Effective and Successful Quantification of Leukemia-Specific Immune Cells in AML Patients’ Blood or Culture, Focusing on Intracellular Cytokine and Degranulation Assays
Abstract
:1. Introduction
1.1. Current Therapy Strategies for Acute Myeloid Leukemia (AML)
1.2. Dendritic Cells for Immune Therapy
1.3. Innate and Adaptive Immune Systems
1.4. Intracellular Cytokine Assay (INCYT), Degranulation Assay (DEG), and Cytotoxicity Fluorolysis Assay (CTX)
1.5. Aims of This Study
2. Results
2.1. Prologue
2.2. Effects of LAA-/SEB-Stimulation in Uncultured (D0) WB (AML and Healthy) on the Intracellular IFNy-Production and Degranulation (CD107a) Positivity of Immune Cell Subtypes
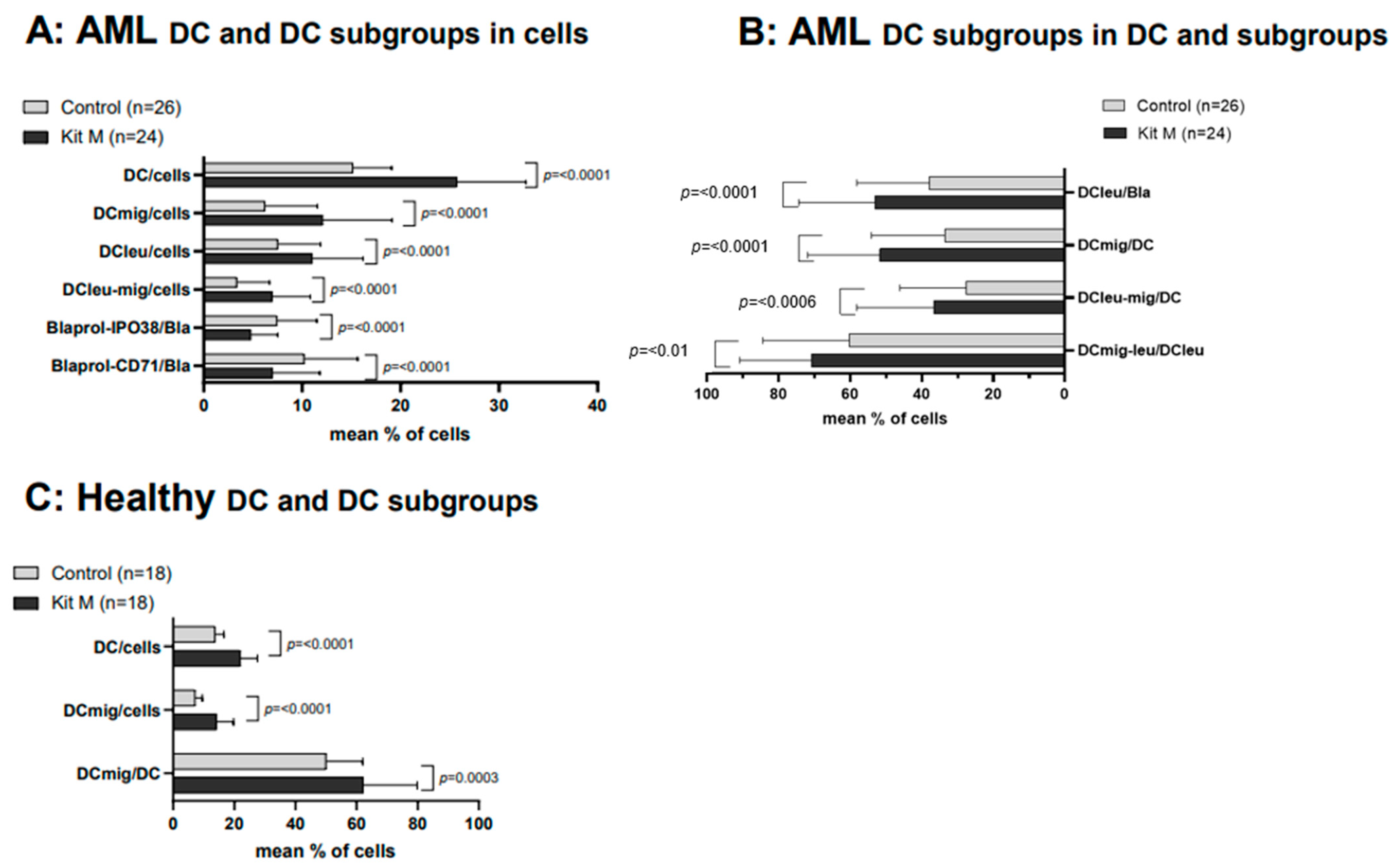
2.3. Effects of Kit M on the Generation of DC/DCleu and Blast Proliferation Using AML and Healthy WB
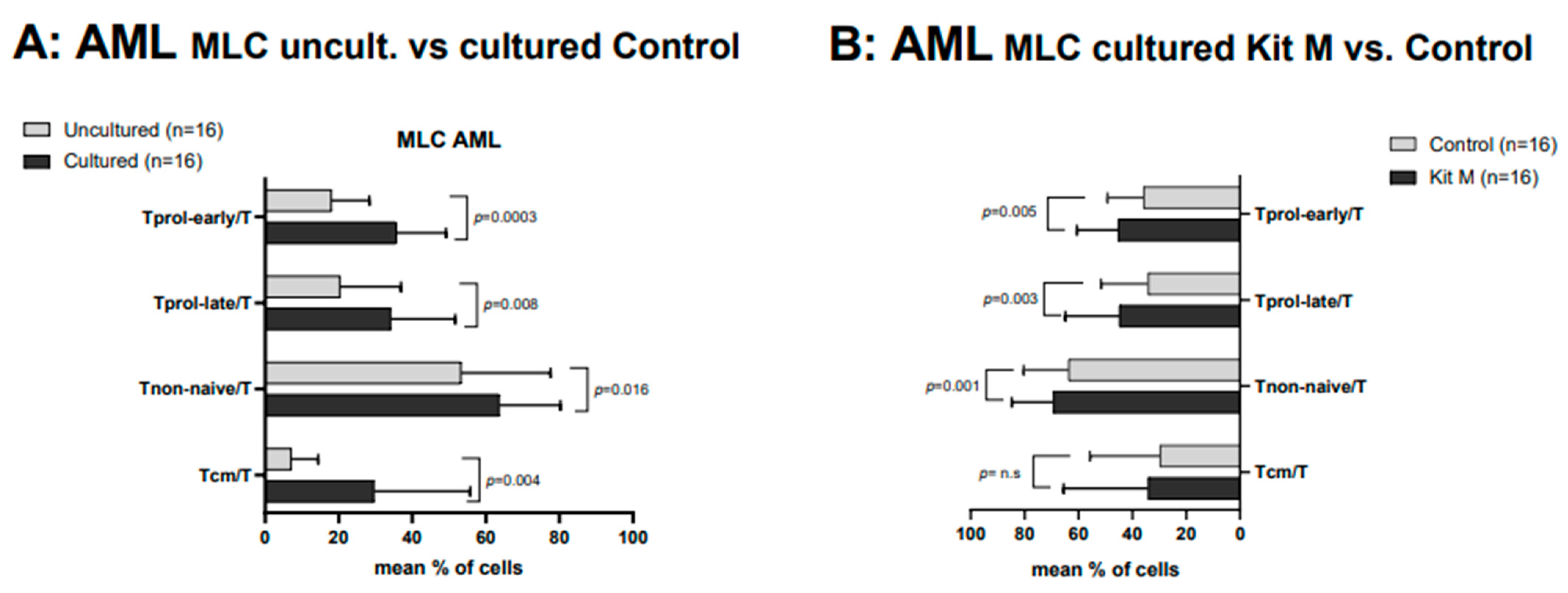
2.4. Effects of Kit M-Treated vs. Untreated AML WB before and after T Cell-Enriched MLC on Immunoreactive Cells
2.5. Composition of IFNy- and TNFa-Producing Immune-Reactive Cells after T Cell-Enriched MLC, Using Leukemic WB with and without (Control) Kit-Pretreatment (Kit M) and with (+LAA) and without (-LAA) LAA-Stimulation
2.6. Effects of Kit M-Treated vs. Untreated AML WB on the Degranulation of Immune Cells after MLC
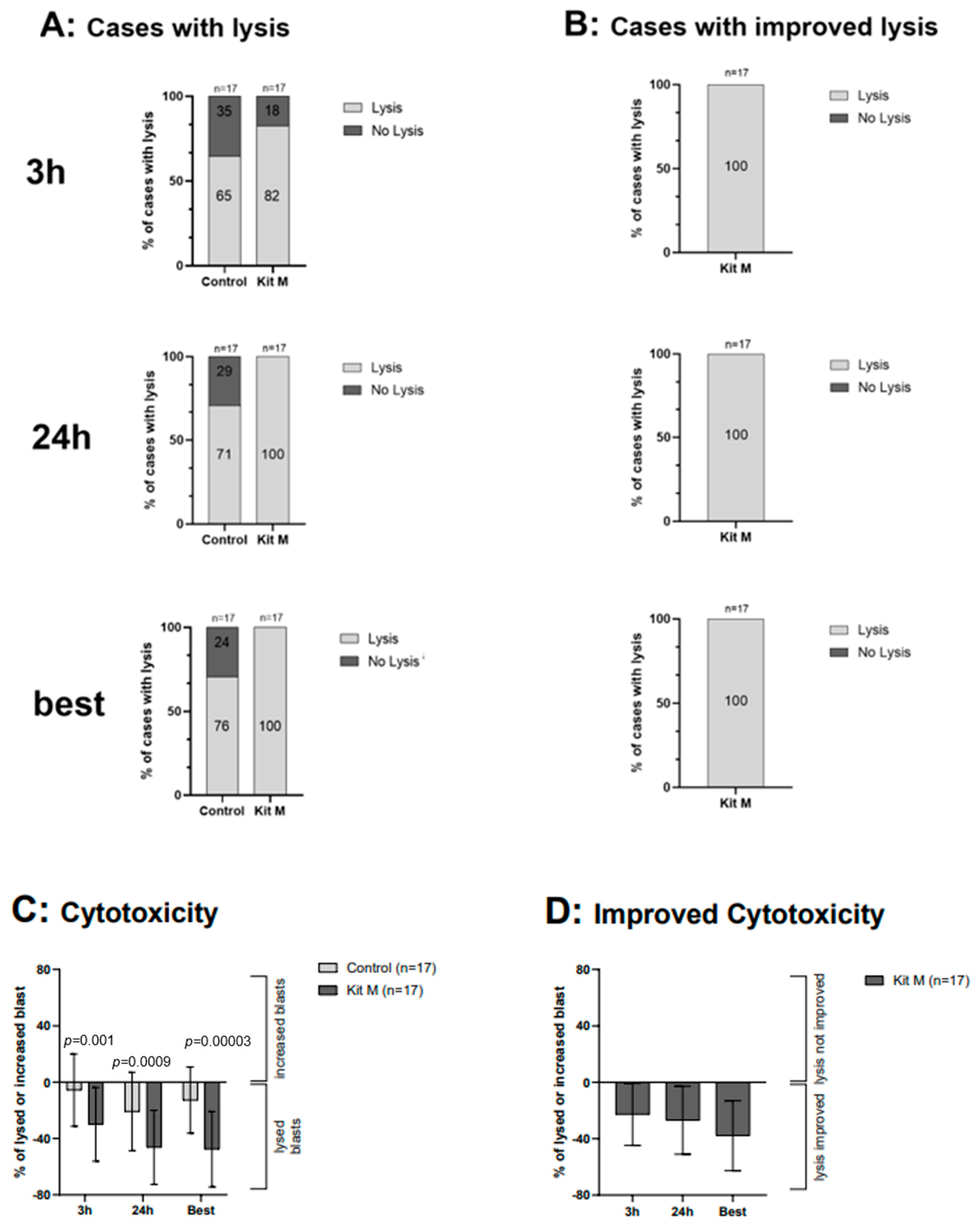
2.7. Effects of Kit M-Treated WB on the Antileukemic and Blastolytic Activity after MLC, Detected via CTX
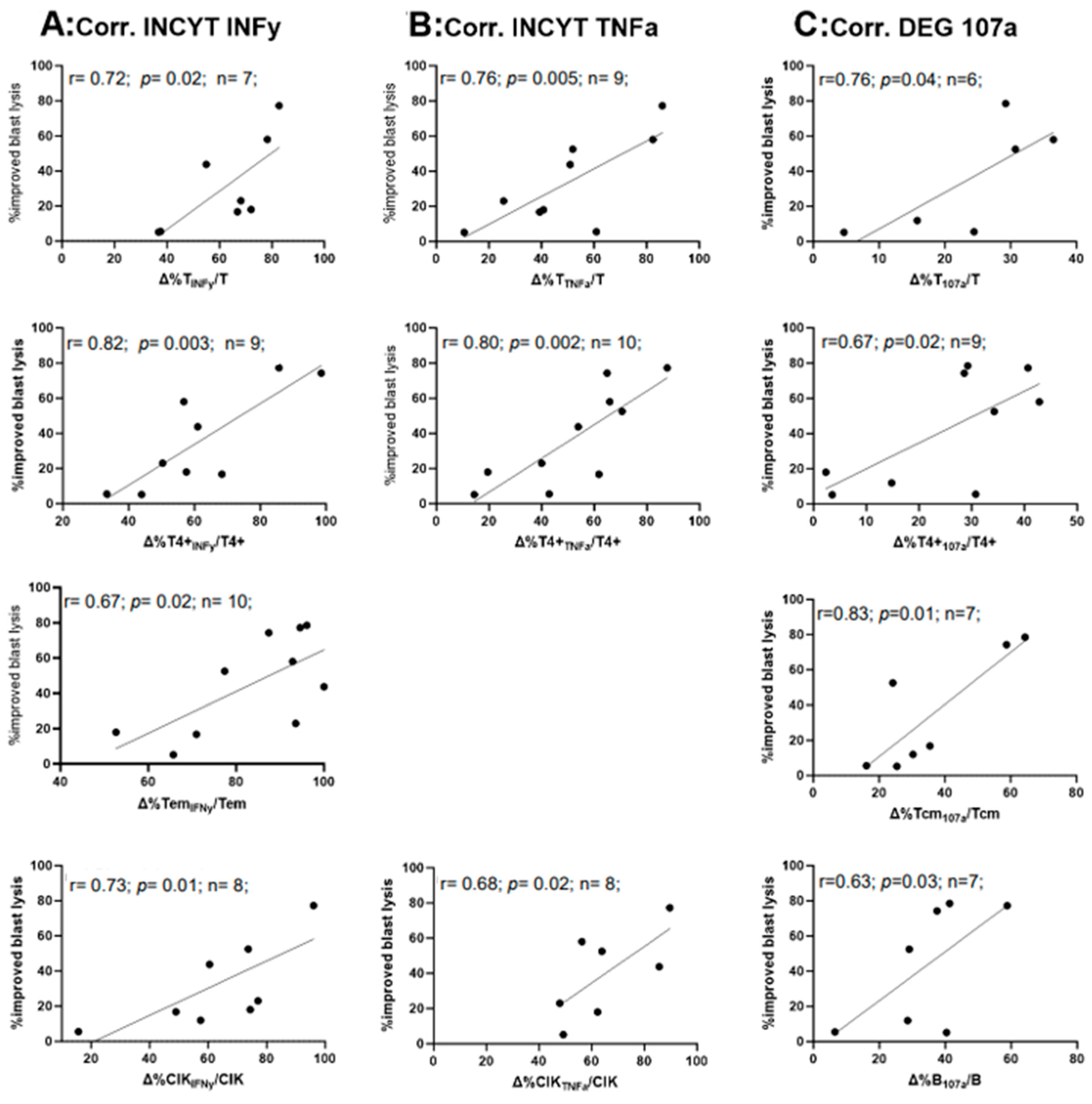
2.8. Correlation of the Relative Improvement of IFNy and TNFa Secretion and the Degranulation with the Relative Improvement of Blast Lysis in Kit M-Treated (vs. Untreated) WB after MLC
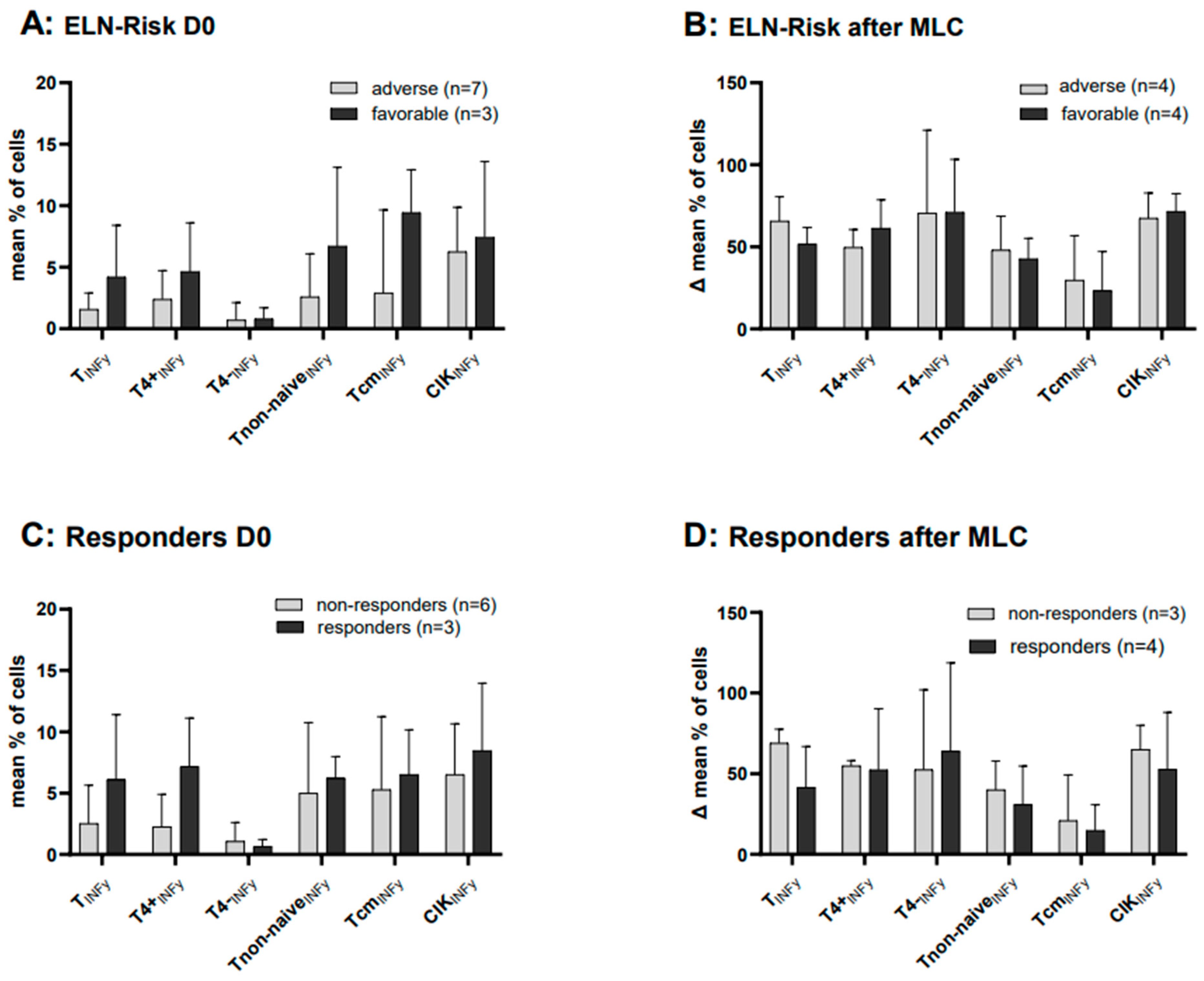
2.9. Cases with Intracellular IFNy Production before and after MLC, Subdivided into Cytogenetic ELN Risk Groups (Favorable and Adverse)
2.10. Cases with Intracellular IFNy Production before and after MLC, Subdivided into Patients’ Response to Induction Chemotherapy
3. Discussion
3.1. Use of DC-Based Immunotherapy in AML
3.2. Generation of DCs Ex Vivo
3.3. Different Age Groups in AML Patients’ WB Samples and Healthy Donors
3.4. Detection of Leukemia-Specific Cells
3.5. Leukemia-Specific Activity of Immune-Reactive Cells
3.6. Correlation of Results of CTX Values and Leukemia-Specific Assays such as INCYT (INFy and TNFa) and DEG
4. Material and Methods
4.1. Sample Acquisition
4.2. Patients’ Characteristics
4.3. Sample Preparation
4.4. Characterization and Quantification of Cells via Flow Cytometry
4.5. Dendritic Cell Culture (DCC)
4.6. Mixed Lymphocyte Culture (MLC)
4.7. Degranulation Assay (DEG) and Intracellular Cytokine Assay (INCYT)
4.8. Intracellular Cytokine Assay (INTCYT)
4.9. Cytotoxicity Fluorolysis Assay (CTX)
4.10. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sato, H.; Wheat, J.C.; Steidl, U.; Ito, K. DNMT3A and TET2 in the Pre-Leukemic Phase of Hematopoietic Disorders. Front. Oncol. 2016, 6, 187. [Google Scholar] [CrossRef] [PubMed]
- Röllig, C. Gemtuzumab ozogamicin in AML: The next chapter. Blood 2023, 142, 1673–1674. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Estey, E.; Döhner, H. Acute myeloid leukaemia. Lancet 2006, 368, 1894–1907. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manero, G.; Chien, K.S.; Montalban-Bravo, G. Myelodysplastic syndromes: 2021 update on diagnosis, risk stratification and management. Am. J. Hematol. 2020, 95, 1399–1420. [Google Scholar] [CrossRef] [PubMed]
- Vago, L.; Gojo, I. Immune escape and immunotherapy of acute myeloid leukemia. J. Clin. Investig. 2020, 130, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- Flörcken, A.; van Lessen, A.; Terwey, T.H.; Dörken, B.; Arnold, R.; Pezzutto, A.; Westermann, J. Anti-leukemia T cells in AML: TNF-α⁺ CD8⁺ T cells may escape detection and possibly reflect a stage of functional impairment. Hum. Vaccines Immunother. 2013, 9, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Klauer, L.K.; Schutti, O.; Ugur, S.; Doraneh-Gard, F.; Amberger, D.C.; Rogers, N.; Krämer, D.; Rank, A.; Schmid, C.; Eiz-Vesper, B.; et al. Interferon Gamma Secretion of Adaptive and Innate Immune Cells as a Parameter to Describe Leukaemia-Derived Dendritic-Cell-Mediated Immune Responses in Acute Myeloid Leukaemia in vitro. Transfus. Med. Hemotherapy Off. Organ Der Dtsch. Ges. Fur Transfusionsmedizin Und Immunhamatol. 2022, 49, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Rackl, E.; Li, L.; Klauer, L.K.; Ugur, S.; Pepeldjiyska, E.; Seidel, C.L.; Gunsilius, C.; Weinmann, M.; Doraneh-Gard, F.; Reiter, N.; et al. Dendritic Cell-Triggered Immune Activation Goes along with Provision of (Leukemia-Specific) Integrin Beta 7-Expressing Immune Cells and Improved Antileukemic Processes. Int. J. Mol. Sci. 2022, 24, 463. [Google Scholar] [CrossRef]
- Amberger, D.C.; Doraneh-Gard, F.; Gunsilius, C.; Weinmann, M.; Möbius, S.; Kugler, C.; Rogers, N.; Böck, C.; Ködel, U.; Werner, J.-O.; et al. PGE1-Containing Protocols Generate Mature (Leukemia-Derived) Dendritic Cells Directly from Leukemic Whole Blood. Int. J. Mol. Sci. 2019, 20, 4590. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Malyguine, A.M.; Strobl, S.; Dunham, K.; Shurin, M.R.; Sayers, T.J. ELISPOT Assay for Monitoring Cytotoxic T Lymphocytes (CTL) Activity in Cancer Vaccine Clinical Trials. Cells 2012, 1, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, J.D. ELISPOT assay: A personal retrospective. Methods Mol. Biol. 2005, 302, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Petrovsky, N.; Schönbach, C.; Koh, J.L.Y.; Brusic, V. Methods for Prediction of Peptide Binding to MHC Molecules: A Comparative Study. Mol. Med. 2002, 8, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Mosmann, T.R. Optimization of the cytokine secretion assay for human IL-2 in single and combination assays. Cytometry. Part A J. Int. Soc. Anal. Cytol. 2015, 87, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Tettamanti, S.; Pievani, A.; Biondi, A.; Dotti, G.; Serafini, M. Catch me if you can: How AML and its niche escape immunotherapy. Leukemia 2022, 36, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, M.; Ma, D. TNF-α, a good or bad factor in hematological diseases? Stem Cell Investig. 2014, 1, 12. [Google Scholar] [CrossRef]
- Shtrichman, R.; Samuel, C.E. The role of gamma interferon in antimicrobial immunity. Curr. Opin. Microbiol. 2001, 4, 251–259. [Google Scholar] [CrossRef]
- Howard, A. Young and Kenneth J. Hardy. Role of interferon-γ in immune cell regulation. J. Leukoc. Biol. 1995, 58, 373–381. [Google Scholar] [CrossRef]
- Jung, T.; Schauer, U.; Heusser, C.; Neumann, C.; Rieger, C. Detection of intracellular cytokines by flow cytometry. J. Immunol. Methods 1993, 159, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Pennock, N.D.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T cell responses: Naive to memory and everything in between. Adv. Physiol. Educ. 2013, 37, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef] [PubMed]
- Wolint, P.; Betts, M.R.; Koup, R.A.; Oxenius, A. Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells. J. Exp. Med. 2004, 199, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Unterfrauner, M.; Rejeski, H.A.; Hartz, A.; Bohlscheid, S.; Baudrexler, T.; Feng, X.; Rackl, E.; Li, L.; Rank, A.; Velázquez, G.F.; et al. Granulocyte-Macrophage-Colony-Stimulating-Factor Combined with Prostaglandin E1 Create Dendritic Cells of Leukemic Origin from AML Patients’ Whole Blood and Whole Bone Marrow That Mediate Antileukemic Processes after Mixed Lymphocyte Culture. Int. J. Mol. Sci. 2023, 24, 17436. [Google Scholar] [CrossRef] [PubMed]
- Schwepcke, C.; Klauer, L.K.; Deen, D.; Amberger, D.C.; Fischer, Z.; Doraneh-Gard, F.; Gunsilius, C.; Hirn-Lopez, A.; Kroell, T.; Tischer, J.; et al. Generation of Leukaemia-Derived Dendritic Cells (DCleu) to Improve Anti-Leukaemic Activity in AML: Selection of the Most Efficient Response Modifier Combinations. Int. J. Mol. Sci. 2022, 23, 8333. [Google Scholar] [CrossRef] [PubMed]
- Aktas, E.; Kucuksezer, U.C.; Bilgic, S.; Erten, G.; Deniz, G. Relationship between CD107a expression and cytotoxic activity. Cell. Immunol. 2009, 254, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Amberger, D.C.; Schmetzer, H.M. Dendritic Cells of Leukemic Origin: Specialized Antigen-Presenting Cells as Potential Treatment Tools for Patients with Myeloid Leukemia. Transfus. Med. Hemotherapy 2020, 47, 432–443. [Google Scholar] [CrossRef] [PubMed]
- van Acker, H.H.; Versteven, M.; Lichtenegger, F.S.; Roex, G.; Campillo-Davo, D.; Lion, E.; Subklewe, M.; van Tendeloo, V.F.; Berneman, Z.N.; Anguille, S. Dendritic Cell-Based Immunotherapy of Acute Myeloid Leukemia. J. Clin. Med. 2019, 8, 579. [Google Scholar] [CrossRef]
- Anguille, S.; Willemen, Y.; Lion, E.; Smits, E.L.; Berneman, Z.N. Dendritic cell vaccination in acute myeloid leukemia. Cytotherapy 2012, 14, 647–656. [Google Scholar] [CrossRef]
- Holzinger, A.; Barden, M.; Abken, H. The growing world of CAR T cell trials: A systematic review. Cancer Immunol. Immunother. CII 2016, 65, 1433–1450. [Google Scholar] [CrossRef] [PubMed]
- Lichtenegger, F.S.; Krupka, C.; Haubner, S.; Köhnke, T.; Subklewe, M. Recent developments in immunotherapy of acute myeloid leukemia. J. Hematol. Oncol. 2017, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Grabrucker, C.; Liepert, A.; Dreyig, J.; Kremser, A.; Kroell, T.; Freudenreich, M.; Schmid, C.; Schweiger, C.; Tischer, J.; Kolb, H.-J.; et al. The quality and quantity of leukemia-derived dendritic cells from patients with acute myeloid leukemia and myelodysplastic syndrome are a predictive factor for the lytic potential of dendritic cells-primed leukemia-specific T cells. J. Immunother. 2010, 33, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Pepeldjiyska, E.; Li, L.; Gao, J.; Seidel, C.L.; Blasi, C.; Özkaya, E.; Schmohl, J.; Kraemer, D.; Schmid, C.; Rank, A.; et al. Leukemia derived dendritic cell (DCleu) mediated immune response goes along with reduced (leukemia-specific) regulatory T-cells. Immunobiology 2022, 227, 152237. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.J.; Meiselman, H.J.; Baskurt, O.K. Blood rheology and aging. J. Geriatr. Cardiol. 2013, 10, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Kim, J.; Metter, E.J.; Nguyen, H.; Truong, T.; Lustig, A.; Ferrucci, L.; Weng, N.-P. Changes in blood lymphocyte numbers with age in vivo and their association with the levels of cytokines/cytokine receptors. Immun. Ageing 2016, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Letsch, A.; Scheibenbogen, C. Quantification and characterization of specific T-cells by antigen-specific cytokine production using ELISPOT assay or intracellular cytokine staining. Methods 2003, 31, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Michelle, N. Wykes and Laurent Renia. ELISPOT Assay to Measure Peptide-specific IFN-γ Production. Bio-Protocol 2017, 7, e2302. [Google Scholar] [CrossRef]
- Chang, J. MHC multimer: A Molecular Toolbox for Immunologists. Mol. Cells 2021, 44, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Dolton, G.; Tungatt, K.; Lloyd, A.; Bianchi, V.; Theaker, S.M.; Trimby, A.; Holland, C.J.; Donia, M.; Godkin, A.J.; Cole, D.K.; et al. More tricks with tetramers: A practical guide to staining T cells with peptide-MHC multimers. Immunology 2015, 146, 11–22. [Google Scholar] [CrossRef]
- Assudani, D.P.; Horton, R.B.V.; Mathieu, M.G.; McArdle, S.E.B.; Rees, R.C. The role of CD4+ T cell help in cancer immunity and the formulation of novel cancer vaccines. Cancer Immunol. Immunother. CII 2007, 56, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Preffer, F.; Dombkowski, D. Advances in complex multiparameter flow cytometry technology: Applications in stem cell research. Cytometry. Part B Clin. Cytom. 2009, 76, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Freer, G.; Rindi, L. Intracellular cytokine detection by fluorescence-activated flow cytometry: Basic principles and recent advances. Methods 2013, 61, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Nomura, L.; Maino, V.C.; Maecker, H.T. Standardization and optimization of multiparameter intracellular cytokine staining. Cytometry. Part A J. Int. Soc. Anal. Cytol. 2008, 73, 984–991. [Google Scholar] [CrossRef] [PubMed]
- O’Neil-Andersen, N.J.; Lawrence, D.A. Differential modulation of surface and intracellular protein expression by T cells after stimulation in the presence of monensin or brefeldin A. Clin. Diagn. Lab. Immunol. 2002, 9, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Koehne, G.; Smith, K.M.; Ferguson, T.L.; Williams, R.Y.; Heller, G.; Pamer, E.G.; Dupont, B.; O’Reilly, R.J. Quantitation, selection, and functional characterization of Epstein-Barr virus-specific and alloreactive T cells detected by intracellular interferon-gamma production and growth of cytotoxic precursors. Blood 2002, 99, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Oelke, M.; Kurokawa, T.; Hentrich, I.; Behringer, D.; Cerundolo, V.; Lindemann, A.; Mackensen, A. Functional Characterization of CD8 + Antigen-Specific Cytotoxic T Lymphocytes after Enrichment Based on Cytokine Secretion: Comparison with the MHC-Tetramer Technology. Scand J. Immunol. 2000, 52, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Mair, F.; Tosevski, V. Intracellular Staining for Cytokines and Transcription Factors; Methods in Molecular Biology; Springer: New York, NY, USA, 2014; pp. 39–49. [Google Scholar] [CrossRef]
- Roggendorf, M.; Yang, D.; Lu, M. The woodchuck: A model for therapeutic vaccination against hepadnaviral infection. Pathol. Biol. 2010, 58, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Alter, G.; Malenfant, J.M.; Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 2004, 294, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Owens, M.A.; Vall, H.G.; Hurley, A.A.; Wormsley, S.B. Validation and quality control of immunophenotyping in clinical flow cytometry. J. Immunol. Methods 2000, 243, 33–50. [Google Scholar] [CrossRef]
- Binder, S.; Luciano, M.; Horejs-Hoeck, J. The cytokine network in acute myeloid leukemia (AML): A focus on pro- and anti-inflammatory mediators. Cytokine Growth Factor Rev. 2018, 43, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Lamble, A.J.; Dietz, M.; Laderas, T.; McWeeney, S.; Lind, E.F. Integrated functional and mass spectrometry-based flow cytometric phenotyping to describe the immune microenvironment in acute myeloid leukemia. J. Immunol. Methods 2018, 453, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Bernal, C.; Sánchez-Herrera, D. Toll-Like Receptors and Natural Killer Cells. In Toll-like Receptors; Rezaei, N., Ed.; InTech: Berlin, Germany, 2020. [Google Scholar] [CrossRef]
- Poli, A.; Michel, T.; Thérésine, M.; Andrès, E.; Hentges, F.; Zimmer, J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology 2009, 126, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Ellegast, J.M.; Alexe, G.; Hamze, A.; Lin, S.; Uckelmann, H.J.; Rauch, P.J.; Pimkin, M.; Ross, L.S.; Dharia, N.V.; Robichaud, A.L.; et al. Unleashing Cell-Intrinsic Inflammation as a Strategy to Kill AML Blasts. Cancer Discov. 2022, 12, 1760–1781. [Google Scholar] [CrossRef] [PubMed]
- Pattengale, P.K.; Sundstrom, C.; Yu, A.L.; Levine, A. Lysis of fresh leukemic blasts by interferon-activated human natural killer cells. Nat. Immun. Cell Growth Regul. 1983, 3, 165–180. [Google Scholar] [PubMed]
- Estey, E.; Gale, R.P. How good are we at predicting the fate of someone with acute myeloid leukaemia? Leukemia 2017, 31, 1255–1258. [Google Scholar] [CrossRef]
- Baudrexler, T.; Boeselt, T.; Li, L.; Bohlscheid, S.; Boas, U.; Schmid, C.; Rank, A.; Schmohl, J.; Koczulla, R.; Schmetzer, H.M. Volatile Phases Derived from Serum, DC, or MLC Culture Supernatants to Deduce a VOC-Based Diagnostic Profiling Strategy for Leukemic Diseases. Biomolecules 2023, 13, 989. [Google Scholar] [CrossRef]
- Price, L.S.; Adamow, M.; Attig, S.; Fecci, P.; Norberg, P.; Reap, E.; Janetzki, S.; McNeil, L.K. Gating Harmonization Guidelines for Intracellular Cytokine Staining Validated in Second International Multiconsortia Proficiency Panel Conducted by Cancer Immunotherapy Consortium (CIC/CRI). Cytometry. Part A J. Int. Soc. Anal. Cytol. 2021, 99, 107–116. [Google Scholar] [CrossRef]



| Cell Type | Name of Subgroups | Abbreviation of Subgroups | Surface Marker | Referred to | Abbreviation | Reference |
|---|---|---|---|---|---|---|
| Subtypes of blasts and DC | ||||||
| Blast cells | Leukemic blasts | Bla | Bla+ (e.g., CD34+, CD177+) | WB | Bla/WB | [26] |
| Proliferating blasts | Blaprol-CD71 | Bla+DC−CD71+ | Bla | Blaprol-CD71/Bla | [26] | |
| Proliferating blasts | Blaprol-IPO38 | Bla+DC−IPO38+ | Bla | Blaprol-IPO38/Bla | [26] | |
| Dendritic cells | Dendritic cells | DC | DC+ (CD80+, CD206+) | WB | DC/cells | [26] |
| Leukemia derived DC | DCleu | DC+Bla+ | WB | DCleu/cells | [26] | |
| Bla | DCleu/Bla | [26] | ||||
| Mature DC | DCmig | DC+CD197+ | WB | DCmig/cells | [26] | |
| Mature DCleu | DCleu-mig | DC+CD197+Bla+ | WB | DCleu-mig/cells | [26] | |
| DC | DCleu-mig/DC | [26] | ||||
| DCleu | DCleu-mig/DCleu | [26] | ||||
| Bla | DCleu-mig/Bla | [26] | ||||
| Subtypes of immune-reactive cells | ||||||
| T cells | CD3+ pan T cells | T | CD3+ | WB | T/cells | [10] |
| CD4+ coexpressing T cells | T4+ | CD3+CD4+ | T | T4+/T | [10] | |
| CD8+ coexpressing T cells | T4- | CD3+CD8+ | T | T4-/T | [10] | |
| Proliferating T cells-early | Tprol-early | CD3+CD69+ | T | Tprol-early/T | [10] | |
| Proliferating T cells-late | Tprol-late | CD3+CD71+ | T | Tprol-late/T | [10] | |
| Non-naive T cells | Tnon-naive | CD3+CD45RO+ | T | Tnon-naive/T | [8] | |
| Central (memory) T cells | Tcm | CD3+CD45RO+CD197+ | T | Tcm/T | [8] | |
| Subtypes of different degranulating (CD107a+) cells | ||||||
| T cells | CD3+ pan T cells | T107a | CD107a+CD3+ | T | T107a/T | [27] |
| CD4+ coexpressing T cells | T4+107a | CD107a+CD3+CD4+ | T | T4+107a/T | [10] | |
| CD8+ coexpressing T cells | T4-107a | CD107a+CD3+CD8+ | T | T4-107a/T | [10] | |
| Non-naive T cells | Tnon-naive107a | CD107a+CD3+CD45RO+ | Tnon-naive | Tnon-naive107a/Tnon-naive | [27] | |
| Effector (memory) T cells | Tem107a | CD107a+CD3+CD45RO+CD197− | Tem | Tem107a/Tem | [27] | |
| Central (memory) T cells | Tcm107a | CD107a+CD3+CD45RO+CD197+ | Tcm | Tcm107a/Tcm | [27] | |
| NK cells | CD3−CD56+ NK cells | NK107a | CD107a+CD3−CD56+ | NK | NK107a/NK | [27] |
| CIK cells | CD3+CD56+ CIK cells | CIK107a | CD107a+CD3+CD56+ | CIK | CIK107a/CIK | [27] |
| B cells | CD19+ | B107a | CD107a+CD19+ | B | B107a/B | [27] |
| Subtypes of different intracellularly IFNy or TNFa-producing cells | ||||||
| T cells | CD3+ pan T cells | TIFNy/TNFa | IFNy+/TNFa+CD3+ | T | TIFNy/TNFa/T | [8] |
| CD4+-coexpressing T cells | T4+IFNy/TNFa | IFNy+/TNFa+CD3+ CD4+ | T4+ | T4+IFNy/TNFa/T4+ | [8] | |
| CD8+-coexpressing T cells | T4-IFNy/TNFa | IFNy+/TNFa+CD3+CD8+ | T4- | T4-IFNy/TNFa/T4- | [8] | |
| Non-naive T cells | Tnon-naive IFNy/TNFa | IFNy+/TNFa+CD3+CD45RO+ | Tnon-naive | Tnon-naiveIFNy/TNFa/Tnon-naive | [8] | |
| Effector (memory) T cells | Tem IFNy/TNFa/Tem | IFNy+/TNFa+CD3+CD45RO+CD197− | Tem | Tem IFNy/TNFa/Tem | [8] | |
| Central (memory) T cells | Tcm IFNy/TNFa | IFNy+/TNFa+CD3+CD45RO+CD197+ | Tcm | Tcm IFNy/TNFa/Tcm | [8] | |
| NK cells | CD3−CD56+ NK cells | NKIFNy/TNFa | IFNy+/TNFa+CD3−CD56+ | NK | NKIFNy/TNFa/NK | [8] |
| CIK cells | CD3+CD56+ CIK cells | CIKIFNy/TNFa | IFNy+/TNFa+CD3+CD56+ | CIK | CIKIFNy/TNFa/CIK | [8] |
| Patient No. | Sex | Age at dgn | FAB type | Stage | Blast in PB (%) * | Blast Phenotype (CD) | ELN-Risk- Stratification | Response to Induction Chemotherapy | Conducted Experiments |
|---|---|---|---|---|---|---|---|---|---|
| AML | |||||||||
| P1509 | m | 60 | pAML/M2 | dgn | 48 | 13,33,34,65,117 | favorable | response | DCC, MLC, INCYT, CSA, CTX |
| P1514 | m | 68 | SAML | dgn | 51 | 33,56,117 | favorable | no response | DCC, MLC, INCYT, DEG |
| P1518 | f | 83 | pAML/M5 | dgn | 72 | 14,15,34,65 | favorable | no response | DCC, MLC, INCYT, CSA |
| P1526 | f | 74 | pAML | dgn | 61 | 15,33,34,56,65,117 | favorable | n.d. | DCC, MLC, DEG, CSA |
| P1602 | m | 67 | pAML | dgn | 16 | 13,33,34,56,117 | favorable | response | DCC, MLC, CTX |
| P1630 | m | 29 | pAML | dgn | 16 | 13,15,33,34,64,117 | Tavorable | response | DCC, MLC, INCYT, DEG, CTX |
| P1512 | f | 80 | pAML | dgn | 40 | 13,34,117 | adverse | no response | DCC, MLC, INCYT, DEG |
| P1567 | f | 98 | SAML | dgn | 16 | 15,34,65,117 | adverse | no response | DCC, MLC, INCYT, DEG, CTX |
| P1572 | f | 63 | SAML | dgn | 12 | 13,33,34,65,117 | adverse | response | DCC, MLC, INCYT, DEG, CTX |
| P1573 | m | 61 | pAML/M6 | dgn | 13 | 13,34,65,71,117 | adverse | no response | DCC, MLC, INCYT, DEG, CTX |
| P1581 | m | 56 | pAML/M4 | dgn | 58 | 13,15,33,34,117 | adverse | no response | DCC, MLC, INCYT, DEG, CTX |
| P1638 | m | 68 | sAML | dgn | 33 | 4,13,33,34,56,117 | adverse | no response | DCC, MLC, INCYT, DEG, CTX |
| P1527 | m | 42 | pAML/M2 | dgn | 28 | 7,13,15,33,34,65,117 | intermediate | n.d. | DCC, MLC, INCYT, DEG, CSA, CTX |
| P1604 | f | 60 | sAML/MDS | dgn | 16 | 7,13,33,34,65,117 | intermediate | n.d. | DCC, MLC, INCYT, DEG, CTX |
| P1635 | m | 51 | pAML | dgn | 25 | 15,33,34,117 | intermediate | response | DCC, MLC, INCYT, DEG |
| P1594 | f | 70 | pAML/M4 | Per. disease | 20 | 13,33,34,65,117 | DCC, MLC, INCYT, DEG, CTX | ||
| P1595 | f | 50 | pAML | Per. disease | 15 | 13,33,34,56,65,117 | DCC, MLC, CTX | ||
| P1597 | f | 83 | sAML | Per. disease | 54 | 15,33,34,56,65,117 | DCC, MLC, INCYT, DEG, CTX | ||
| P1603 | f | 32 | pAML | Per. disease | 50 | 15,33,34,117 | DCC, MLC, INCYT, DEG, CTX | ||
| P1616 | f | 69 | pAML | Per. disease | 16 | 33,34,117 | DCC, MLR, INCYT, DEG | ||
| P1497(2) | m | 74 | SAML | relapse | 59 | 13,34,65,117 | DCC, MLR, INCYT, DEG, CTX | ||
| P1516 | f | 52 | SAML | relapse | 70 | 15,33,34,65,117 | DCC, MLR, INCYT, DEG, CTX | ||
| P1522 | m | 47 | SAML | relapse | 55 | 13,34,71,117 | DCC, MLR, INCYT, DEG | ||
| P1598 | f | 61 | sAML | relapse | 25 | 13,33,34,117 | DCC, MLR, INCYT, DEG, CTX | ||
| P1599 | f | 71 | pAML/M4 | relapse | 79 | 7,13,33,34,117 | DCC, MLC, DEG, CTX | ||
| P1632 | f | 56 | pAML | relapse | 65 | 13,33,34,56,65,117 | DCC, MLC, INCYT, DEG, CTX | ||
| HEALTHY | |||||||||
| P1510 | m | 22 | DCC, MLC, INCYT, DEG | ||||||
| P1513 | m | 27 | DCC, MLC, INCYT, DEG | ||||||
| P1517 | m | 39 | DCC, MLC, INCYT, DEG | ||||||
| P1523 | m | 17 | DCC, MLC, INCYT, DEG, CSA | ||||||
| P1566 | f | 54 | DCC, MLC, INCYT, DEG | ||||||
| P1579 | m | 30 | DCC, MLC, INCYT, DEG | ||||||
| P1580 | f | 24 | DCC, MLC, INCYT, DEG | ||||||
| P1582 | m | 27 | DCC, MLC, INCYT, DEG | ||||||
| P1583 | m | 28 | DCC, MLC, INCYT, DEG | ||||||
| P1585 | m | 29 | DCC, MLC, INCYT, DEG | ||||||
| P1586 | m | 29 | DCC, MLC | ||||||
| P1590 | f | 23 | DCC, MLC | ||||||
| P1592 | f | 58 | DCC, MLC, INCYT, DEG | ||||||
| P1596 | m | 26 | DCC, MLC | ||||||
| P1611 | f | 27 | DCC, MLC, INCYT, DEG | ||||||
| P1613 | f | 24 | DCC, MLC, INCYT, DEG | ||||||
| P1636 | m | 22 | DCC, MLC, INCYT, DEG | ||||||
| P1637 | f | 22 | DCC, MLC, INCYT, DEG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schutti, O.; Klauer, L.; Baudrexler, T.; Burkert, F.; Schmohl, J.; Hentrich, M.; Bojko, P.; Kraemer, D.; Rank, A.; Schmid, C.; et al. Effective and Successful Quantification of Leukemia-Specific Immune Cells in AML Patients’ Blood or Culture, Focusing on Intracellular Cytokine and Degranulation Assays. Int. J. Mol. Sci. 2024, 25, 6983. https://doi.org/10.3390/ijms25136983
Schutti O, Klauer L, Baudrexler T, Burkert F, Schmohl J, Hentrich M, Bojko P, Kraemer D, Rank A, Schmid C, et al. Effective and Successful Quantification of Leukemia-Specific Immune Cells in AML Patients’ Blood or Culture, Focusing on Intracellular Cytokine and Degranulation Assays. International Journal of Molecular Sciences. 2024; 25(13):6983. https://doi.org/10.3390/ijms25136983
Chicago/Turabian StyleSchutti, Olga, Lara Klauer, Tobias Baudrexler, Florian Burkert, Joerg Schmohl, Marcus Hentrich, Peter Bojko, Doris Kraemer, Andreas Rank, Christoph Schmid, and et al. 2024. "Effective and Successful Quantification of Leukemia-Specific Immune Cells in AML Patients’ Blood or Culture, Focusing on Intracellular Cytokine and Degranulation Assays" International Journal of Molecular Sciences 25, no. 13: 6983. https://doi.org/10.3390/ijms25136983





