The Human Neonatal Skin Fibroblast, an Available Cell Source for Tissue Production and Transplantation, Exhibits Low Risk of Immunogenicity In Vitro
Abstract
1. Introduction
2. Results
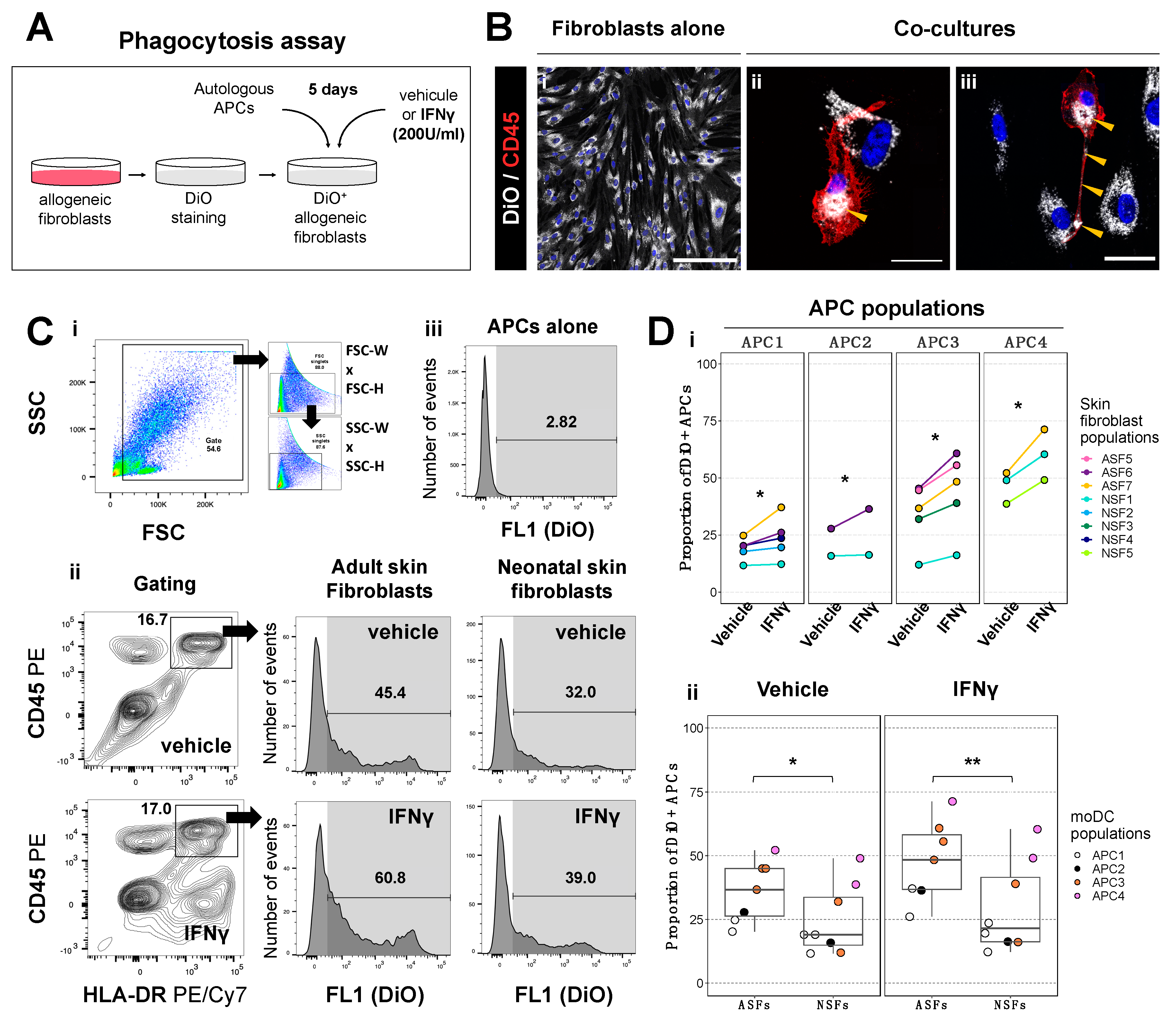
2.1. Phagocytic Activity of Antigen Presenting Cells Is Higher in Co-Cultures with Adult Skin Fibroblasts Than in Those with Neonatal Skin Fibroblasts
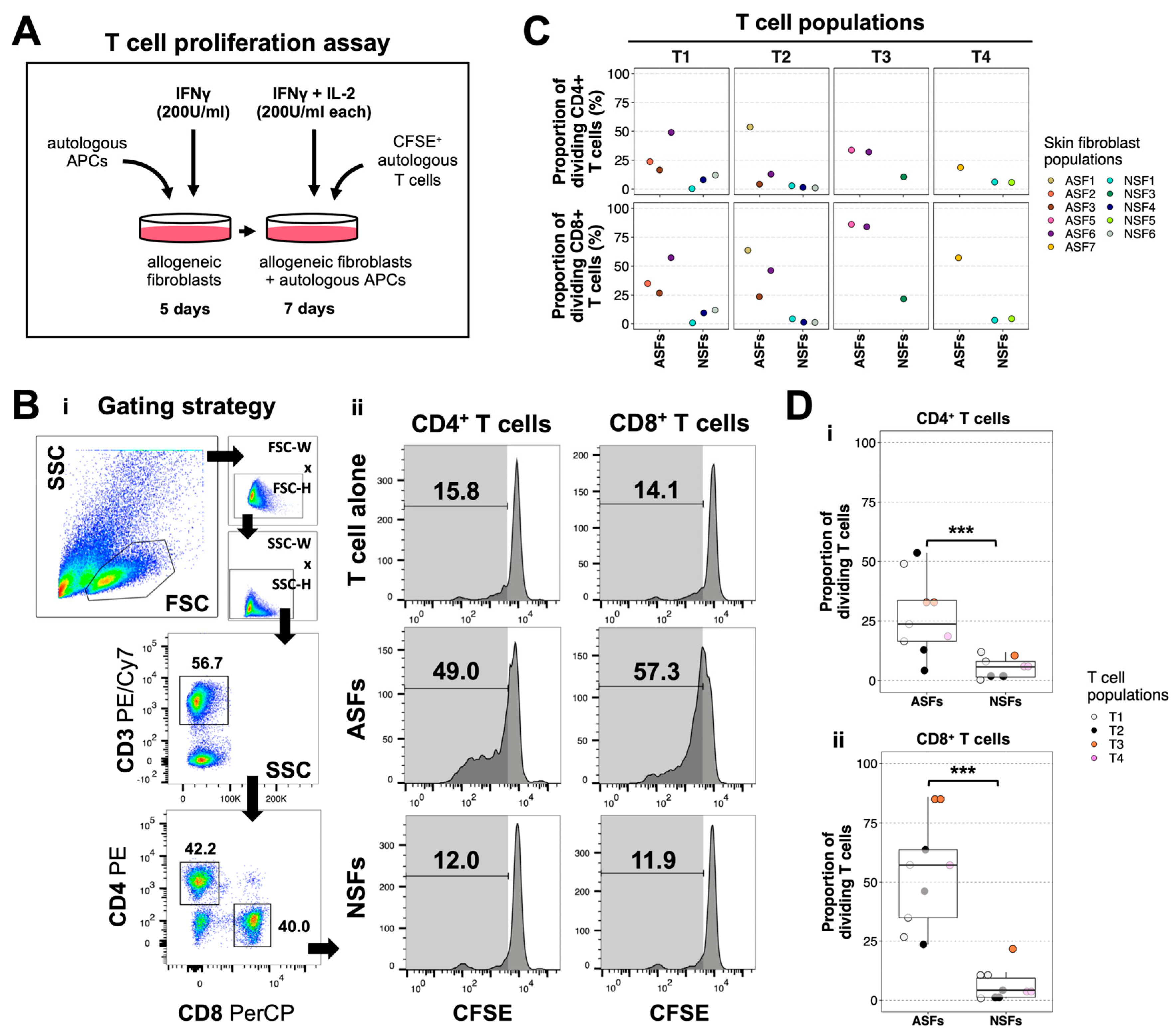
2.2. ASFs Induce More T Cell Proliferation Than NSFs
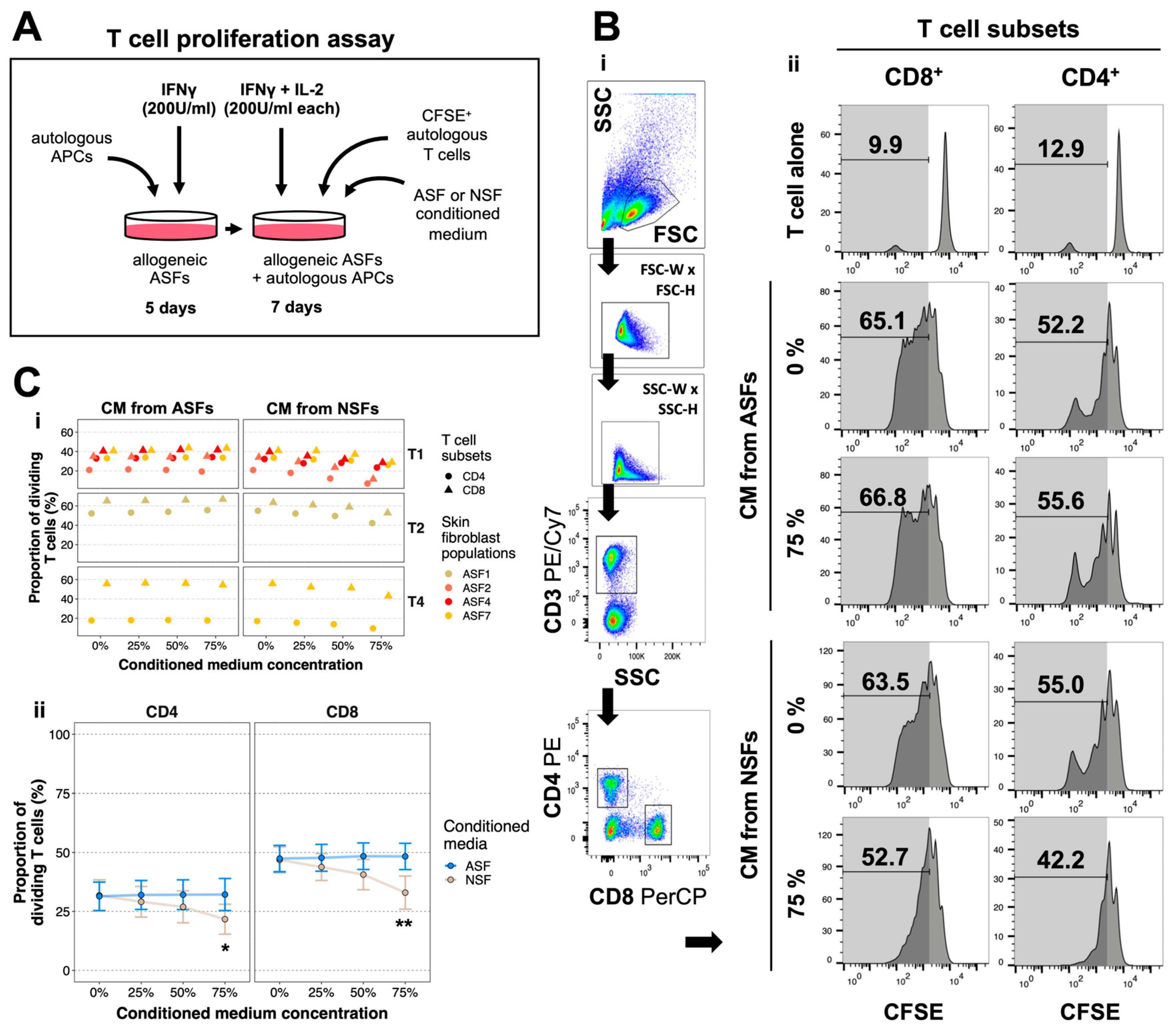
2.3. NSFs Secrete Paracrine Factors That Reduce Alloreactive T Cell Proliferation
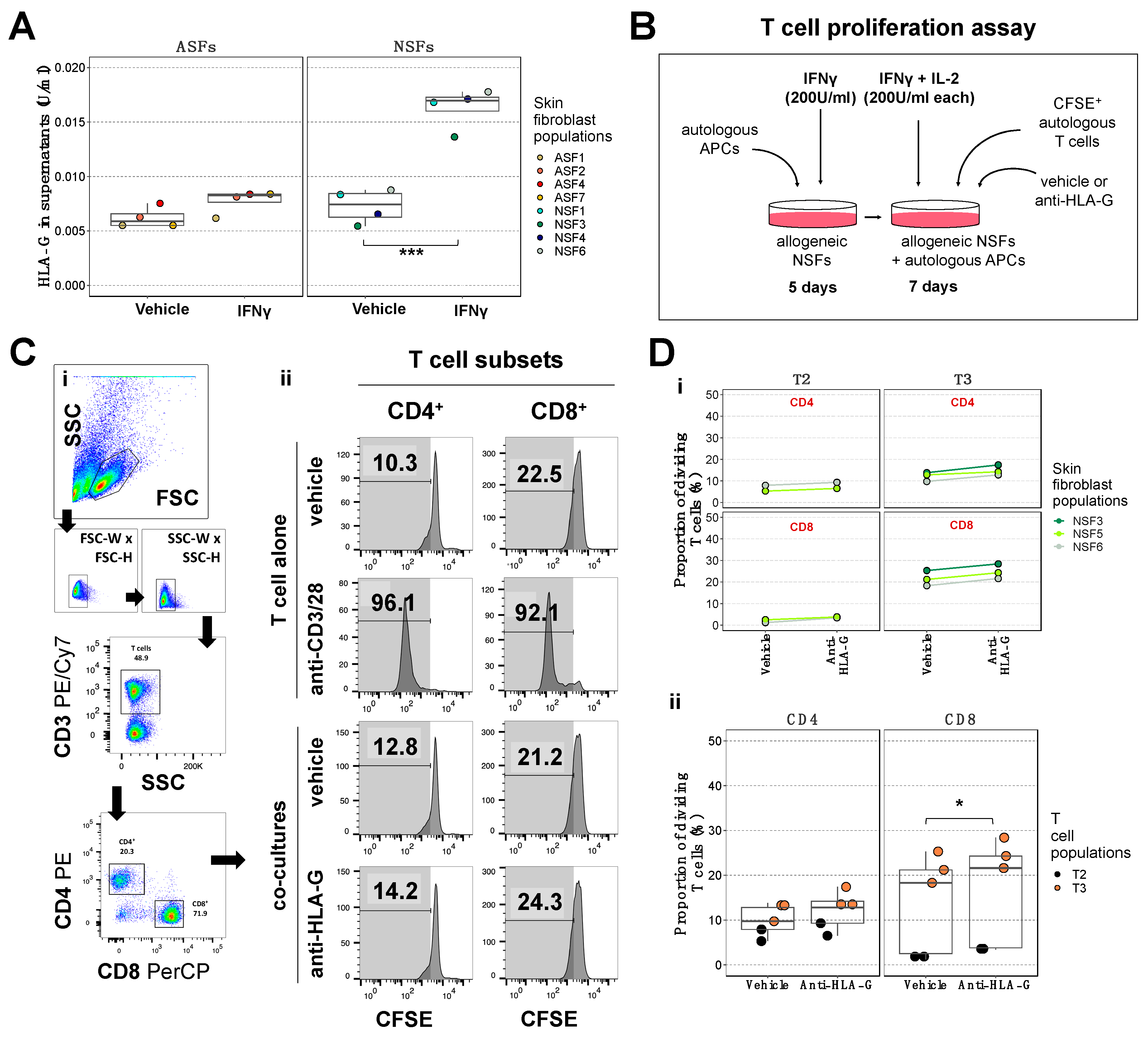
2.4. Blockade of the NSF-Secreted Immune Suppressive Cytokine HLA-G Does Not Reduce T Cell Proliferation
2.5. NSFs Display a Phenotype That Could Facilitate Immune Evasion in Response to Inflammation
3. Discussion
4. Materials and Methods
4.1. Skin Fibroblast Isolation and Culture
4.2. Peripheral Blood Mononuclear Cell Isolation, Culture, and Differentiation
4.3. Phagocytosis Assay
4.4. T Cell Proliferation Assay
4.5. Flow Cytometry
4.6. Immunofluorescence
4.7. Western Blots
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Germain, L.; Larouche, D.; Nedelec, B.; Perreault, I.; Duranceau, L.; Bortoluzzi, P.; Beaudoin Cloutier, C.; Genest, H.; Caouette-Laberge, L.; Dumas, A.; et al. Autologous Bilayered Self-Assembled Skin Substitutes (SASSs) as Permanent Grafts: A Case Series of 14 Severely Burned Patients Indicating Clinical Effectiveness. Eur. Cells Mater. 2018, 36, 128–141. [Google Scholar] [CrossRef]
- Burke, J.F.; Yannas, O.V.; Quinby, W.C.; Bondoc, C.C.; Jung, W.K. Successful Use of a Physiologically Acceptable Artificial Skin in the Treatment of Extensive Burn Injury. Ann. Surg. 1981, 194, 413–427. [Google Scholar] [CrossRef]
- Yannas, I.V.; Burke, J.F.; Orgill, D.P.; Skrabut, E.M. Wound Tissue Can Utilize a Polymeric Template to Synthesize a Functional Extension of Skin. Science 1982, 215, 174–176. [Google Scholar] [CrossRef]
- Yannas, I.V.; Orgill, D.P.; Burke, J.F. Template for Skin Regeneration. Plast. Reconstr. Surg. 2011, 127, 60–70. [Google Scholar] [CrossRef]
- Downer, M.; Berry, C.E.; Parker, J.B.; Kameni, L.; Griffin, M. Current Biomaterials for Wound Healing. Bioengineering 2023, 10, 1378. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Ahmadian, N.; Wheatley, S.; Alizadeh Sardroud, H.; Nasrollah, S.A.S.; Naseri, E.; Ahmadi, A. Advancements in 3D-Printable Polysaccharides, Proteins, and Synthetic Polymers for Wound Dressing and Skin Scaffolding—A Review. Int. J. Biol. Macromol. 2024, 266, 131207. [Google Scholar] [CrossRef]
- Brown, J.B.; McDowell, F. Massive Repairs of Burns With Thick Split-Skin Grafts. Ann. Surg. 1942, 115, 658–674. [Google Scholar] [CrossRef]
- Gibson, T.; Medawar, P.B. The Fate of Skin Homografts in Man. J. Anat. 1943, 77, 299–310.4. [Google Scholar]
- Rapaport, F.T.; Thomas, L.; Converse, J.M.; Lawrence, H.S. The Specificity of Skin Homograft Rejection in Man. Ann. N. Y. Acad. Sci. 1960, 87, 217–222. [Google Scholar] [CrossRef]
- Burt, A.M.; Pallett, C.D.; Sloane, J.P.; O’Hare, M.J.; Schafler, K.; Yardeni, P.; Eldad, A.; Clarke, J.A.; Gusterson, B.A. Survival of Cultured Allografts in Patients with Burns Assessed with Probe Specific for Y Chromosome. Br. Med. J. 1989, 298, 915–917. [Google Scholar] [CrossRef]
- Climov, M.; Matar, A.J.; Farkash, E.A.; Medeiros, E.; Qiao, J.; Harrington, E.; Gusha, A.; Al-Musa, A.; Sachs, D.H.; Randolph, M.; et al. Survival of Allogeneic Self-Assembled Cultured Skin. Transplantation 2016, 100, 2071–2078. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Mihm, M.C.; Dvorak, A.M.; Barnes, B.A.; Manseau, E.J.; Galli, S.J. Rejection of First-Set Skin Allografts in Man. the Microvasculature Is the Critical Target of the Immune Response. J. Exp. Med. 1979, 150, 322–337. [Google Scholar] [CrossRef]
- Larsen, C.P.; Steinman, R.M.; Witmer-Pack, M.; Hankins, D.F.; Morris, P.J.; Austyn, J.M. Migration and Maturation of Langerhans Cells in Skin Transplants and Explants. J. Exp. Med. 1990, 172, 1483–1493. [Google Scholar] [CrossRef]
- He, C.; Schenk, S.; Zhang, Q.; Valujskikh, A.; Bayer, J.; Fairchild, R.L.; Heeger, P.S. Effects of T Cell Frequency and Graft Size on Transplant Outcome in Mice. J. Immunol. 2004, 172, 240–247. [Google Scholar] [CrossRef]
- Rapaport, F.T.; Dausset, J. Behavior of HLA-Compatible and Incompatible Skin Allografts in Human Recipients Preimmunized with Pooled Leukocyte Extracts Obtained from Randomly Selected Donors. Transplantation 1983, 36, 592–594. [Google Scholar]
- Boyce, S.T.; Kagan, R.J.; Greenhalgh, D.G.; Warner, P.; Yakuboff, K.P.; Palmieri, T.; Warden, G.D. Cultured Skin Substitutes Reduce Requirements for Harvesting of Skin Autograft for Closure of Excised, Full-Thickness Burns. J. Trauma-Inj. Infect. Crit. Care 2006, 60, 821–829. [Google Scholar] [CrossRef]
- Larouche, D.; Cantin-Warren, L.; Desgagné, M.; Guignard, R.; Martel, I.; Ayoub, A.; Lavoie, A.; Gauvin, R.; Auger, F.A.; Moulin, V.J.; et al. Improved Methods to Produce Tissue-Engineered Skin Substitutes Suitable for the Permanent Closure of Full-Thickness Skin Injuries. Biores. Open Access 2016, 5, 320–329. [Google Scholar] [CrossRef]
- Parker, J.B.; Valencia, C.; Akras, D.; DiIorio, S.E.; Griffin, M.F.; Longaker, M.T.; Wan, D.C. Understanding Fibroblast Heterogeneity in Form and Function. Biomedicines 2023, 11, 2264. [Google Scholar] [CrossRef]
- Driskell, R.R.; Watt, F.M. Understanding Fibroblast Heterogeneity in the Skin. Trends Cell Biol. 2015, 25, 92–99. [Google Scholar] [CrossRef]
- Cavagnero, K.J.; Gallo, R.L. Essential Immune Functions of Fibroblasts in Innate Host Defense. Front. Immunol. 2022, 13, 1058862. [Google Scholar] [CrossRef]
- Buechler, M.B.; Pradhan, R.N.; Krishnamurty, A.T.; Cox, C.; Calviello, A.K.; Wang, A.W.; Yang, Y.A.; Tam, L.; Caothien, R.; Roose-Girma, M.; et al. Cross-Tissue Organization of the Fibroblast Lineage. Nature 2021, 593, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Proost, P.; Vynckier, A.K.; Mahieu, F.; Put, W.; Grillet, B.; Struyf, S.; Wuyts, A.; Opdenakker, G.; Van Damme, J. Microbial Toll-like Receptor Ligands Differentially Regulate CXCL10/IP-10 Expression in Fibroblasts and Mononuclear Leukocytes in Synergy with IFN-γ and Provide a Mechanism for Enhanced Synovial Chemokine Levels in Septic Arthritis. Eur. J. Immunol. 2003, 33, 3146–3153. [Google Scholar] [CrossRef] [PubMed]
- Farina, G.A.; York, M.R.; Di Marzio, M.; Collins, C.A.; Meller, S.; Homey, B.; Rifkin, I.R.; Marshak-Rothstein, A.; Radstake, T.R.D.J.; Lafyatis, R. Poly(I:C) Drives Type i IFN- and TGFΒ-Mediated Inflammation and Dermal Fibrosis Simulating Altered Gene Expression in Systemic Sclerosis. J. Investig. Dermatol. 2010, 130, 2583–2593. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, P.; Steurer, W.; Steiger, J.; Zheng, X.; Steele, A.W.; Strom, T.B. Cytokines and the Th1/Th2 Paradigm in Transplantation. Curr. Opin. Immunol. 1994, 6, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Strom, T.B.; Roy-Chaudhury, P.; Manfro, R.; Xheng, X.X.; Nickerson, P.W.; Wood, K.; Bushell, A. The Th1/Th2 Paradigm and the Allograft Response. Curr. Opin. Immunol. 1996, 8, 688–693. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, D.; Hu, Y.; Jiang, K.; Huang, H.; Du, Y.; Wu, W.; Wang, J.; Sui, J.; Wang, W.; et al. Anatomically Distinct Fibroblast Subsets Determine Skin Autoimmune Patterns. Nature 2022, 601, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yu, Q.; Zhang, H.; Wang, Z.; Zhang, T.; Xiang, J.; Yu, S.; Zhang, S.; Wu, H.; Xu, Y.; et al. A Resident Stromal Cell Population Actively Restrains Innate Immune Response in the Propagation Phase of Colitis Pathogenesis in Mice. Sci. Transl. Med. 2021, 13, eabb5071. [Google Scholar] [CrossRef] [PubMed]
- Vancheri, C.; Mastruzzo, C.; Tomaselli, V.; Sortino, M.A.; D’Amico, L.; Bellistrí, G.; Pistorio, M.P.; Salinaro, E.T.; Palermo, F.; Mistretta, A.; et al. Normal Human Lung Fibroblasts Differently Modulate Interleukin-10 and Interleukin-12 Production by Monocytes: Implications for an Altered Immune Response in Pulmonary Chronic Inflammation. Am. J. Respir. Cell Mol. Biol. 2001, 25, 592–599. [Google Scholar] [CrossRef]
- Hultman, C.S.; Brinson, G.M.; Siltharm, S.; DeSerres, S.; Cairns, B.A.; Peterson, H.D.; Meyer, A.A. Allogeneic Fibroblasts Used to Grow Cultured Epidermal Autografts Persist in Vivo and Sensitize the Graft Recipient for Accelerated Second-Set Rejection. J. Trauma-Inj. Infect. Crit. Care 1996, 41, 51–60. [Google Scholar] [CrossRef]
- Farrokhi, A.; Rahavi, M.; Jo, S.; Jalili, R.; Lim, C.J.; Ghahsary, A.; Reid, G.S. Inflammatory Immune Responses Trigger Rejection of Allogeneic Fibroblasts Transplanted into Mouse Skin. Cell Transplant. 2022, 31, 09636897221113803. [Google Scholar] [CrossRef]
- Hansbrough, J.F.; Doré, C.; Hansbrough, W.B. Clinical Trials of a Living Dermal Tissue Replacement Placed beneath Meshed, Split-Thickness Skin Grafts on Excised Burn Wounds. J. Burn Care Rehabil. 1992, 13, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Theobald, V.A.; Lauer, J.D.; Kaplan, F.A.; Baker, K.B.; Rosenberg, M. “Neutral Allografts”—Lack of Allogeneic Stimulation by Cultured Human Cells Expressing MHC Class I and Class II Antigens. Transplantation 1993, 55, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.L.; Rose, M.L.; Yacoub, M.H. Immunogenicity of Human Heart Valve Endothelial Cells and Fibroblasts. Transplant. Proc. 1997, 29, 984–985. [Google Scholar] [CrossRef] [PubMed]
- Maurer, D.H.; Collins, W.E.; Hanke, J.H.; Van, M.; Rich, R.R.; Pollack, M.S. Class II Positive Human Dermal Fibroblasts Restimulate Cloned Allospecific T Cells but Fail to Stimulate Primary Allogeneic Lymphoproliferation. Hum. Immunol. 1985, 14, 245–258. [Google Scholar] [CrossRef]
- Samsonov, D.; Geehan, C.; Woda, C.B.; Briscoe, D.M. Differential Activation of Human T Cells to Allogeneic Endothelial Cells, Epithelial Cells and Fibroblasts in Vitro. Transplant. Res. 2012, 1, 4. [Google Scholar] [CrossRef]
- Shimabukuro, Y.; Murakami, S.; Okada, H. Antigen-Presenting-Cell Function of Interferon γ-Treated Human Gingival Fibroblasts. J. Periodontal Res. 1996, 31, 217–228. [Google Scholar] [CrossRef]
- Benichou, G.; Yamada, Y.; Yun, S.; Lin, C.; Fray, M.; Tocco, G. Immune Recognition and Rejection of Allogeneic Skin Grafts. Immunotherapy 2011, 3, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.B.; Aßmann, C.U.; Girolomoni, G.; Ricciardi-Castagnoli, P. Different Cytokines Regulate Antigen Uptake and Presentation of a Precursor Dendritic Cell Line. Eur. J. Immunol. 1996, 26, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Banyer, J.L.; Halliday, D.C.T.; Thomson, S.A.; Hamilton, N.H.R. Combinations of IFN-γ and IL-4 Induce Distinct Profiles of Dendritic Cell-Associated Immunoregulatory Properties. Genes Immun. 2003, 4, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.E.; Wani, M.C. Camptothecin and Taxol: Discovery to Clinic—Thirteenth Bruce F. Cain Memorial Award Lecture. Cancer Res. 1995, 55, 753–760. [Google Scholar]
- Savill, J. Recognition and Phagocytosis of Cells Undergoing Apoptosis. Br. Med. Bull. 1997, 53, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Ochando, J.; Ordikhani, F.; Jordan, S.; Boros, P.; Thomson, A.W. Tolerogenic Dendritic Cells in Organ Transplantation. Transpl. Int. 2019, 33, 113–127. [Google Scholar] [CrossRef]
- Rouas-Freiss, N.; LeMaoult, J.; Moreau, P.; Dausset, J.; Carosella, E.D. HLA-G in Transplantation: A Relevant Molecule for Inhibition of Graft Rejection? Am. J. Transplant. 2003, 3, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Chapoval, A.I.; Ni, J.; Lau, J.S.; Wilcox, R.A.; Flies, D.B.; Liu, D.; Dong, H.; Sica, G.L.; Zhu, G.; Tamada, K.; et al. B7-H3: A Costimulatory Molecule for T Cell Activation and IFN-γ Production. Nat. Immunol. 2001, 2, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Cappellesso-Fleury, S.; Puissant-Lubrano, B.; Apoil, P.A.; Titeux, M.; Winterton, P.; Casteilla, L.; Bourin, P.; Blancher, A. Human Fibroblasts Share Immunosuppressive Properties with Bone Marrow Mesenchymal Stem Cells. J. Clin. Immunol. 2010, 30, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Leffell, M.S.; Steinberg, A.G.; Bias, W.B.; Machan, C.H.; Zachary, A.A. The Distribution of HLA Antigens and Phenotypes among Donors and Patients in the UNOS Registry. Transplantation 1994, 58, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Ting, A.; Edwards, L.B. Human Leukocyte Antigen in the Allocation of Kidneys from Cadaveric Donors in the United States. Transplantation 2004, 77, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.T.; Simpson, P.S.; Rieman, M.T.; Warner, P.M.; Yakuboff, K.P.; Bailey, J.K.; Nelson, J.K.; Fowler, L.A.; Kagan, R.J. Randomized, Paired-Site Comparison of Autologous Engineered Skin Substitutes and Split-Thickness Skin Graft for Closure of Extensive, Full-Thickness Burns. J. Burn Care Res. 2017, 38, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, A.; Galbraith, T.; Fauvel, C.M.; Khuong, H.T.; Berthod, F. Repair of Peripheral Nerve Injuries Using a Prevascularized Cell-Based Tissue-Engineered Nerve Conduit. Biomaterials 2022, 280, 121269. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, T.; Roy, V.; Bourget, J.M.; Tsutsumi, T.; Picard-Deland, M.; Morin, J.F.; Gauvin, R.; Ismail, A.A.; Auger, F.A.; Gros-Louis, F. Cell Seeding on UV-C-Treated 3D Polymeric Templates Allows for Cost-Effective Production of Small-Caliber Tissue-Engineered Blood Vessels. Biotechnol. J. 2019, 14, e1800306. [Google Scholar] [CrossRef]
- Dubé, J.; Bourget, J.M.; Gauvin, R.; Lafrance, H.; Roberge, C.J.; Auger, F.A.; Germain, L. Progress in Developing a Living Human Tissue-Engineered Tri-Leaflet Heart Valve Assembled from Tissue Produced by the Self-Assembly Approach. Acta Biomater. 2014, 10, 3563–3570. [Google Scholar] [CrossRef] [PubMed]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a Marker of Self on Red Blood Cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Jaiswal, S.; Chao, M.P.; Majeti, R.; Weissman, I.L. Macrophages as Mediators of Tumor Immunosurveillance. Trends Immunol. 2010, 31, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Hoek, R.H.; Ruuls, S.R.; Murphy, C.A.; Wright, G.J.; Goddard, R.; Zurawski, S.M.; Blom, B.; Homola, M.E.; Streit, W.J.; Brown, M.H.; et al. Down-Regulation of the Macrophage Lineage through Interaction with OX2 (CD200). Science 2000, 290, 1768–1771. [Google Scholar] [CrossRef]
- Lerbs, T.; Cui, L.; King, M.E.; Chai, T.; Muscat, C.; Chung, L.; Brown, R.; Rieger, K.; Shibata, T.; Wernig, G. CD47 Prevents the Elimination of Diseased Fibroblasts in Scleroderma. JCI Insight 2020, 5, e140458. [Google Scholar] [CrossRef]
- Tresini, M.; Pignolo, R.J.; Allen, R.G.; Cristofalo, V.J. Effects of Donor Age on the Expression of a Marker of Replicative Senescence (EPC-1) in Human Dermal Fibroblasts. J. Cell. Physiol. 1999, 179, 11–17. [Google Scholar] [CrossRef]
- Ressler, S.; Bartkova, J.; Niederegger, H.; Bartek, J.; Scharffetter-Kochanek, K.; Jansen-Dürr, P.; Wlaschek, M. P16INK4A Is a Robust in Vivo Biomarker of Cellular Aging in Human Skin. Aging Cell 2006, 5, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Popoola, J.; Khandwala, S.; Vadivel, N.; Vanguri, V.; Yuan, X.; Dada, S.; Guleria, I.; Tian, C.; Ansari, M.J.; et al. Critical Role of Donor Tissue Expression of Programmed Death Ligand-1 in Regulating Cardiac Allograft Rejection and Vasculopathy. Circulation 2008, 117, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Albin, M.J.; Yuan, X.; Yamaura, K.; Habicht, A.; Murayama, T.; Grimm, M.; Waaga, A.M.; Ueno, T.; Padera, R.F.; et al. PDL1 Is Required for Peripheral Transplantation Tolerance and Protection from Chronic Allograft Rejection. J. Immunol. 2007, 179, 5204–5210. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, Definitions, and Functions in Health and Disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef]
- Nunez, G.; Ball, E.J.; Stastny, P. Antigen Presentation by Adherent Cells from Human Peripheral Blood. Correlation between T-Cell Activation and Expression of HLA-DQ and -DR Antigens. Hum. Immunol. 1987, 19, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Kesikli, S.A.; Guc, D. Cancer Associated Fibroblasts Have Phenotypic and Functional Characteristics Similar to the Fibrocytes That Represent a Novel MDSC Subset. Oncoimmunology 2015, 4, e1034918. [Google Scholar] [CrossRef] [PubMed]
- Ngwenyama, N.; Kaur, K.; Bugg, D.; Theall, B.; Aronovitz, M.; Berland, R.; Panagiotidou, S.; Genco, C.; Perrin, M.A.; Davis, J.; et al. Antigen Presentation by Cardiac Fibroblasts Promotes Cardiac Dysfunction. Nat. Cardiovasc. Res. 2022, 1, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Piskurich, J.F.; Gilbert, C.A.; Ashley, B.D.; Zhao, M.; Chen, H.; Wu, J.; Bolick, S.C.; Wright, K.L. Expression of the MHC Class II Transactivator (CIITA) Type IV Promoter in B Lymphocytes and Regulation by IFN-γ. Mol. Immunol. 2006, 43, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Goyer, B.; Larouche, D.; Kim, D.H.; Veillette, N.; Pruneau, V.; Bernier, V.; Auger, F.A.; Germain, L. Immune Tolerance of Tissue-Engineered Skin Produced with Allogeneic or Xenogeneic Fibroblasts and Syngeneic Keratinocytes Grafted on Mice. Acta Biomater. 2019, 92, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Gauglitz, G.G.; Kulp, G.A.; Finnerty, C.C.; Williams, F.N.; Kraft, R.; Suman, O.E.; Mlcak, R.P.; Herndon, D.N. Long-Term Persistance of the Pathophysiologic Response to Severe Burn Injury. PLoS ONE 2011, 6, e21245. [Google Scholar] [CrossRef]
- Hu, S.; Kirsner, R.S.; Falanga, V.; Phillips, T.; Eaglstein, W.H. Evaluation of Apligraf® Persistence and Basement Membrane Restoration in Donor Site Wounds: A Pilot Study. Wound Repair Regen. 2006, 14, 427–433. [Google Scholar] [CrossRef]
- Centanni, J.M.; Straseski, J.A.; Wicks, A.; Hank, J.A.; Rasmussen, C.A.; Lokuta, M.A.; Schurr, M.J.; Foster, K.N.; Faucher, L.D.; Caruso, D.M.; et al. Stratagraft Skin Substitute Is Well-Tolerated and Is Not Acutely Immunogenic in Patients with Traumatic Wounds: Results from a Prospective, Randomized, Controlled Dose Escalation Trial. Ann. Surg. 2011, 253, 672–683. [Google Scholar] [CrossRef]

| Cell Type | Identifier | Source | Sex | Age | Code | Color |
|---|---|---|---|---|---|---|
| Fibroblast | FAabF2146X | Abdomen | F | 46 | ASF1 | khaki |
| FAkI2064X | Abdomen | F | 64 | ASF2 | coral | |
| FArJ2050X | Abdomen | F | 50 | ASF3 | brown | |
| FAyE2147X | Abdomen | F | 47 | ASF4 | red | |
| FAyB2143X | Abdomen | F | 43 | ASF5 | pink | |
| FMaaH1369X | Breast | F | 69 | ASF6 | violet | |
| FMqC1245X | Breast | F | 45 | ASF7 | yellow | |
| FPEccG170.1Y | Foreskin | M | <1 | NSF1 | turquoise | |
| FPEgI10.1Y | Foreskin | M | <1 | NSF2 | sky blue | |
| FPEsE130.1Y | Foreskin | M | <1 | NSF3 | dark green | |
| FPEvC200.1Y | Foreskin | M | <1 | NSF4 | navy blue | |
| FPEzE130.1Y | Foreskin | M | <1 | NSF5 | light green | |
| FPEeeH170.2Y | Foreskin | M | <1 | NSF6 | grey | |
| PBMC | SAabF2146XT | Blood | F | 46 | T1 | white |
| SAabF2146XA | Blood | F | 46 | APC1 | white | |
| SAkI2064XT | Blood | F | 64 | T2 | black | |
| SAkI2064XA | Blood | F | 64 | APC2 | black | |
| SArJ2050XT | Blood | F | 50 | T3 | orange | |
| SArJ2050XA | Blood | F | 50 | APC3 | orange | |
| SAyE2147XT | Blood | F | 47 | T4 | lilac | |
| SAyE2147XA | Blood | F | 47 | APC4 | lilac |
| Use | Target | Clone | Specie | Conjugate | Supplier | Cat. # | Dilution |
|---|---|---|---|---|---|---|---|
| FC | CD3 | UCHT-1 | Ms | PE/Cy7 | BD, Mississauga, CA, USA | 563423 | 1/20 |
| CD3 | HIT3a | Ms | BB700 | BD | 742207 | 1/20 | |
| CD4 | OKT4 | Ms | PE | Biolegend, San Diego, CA, USA | 317410 | 1/20 | |
| CD8 | SK1 | Ms | PerCP | Biolegend | 344708 | 1/20 | |
| CD11c | 3.9 | Ms | FITC | Biolegend | 301604 | 1/20 | |
| CD14 | M5E2 | Ms | PE | Biolegend | 301806 | 1/20 | |
| CD45 | 2D1 | Ms | PE | Biolegend | 368510 | 1/20 | |
| HLADR | L243 | Ms | APC | Biolegend | 307610 | 1/20 | |
| HLADR | L243 | Ms | PE/Cy7 | BD | 560651 | 1/20 | |
| N | HLA-G | 87G | Ms | - | Thermofisher, Ottawa, ON, Canada | MA110356 | 2 µg/mL |
| IF | CD45 | 2D1 | Ms | PE | Biolegend | 368510 | 1/200 |
| WB | HLAABCE | TP2599SF | Ms | - | Novus Bio, Littleton, CO, USA | NBP268006 | 1/500 |
| HLADPDQDR | CR3/43 | Ms | - | Abcam, Cambridge, MA, USA | ab7856 | 1/500 | |
| B7-H3 | D9M2L | Rb | - | Cell Signaling, Cambridge, MA, USA | 14058S | 1/1000 | |
| α-Tubulin | DM1A | Ms | - | Sigma, St. Louis, MO, USA | T9026 | 1/2000 | |
| Rabbit | - | Gt | HRP | Invitrogen, Carlsbad, CA, USA | 62-6120 | 1/5000 | |
| Mouse | - | Gt | HRP | Invitrogen | 62-6520 | 1/5000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magne, B.; Ferland, K.; Savard, É.; Barbier, M.A.; Morissette, A.; Larouche, D.; Beaudoin-Cloutier, C.; Germain, L. The Human Neonatal Skin Fibroblast, an Available Cell Source for Tissue Production and Transplantation, Exhibits Low Risk of Immunogenicity In Vitro. Int. J. Mol. Sci. 2024, 25, 6965. https://doi.org/10.3390/ijms25136965
Magne B, Ferland K, Savard É, Barbier MA, Morissette A, Larouche D, Beaudoin-Cloutier C, Germain L. The Human Neonatal Skin Fibroblast, an Available Cell Source for Tissue Production and Transplantation, Exhibits Low Risk of Immunogenicity In Vitro. International Journal of Molecular Sciences. 2024; 25(13):6965. https://doi.org/10.3390/ijms25136965
Chicago/Turabian StyleMagne, Brice, Karel Ferland, Étienne Savard, Martin A. Barbier, Amélie Morissette, Danielle Larouche, Chanel Beaudoin-Cloutier, and Lucie Germain. 2024. "The Human Neonatal Skin Fibroblast, an Available Cell Source for Tissue Production and Transplantation, Exhibits Low Risk of Immunogenicity In Vitro" International Journal of Molecular Sciences 25, no. 13: 6965. https://doi.org/10.3390/ijms25136965
APA StyleMagne, B., Ferland, K., Savard, É., Barbier, M. A., Morissette, A., Larouche, D., Beaudoin-Cloutier, C., & Germain, L. (2024). The Human Neonatal Skin Fibroblast, an Available Cell Source for Tissue Production and Transplantation, Exhibits Low Risk of Immunogenicity In Vitro. International Journal of Molecular Sciences, 25(13), 6965. https://doi.org/10.3390/ijms25136965







