Comparison of the Influence of Bisphenol A and Bisphenol S on the Enteric Nervous System of the Mouse Jejunum
Abstract
1. Introduction
2. Results
3. Discussion
4. Material and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mikolajewska, K.; Stragierowicz, J.; Gromadzinska, J. Bisphenol A—Application, sources of exposure and potential risks in infants, children and pregnant women. Int. J. Occup. Med. Environ. Health 2015, 28, 209–241. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid. Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Wang, D.; Yan, S.; Li, R.; Yan, J.; Teng, M.; Zhou, Z.; Zhu, W. Effects of perinatal exposure to BPA and its alternatives (BPS, BPF and BPAF) on hepatic lipid and glucose homeostasis in female mice adolescent offspring. Chemosphere 2018, 212, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Adeyi, A.A. Distribution and bioaccumulation of Endocrine Disrupting Chemicals (EDCS) in Lagos Lagoon water, sediment and fish. Ife J. Sci. 2020, 22, 57–74. [Google Scholar] [CrossRef]
- Pivnenko, K.; Pedersen, G.A.; Eriksson, E.; Astrup, T.F. Bisphenol A and its structural analogues in household waste paper. Waste Manag. 2015, 44, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.; Raposo, A.; Almeida-Gonzales, M.; Carrascosa, C. Bisphenol A: Food exposure and impact on human health. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1503–1517. [Google Scholar] [CrossRef] [PubMed]
- Michałowicz, J. Bisphenol A—Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef] [PubMed]
- Frankowski, R.; Zgoła-Grześkowiak, A.; Grześkowiak, T.; Sójka, K. The presence of bisphenol A in the thermal paper in the face of changing European regulations–A comparative global research. Environ. Pollut. 2020, 265, 114879. [Google Scholar] [CrossRef]
- Thoene, M.; Dzika, E.; Gonkowski, S.; Wojtkiewicz, J. Bisphenol S in food causes hormonal and obesogenic effects comparable to or worse than bisphenol A: A literature review. Nutrients 2020, 12, 532. [Google Scholar] [CrossRef]
- Winkler, J.; Liu, P.; Phong, K.; Hinrichs, J.H.; Ataii, N.; Williams, K.; Hadler-Olsen, E.; Samson, S.; Gartner, Z.J.; Fisher, S.; et al. Bisphenol A replacement chemicals, BPF and BPS, induce protumorigenic changes in human mammary gland organoid morphology and proteome. Proc. Natl. Acad. Sci. USA 2022, 119, e2115308119. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Fouyet, S.; Olivier, E.; Leproux, P.; Dutot, M.; Rat, P. Bisphenol A, Bisphenol F, and Bisphenol S: The Bad and the Ugly. Where Is the Good? Life 2021, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhang, X.; Bai, J.; Li, J.; Zhang, C.; Zhao, Y.; Zhu, Y.; Zhang, J.; Zhou, X. Bisphenol S increases the obesogenic effects of a high-glucose diet through regulating lipid metabolism in Caenorhabditis elegans. Food Chem. 2021, 339, 127813. [Google Scholar] [CrossRef]
- Qiu, W.; Chen, B.; Greer, J.B.; Magnuson, J.T.; Xiong, Y.; Zhong, H.; Andrzejczyk, N.E.; Zheng, C.; Schlenk, D. Transcriptomic Responses of Bisphenol S Predict Involvement of Immune Function in the Cardiotoxicity of Early Life-Stage Zebrafish (Danio rerio). Environ. Sci. Technol. 2020, 54, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Hamnett, R.; Dershowitz, L.B.; Sampathkumar, V.; Wang, Z.; Gomez-Frittelli, J.; De Andrade, V.; Kasthuri, N.; Druckmann, S.; Kaltschmidt, J.A. Regional cytoarchitecture of the adult and developing mouse enteric nervous system. Curr Biol. 2022, 32, 4483–4492.e5. [Google Scholar] [CrossRef] [PubMed]
- Vasina, V.; Barbara, G.; Talamonti, L.; Stanghellini, V.; Corinaldesi, R.; Tonini, M.; De Ponti, F.; De Giorgio, R. Enteric neuroplasticity evoked by inflammation. Auton. Neurosci. 2006, 126–127, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, C.; Bessard, A.; Aubert, P.; Joussain, C.; Giuliano, F.; Behr-Roussel, D.; Perrouin-Verbe, M.A.; Perrouin-Verbe, B.; Brochard, C.; Neunlist, M. Enteric Nervous System Remodeling in a Rat Model of Spinal Cord Injury: A Pilot Study. Neurotrauma Rep. 2020, 1, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar]
- Timmermans, J.P.; Barbiers, M.; Scheuermann, D.W.; Stach, W.; Adriaensen, D.; Mayer, B.; De Groodt-Lasseel, M.H. Distribution pattern, neurochemical features and projections of nitrergic neurons in the pig small intestine. Ann Anat. 1994, 176, 515–525. [Google Scholar] [CrossRef]
- Volk, N.; Lacy, B. Anatomy and Physiology of the Small Bowel. Gastrointest. Endosc. Clin. N. Am. 2017, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Yokota, H.; Kibe, R.; Sayama, Y.; Yuasa, A. Excretion of bisphenol A-glucuronide into the small intestine and deconjugation in the cecum of the rat. Biochim. Biophys. Acta. 2002, 1573, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Peillex, C.; Kerever, A.; Lachhab, A.; Pelletier, M. Bisphenol A, bisphenol S and their glucuronidated metabolites modulate glycolysis and functional responses of human neutrophils. Environ. Res. 2021, 196, 110336. [Google Scholar] [CrossRef]
- Rząp, D.; Czajkowska, M.; Całka, J. Neurochemical Plasticity of nNOS-, VIP- and CART-Immunoreactive Neurons Following Prolonged Acetylsalicylic Acid Supplementation in the Porcine Jejunum. Int. J. Mol. Sci. 2020, 21, 2157. [Google Scholar] [CrossRef]
- Szymanska, K.; Gonkowski, S. Neurochemical characterization of the enteric neurons within the porcine jejunum in physiological conditions and under the influence of bisphenol A (BPA). Neurogastroenterol. Motil. 2019, 31, e13580. [Google Scholar] [CrossRef] [PubMed]
- Serzysko, T.; Skwarek, A.; Chudziak, E.; Malina, M.; Kaleczyc, J.; Sienkiewicz, W. Enteric neuronal development in canine small intestine—An immunohistochemical study. Pol. J. Vet. Sci. 2021, 24, 293–301. [Google Scholar] [CrossRef]
- Schuy, J.; Schlabrakowski, A.; Neuhuber, W.; Brehmer, A. Quantitative estimation and chemical coding of spiny type I neurons in human intestines. Cells Tissues Organs 2011, 193, 195–206. [Google Scholar] [CrossRef]
- Spencer, N.J.; Hu, H. Enteric nervous system: Sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 338–351. [Google Scholar] [CrossRef]
- Herath, M.; Hosie, S.; Bornstein, J.C.; Franks, A.E.; Hill-Yardin, E.L. The Role of the Gastrointestinal Mucus System in Intestinal Homeostasis: Implications for Neurological Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 248. [Google Scholar] [CrossRef]
- Palus, K.; Makowska, K.; Całka, J. Alterations in Galanin-Like Immunoreactivity in the Enteric Nervous System of the Porcine Stomach Following Acrylamide Supplementation. Int. J. Mol. Sci. 2019, 20, 3345. [Google Scholar] [CrossRef]
- Gonkowski, S.; Gajęcka, M.; Makowska, K. Mycotoxins and the Enteric Nervous System. Toxins 2020, 12, 461. [Google Scholar] [CrossRef] [PubMed]
- Palus, K.; Bulc, M.; Całka, J. Glyphosate affects the neurochemical phenotype of the intramural neurons in the duodenum in the pig. Neurogastroenterol. Motil. 2023, 35, e14507. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Lepiarczyk, E.; Gonkowski, S. The comparison of the influence of bisphenol A (BPA) and its analogue bisphenol S (BPS) on the enteric nervous system of the distal colon in mice. Nutrients 2023, 15, 200. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Lech, P.; Gonkowski, S. Bisphenol A Effects on Neurons’ Neurochemical Character in the Urinary Bladder Intramural Ganglia of Domestic Pigs. Int. J. Mol. Sci. 2023, 24, 16792. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Gonkowski, S. Changes Caused by Low Doses of Bisphenol A (BPA) in the Neuro-Chemistry of Nerves Located in the Porcine Heart. Animals 2021, 11, 780. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Furness, J.B. Immunohistochemical localisation of cholinergic markers in putative intrinsic primary afferent neurons of the guinea-pig small intestine. Cell Tissue Res. 1998, 294, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Gonkowski, S. Changes Caused by Bisphenols in the Chemical Coding of Neurons of the Enteric Nervous System of Mouse Stomach. Int. J. Environ. Res. Public Health 2023, 20, 5125. [Google Scholar] [CrossRef]
- Ambreen, S.; Akhtar, T.; Hameed, N.; Ashfaq, I.; Sheikh, N. In Vivo Evaluation of Histopathological Alterations and Trace Metals Estimation of the Small Intestine in Bisphenol A-Intoxicated Rats. Can. J. Gastroenterol. Hepatol. 2019, 2019, 9292316. [Google Scholar] [CrossRef]
- Xu, X.; Xie, L.; Hong, X.; Ruan, Q.; Lu, H.; Zhang, Q.; Zhang, G.; Liu, X. Perinatal exposure to bisphenol-A inhibits synaptogenesis and affects the synaptic morphological development in offspring male mice. Chemosphere 2013, 91, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, K.; Makowska, K.; Gonkowski, S. The Influence of High and Low Doses of Bisphenol A (BPA) on the Enteric Nervous System of the Porcine Ileum. Int. J. Mol. Sci. 2018, 19, 917. [Google Scholar] [CrossRef]
- Wright, E.C.; Johnson, S.A.; Hao, R.; Kowalczyk, A.S.; Greenberg, G.D.; Ordoñes Sanchez, E.; Laman-Maharg, A.; Trainor, B.C.; Rosenfeld, C.S. Exposure to extrinsic stressors, social defeat or bisphenol A, eliminates sex differences in DNA methyltransferase expression in the amygdala. J. Neuroendocrinol. 2017, 29, 10. [Google Scholar] [CrossRef] [PubMed]
- Mokra, K.; Kocia, M.; Michałowicz, J. Bisphenol A and its analogs exhibit different apoptotic potential in peripheral blood mononuclear cells (in vitro study). Food Chem. Toxicol. 2015, 84, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Yu, M.; Wan, D.; Zhang, L.; Han, L.; Shen, Z.; Shi, M.; Zhu, Y.; Zhang, Z.; Bo, P. Regulatory effects of galanin system on development of several age-related chronic diseases. Exp. Gerontol. 2017, 95, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; MacTavish, D.; Kar, S.; Jhamandas, J.H. Galanin attenuates beta-amyloid (Abeta) toxicity in rat cholinergic basal forebrain neurons. Neurobiol. Dis. 2006, 21, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra Rao, V.L.; Bowen, K.K.; Dhodda, V.K.; Song, G.; Franklin, J.L.; Gavva, N.R.; Dempsey, R.J. Gene expression analysis of spontaneously hypertensive rat cerebral cortex following transient focal cerebral ischemia. J. Neurochem. 2002, 83, 1072–1086. [Google Scholar] [CrossRef] [PubMed]
- Piqueras, L.; Taché, Y.; Martinez, V. Galanin inhibits gastric acid secretion through a somatostatin-independent mechanism in mice. Peptides 2004, 25, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Psichas, A.; Glass, L.L.; Sharp, S.J.; Reimann, F.; Gribble, F.M. Galanin inhibits GLP-1 and GIP secretion via the GAL1 receptor in enteroendocrine L and K cells. Br. J. Pharmacol. 2016, 173, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Gundlach, A.L.; Kofler, B. The galanin peptide family: Receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol. Ther. 2007, 115, 177–207. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Yamazaki, S.; Kumakura, S.; Someya, A.; Iseki, M.; Inada, E.; Nagaoka, I. Yokukansan, a Japanese Herbal Medicine, suppresses Substance P-induced Production of Interleukin-6 and Interleukin-8 by Human U373 MG Glioblastoma Astrocytoma Cells. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1073–1080. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, L.; Xue, Y.; Xia, Y.J. Substance P prevents 1-methyl-4-phenylpyridinium-induced cytotoxicity through inhibition of apoptosis via neurokinin-1 receptors in MES23.5 cells. Mol. Med. Rep. 2015, 12, 8085–8092. [Google Scholar] [CrossRef]
- Ye, X.; Liu, Z.; Han, H.W.; Noh, J.Y.; Shen, Z.; Kim, D.M.; Wang, H.; Guo, H.; Ballard, J.; Golovko, A.; et al. Nutrient-Sensing Ghrelin Receptor in Macrophages Modulates Bisphenol A-Induced Intestinal Inflammation in Mice. Genes 2023, 14, 1455. [Google Scholar] [CrossRef] [PubMed]
- Zalecki, M. Gastric ulcer induced changes in substance P and Nk1, Nk2, Nk3 receptors expression in different stomach localizations with regard to intrinsic neuronal system. Histochem. Cell Biol. 2019, 151, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Kasparek, M.S.; Fatima, J.; Iqbal, C.W.; Duenes, J.A.; Sarr, M.G. Long-term effects of extrinsic denervation on VIP and substance P innervation in circular muscle of rat jejunum. J. Gastrointest. Surg. 2007, 11, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- de Souza, F.R.O.; Ribeiro, F.M.; Lima, P.M.D. Implications of VIP and PACAP in Parkinson’s Disease: What do we Know So Far? Curr. Med. Chem. 2021, 28, 1703–1715. [Google Scholar] [CrossRef] [PubMed]
- Vota, D.; Aguero, M.; Grasso, E.; Hauk, V.; Gallino, L.; Soczewski, E.; Pérez Leirós, C.; Ramhorst, R. Progesterone and VIP cross-talk enhances phagocytosis and anti-inflammatory profile in trophoblast-derived cells. Mol. Cell Endocrinol. 2017, 443, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Pan, L.; Qian, W. Associations between the severity of reflux esophagitis in children and changes in oxidative stress, serum inflammation, vasoactive intestinal peptide and motilin. Exp. Ther. Med. 2019, 18, 3509–3513. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, O.T.; Tunçel, N. Advantages of Vasoactive Intestinal Peptide for the Future Treatment of Parkinson’s Disease. Curr. Pharm. Des. 2018, 24, 4693–4701. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, K.; Tarafder, P.; Paul, G. Bisphenol A inhibits duodenal movement ex vivo of rat through nitric oxide-mediated soluble guanylyl cyclase and α-adrenergic signaling pathways. J. Appl. Toxicol. 2016, 36, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, S.; Zhang, L.; Qu, W.; Chen, Z. Bisphenol A increases intestinal permeability through disrupting intestinal barrier function in mice. Environ. Pollut. 2019, 254, 112960. [Google Scholar] [CrossRef]
- Inoue, A.; Hayashi, S.; Aoyagi, K.; Nishigaki, M.; Sasaki, H.; Kiyama, R. A reporter gene assay for evaluation of tissue-specific responses to estrogens based on the differential use of promoters A to F of the human estrogen receptor alpha gene. J. Pharmacol. Toxicol. Methods 2002, 47, 129–135. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, P.; Huang, Q.; Chi, Y.; Dong, S.; Fan, J. Bisphenol-A induces neurodegeneration through disturbance of intracellular calcium homeostasis in human embryonic stem cells-derived cortical neurons. Chemosphere 2019, 229, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.H.; Oh, J.E.; Shim, J.K.; Kwak, Y.L.; Cho, J.S. Effects of bisphenol A on the proliferation, migration, and tumor growth of colon cancer cells: In vitro and in vivo evaluation with mechanistic insights related to ERK and 5-HT3. Food Chem. Toxicol. 2021, 158, 112662. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rui, M.; Nie, Y.; Lu, G. Influence of gastrointestinal tract on metabolism of bisphenol A as determined by in vitro simulated system. J. Hazard. Mater. 2018, 355, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Tyl, R.W.; Myers, C.B.; Marr, M.C.; Thomas, B.F.; Keimowitz, A.R.; Brine, D.R.; Veselica, M.M.; Fail, P.A.; Chang, T.Y.; Seely, J.C.; et al. Three generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol. Sci. 2002, 68, 121–146. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.W.; Jeong, J.Y.; Hwang, M.S.; Jung, K.K.; Lee, K.H.; Lee, H.M. Establishment of the korean tolerable daily intake of bisphenol a based on risk assessments by an expert committee. Toxicol. Res. 2010, 26, 285–291. [Google Scholar] [CrossRef]
- Zielinska, M.; Wojnowska-Baryla, I.; Cydzik-Kwiatkowska, A. Bisphenol A Removal from Water and Wastewater; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-319-92361-1. [Google Scholar]
- Wojtkiewicz, J.; Makowska, K.; Bejer-Olenska, E.; Gonkowski, S. Zinc Transporter 3 (Znt3) as an Active Substance in the Enteric Nervous System of the Porcine Esophagus. J. Mol. Neurosci. 2017, 61, 315–324. [Google Scholar] [CrossRef]
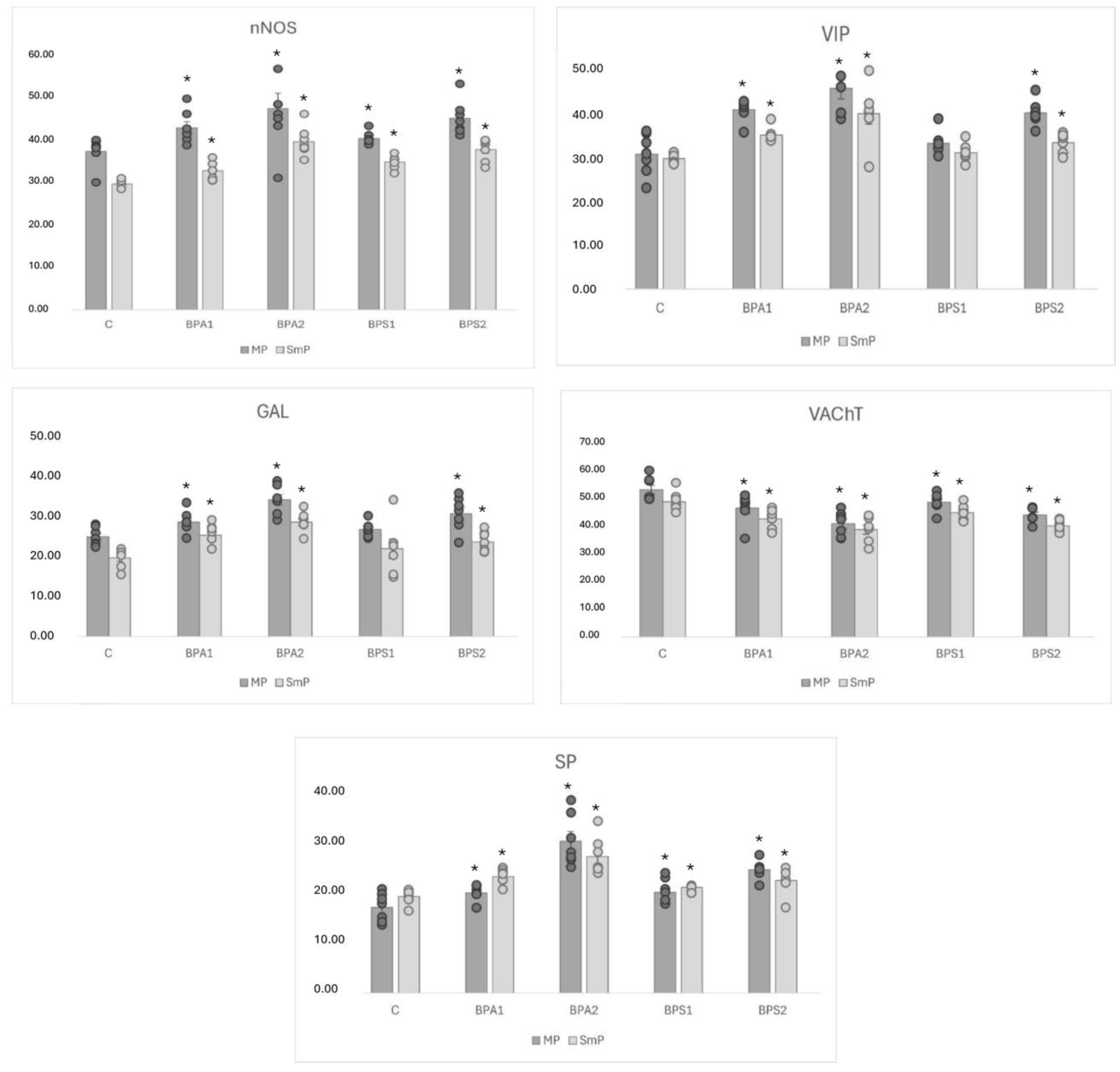
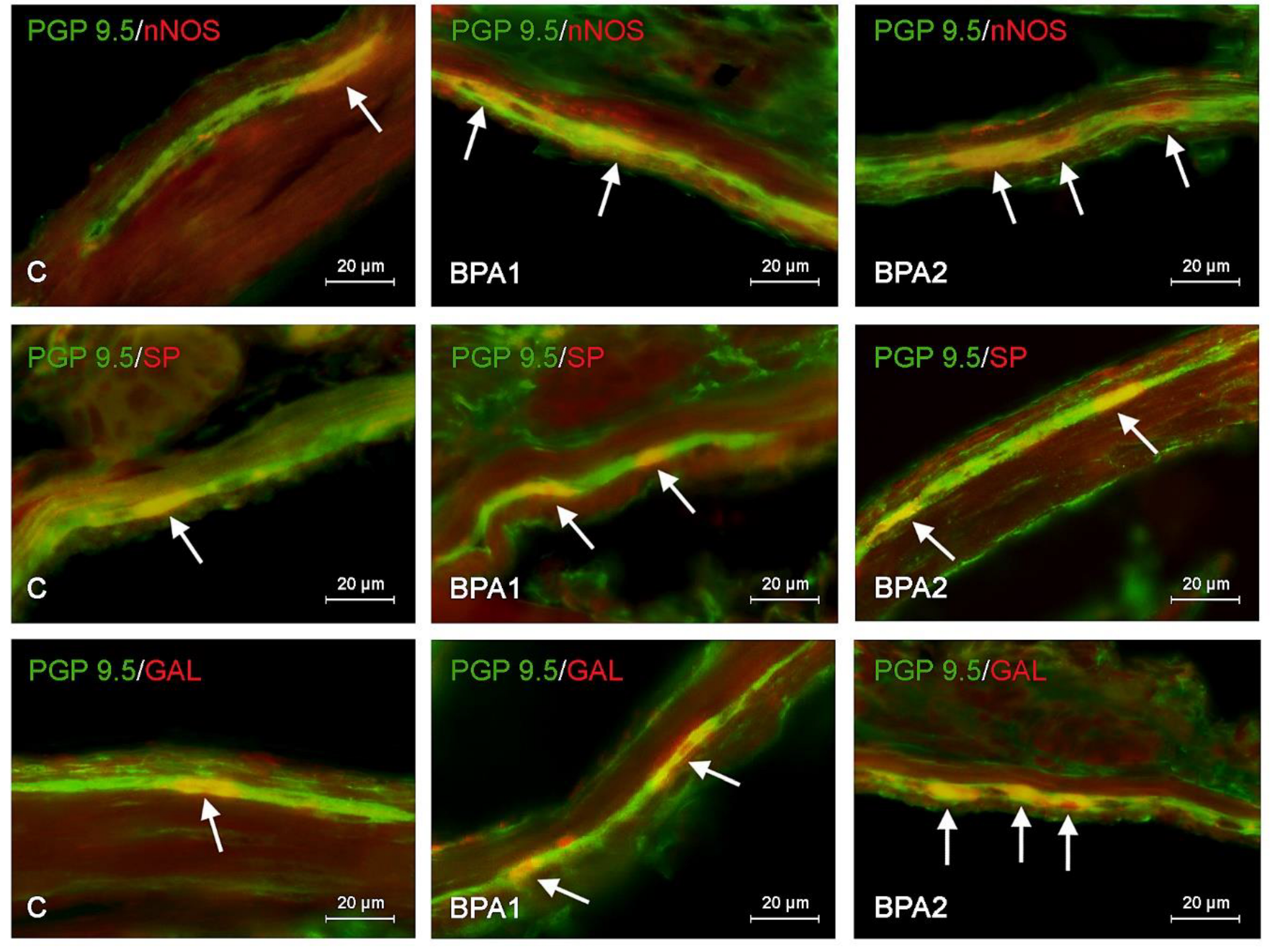
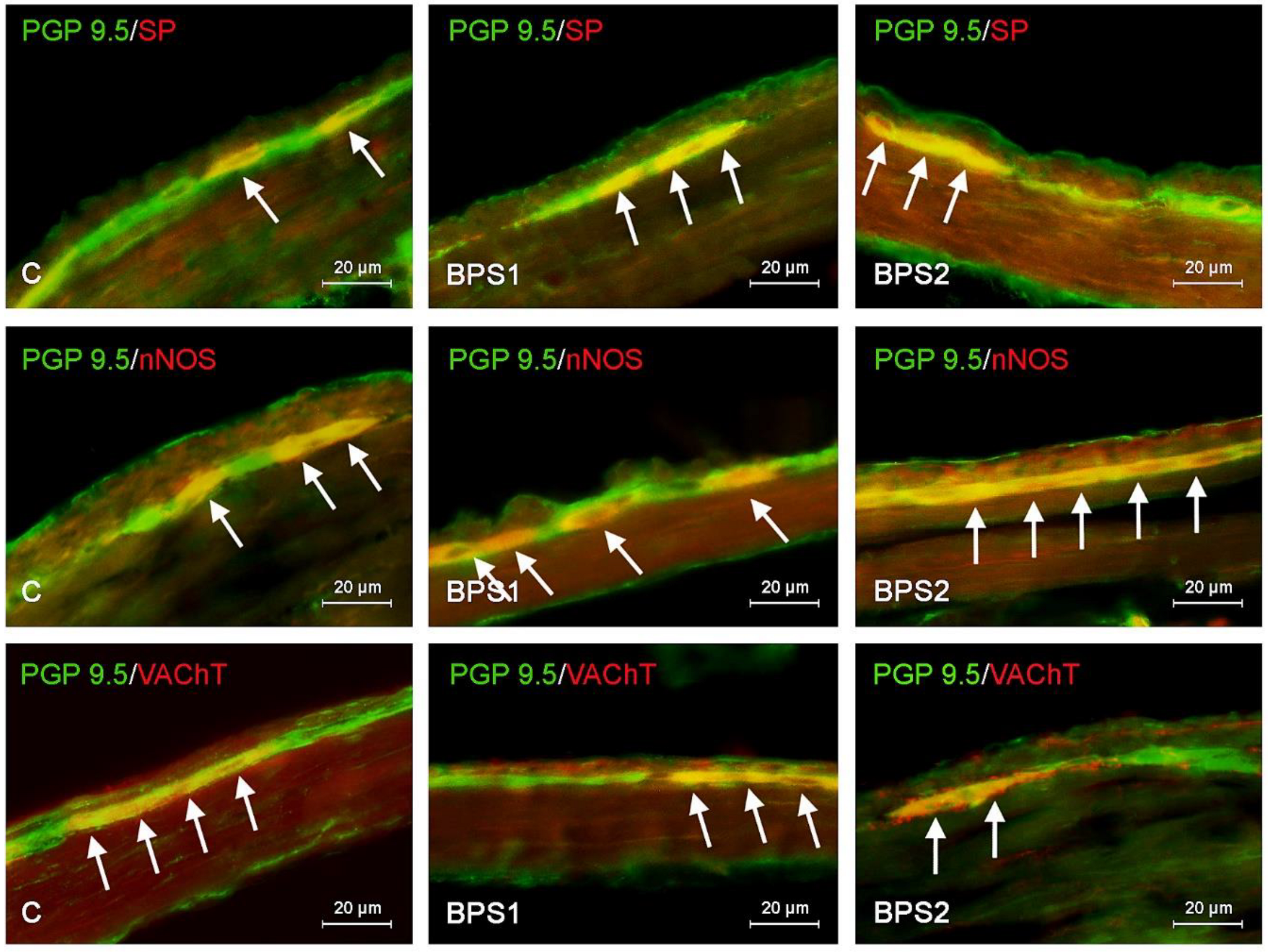

| nNOS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | C | BPA1 | BPA2 | BPS1 | BPS2 | ||||||
| Ganglion | MP | SmP | MP | SmP | MP | SmP | MP | SmP | MP | SmP | |
| Animal 1 | A | 506/200 | 498/141 | 505/209 | 508/174 | 504/307 | 475/167 | 501/216 | 503/178 | 506/215 | 500/194 |
| B | 39.53 | 28.31 | 41.39 | 34.25 | 60.91 | 35.16 | 43.11 | 35.39 | 42.49 | 38.8 | |
| Animal 2 | A | 502/185 | 500/148 | 505/250 | 504/156 | 504/285 | 509/192 | 502/200 | 504/169 | 503/206 | 507/190 |
| B | 36.85 | 29.6 | 49.5 | 30.95 | 56.55 | 37.72 | 39.84 | 33.53 | 40.95 | 37.47 | |
| Animal 3 | A | 502/200 | 502/147 | 502/201 | 508/174 | 512/235 | 503/194 | 503/206 | 502/178 | 509/214 | 505/199 |
| B | 39.84 | 29.28 | 40.04 | 34.25 | 45.9 | 38.57 | 40.95 | 35.46 | 42.04 | 39.41 | |
| Animal 4 | A | 502/150 | 498/147 | 502/207 | 502/164 | 506/227 | 500/190 | 500/194 | 500/176 | 505/231 | 500/199 |
| B | 29.88 | 28.31 | 41.24 | 32.67 | 44.86 | 38 | 38.8 | 35.2 | 45.74 | 39.8 | |
| Animal 5 | A | 509/196 | 498/150 | 503/194 | 505/135 | 502/242 | 502/201 | 505/200 | 503/185 | 509/225 | 502/200 |
| B | 38.51 | 30.12 | 38.57 | 30.3 | 48.21 | 40.04 | 39.6 | 36.78 | 44.2 | 39.84 | |
| Animal 6 | A | 500/191 | 503/152 | 508/216 | 501/179 | 01/155 | 502/207 | 502/201 | 500/160 | 517/242 | 502/174 |
| B | 38.2 | 30.22 | 42.52 | 35.73 | 30.94 | 41.24 | 40.04 | 32 | 46.81 | 34.66 | |
| Animal 7 | A | 509/193 | 505/156 | 512/235 | 500/152 | 501/216 | 512/235 | 507/201 | 504/174 | 504/269 | 501/167 |
| B | 37.92 | 30.89 | 45.9 | 30.4 | 43.11 | 45.9 | 39.65 | 34.52 | 52.98 | 33.33 | |
| Total | C | 37.25 ± 1.28 | 29.53 ± 0.37 | 42.74 ± 1.42 * | 32.65 ± 0.82 * | 47.21 ± 3.67 * | 39.52 ± 1.29 * | 40.28 ± 0.53 * | 34.70 ± 0.58 * | 45.03 ± 1.54 * | 37.62 ± 0.99 * |
| VIP | |||||||||||
| Animal 1 | A | 503/115 | 508/157 | 506/215 | 502/174 | 501/268 | 505/250 | 500/160 | 504/174 | 502/226 | 504/172 |
| B | 22.86 | 30.91 | 42.49 | 34.66 | 53.49 | 49.5 | 32 | 34.52 | 45.02 | 34.13 | |
| Animal 2 | A | 500/134 | 501/148 | 503/206 | 502/170 | 503/194 | 506/140 | 508/164 | 502/152 | 499/200 | 502/155 |
| B | 26.8 | 29.54 | 40.95 | 33.86 | 38.57 | 27.67 | 32.28 | 30.28 | 40.08 | 30.88 | |
| Animal 3 | A | 503/178 | 501/155 | 502/201 | 502/174 | 502/200 | 500/200 | 504/169 | 502/141 | 506/196 | 502/178 |
| B | 35.39 | 30.94 | 40.04 | 34.66 | 39.84 | 40 | 33.53 | 28.09 | 38.74 | 35.46 | |
| Animal 4 | A | 501/179 | 500/143 | 502/207 | 509/196 | 502/200 | 501/201 | 507/161 | 500/160 | 506/208 | 498/165 |
| B | 35.73 | 28.6 | 41.24 | 38.51 | 39.84 | 40.12 | 31.76 | 32 | 41.11 | 33.13 | |
| Animal 5 | A | 507/168 | 498/150 | 502/210 | 509/171 | 502/242 | 507/206 | 508/167 | 500/150 | 500/200 | 507/174 |
| B | 33.14 | 30.12 | 41.83 | 33.6 | 48.21 | 40.63 | 32.87 | 30 | 40 | 34.32 | |
| Animal 6 | A | 502/147 | 502/144 | 498/177 | 502/176 | 504/267 | 509/214 | 498/150 | 501/152 | 485/190 | 504/150 |
| B | 29.28 | 28.69 | 35.54 | 35.06 | 52.98 | 42.04 | 30.12 | 30.34 | 39.18 | 29.76 | |
| Animal 7 | A | 508/156 | 498/141 | 508/216 | 504/174 | 505/231 | 509/197 | 503/194 | 504/156 | 501/179 | 500/174 |
| B | 30.71 | 28.31 | 42.52 | 34.52 | 45.74 | 38.7 | 38.57 | 30.95 | 35.73 | 34.8 | |
| Total | C | 30.56 ± 1.77 | 29.59 ± 0.42 | 40.66 ± 0.92 * | 34.98 ± 0.62 * | 45.52 ± 2.39 * | 39.81 ± 2.43 * | 33.02 ± 1.01 | 30.88 ± 0.75 | 39.98 ± 1.06 * | 33.21 ± 0.8 * |
| GAL | |||||||||||
| Animal 1 | A | 504/134 | 500/110 | 509/128 | 509/114 | 503/178 | 500/143 | 509/128 | 509/122 | 500/143 | 500/120 |
| B | 26.59 | 22 | 25.15 | 22.4 | 35.39 | 28.6 | 25.15 | 23.97 | 28.6 | 24 | |
| Animal 2 | A | 500/125 | 500/112 | 502/141 | 505/113 | 502/200 | 504/145 | 502/141 | 507/121 | 498/150 | 506/109 |
| B | 25 | 22.4 | 28.09 | 22.38 | 39.84 | 28.77 | 28.09 | 23.86 | 30.12 | 21.54 | |
| Animal 3 | A | 502/144 | 500/104 | 504/156 | 507/127 | 502/150 | 499/166 | 500/134 | 504/117 | 500/176 | 505/112 |
| B | 28.69 | 20.8 | 30.95 | 25.05 | 29.88 | 33.27 | 26.8 | 23.21 | 35.2 | 22.18 | |
| Animal 4 | A | 498/141 | 507/109 | 508/174 | 500/134 | 506/159 | 500/125 | 500/128 | 502/176 | 503/185 | 509/135 |
| B | 28.31 | 21.5 | 34.25 | 26.8 | 31.42 | 25 | 25.6 | 35.06 | 36.78 | 26.52 | |
| Animal 5 | A | 505/120 | 487/101 | 502/141 | 504/141 | 501/174 | 498/150 | 507/139 | 487/101 | 508/164 | 504/141 |
| B | 23.76 | 20.74 | 28.09 | 27.98 | 34.73 | 30.12 | 27.42 | 20.74 | 32.28 | 27.98 | |
| Animal 6 | A | 501/118 | 504/90 | 504/148 | 500/138 | 502/178 | 502/144 | 502/141 | 489/74 | 504/169 | 504/131 |
| B | 23.55 | 17.86 | 29.37 | 27.6 | 35.46 | 28.69 | 28.09 | 15.13 | 33.53 | 25.99 | |
| Animal 7 | A | 498/114 | 500/79 | 502/147 | 506/151 | 507/197 | 503/155 | 504/156 | 500/79 | 500/120 | 502/109 |
| B | 22.89 | 15.8 | 29.28 | 29.84 | 38.86 | 30.82 | 30.95 | 15.8 | 24 | 21.71 | |
| Total | C | 25.54 ± 0.89 | 20.16 ± 0.92 | 29.31 ± 1.06 * | 26.01 ± 1.08 * | 35.08 ± 1.36 * | 29.32 ± 0.96 * | 27.44 ± 0.73 | 22.54 ± 2.51 | 31.50 ± 1.64 * | 24.27 ± 0.98 * |
| VAChT | |||||||||||
| Animal 1 | A | 500/250 | 502/242 | 500/242 | 500/196 | 514/241 | 502/199 | 504/269 | 508/216 | 503/235 | 506/208 |
| B | 50 | 48.21 | 48.4 | 39.2 | 46.88 | 39.64 | 52.98 | 42.52 | 46.72 | 41.11 | |
| Animal 2 | A | 501/250 | 610/286 | 514/241 | 501/218 | 505/180 | 503/217 | 504/252 | 512/235 | 500/215 | 500/194 |
| B | 49.9 | 46.88 | 46.88 | 43.51 | 35.64 | 43.14 | 50 | 45.9 | 43 | 38.8 | |
| Animal 3 | A | 548/279 | 548/279 | 505/180 | 503/215 | 505/182 | 510/200 | 505/241 | 506/227 | 507/220 | 507/190 |
| B | 50.86 | 50.86 | 35.64 | 42.74 | 36.04 | 39.21 | 47.72 | 44.86 | 43.39 | 37.47 | |
| Animal 4 | A | 504/285 | 513/256 | 500/246 | 519/231 | 503/194 | 506/161 | 504/216 | 513/241 | 501/216 | 517/27 |
| B | 56.55 | 49.9 | 49.2 | 44.51 | 38.57 | 31.82 | 42.86 | 46.98 | 43.11 | 40.04 | |
| Animal 5 | A | 500/250 | 501/280 | 500/250 | 505/190 | 519/231 | 511/225 | 500/250 | 510/213 | 502/200 | 507/201 |
| B | 50 | 55.88 | 50 | 37.62 | 44.51 | 44.03 | 50 | 41.76 | 39.84 | 39.65 | |
| Animal 6 | A | 503/286 | 514/241 | 500/257 | 610/286 | 501/218 | 504/175 | 502/242 | 506/227 | 513/241 | 508/218 |
| B | 56.86 | 46.88 | 51.4 | 46.88 | 43.51 | 34.72 | 48.21 | 44.86 | 46.98 | 42.91 | |
| Animal 7 | A | 509/307 | 503/227 | 502/230 | 503/230 | 508/216 | 502/201 | 500/256 | 503/250 | 503/235 | 508/216 |
| B | 60.31 | 45.13 | 45.82 | 45.73 | 42.52 | 40.04 | 51.2 | 49.7 | 46.72 | 42.52 | |
| Total | C | 53.50 ± 1.63 | 49.11 ± 1.35 | 46.74 ± 1.98 * | 42.88 ± 1.27 * | 41.10 ± 1.62 * | 38.94 ± 1.65 * | 49.00 ± 1.22 * | 45.22 ± 1.01 * | 44.25 ± 1.01 * | 40.36 ± 0.74 * |
| SP | |||||||||||
| Animal 1 | A | 489/74 | 505/101 | 509/105 | 500/125 | 505/182 | 507/174 | 487/101 | 507/109 | 509/128 | 507/122 |
| B | 15.13 | 20 | 20.63 | 25 | 36.04 | 34.32 | 20.74 | 21.5 | 25.15 | 24.06 | |
| Animal 2 | A | 499/68 | 505/101 | 500/85 | 507/105 | 503/194 | 504/150 | 504/90 | 501/106 | 500/124 | 500/125 |
| B | 13.63 | 20 | 17 | 20.71 | 38.57 | 29.76 | 1786 | 21.16 | 24.8 | 25 | |
| Animal 3 | A | 501/71 | 50/103 | 504/100 | 489/120 | 509/128 | 505/125 | 503/101 | 505/101 | 509/128 | 500/113 |
| B | 14.17 | 20.6 | 19.84 | 24.53 | 25.15 | 24.75 | 20.08 | 20 | 25.15 | 22.6 | |
| Animal 4 | A | 487/101 | 500/97 | 507/109 | 500/113 | 502/141 | 500/141 | 500/89 | 505/101 | 505/21 | 500/114 |
| B | 20.74 | 19.4 | 21.5 | 22.6 | 28.09 | 28.2 | 17.8 | 20 | 23.96 | 22.8 | |
| Animal 5 | A | 504/90 | 52/101 | 506/109 | 500/120 | 503/156 | 500/120 | 505/94 | 506/112 | 504/139 | 500/110 |
| B | 17.86 | 20.12 | 21.54 | 24 | 31.01 | 24 | 18.61 | 20.16 | 27.58 | 22 | |
| Animal 6 | A | 500/99 | 498/93 | 500/99 | 500/113 | 506/135 | 509/128 | 503/116 | 500/106 | 507/109 | 509/122 |
| B | 19.8 | 18.67 | 19.8 | 22.6 | 26.68 | 25.15 | 23.06 | 21.2 | 21.5 | 23.97 | |
| Animal 7 | A | 501/94 | 499/82 | 503/99 | 505/120 | 501/136 | 500/124 | 500/120 | 501/100 | 505/125 | 503/86 |
| B | 18.76 | 16.43 | 19.68 | 23.76 | 27.15 | 24.8 | 24 | 19.96 | 24.75 | 17.1 | |
| Total | C | 17.16 ± 1.07 | 19.32 ± 0.53 | 20.00 ± 0.58 * | 23.31 ± 0.55 * | 30.38 ± 1.93 * | 27.28 ± 1.42 * | 20.15 ± 0.84 * | 21.15 ± 0.53 * | 24.70 ± 0.68 * | 22.50 ± 0.98 * |
| nNOS | |||||
| C | BPA1 | BPA2 | BPS1 | BPS2 | |
| MP | 37.25 ± 1.28 | 42.74 ± 1.42 * | 47.21 ± 3.67 * | 40.28 ± 0.53 * | 45.03 ± 1.54 * |
| SmP | 29.53 ± 0.37 | 32.65 ± 0.82 * | 39.52 ± 1.29 * | 34.70 ± 0.58 * | 37.62 ± 0.99 * |
| VIP | |||||
| C | BPA1 | BPA2 | BPS1 | BPS2 | |
| MP | 30.56 ± 1.77 | 40.66 ± 0.92 * | 45.52 ± 2.39 * | 33.02 ± 1.01 | 39.98 ± 1.06 * |
| SmP | 29.59 ± 0.42 | 34.98 ± 0.62 * | 39.81 ± 2.43 * | 30.88 ± 0.75 | 33.21 ± 0.8 * |
| GAL | |||||
| C | BPA1 | BPA2 | BPS1 | BPS2 | |
| MP | 25.54 ± 0.89 | 29.31 ± 1.06 * | 35.08 ± 1.36 * | 27.44 ± 0.73 | 31.50 ± 1.64 * |
| SmP | 20.16 ± 0.92 | 26.01 ± 1.08 * | 29.32 ± 0.96 * | 22.54 ± 2.51 | 24.27 ± 0.98 * |
| VAChT | |||||
| C | BPA1 | BPA2 | BPS1 | BPS2 | |
| MP | 53.50 ± 1.63 | 46.74 ± 1.98 * | 41.10 ± 1.62 * | 49.00 ± 1.22 * | 44.25 ± 1.01 * |
| SmP | 49.11 ± 1.35 | 42.88 ± 1.27 * | 38.94 ± 1.65 * | 45.22 ± 1.01 * | 40.36 ± 0.74 * |
| SP | |||||
| C | BPA1 | BPA2 | BPS1 | BPS2 | |
| MP | 17.16 ± 1.07 | 20.00 ± 0.58 * | 30.38 ± 1.93 * | 20.15 ± 0.84 * | 24.70 ± 0.68 * |
| SmP | 19.32 ± 0.53 | 23.31 ± 0.55 * | 27.28 ± 1.42 * | 21.15 ± 0.53 * | 22.50 ± 0.98 * |
| Primary Antibodies | ||||
| Antigen | Code | Species | Working Dilution | Supplier |
| PGP 9.5 | 7863-2004 | Mouse | 1:1000 | BioRad, Hercules, CA, USA |
| VIP | 11428 | Rabbit | 1:4000 | Cappel, Aurora, OH, USA, 11428, working dilution 1:5000 |
| SP | 8450-0505 | Rat | 1:1000 | BioRad |
| nNOS | AB5380 | Rabbit | 1:2000 | Sigma-Aldrich, Saint Louis, MO, USA |
| GAL | T-5036 | Guinea pig | 1:2000 | Peninsula Labs, San Carlos, CA, USA |
| VAChT | H-V006 | Rabbit | 1:2000 | Phoenix Pharmaceuticals, Burlingame, CA, USA |
| Secondary Antibodies Reagents | ||||
| Reagents | Working Dilution | Supplier | ||
| Alexa fluor 488 donkey anti-mouse IgG | 1:1000 | ThermoFisher Scientific, Waltham, MA, USA | ||
| Alexa fluor 546 donkey anti-rabbit IgG | 1:1000 | ThermoFisher Scientific, | ||
| Alexa fluor 548 donkey anti-rat IgG | 1:1000 | ThermoFisher Scientific, | ||
| Alexa fluor 546 donkey anti-guinea pig IgG | 1:1000 | ThermoFisher Scientific, | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makowska, K.; Gonkowski, S. Comparison of the Influence of Bisphenol A and Bisphenol S on the Enteric Nervous System of the Mouse Jejunum. Int. J. Mol. Sci. 2024, 25, 6941. https://doi.org/10.3390/ijms25136941
Makowska K, Gonkowski S. Comparison of the Influence of Bisphenol A and Bisphenol S on the Enteric Nervous System of the Mouse Jejunum. International Journal of Molecular Sciences. 2024; 25(13):6941. https://doi.org/10.3390/ijms25136941
Chicago/Turabian StyleMakowska, Krystyna, and Sławomir Gonkowski. 2024. "Comparison of the Influence of Bisphenol A and Bisphenol S on the Enteric Nervous System of the Mouse Jejunum" International Journal of Molecular Sciences 25, no. 13: 6941. https://doi.org/10.3390/ijms25136941
APA StyleMakowska, K., & Gonkowski, S. (2024). Comparison of the Influence of Bisphenol A and Bisphenol S on the Enteric Nervous System of the Mouse Jejunum. International Journal of Molecular Sciences, 25(13), 6941. https://doi.org/10.3390/ijms25136941







