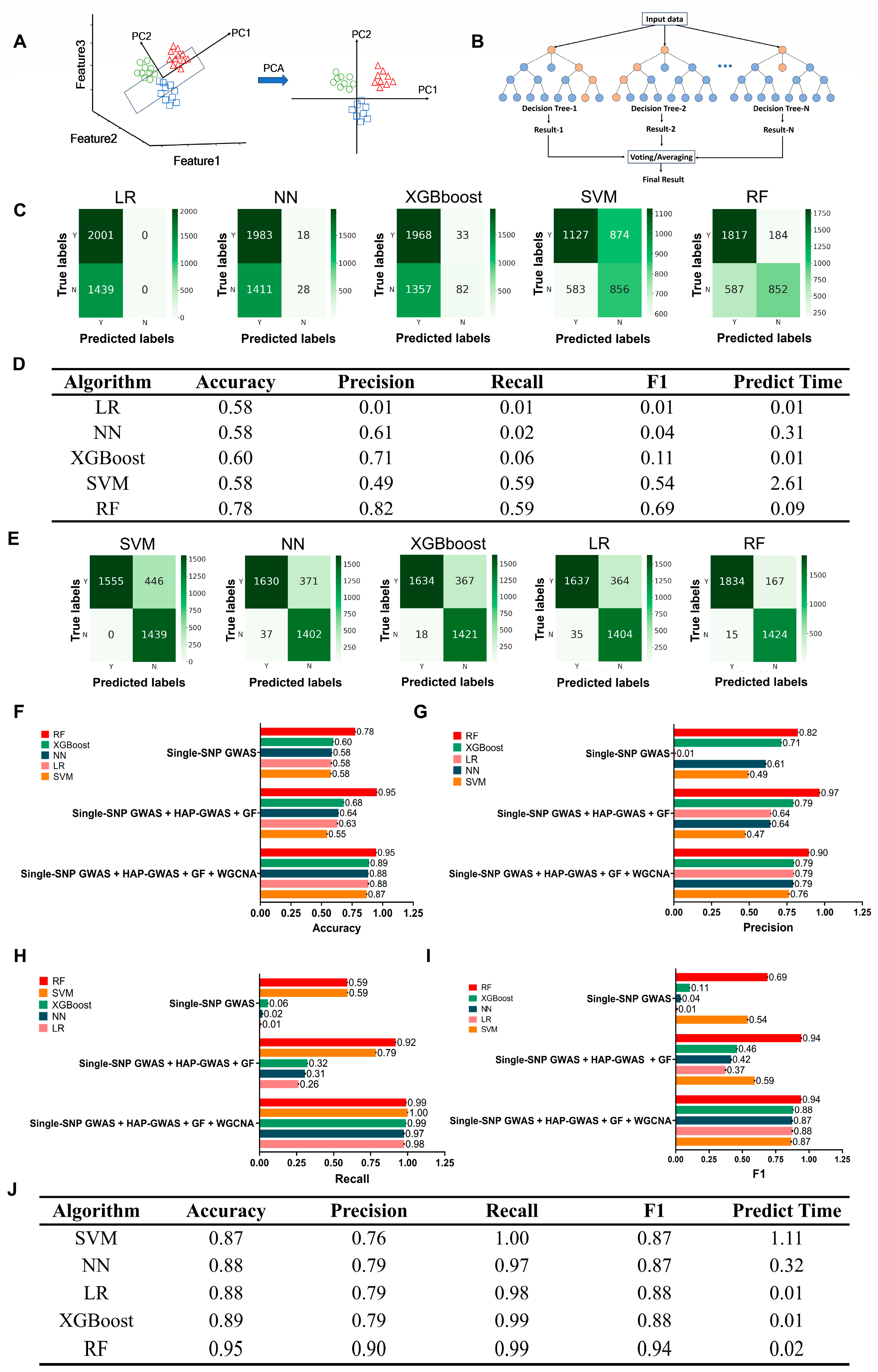
2.3. Identification of RALs and Genes by Integrated Assays of Single-SNP GWAS, Hap-GWAS, WGCNA, and DEGs
2.4. iMAP Predicts the Involvement of Calcium Signaling Genes in Resistance to Ss
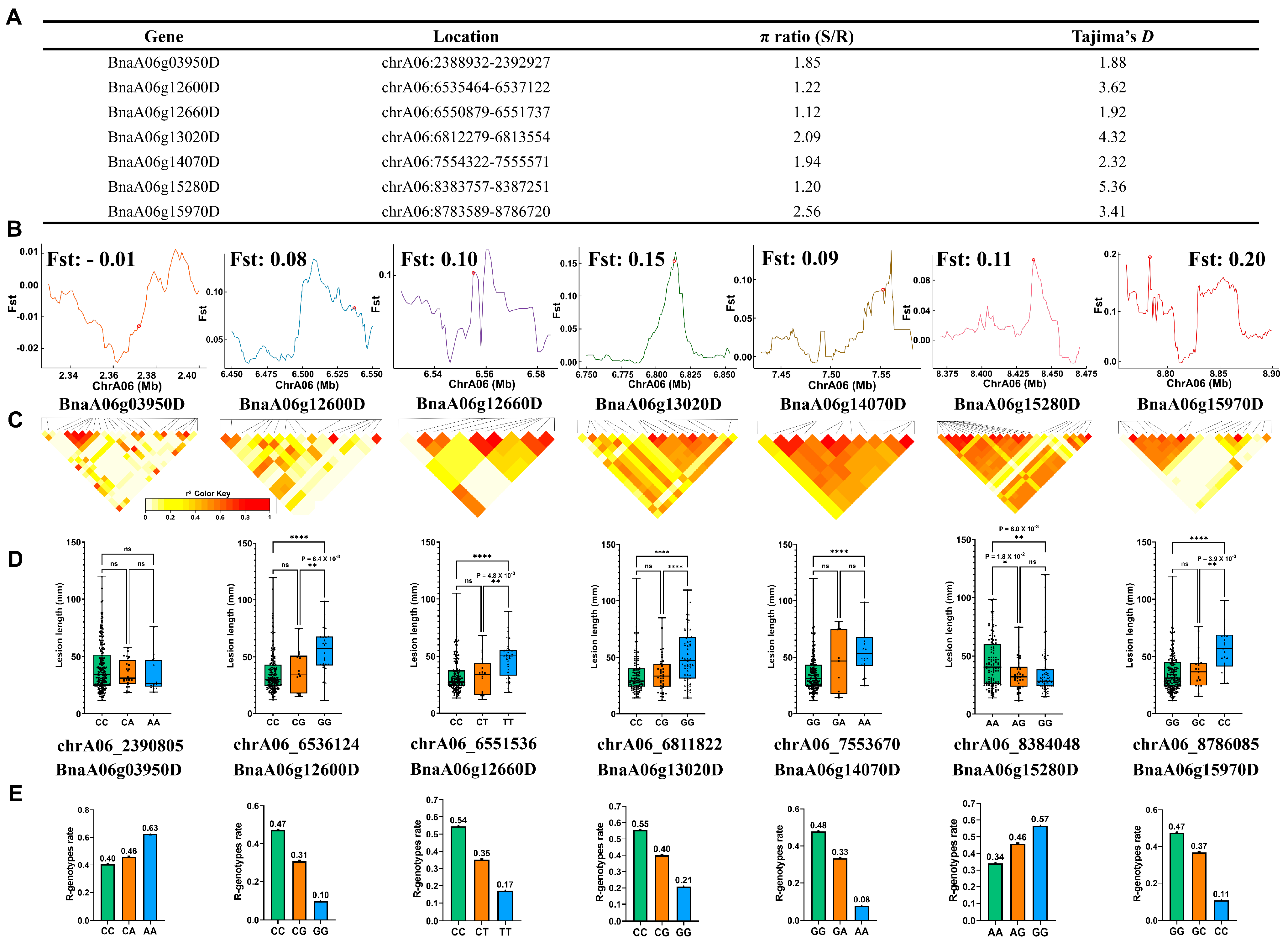
2.5. Positive Selection of Multiple Calcium Signaling Genes in the Population Evolution for Ss Resistance in Oilseed Rape
3. Discussion
4. Materials and Methods
4.1. Plant Cultivation and Field Inoculation
4.2. Data Quality Control and Single-SNP GWAS Analysis
4.3. RAL Identification and Enrichment Analysis
4.4. Hap-GWAS, WGCNA and DEGs Analysis
4.5. Machine Learning
4.6. Selective Sweep Scans
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolton, M.D.; Thomma, B.P.H.J.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and Molecular Traits of a Cosmopolitan Pathogen. Mol. Plant Pathol. 2006, 7, 1–16. [Google Scholar] [CrossRef]
- Ding, L.N.; Li, T.; Guo, X.J.; Li, M.; Liu, X.Y.; Cao, J.; Tan, X.L. Sclerotinia Stem Rot Resistance in Rapeseed: Recent Progress and Future Prospects. J. Agric. Food Chem. 2021, 69, 2965–2978. [Google Scholar] [CrossRef]
- Adams, P.B. Ecology of Sclerotinia Species. Phytopathology 1979, 69, 896. [Google Scholar] [CrossRef]
- Alkooranee, J.T.; Aledan, T.R.; Ali, A.K.; Lu, G.Y.; Zhang, X.K.; Wu, J.S.; Fu, C.H.; Li, M.T. Detecting the Hormonal Pathways in Oilseed Rape behind Induced Systemic Resistance by Trichoderma Harzianum TH12 to Sclerotinia sclerotiorum. PLoS ONE 2017, 12, e0168850. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Cowling, W.A.; Banga, S.S.; Barbetti, M.J.; Cantila, A.Y.; Amas, J.C.; Thomas, W.J.W.; You, M.P.; Tyagi, V.; Bharti, B.; et al. Genetic and Molecular Analysis of Stem Rot (Sclerotinia sclerotiorum) Resistance in Brassica Napus (Canola Type). Heliyon 2023, 9, e19237. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, Y.; Zhang, X.; Gill, R.A.; Hu, M.; Bai, Z.; Zhao, C.J.; Zhang, Y.; Liu, Y.Y.; Hu, Q.; et al. Functional and Evolutionary Study of MLO Gene Family in the Regulation of Sclerotinia Stem Rot Resistance in Brassica napus L. Biotechnol. Biofuels Bioprod. 2023, 16, 86. [Google Scholar] [CrossRef]
- Corwin, J.A.; Kliebenstein, D.J. Quantitative Resistance: More Than Just Perception of a Pathogen. Plant Cell 2017, 29, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Roux, F.; Voisin, D.; Badet, T.; Balagué, C.; Barlet, X.; Huard-Chauveau, C.; Roby, D.; Raffaele, S. Resistance to Phytopathogens e Tutti quanti: Placing Plant Quantitative Disease Resistance on the Map. Mol. Plant Pathol. 2014, 15, 427–432. [Google Scholar] [CrossRef]
- Wu, J.; Cai, G.Q.; Tu, J.Y.; Li, L.X.; Liu, S.; Luo, X.P.; Zhou, L.P.; Fan, C.C.; Zhou, Y.M. Identification of QTLs for Resistance to Sclerotinia Stem Rot and BnaC.IGMT5.a as a Candidate Gene of the Major Resistant QTL SRC6 in Brassica napus. PLoS ONE 2013, 8, e67740. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Q.; Yang, Q.Y.; Liu, H.; Li, Q.Y.; Yi, X.Q.; Cheng, Y.; Guo, L.; Fan, C.C.; Zhou, Y.M. Comparative Transcriptomic Analysis Uncovers the Complex Genetic Network for Resistance to Sclerotinia sclerotiorum in Brassica Napus. Sci. Rep. 2016, 6, 19007. [Google Scholar] [CrossRef]
- Wei, L.J.; Jian, H.J.; Lu, K.; Filardo, F.; Yin, N.; Liu, L.Z.; Qu, C.M.; Li, W.; Du, H.; Li, J.N. Genome-wide Association Analysis and Differential Expression Analysis of Resistance to Sclerotinia Stem Rot in Brassica napus. Plant Biotechnol. J. 2016, 14, 1368–1380. [Google Scholar] [CrossRef] [PubMed]
- Bazakos, C.; Hanemian, M.; Trontin, C.; Jiménez-Gómez, J.M.; Loudet, O. New Strategies and Tools in Quantitative Genetics: How to Go from the Phenotype to the Genotype. Annu. Rev. Plant Biol. 2017, 68, 435–455. [Google Scholar] [CrossRef]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; De Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-Wide Association Studies. Nat. Rev. Methods Primers 2021, 1, 59. [Google Scholar] [CrossRef]
- Ding, L.N.; Li, M.; Guo, X.J.; Tang, M.Q.; Cao, J.; Wang, Z.; Liu, R.; Zhu, K.M.; Guo, L.; Liu, S.Y.; et al. Arabidopsis GDSL1 Overexpression Enhances Rapeseed Sclerotinia sclerotiorum Resistance and the Functional Identification of Its Homolog in Brassica napus. Plant Biotechnol. J. 2020, 18, 1255–1270. [Google Scholar] [CrossRef]
- Lorenz, A.J.; Hamblin, M.T.; Jannink, J.L. Performance of Single Nucleotide Polymorphisms versus Haplotypes for Genome-Wide Association Analysis in Barley. PLoS ONE 2010, 5, e14079. [Google Scholar] [CrossRef] [PubMed]
- N’Diaye, A.; Haile, J.K.; Cory, A.T.; Clarke, F.R.; Clarke, J.M.; Knox, R.E.; Pozniak, C.J. Single Marker and Haplotype-Based Association Analysis of Semolina and Pasta Colour in Elite Durum Wheat Breeding Lines Using a High-Density Consensus Map. PLoS ONE 2017, 12, e0170941. [Google Scholar] [CrossRef]
- Xiao, Y.J.; Liu, H.J.; Wu, L.J.; Warburton, M.; Yan, J.B. Genome-Wide Association Studies in Maize: Praise and Stargaze. Mol. Plant 2017, 10, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.M. Epigenome-Wide Association Studies (EWAS): Past, Present, and Future. In Cancer Epigenetics; Verma, M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; Volume 1238, pp. 51–63. ISBN 978-1-4939-1803-4. [Google Scholar]
- Gusev, A.; Ko, A.; Shi, H.; Bhatia, G.; Chung, W.; Penninx, B.W.J.H.; Jansen, R.; De Geus, E.J.C.; Boomsma, D.I.; Wright, F.A.; et al. Integrative Approaches for Large-Scale Transcriptome-Wide Association Studies. Nat. Genet. 2016, 48, 245–252. [Google Scholar] [CrossRef]
- Kastenmüller, G.; Raffler, J.; Gieger, C.; Suhre, K. Genetics of Human Metabolism: An Update. Hum. Mol. Genet. 2015, 24, R93–R101. [Google Scholar] [CrossRef]
- Song, Y.P.; Chen, P.F.; Xuan, A.R.; Bu, C.H.; Liu, P.; Ingvarsson, P.K.; El-Kassaby, Y.A.; Zhang, D.Q. Integration of Genome Wide Association Studies and Co-expression Networks Reveal Roles of PtoWRKY 42-PtoUGT76C1-1 in Trans -zeatin Metabolism and Cytokinin Sensitivity in Poplar. New Phytol. 2021, 231, 1462–1477. [Google Scholar] [CrossRef]
- Zhu, C.S.; Li, X.R.; Yu, J.M. Integrating Rare-Variant Testing, Function Prediction, and Gene Network in Composite Resequencing-Based Genome-Wide Association Studies (CR-GWAS). G3 2011, 1, 233–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roy, A. A Classification Algorithm for High-Dimensional Data. Procedia Comput. Sci. 2015, 53, 345–355. [Google Scholar] [CrossRef]
- Thottakkara, P.; Ozrazgat-Baslanti, T.; Hupf, B.B.; Rashidi, P.; Pardalos, P.; Momcilovic, P.; Bihorac, A. Application of Machine Learning Techniques to High-Dimensional Clinical Data to Forecast Postoperative Complications. PLoS ONE 2016, 11, e0155705. [Google Scholar] [CrossRef] [PubMed]
- De Luis Balaguer, M.A.; Fisher, A.P.; Clark, N.M.; Fernandez-Espinosa, M.G.; Möller, B.K.; Weijers, D.; Lohmann, J.U.; Williams, C.; Lorenzo, O.; Sozzani, R. Predicting Gene Regulatory Networks by Combining Spatial and Temporal Gene Expression Data in Arabidopsis Root Stem Cells. Proc. Natl. Acad. Sci. USA 2017, 114, E7632–E7640. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, H.H.; Wang, X.F. Machine Learning for Big Data Analytics in Plants. Trends Plant. Sci. 2014, 19, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Xu, Y.T.; Cheng, Q.; Jiang, S.Q.; Wang, Q.; Xiao, Y.J.; Ma, C.; Yan, J.B.; Wang, X.F. LightGBM: Accelerated Genomically Designed Crop Breeding through Ensemble Learning. Genome Biol. 2021, 22, 271. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Fan, J.; Rhee, S.Y. QTG-Finder: A Machine-Learning Based Algorithm to Prioritize Causal Genes of Quantitative Trait Loci in Arabidopsis and Rice. G3 2019, 9, 3129–3138. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Lazarus, E.Z.; Rhee, S.Y. QTG-Finder2: A Generalized Machine-Learning Algorithm for Prioritizing QTL Causal Genes in Plants. G3 2020, 10, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Singleton, A.B.; Hardy, J.; Traynor, B.J.; Houlden, H. Towards a Complete Resolution of the Genetic Architecture of Disease. Trends Genet. 2010, 26, 438–442. [Google Scholar] [CrossRef]
- Tang, S.; Zhao, H.; Lu, S.P.; Yu, L.Q.; Zhang, G.F.; Zhang, Y.T.; Yang, Q.Y.; Zhou, Y.M.; Wang, X.M.; Ma, W.; et al. Genome- and Transcriptome-Wide Association Studies Provide Insights into the Genetic Basis of Natural Variation of Seed Oil Content in Brassica napus. Mol. Plant 2021, 14, 470–487. [Google Scholar] [CrossRef]
- Dangl, J.L.; Dietrich, R.A.; Richberg, M.H. Death Don’t Have No Mercy: Cell Death Programs in Plant-Microbe Interactions. Plant Cell 1996, 8, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Luan, S.; Wang, C. Calcium Signaling Mechanisms Across Kingdoms. Annu. Rev. Cell Dev. Biol. 2021, 37, 311–340. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Ngou, B.P.M.; Ding, P.T.; Xin, X.F. PTI-ETI Crosstalk: An Integrative View of Plant Immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef] [PubMed]
- Schönknecht, G. Calcium Signals from the Vacuole. Plants 2013, 2, 589–614. [Google Scholar] [CrossRef] [PubMed]
- Dodd, A.N.; Kudla, J.; Sanders, D. The Language of Calcium Signaling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef] [PubMed]
- Hannan Parker, A.; Wilkinson, S.W.; Ton, J. Epigenetics: A Catalyst of Plant Immunity against Pathogens. New Phytol. 2022, 233, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Liang, Z.; Yan, T.; Xu, Y.; Xuan, L.; Tang, J.; Zhou, G.; Lohwasser, U.; Hua, S.; Wang, H.; et al. Whole-Genome Resequencing of a Worldwide Collection of Rapeseed Accessions Reveals the Genetic Basis of Ecotype Divergence. Mol. Plant 2019, 12, 30–43. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, H.; Tang, Z.; Xu, J.; Yin, D.; Zhang, Z.; Yuan, X.; Zhu, M.; Zhao, S.; Li, X.; et al. rMVP: A Memory-Efficient, Visualization-Enhanced, and Parallel-Accelerated Tool for Genome-Wide Association Study. Genom. Proteom. Bioinform. 2021, 19, 619–628. [Google Scholar] [CrossRef]
- Qasim, M.U.; Zhao, Q.; Shahid, M.; Samad, R.A.; Ahmar, S.; Wu, J.; Fan, C.C.; Zhou, Y.M. Identification of QTLs Containing Resistance Genes for Sclerotinia Stem Rot in Brassica napus Using Comparative Transcriptomic Studies. Front. Plant Sci. 2020, 11, 776. [Google Scholar] [CrossRef]
- Wu, J.; Chen, P.P.; Zhao, Q.; Cai, G.Q.; Hu, Y.; Xiang, Y.; Yang, Q.Y.; Wang, Y.P.; Zhou, Y.M. Co-location of QTL for Sclerotinia Stem Rot Resistance and Flowering Time in Brassica napus. Crop J. 2019, 7, 227–237. [Google Scholar] [CrossRef]
- Zhao, J.W.; Udall, J.A.; Quijada, P.A.; Grau, C.R.; Meng, J.L.; Osborn, T.C. Quantitative Trait Loci for Resistance to Sclerotinia Sclerotiorum and Its Association with a Homeologous Non-reciprocal Transposition in Brassica napus L. Theor. Appl. Genet. 2006, 112, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, Q.; Liu, S.; Shahid, M.; Lan, L.; Cai, G.; Zhang, C.; Fan, C.; Wang, Y.; Zhou, Y. Genome-Wide Association Study Identifies New Loci for Resistance to Sclerotinia Stem Rot in Brassica napus. Front. Plant Sci. 2016, 7, 225163. [Google Scholar] [CrossRef] [PubMed]
- Maćkiewicz, A.; Ratajczak, W. Principal Components Analysis (PCA). Comput. Geosci. 1993, 19, 303–342. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Boland, G.J.; Hall, R. Index of Plant Hosts of Sclerotinia sclerotiorum. Can. J. Plant Pathol. 1994, 16, 93–108. [Google Scholar] [CrossRef]
- Derbyshire, M.C.; Khentry, Y.; Severn-Ellis, A.; Mwape, V.; Saad, N.S.M.; Newman, T.E.; Taiwo, A.; Regmi, R.; Buchwaldt, L.; Denton-Giles, M.; et al. Modeling First Order Additive × Additive Epistasis Improves Accuracy of Genomic Prediction for Sclerotinia Stem Rot Resistance in Canola. Plant Genome 2021, 14, e20088. [Google Scholar] [CrossRef] [PubMed]
- Badet, T.; Léger, O.; Barascud, M.; Voisin, D.; Sadon, P.; Vincent, R.; Le Ru, A.; Balagué, C.; Roby, D.; Raffaele, S. Expression Polymorphism at the ARPC 4 Locus Links the Actin Cytoskeleton with Quantitative Disease Resistance to Sclerotinia sclerotiorum in Arabidopsis thaliana. New Phytol. 2019, 222, 480–496. [Google Scholar] [CrossRef]
- Iakovidis, M.; Teixeira, P.J.P.L.; Exposito-Alonso, M.; Cowper, M.G.; Law, T.F.; Liu, Q.; Vu, M.C.; Dang, T.M.; Corwin, J.A.; Weigel, D.; et al. Effector-Triggered Immune Response in Arabidopsis thaliana Is a Quantitative Trait. Genetics 2016, 204, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Shahoveisi, F.; Oladzad, A.; Del Río Mendoza, L.E.; Hosseinirad, S.; Ruud, S.; Rissato, B. Assessing the Effect of Phenotyping Scoring Systems and SNP Calling and Filtering Parameters on Detection of QTL Associated with Reaction of Brassica napus to Sclerotinia sclerotiorum. PhytoFrontiers 2021, 1, 135–148. [Google Scholar] [CrossRef]
- Wei, D.Y.; Mei, J.Q.; Fu, Y.; Disi, J.O.; Li, J.; Qian, W. Quantitative Trait Loci Analyses for Resistance to Sclerotinia sclerotiorum and Flowering Time in Brassica napus. Mol. Breeding 2014, 34, 1797–1804. [Google Scholar] [CrossRef]
- Murphy, K.P. Machine Learning: A Probabilistic Perspective; Adaptive Computation and Machine Learning Series; MIT Press: Cambridge, MA, USA, 2012; ISBN 978-0-262-01802-9. [Google Scholar]
- Szymczak, S.; Biernacka, J.M.; Cordell, H.J.; González-Recio, O.; König, I.R.; Zhang, H.P.; Sun, Y.V. Machine Learning in Genome-Wide Association Studies. Genet. Epidemiol. 2009, 33 (Suppl. S1), S51–S57. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Bagging Predictors. Mach. Learn 1996, 24, 123–140. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, H.Y.; Wang, X.T.; Zhao, Y.Y.; Wang, L.Y.; Pan, J.Y.; Mei, H.A.; Han, J.; Wang, S.Y.; Lu, K.N.; et al. Integration of eQTL and Machine Learning to Dissect Causal Genes with Pleiotropic Effects in Genetic Regulation Networks of Seed Cotton Yield. Cell Rep. 2023, 42, 113111. [Google Scholar] [CrossRef] [PubMed]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth-Defense Tradeoffs in Plants: A Balancing Act to Optimize Fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.H.; Jiang, Z.Y.; Bi, G.Z.; Nomura, K.; Liu, M.H.; Wang, Y.P.; Cai, B.Y.; Zhou, J.M.; He, S.Y.; Xin, X.F. Pattern-Recognition Receptors Are Required for NLR-Mediated Plant Immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Bi, G.Z.; Su, M.; Li, N.; Liang, Y.; Dang, S.; Xu, J.C.; Hu, M.J.; Wang, J.Z.; Zou, M.X.; Deng, Y.A.; et al. The ZAR1 Resistosome Is a Calcium-Permeable Channel Triggering Plant Immune Signaling. Cell 2021, 184, 3528–3541. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.; Kim, N.H.; Wu, F.H.; El-Kasmi, F.; Chi, Y.; Walton, W.G.; Furzer, O.J.; Lietzan, A.D.; Sunil, S.; Kempthorn, K.; et al. Plant “Helper” Immune Receptors Are Ca2+-Permeable Nonselective Cation Channels. Science 2021, 373, 420–425. [Google Scholar] [CrossRef]
- Tian, W.; Hou, C.C.; Ren, Z.J.; Wang, C.; Zhao, F.G.; Dahlbeck, D.; Hu, S.P.; Zhang, L.Y.; Niu, Q.; Li, L.G.; et al. A Calmodulin-Gated Calcium Channel Links Pathogen Patterns to Plant Immunity. Nature 2019, 572, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kang, Y.; Ma, C.; Miao, R.; Wu, C.; Long, Y.; Ge, T.; Wu, Z.; Hou, X.; Zhang, J.; et al. CNGC2 Is a Ca2+ Influx Channel That Prevents Accumulation of Apoplastic Ca2+ in the Leaf. Plant Physiol. 2017, 173, 1342–1354. [Google Scholar] [CrossRef]
- Yang, D.L.; Yang, Y.N.; He, Z.H. Roles of Plant Hormones and Their Interplay in Rice Immunity. Mol. Plant 2013, 6, 675–685. [Google Scholar] [CrossRef]
- Wang, C.; Tang, R.J.; Kou, S.H.; Xu, X.S.; Lu, Y.; Rauscher, K.; Voelker, A.; Luan, S. Mechanisms of Calcium Homeostasis Orchestrate Plant Growth and Immunity. Nature 2024, 627, 382–388. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Robe, E.; Jomat, L.; Aldon, D.; Mazars, C.; Galaud, J.P. CML8, an Arabidopsis Calmodulin-Like Protein, Plays a Role in Pseudomonas Syringae Plant Immunity. Plant Cell Physiol. 2017, 58, 307–319. [Google Scholar] [CrossRef][Green Version]
- He, F.X.; Wang, C.; Sun, H.L.; Tian, S.X.; Zhao, G.S.; Liu, C.; Wan, C.P.; Guo, J.; Huang, X.L.; Zhan, G.M.; et al. Simultaneous Editing of Three Homoeologues of TaCIPK14 Confers Broad-Spectrum Resistance to Stripe Rust in Wheat. Plant Biotechnol. J. 2023, 21, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wang, X.Q.; Yang, C.X.; Wang, G.Y.; Fan, B.L.; Shang, Y.T.; Dang, C.; Xie, C.J.; Wang, Z.Y. Genome-Wide Identification of TaCIPK Gene Family Members in Wheat and Their Roles in Host Response to Blumeria graminis f. sp. Tritici Infection. Int. J. Biol. Macromol. 2023, 248, 125691. [Google Scholar] [CrossRef]
- Xie, Q.; Yin, X.C.; Wang, Y.; Qi, Y.T.; Pan, C.C.; Sulaymanov, S.; Qiu, Q.S.; Zhou, Y.; Jiang, X.Y. The Signalling Pathways, Calcineurin B-like Protein 5 (CBL5)-CBL-interacting Protein Kinase 8 (CIPK8)/CIPK24-salt Overly Sensitive 1 (SOS1), Transduce Salt Signals in Seed Germination in Arabidopsis. Plant Cell Environ. 2024, 47, 1486–1502. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Wang, X.Y.; Xu, Y.P.; He, Y.H.; Cai, X.Z. Characterization of Tomato Protein Kinases Embedding Guanylate Cyclase Catalytic Center Motif. Sci. Rep. 2020, 10, 4078. [Google Scholar] [CrossRef]
- Saand, M.A.; Xu, Y.P.; Munyampundu, J.P.; Li, W.; Zhang, X.R.; Cai, X.Z. Phylogeny and Evolution of Plant Cyclic Nucleotide-Gated Ion Channel (CNGC) Gene Family and Functional Analyses of Tomato CNGCs. DNA Res. 2015, 22, 471–483. [Google Scholar] [CrossRef]
- Saand, M.A.; Xu, Y.P.; Li, W.; Wang, J.P.; Cai, X.Z. Cyclic Nucleotide Gated Channel Gene Family in Tomato: Genome-Wide Identification and Functional Analyses in Disease Resistance. Front. Plant Sci. 2015, 06, 303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, W.; Xu, Y.P.; Cao, J.Y.; Braam, J.; Cai, X.Z. Genome-Wide Identification and Functional Analyses of Calmodulin Genes in Solanaceous species. BMC Plant Biol. 2013, 13, 70. [Google Scholar] [CrossRef]
- Wang, J.P.; Xu, Y.P.; Munyampundu, J.P.; Liu, T.Y.; Cai, X.Z. Calcium-Dependent Protein Kinase (CDPK) and CDPK-Related Kinase (CRK) Gene Families in Tomato: Genome-Wide Identification and Functional Analyses in Disease Resistance. Mol. Genet. Genom. 2016, 291, 661–676. [Google Scholar] [CrossRef]
- Wang, J.P.; Xu, Y.P.; Cai, X.Z. Phylogeny of Plant Calcium and Calmodulin-Dependent Protein Kinases (CCaMKs) and Functional Analyses of Tomato CCaMK in Disease Resistance. Front. Plant Sci. 2015, 6, 1075. [Google Scholar] [CrossRef]
- Rahman, H.; Xu, Y.P.; Zhang, X.R.; Cai, X.Z. Brassica Napus Genome Possesses Extraordinary High Number of CAMTA Genes and CAMTA3 Contributes to PAMP Triggered Immunity and Resistance to Sclerotinia sclerotiorum. Front. Plant Sci. 2016, 7, 581. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Yang, J.; Xu, Y.P.; Wang, J.P.; Cai, X.Z. Phylogeny of Plant CAMTAs and Role of AtCAMTAs in Nonhost Resistance to Xanthomonas oryzae Pv. Oryzae. Front. Plant Sci. 2016, 7, 177. [Google Scholar] [CrossRef]
- Yan, T.; Wang, Q.; Maodzeka, A.; Wu, D.Z.; Jiang, L.X. BnaSNPDB: An Interactive Web Portal for the Efficient Retrieval and Analysis of SNPs among 1,007 Rapeseed Accessions. Comput. Struct. Biotechnol. J. 2020, 18, 2766–2773. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-Generation PLINK: Rising to the Challenge of Larger and Richer Datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- Zhang, C.; Dong, S.S.; Xu, J.Y.; He, W.M.; Yang, T.L. PopLDdecay: A Fast and Effective Tool for Linkage Disequilibrium Decay Analysis Based on Variant Call Format Files. Bioinformatics 2019, 35, 1786–1788. [Google Scholar] [CrossRef]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal Components Analysis Corrects for Stratification in Genome-Wide Association Studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.T.; Li, Z.Y.; Sun, Y.H.; Aluko, O.O.; Wu, X.R.; Wang, Q.; Liu, G.S. MG2C: A User-Friendly Online Tool for Drawing Genetic Maps. Mol. Hortic. 2021, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Han, F.; Ge, C.; Mao, W.; Chen, L.; Hu, H.; Chen, G.; Lang, Q.; Fang, C. OmicStudio: A Composable Bioinformatics Cloud Platform with Real-time Feedback That Can Generate High-quality Graphs for Publication. iMeta 2023, 2, e85. [Google Scholar] [CrossRef]
- Hamazaki, K.; Iwata, H. RAINBOW: Haplotype-Based Genome-Wide Association Study Using a Novel SNP-Set Method. PLoS Comput. Biol. 2020, 16, e1007663. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The Variant Call Format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]


| RAL Name 1 | Chr 2 | Start | End | Recurring QTLs in the Reference | Reference |
|---|---|---|---|---|---|
| RSSA01a | chrA01 | 29,310 | 7,115,091 | ||
| RSSA01b | chrA01 | 13,304,776 | 22,353,741 | chrA01:12,444,829–19,857,639 | Wu et al., 2013 [9] |
| RSSA02a | chrA02 | 6,327,197 | 8,672,595 | chrA02:5,418,060–7,389,046 | Qasim et al., 2020 [40] |
| chrA02:8,152,495–9,281,526 | Qasim et al., 2020 [40] | ||||
| RSSA02b | chrA02 | 17,499,716 | 19,951,552 | chrA02:16,670,964–20,474,897 | Wu et al., 2013 [9] |
| RSSA03a | chrA03 | 178,592 | 4,297,931 | chrA03:2,702,453–4,262,421 | Wu et al., 2019 [41] |
| RSSA03b | chrA03 | 8,896,717 | 11,278,534 | ||
| RSSA03c | chrA03 | 15,124,067 | 22,211,498 | chrA03:15,547,362–16,064,878 | Zhao et al., 2006 [42] |
| RSSA04a | chrA04 | 9,608,450 | 12,922,254 | ||
| RSSA04b | chrA04 | 14,872,047 | 18,447,087 | ||
| RSSA05 | chrA05 | 17,966,325 | 20,397,420 | ||
| RSSA06a | chrA06 | 1,853,642 | 2,407,529 | ||
| RSSA06b | chrA06 | 6,152,638 | 8,944,061 | ||
| RSSA06c | chrA06 | 17,880,852 | 21,689,696 | chrA06:20,965,425–23,324,273 chrA06:21,629,047–23,499,018 | Wu et al., 2013 [9] Wu et al., 2019 [41] |
| RSSA07a | chrA07 | 10,082,564 | 14,250,889 | ||
| RSSA07b | chrA07 | 20,186,060 | 21,609,303 | ||
| RSSA09a | chrA09 | 6,448,538 | 6,596,656 | ||
| RSSA09b | chrA09 | 32,071,202 | 32,105,505 | chrA09:28,638,676–31,720,464 | Qasim et al., 2020 [40] |
| RSSA10a | chrA10 | 1,236,286 | 4,041,051 | ||
| RSSA10b | chrA10 | 10,486,133 | 14,839,131 | ||
| RSSAnn_random_a | chrAnn_random | 15,679,614 | 21,810,062 | ||
| RSSAnn_random_b | chrAnn_random | 25,598,042 | 33,228,887 | ||
| RSSAnn_random_c | chrAnn_random | 39,652,449 | 46,732,598 | ||
| RSSC01 | chrC01 | 19,283,445 | 34,365,756 | ||
| RSSC02a | chrC02 | 14,388,409 | 18,630,147 | ||
| RSSC02b | chrC02 | 26,296,409 | 28,749,960 | ||
| RSSC03a | chrC03 | 4,693,455 | 9,737,761 | ||
| RSSC03b | chrC03 | 20,346,765 | 33,132,785 | chrC03:22,217,518–30,597,169 chrC03:30,597,169–47,861,855 | Qasim et al., 2020 [40] Qasim et al., 2020 [40] |
| RSSC03_random | chrC03_random | 1,845,659 | 1,847,988 | ||
| RSSC04a | chrC04 | 19,676,382 | 20,444,349 | chrC04:11,691,778–28,720,453 | Zhao et al., 2006 [42] |
| RSSC04b | chrC04 | 23,959,219 | 26,737,257 | chrC04:11,691,778–28,720,453 | Zhao et al., 2006 [42] |
| RSSC04c | chrC04 | 39,374,823 | 43,990,961 | chrC04:40,419,964–41,916,428 | Wu et al., 2016 [43] |
| RSSC05a | chrC05 | 1,079,675 | 8,269,010 | ||
| RSSC05b | chrC05 | 10,013,537 | 17,710,073 | ||
| RSSC05c | chrC05 | 33,795,591 | 39,750,069 | ||
| RSSC06a | chrC06 | 2,873,709 | 4,819,806 | ||
| RSSC06b | chrC06 | 12,871,211 | 17,346,441 | ||
| RSSC07a | chrC07 | 22,967,050 | 27,205,509 | ||
| RSSC07b | chrC07 | 30,140,825 | 33,700,961 | chrC07:29,634,609–31,761,057 | Wu et al., 2013 [9] |
| RSSC07c | chrC07 | 40,473,495 | 44,450,958 | ||
| RSSC08a | chrC08 | 14,471,405 | 23,488,895 | chrC08:20,654,840–20,788,744 | Wu et al., 2016 [43] |
| RSSC08b | chrC08 | 29,321,327 | 33,960,528 | chrC08:31,404,544–32,040,944 chrC08:31,404,544–33,506,513 | Wu et al., 2013 [9] Wu et al., 2013 [9] |
| RSSC09a | chrC09 | 3,611,793 | 21,444,314 | ||
| RSSC09b | chrC09 | 33,468,285 | 33,556,099 | ||
| RSSC09c | chrC09 | 41,569,762 | 45,686,680 | chrC09:43,326,113–43,593,795 | Zhao et al., 2006 [42] |
| RSSCnn_random_c | chrCnn_random | 19,417,331 | 25,686,759 | ||
| RSSCnn_random_d | chrCnn_random | 41,618,220 | 49,282,425 | ||
| RSSCnn_random_e | chrCnn_random | 55,253,392 | 60,160,894 | ||
| RSSCnn_random_f | chrCnn_random | 62,592,394 | 69,187,154 |
| RAL_Name 1 | Gene_ID | Chr 2 | Start | End | At_Gene 3 | Symbol | Description | Score |
|---|---|---|---|---|---|---|---|---|
| RSSA06a | BnaA06g03950D | chrA06 | 2,392,927 | 2,389,029 | AT1G48260 | CIPK17 | CBL interacting protein kinase 17 | 0.78 |
| RSSA06b | BnaA06g12600D | chrA06 | 6,535,463 | 6,537,122 | AT1G18480 | SLP2 | Calcineurin like metallo phosphoesterase superfamily protein | 0.97 |
| RSSA06b | BnaA06g12660D | chrA06 | 6,551,737 | 6,551,264 | AT1G18530 | CML15 | Calmodulin-like 15 | 0.92 |
| RSSA06b | BnaA06g13020D | chrA06 | 6,809,184 | 6,812,279 | AT1G18840 | IQD30 | IQ domain 30 | 0.98 |
| RSSA06b | BnaA06g14070D | chrA06 | 7,550,927 | 7,554,322 | AT1G19870 | IQD32 | IQ domain 32 | 0.97 |
| RSSA06b | BnaA06g15280D | chrA06 | 8,382,965 | 8,383,757 | AT1G21550 | CML44 | Calcium binding EF hand family protein | 1.00 |
| RSSA06b | BnaA06g15970D | chrA06 | 8,783,588 | 8,786,720 | AT5G24430 | CPK4 | Calcium dependent protein kinase | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-Y.; Ren, C.-X.; Fan, Q.-W.; Xu, Y.-P.; Wang, L.-W.; Mao, Z.-L.; Cai, X.-Z. Integrated Assays of Genome-Wide Association Study, Multi-Omics Co-Localization, and Machine Learning Associated Calcium Signaling Genes with Oilseed Rape Resistance to Sclerotinia sclerotiorum. Int. J. Mol. Sci. 2024, 25, 6932. https://doi.org/10.3390/ijms25136932
Wang X-Y, Ren C-X, Fan Q-W, Xu Y-P, Wang L-W, Mao Z-L, Cai X-Z. Integrated Assays of Genome-Wide Association Study, Multi-Omics Co-Localization, and Machine Learning Associated Calcium Signaling Genes with Oilseed Rape Resistance to Sclerotinia sclerotiorum. International Journal of Molecular Sciences. 2024; 25(13):6932. https://doi.org/10.3390/ijms25136932
Chicago/Turabian StyleWang, Xin-Yao, Chun-Xiu Ren, Qing-Wen Fan, You-Ping Xu, Lu-Wen Wang, Zhou-Lu Mao, and Xin-Zhong Cai. 2024. "Integrated Assays of Genome-Wide Association Study, Multi-Omics Co-Localization, and Machine Learning Associated Calcium Signaling Genes with Oilseed Rape Resistance to Sclerotinia sclerotiorum" International Journal of Molecular Sciences 25, no. 13: 6932. https://doi.org/10.3390/ijms25136932
APA StyleWang, X.-Y., Ren, C.-X., Fan, Q.-W., Xu, Y.-P., Wang, L.-W., Mao, Z.-L., & Cai, X.-Z. (2024). Integrated Assays of Genome-Wide Association Study, Multi-Omics Co-Localization, and Machine Learning Associated Calcium Signaling Genes with Oilseed Rape Resistance to Sclerotinia sclerotiorum. International Journal of Molecular Sciences, 25(13), 6932. https://doi.org/10.3390/ijms25136932






