Overexpression of Liriodendron Hybrid LhGLK1 in Arabidopsis Leads to Excessive Chlorophyll Synthesis and Improved Growth
Abstract
1. Introduction
2. Results
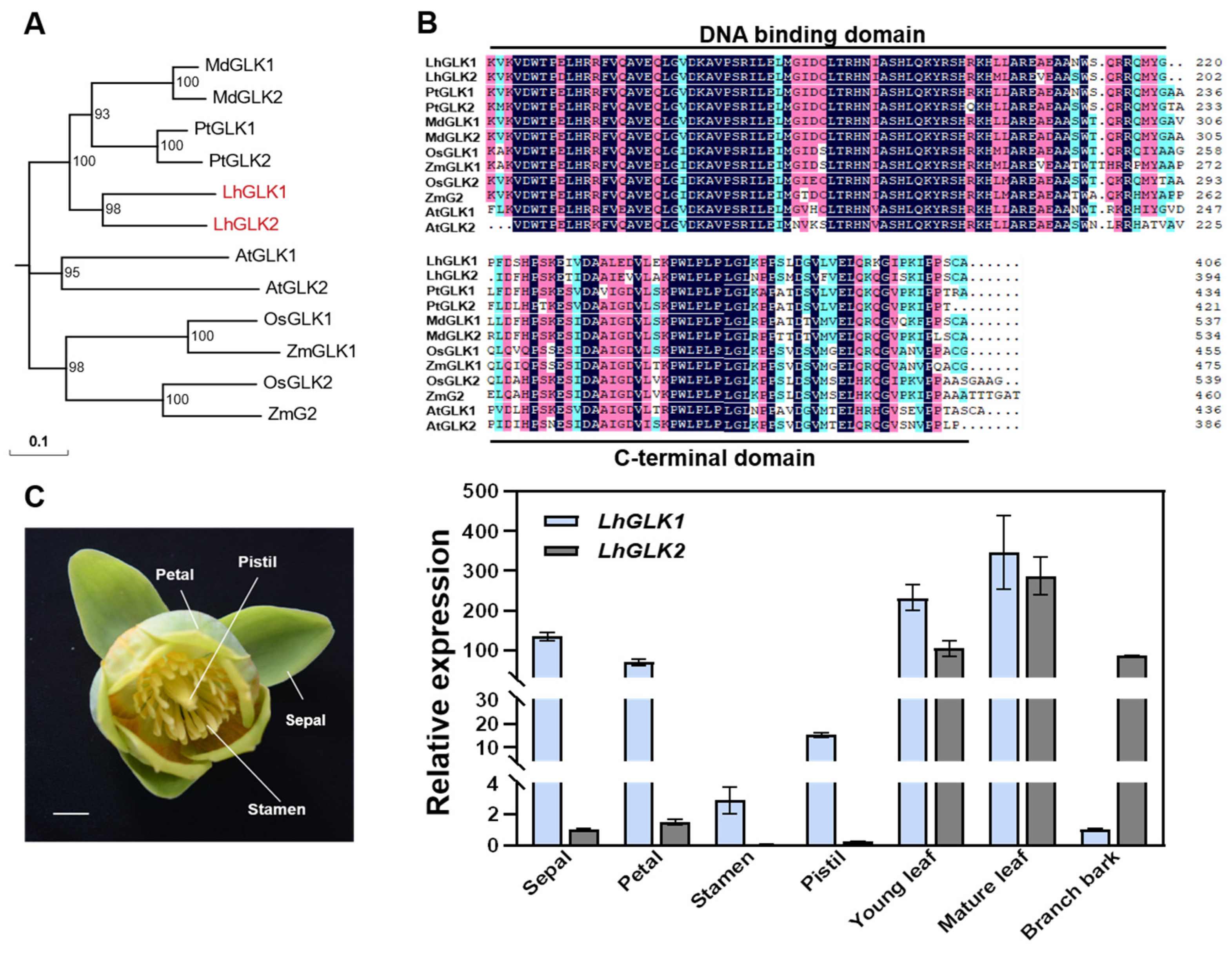
2.1. Identification and Tissue Expression Pattern Analysis of LhGLKs
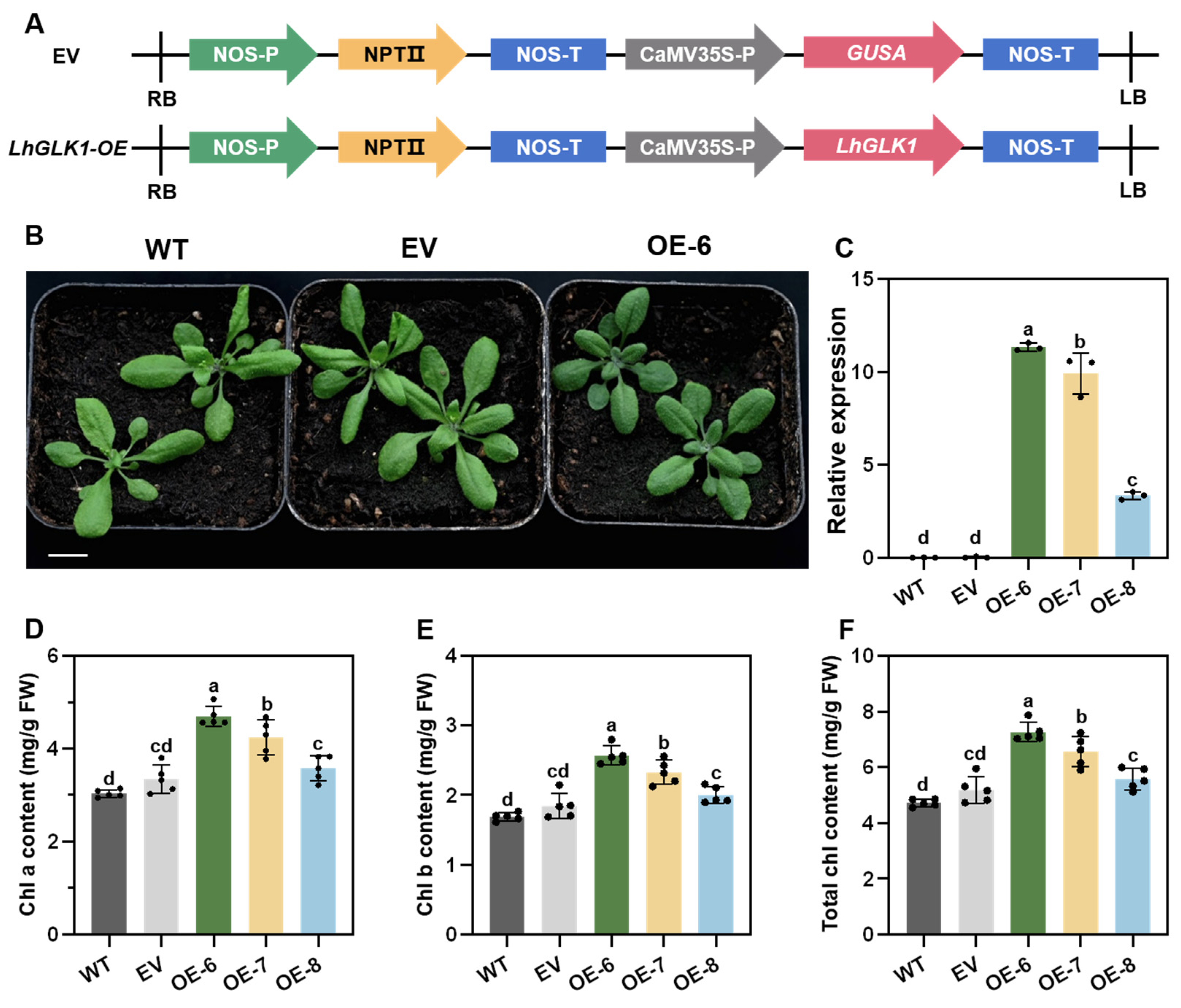
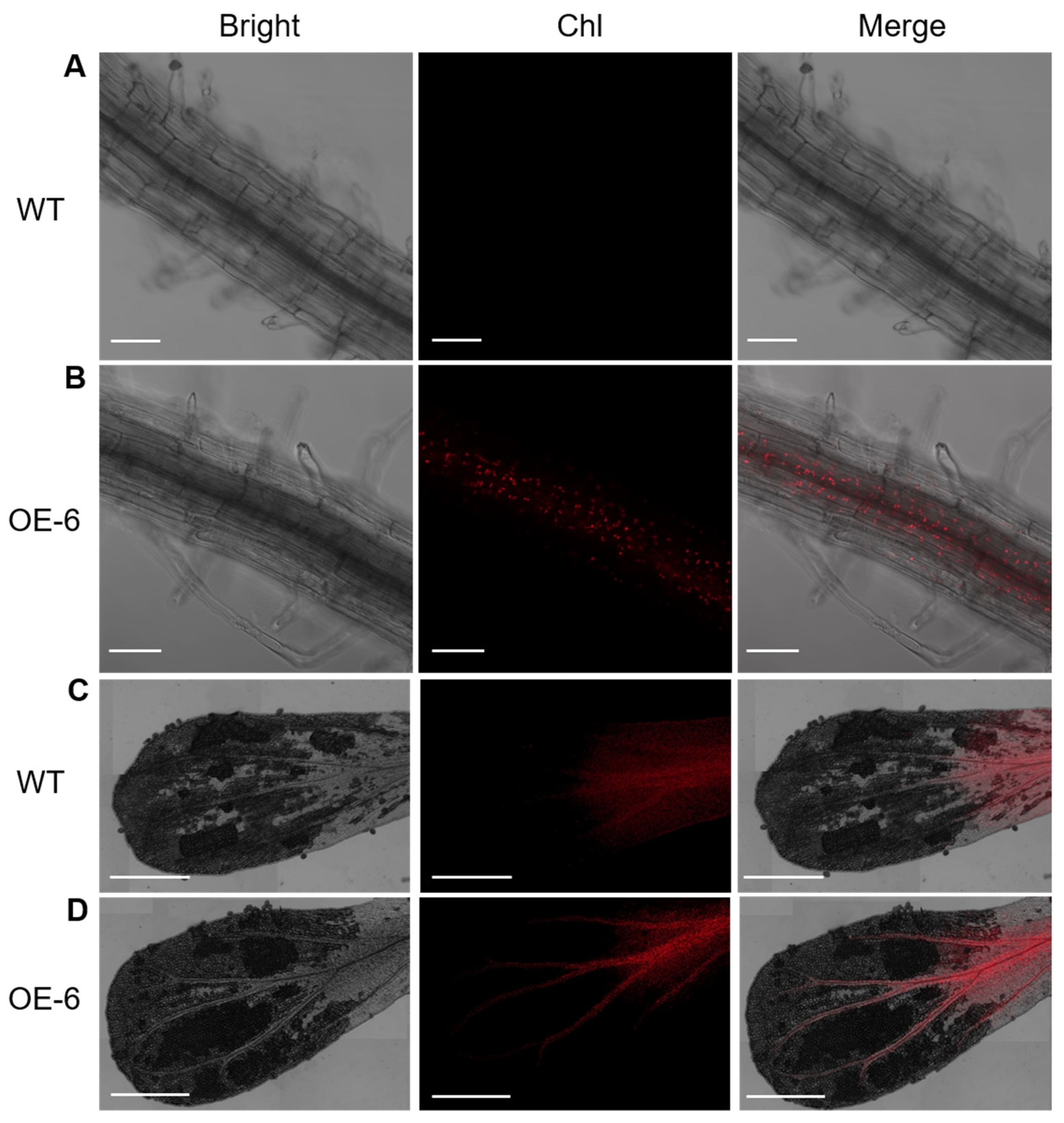
2.2. Overexpression of LhGLK1 in Arabidopsis Leads to More Chlorophyll Accumulation and Promotes Chloroplast Formation in Non-Green Tissue
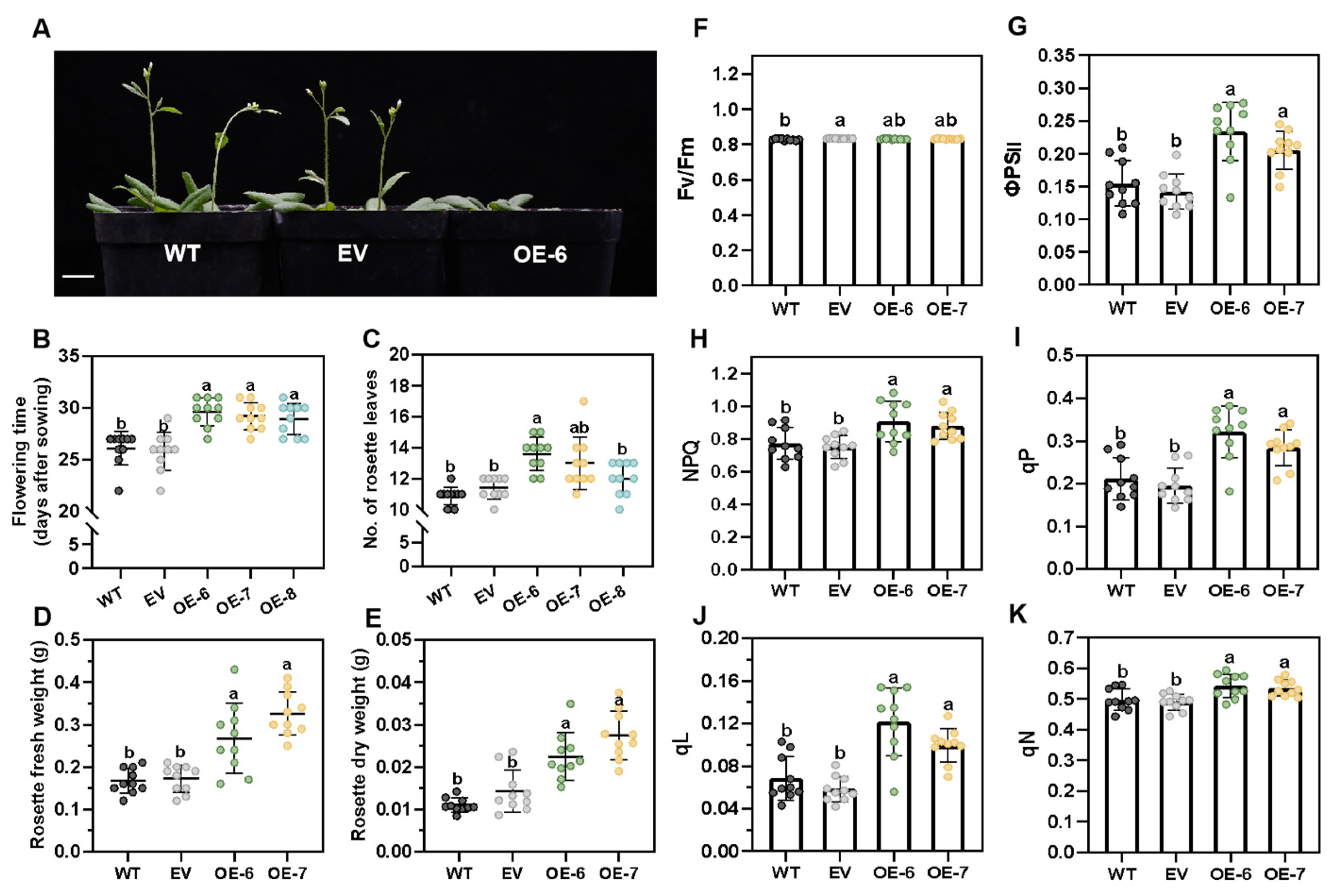
2.3. LhGLK1 Overexpression Changes Photosynthetic Characteristics and Flowering Time and Improved Growth
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Generation of Overexpressing LhGLK1 Lines
4.3. Identification of the LhGLK Genes
4.4. Transmission Electron Microscopy
4.5. qRT-PCR Analysis
4.6. Western Blot Analysis
4.7. Chlorophyll Content and Measurement
4.8. Chlorophyll Autofluorescence Analysis
4.9. Flowering Time and Rosette Leaf Mass Measurements
4.10. Chlorophyll Fluorescence Measurement
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fitter, D.W.; Martin, D.J.; Copley, M.J.; Scotland, R.W.; Langdale, J.A. GLK gene pairs regulate chloroplast development in diverse plant species. Plant J. 2002, 31, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Yasumura, Y.; Moylan, E.C.; Langdale, J.A. A Conserved Transcription Factor Mediates Nuclear Control of Organelle Biogenesis in Anciently Diverged Land Plants. Plant Cell 2005, 17, 1894–1907. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.T.; Moylan, E.C.; Langdale, J.A. GLK transcription factors regulate chloroplast development in a cell-autonomous manner. Plant J. 2008, 56, 432–444. [Google Scholar] [CrossRef]
- Nakamura, H.; Muramatsu, M.; Hakata, M.; Ueno, O.; Nagamura, Y.; Hirochika, H.; Takano, M.; Ichikawa, H. Ectopic Overexpression of The Transcription Factor OsGLK1 Induces Chloroplast Development in Non-Green Rice Cells. Plant Cell Physiol. 2009, 50, 1933–1949. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.T.; Langdale, J.A. The making of a chloroplast. Embo J. 2009, 28, 2861–2873. [Google Scholar] [CrossRef]
- Li, M.; Lee, K.P.; Liu, T.; Dogra, V.; Duan, J.; Li, M.; Xing, W.; Kim, C. Antagonistic modules regulate photosynthesis-associated nuclear genes via GOLDEN2-LIKE transcription factors. Plant Physiol. 2022, 188, 2308–2324. [Google Scholar] [CrossRef]
- Zubo, Y.O.; Blakley, I.C.; Franco-Zorrilla, J.M.; Yamburenko, M.V.; Solano, R.; Kieber, J.J.; Loraine, A.E.; Schaller, G.E. Coordination of Chloroplast Development through the Action of the GNC and GLK Transcription Factor Families. Plant Physiol. 2018, 178, 130–147. [Google Scholar] [CrossRef] [PubMed]
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.J.; Samaha, R.R.; et al. Arabidopsis Transcription Factors: Genome-Wide Comparative Analysis among Eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef]
- Chen, M.; Ji, M.; Wen, B.; Liu, L.; Li, S.; Chen, X.; Gao, D.; Li, L. GOLDEN 2-LIKE Transcription Factors of Plants. Front. Plant Sci. 2016, 7, 1509. [Google Scholar] [CrossRef]
- Jenkins, M.T. A Second Gene Producing Golden Plant Color in Maize. Am. Nat. 1926, 60, 484–488. [Google Scholar] [CrossRef]
- Hall, L.N.; Rossini, L.; Cribb, L.; Langdale, J.A. GOLDEN 2: A Novel Transcriptional Regulator of Cellular Differentiation in the Maize Leaf. Plant Cell 1998, 10, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Langdale, J.A.; Kidner, C.A. bundle sheath defective, a mutation that disrupts cellular differentiation in maize leaves. Development 1994, 120, 673–681. [Google Scholar] [CrossRef]
- Rossini, L.; Cribb, L.; Martin, D.J.; Langdale, J.A. The Maize Golden2 Gene Defines a Novel Class of Transcriptional Regulators in Plants. Plant Cell 2001, 13, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.-P.; Wu, P.-J.; Zhang, T.-Y.; Song, H.-L.; Zhang, Y.-F.; Chen, J.-F.; Yue, C.-P.; Huang, J.-Y.; Sun, T.; Zhou, T. Genome-Scale Investigation of GARP Family Genes Reveals Their Pivotal Roles in Nutrient Stress Resistance in Allotetraploid Rapeseed. Int. J. Mol. Sci. 2022, 23, 14484. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Zhu, J.; Wang, Z. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat. Cell Biol. 2012, 14, 802–809. [Google Scholar] [CrossRef]
- Schreiber, K.J.; Nasmith, C.G.; Allard, G.; Singh, J.; Subramaniam, R.; Desveaux, D. Found in Translation: High-Throughput Chemical Screening in Arabidopsis thaliana Identifies Small Molecules That Reduce Fusarium Head Blight Disease in Wheat. Mol. Plant-Microbe Interact.® 2011, 24, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Savitch, L.V.; Subramaniam, R.; Allard, G.C.; Singh, J. The GLK1 ‘regulon’ encodes disease defense related proteins and confers resistance to Fusarium graminearum in Arabidopsis. Biochem. Biophys. Res. Commun. 2007, 359, 234–238. [Google Scholar] [CrossRef]
- Hosoda, K.; Imamura, A.; Katoh, E.; Hatta, T.; Tachiki, M.; Yamada, H.; Mizuno, T.; Yamazaki, T. Molecular Structure of the GARP Family of Plant Myb-Related DNA Binding Motifs of the Arabidopsis Response Regulators. Plant Cell 2002, 14, 2015–2029. [Google Scholar] [CrossRef]
- Safi, A.; Medici, A.; Szponarski, W.; Ruffel, S.; Lacombe, B.; Krouk, G. The world according to GARP transcription factors. Curr. Opin. Plant Biol. 2017, 39, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.T.; Wang, P.; Korkaric, M.; Capper, R.G.; Saunders, N.J.; Langdale, J.A. GLK Transcription Factors Coordinate Expression of the Photosynthetic Apparatus in Arabidopsis. Plant Cell 2009, 21, 1109–1128. [Google Scholar] [CrossRef]
- Larkin, R.M.; Alonso, J.M.; Ecker, J.R.; Chory, J. GUN4, a Regulator of Chlorophyll Synthesis and Intracellular Signaling. Science 2003, 299, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, P.; Li, J.; Wei, S.; Yan, Y.; Yang, J.; Zhao, M.; Langdale, J.A.; Zhou, W. Maize GOLDEN2-LIKE genes enhance biomass and grain yields in rice by improving photosynthesis and reducing photoinhibition. Commun. Biol. 2020, 3, 151. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.V.; Vrebalov, J.T.; Gapper, N.E.; Zheng, Y.; Zhong, S.; Fei, Z.; Giovannoni, J.J. Tomato GOLDEN2-LIKE Transcription Factors Reveal Molecular Gradients That Function during Fruit Development and Ripening. Plant Cell 2014, 26, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.-Y.; Lin, H.-H.; Chang, Y.-M.; Chang, Y.-L.; Chang, C.-K.; Huang, Y.-C.; Ho, Y.-W.; Lin, C.-Y.; Zheng, J.-Z.; Jane, W.-N.; et al. Maize Golden2-like transcription factors boost rice chloroplast development, photosynthesis, and grain yield. Plant Physiol. 2022, 188, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, X.; Zhang, S.; Xie, X.; Li, M.; Liu, Y.; Wang, S. Tea GOLDEN2-LIKE genes enhance catechin biosynthesis through activating R2R3-MYB transcription factor. Hortic. Res. 2022, 9, uhac117. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Sasaki, D.; Noguchi, K.; Fujinuma, D.; Komatsu, H.; Kobayashi, M.; Sato, M.; Toyooka, K.; Sugimoto, K.; Niyogi, K.K.; et al. Photosynthesis of Root Chloroplasts Developed in Arabidopsis Lines Overexpressing GOLDEN2-LIKE Transcription Factors. Plant Cell Physiol. 2013, 54, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.L.T.; Nguyen, C.V.; Hill, T.; Cheng, K.L.; Figueroa-Balderas, R.; Aktas, H.; Ashrafi, H.; Pons, C.; Fernández-Muñoz, R.; Vicente, A.; et al. Uniform ripening Encodes a Golden 2-like Transcription Factor Regulating Tomato Fruit Chloroplast Development. Science 2012, 336, 1711–1715. [Google Scholar] [CrossRef]
- Bravo-Garcia, A.; Yasumura, Y.; Langdale, J.A. Specialization of the Golden2-like regulatory pathway during land plant evolution. New Phytol. 2009, 183, 133–141. [Google Scholar] [CrossRef]
- Tu, X.; Ren, S.; Shen, W.; Li, J.; Li, Y.; Li, C.; Li, Y.; Zong, Z.; Xie, W.; Grierson, D.; et al. Limited conservation in cross-species comparison of GLK transcription factor binding suggested wide-spread cistrome divergence. Nat. Commun. 2022, 13, 7632. [Google Scholar] [CrossRef]
- Li, M.; Wang, D.; Long, X.; Hao, Z.; Lu, Y.; Zhou, Y.; Peng, Y.; Cheng, T.; Shi, J.; Chen, J. Agrobacterium-Mediated Genetic Transformation of Embryogenic Callus in a Liriodendron Hybrid (L. chinense × L. tulipifera). Front. Plant Sci. 2022, 13, 802128. [Google Scholar] [CrossRef]
- Chen, J.; Hao, Z.; Guang, X.; Zhao, C.; Wang, P.; Xue, L.; Zhu, Q.; Yang, L.; Sheng, Y.; Zhou, Y.; et al. Liriodendron genome sheds light on angiosperm phylogeny and species–pair differentiation. Nat. Plants 2019, 5, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Gang, H.; Li, R.; Zhao, Y.; Liu, G.; Chen, S.; Jiang, J. Loss of GLK1 transcription factor function reveals new insights in chlorophyll biosynthesis and chloroplast development. J. Exp. Bot. 2019, 70, 3125–3138. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ma, X.; Hao, Z.; Long, X.; Shi, J.; Chen, J. Overexpression of Liriodenron WOX5 in Arabidopsis Leads to Ectopic Flower Formation and Altered Root Morphology. Int. J. Mol. Sci. 2023, 24, 906. [Google Scholar] [CrossRef] [PubMed]
- Barton, N.H.; Keightley, P.D. Understanding quantitative genetic variation. Nat. Rev. Genet. 2002, 3, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Lan, J.; Yu, H.; Zhang, J.; Zhang, Y.; Qin, Y.; Su, X.-D.; Qin, G. Arabidopsis transcription factor TCP4 represses chlorophyll biosynthesis to prevent petal greening. Plant Commun. 2022, 3, 100309. [Google Scholar] [CrossRef] [PubMed]
- López-Juez, E. Plastid biogenesis, between light and shadows. J. Exp. Bot. 2007, 58, 11–26. [Google Scholar] [CrossRef]
- Kobayashi, K.; Narise, T.; Sonoike, K.; Hashimoto, H.; Sato, N.; Kondo, M.; Nishimura, M.; Sato, M.; Toyooka, K.; Sugimoto, K.; et al. Role of galactolipid biosynthesis in coordinated development of photosynthetic complexes and thylakoid membranes during chloroplast biogenesis in Arabidopsis. Plant J. 2013, 73, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.J. Consistent Sets of Spectrophotometric Chlorophyll Equations for Acetone, Methanol and Ethanol Solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Shihan, M.H.; Novo, S.G.; Le Marchand, S.J.; Wang, Y.; Duncan, M.K. A simple method for quantitating confocal fluorescent images. Biochem. Biophys. Rep. 2021, 25, 100916. [Google Scholar] [CrossRef] [PubMed]
- Boyes, D.C.; Zayed, A.M.; Ascenzi, R.; McCaskill, A.J.; Hoffman, N.E.; Davis, K.R.; Görlach, J. Growth Stage–Based Phenotypic Analysis of Arabidopsis: A Model for High Throughput Functional Genomics in Plants. Plant Cell 2001, 13, 1499–1510. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, H.; Liang, S.; Hu, L.; Yu, L.; Liang, P.; Hao, Z.; Peng, Y.; Yang, J.; Shi, J.; Chen, J. Overexpression of Liriodendron Hybrid LhGLK1 in Arabidopsis Leads to Excessive Chlorophyll Synthesis and Improved Growth. Int. J. Mol. Sci. 2024, 25, 6968. https://doi.org/10.3390/ijms25136968
Qu H, Liang S, Hu L, Yu L, Liang P, Hao Z, Peng Y, Yang J, Shi J, Chen J. Overexpression of Liriodendron Hybrid LhGLK1 in Arabidopsis Leads to Excessive Chlorophyll Synthesis and Improved Growth. International Journal of Molecular Sciences. 2024; 25(13):6968. https://doi.org/10.3390/ijms25136968
Chicago/Turabian StyleQu, Haoxian, Shuang Liang, Lingfeng Hu, Long Yu, Pengxiang Liang, Zhaodong Hao, Ye Peng, Jing Yang, Jisen Shi, and Jinhui Chen. 2024. "Overexpression of Liriodendron Hybrid LhGLK1 in Arabidopsis Leads to Excessive Chlorophyll Synthesis and Improved Growth" International Journal of Molecular Sciences 25, no. 13: 6968. https://doi.org/10.3390/ijms25136968
APA StyleQu, H., Liang, S., Hu, L., Yu, L., Liang, P., Hao, Z., Peng, Y., Yang, J., Shi, J., & Chen, J. (2024). Overexpression of Liriodendron Hybrid LhGLK1 in Arabidopsis Leads to Excessive Chlorophyll Synthesis and Improved Growth. International Journal of Molecular Sciences, 25(13), 6968. https://doi.org/10.3390/ijms25136968





