Proteomics Analyses of Small Extracellular Vesicles of Aqueous Humor: Identification and Validation of GAS6 and SPP1 as Glaucoma Markers
Abstract
:1. Introduction
2. Results
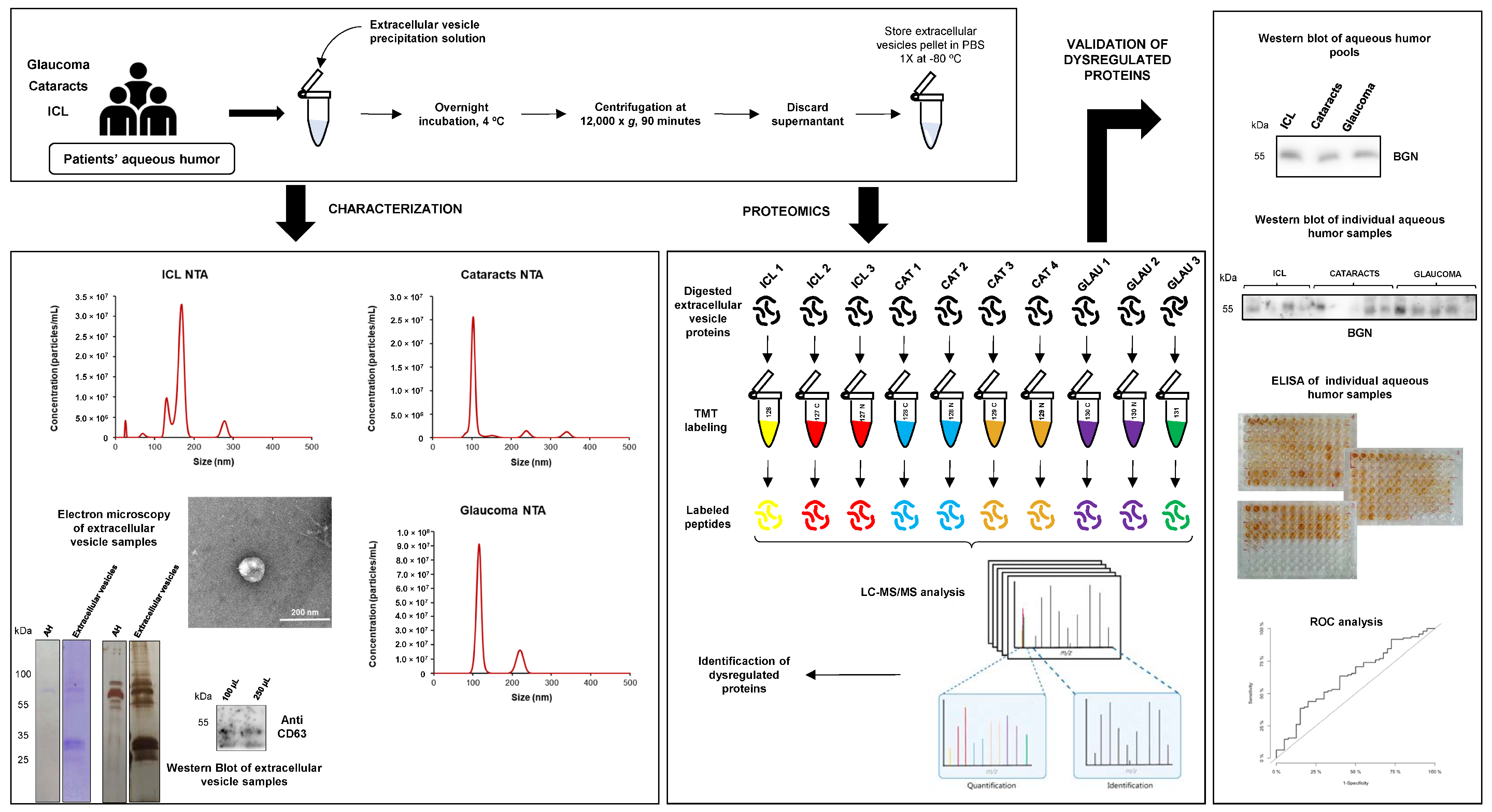
2.1. Workflow of Aqueous Humor sEVs Proteome Analysis
2.2. Characterization of sEVs Present in Aqueous Humors
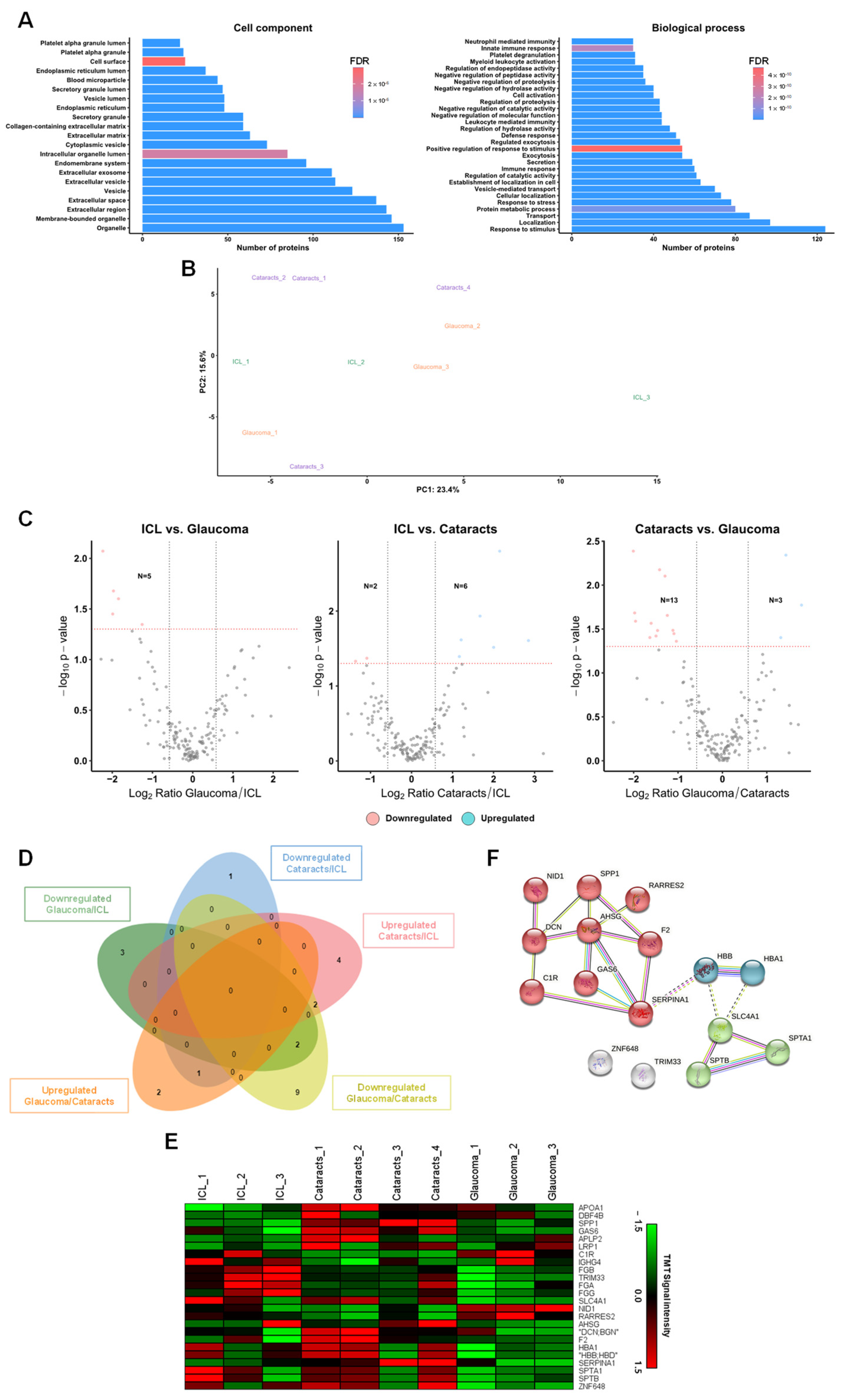
2.3. Identification of Dysregulated Proteins from Aqueous Humor sEVs
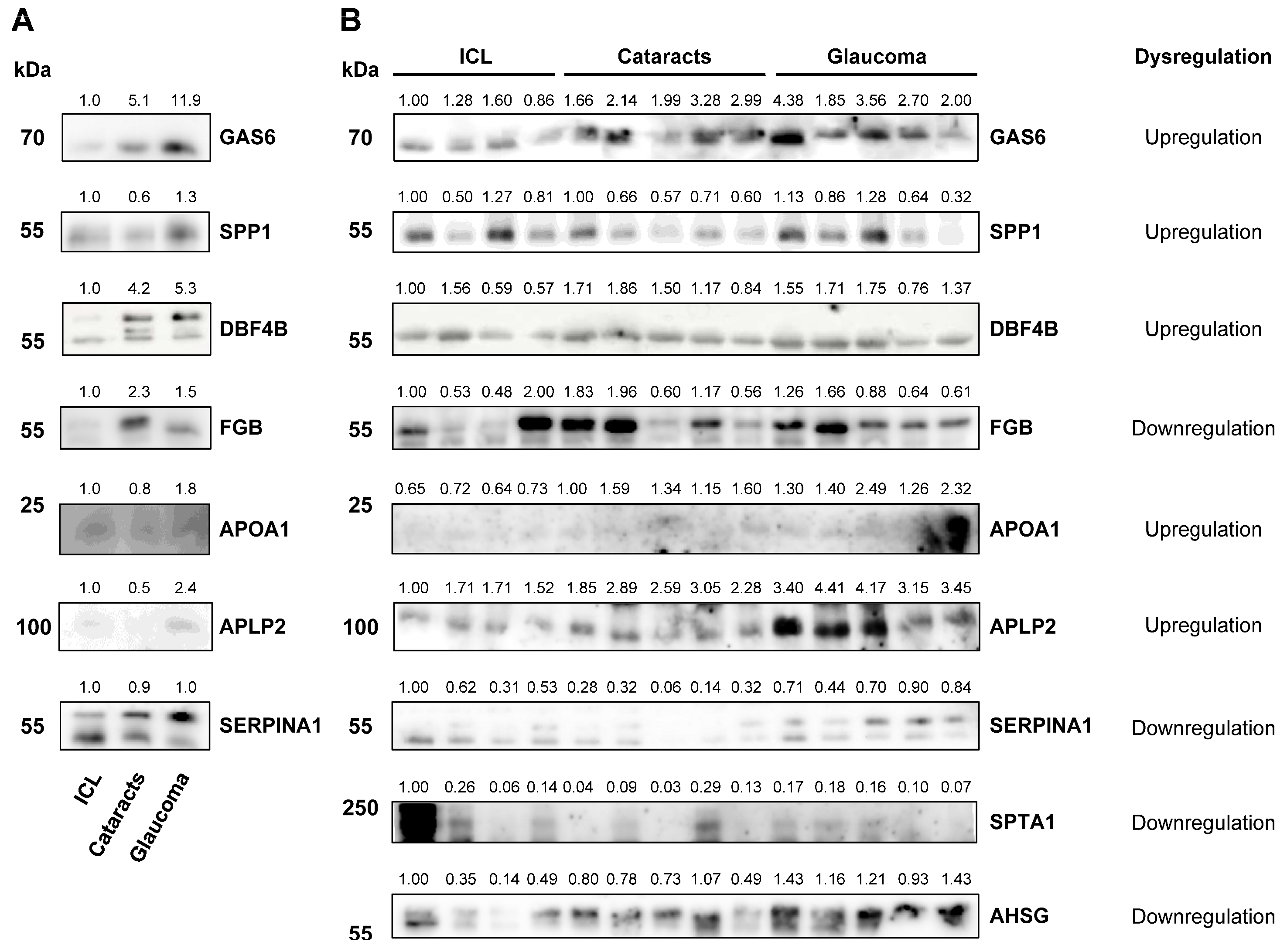
2.4. Validation
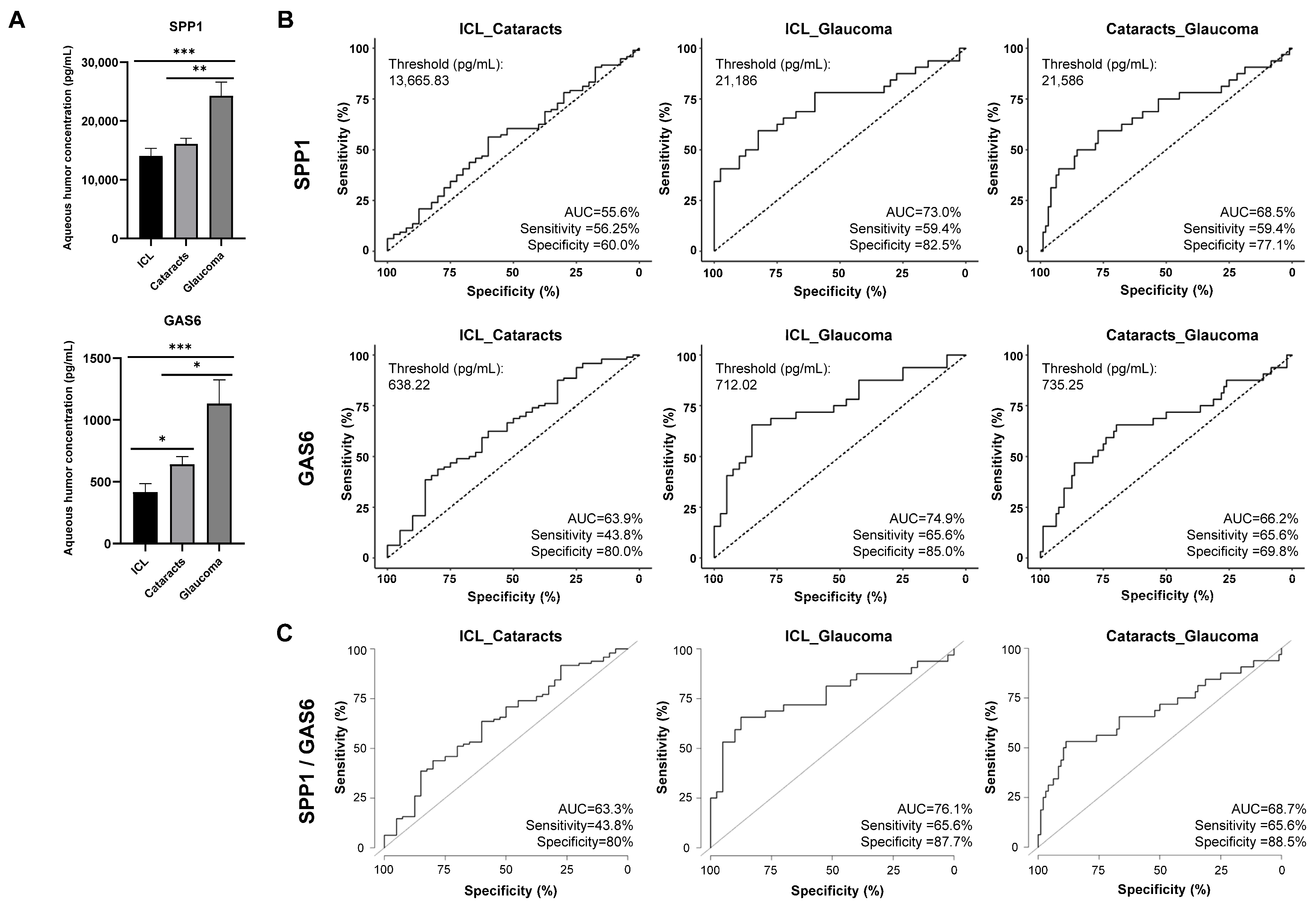
2.5. GAS6 and SPP1 Analysis as Humor Aqueous Biomarkers for Glaucoma Diagnosis
3. Discussion
4. Materials and Methods
4.1. Aqueous Humor Sample Collection
4.2. Small EVs Isolation and Purification
4.3. Electron Microscopy
4.4. Mass Spectrometry and Data Analysis
4.5. Normalization and Bioinformatics Analysis
4.6. SDS-PAGE and WB
4.7. ELISA Tests
4.8. Statistical Analyses
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A. Glaucoma. Lancet 2011, 377, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012, 96, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, K.; Sotozono, C.; Koizumi, N.; Nagata, K.; Inatomi, T.; Sasaki, H.; Kinoshita, S. Safety of anterior chamber paracentesis using a 30-gauge needle integrated with a specially designed disposable pipette. Br. J. Ophthalmol. 2017, 101, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Lee, C.H.; Chiou, S.H.; Liao, C.C.; Cheng, C.W. Proteomic Analysis of Aqueous Humor Proteins in Association with Cataract Risks: Diabetes and Smoking. J. Clin. Med. 2021, 10, 5731. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Rong, X.; Ye, H.; Zhang, K.; Lu, Y. Proteomic analysis of aqueous humor proteins associated with cataract development. Clin. Biochem. 2015, 48, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Beutgen, V.M.; Graumann, J. Advances in aqueous humor proteomics for biomarker discovery and disease mechanisms exploration: A spotlight on primary open angle glaucoma. Front. Mol. Neurosci. 2024, 17, 1397461. [Google Scholar] [CrossRef] [PubMed]
- Hubens, W.H.G.; Mohren, R.J.C.; Liesenborghs, I.; Eijssen, L.M.T.; Ramdas, W.D.; Webers, C.A.B.; Gorgels, T. The aqueous humor proteome of primary open angle glaucoma: An extensive review. Exp. Eye Res. 2020, 197, 108077. [Google Scholar] [CrossRef] [PubMed]
- Kersten, E.; Paun, C.C.; Schellevis, R.L.; Hoyng, C.B.; Delcourt, C.; Lengyel, I.; Peto, T.; Ueffing, M.; Klaver, C.C.W.; Dammeier, S.; et al. Systemic and ocular fluid compounds as potential biomarkers in age-related macular degeneration. Surv. Ophthalmol. 2018, 63, 9–39. [Google Scholar] [CrossRef]
- Midena, E.; Frizziero, L.; Midena, G.; Pilotto, E. Intraocular fluid biomarkers (liquid biopsy) in human diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 3549–3560. [Google Scholar] [CrossRef]
- Xiao, H.; Xin, W.; Sun, L.M.; Li, S.S.; Zhang, T.; Ding, X.Y. Comprehensive Proteomic Profiling of Aqueous Humor Proteins in Proliferative Diabetic Retinopathy. Transl. Vis. Sci. Technol. 2021, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Wu, L.; An, J.; Liu, Z.; Zhang, K.; Zhou, L.; Zhang, X. Proteomic analysis of human aqueous humor from fuchs uveitis syndrome. Exp. Eye Res. 2024, 239, 109752. [Google Scholar] [CrossRef] [PubMed]
- Galardi, A.; Stathopoulos, C.; Colletti, M.; Lavarello, C.; Russo, I.; Cozza, R.; Romanzo, A.; Carcaboso, A.M.; Locatelli, F.; Petretto, A.; et al. Proteomics of Aqueous Humor as a Source of Disease Biomarkers in Retinoblastoma. Int. J. Mol. Sci. 2022, 23, 13458. [Google Scholar] [CrossRef]
- Adav, S.S.; Wei, J.; Terence, Y.; Ang, B.C.H.; Yip, L.W.L.; Sze, S.K. Proteomic Analysis of Aqueous Humor from Primary Open Angle Glaucoma Patients on Drug Treatment Revealed Altered Complement Activation Cascade. J. Proteome Res. 2018, 17, 2499–2510. [Google Scholar] [CrossRef] [PubMed]
- Kaeslin, M.A.; Killer, H.E.; Fuhrer, C.A.; Zeleny, N.; Huber, A.R.; Neutzner, A. Changes to the Aqueous Humor Proteome during Glaucoma. PLoS ONE 2016, 11, e0165314. [Google Scholar] [CrossRef]
- Liu, H.; Anders, F.; Funke, S.; Mercieca, K.; Grus, F.; Prokosch, V. Proteome alterations in aqueous humour of primary open angle glaucoma patients. Int. J. Ophthalmol. 2020, 13, 176–179. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Wang, Y.; Sun, H.; Guo, Z.; Tang, X.; Li, J.; Xiao, X.; Zheng, S.; Yu, M.; et al. Proteome Characterization of Glaucoma Aqueous Humor. Mol. Cell. Proteom. 2021, 20, 100117. [Google Scholar] [CrossRef]
- Sharma, S.; Bollinger, K.E.; Kodeboyina, S.K.; Zhi, W.; Patton, J.; Bai, S.; Edwards, B.; Ulrich, L.; Bogorad, D.; Sharma, A. Proteomic Alterations in Aqueous Humor from Patients with Primary Open Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2635–2643. [Google Scholar] [CrossRef]
- Nikhalashree, S.; George, R.; Shantha, B.; Lingam, V.; Vidya, W.; Panday, M.; Sulochana, K.N.; Coral, K. Detection of Proteins Associated with Extracellular Matrix Regulation in the Aqueous Humour of Patients with Primary Glaucoma. Curr. Eye Res. 2019, 44, 1018–1025. [Google Scholar] [CrossRef]
- Kliuchnikova, A.A.; Samokhina, N.I.; Ilina, I.Y.; Karpov, D.S.; Pyatnitskiy, M.A.; Kuznetsova, K.G.; Toropygin, I.Y.; Kochergin, S.A.; Alekseev, I.B.; Zgoda, V.G.; et al. Human aqueous humor proteome in cataract, glaucoma, and pseudoexfoliation syndrome. Proteomics 2016, 16, 1938–1946. [Google Scholar] [CrossRef]
- Kodeboyina, S.K.; Lee, T.J.; Bollinger, K.; Ulrich, L.; Bogorad, D.; Estes, A.; Zhi, W.; Sharma, S.; Sharma, A. Aqueous Humor Proteomic Alterations Associated with Visual Field Index Parameters in Glaucoma Patients: A Pilot Study. J. Clin. Med. 2021, 10, 1180. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Feng, X.; Peng, Z.; Yuan, L.; Jin, M.; Hu, H.; Peng, X.; Wang, Y.; Zhang, C.; Luo, Z.; Liao, H. Research progress of exosomes in pathogenesis, diagnosis, and treatment of ocular diseases. Front. Bioeng. Biotechnol. 2023, 11, 1100310. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Cho, H.K.; Song, D.H.; Kee, C. Quantitative analysis of exosomes in the aqueous humor of Korean patients with pseudoexfoliation glaucoma. Sci. Rep. 2022, 12, 12875. [Google Scholar] [CrossRef]
- Hsiao, Y.P.; Chen, C.; Lee, C.M.; Chen, P.Y.; Chung, W.H.; Wang, Y.P.; Hung, Y.C.; Cheng, C.M.; Ko, B.H.; Hsu, M.Y. Differences in the Quantity and Composition of Extracellular Vesicles in the Aqueous Humor of Patients with Retinal Neovascular Diseases. Diagnostics 2021, 11, 1276. [Google Scholar] [CrossRef]
- Gao, C.; Fan, F.; Liu, X.; Yang, J.; Zhou, X.; Mei, H.; Lin, X.; Luo, Y. Exosomal miRNA Analysis of Aqueous Humour of Diabetes and Cataract Patients. Curr. Eye Res. 2021, 46, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.Y.; Bang, J.Y.; Choi, A.J.; Yoon, J.; Lee, W.C.; Choi, S.; Yoon, S.; Kim, H.C.; Baek, J.H.; Park, H.S.; et al. Exosomal proteins in the aqueous humor as novel biomarkers in patients with neovascular age-related macular degeneration. J. Proteome Res. 2014, 13, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Chen, C.T.; Wu, H.H.; Liao, C.C.; Hua, K.; Hsu, C.H.; Chen, C.F. Proteomic Profiling of Aqueous Humor Exosomes from Age-related Macular Degeneration Patients. Int. J. Med. Sci. 2022, 19, 893–900. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Chen, C.T.; Lin, C.H.; Liao, C.C.; Hua, K.; Hsu, C.H.; Chen, C.F. Proteomic analysis of Exosomes derived from the Aqueous Humor of Myopia Patients. Int. J. Med. Sci. 2021, 18, 2023–2029. [Google Scholar] [CrossRef]
- Hsu, M.Y.; Chiu, C.C.; Wang, J.Y.; Huang, C.T.; Huang, Y.F.; Liou, J.C.; Chen, C.; Chen, H.C.; Cheng, C.M. Paper-Based Microfluidic Platforms for Understanding the Role of Exosomes in the Pathogenesis of Major Blindness-Threatening Diseases. Nanomaterials 2018, 8, 310. [Google Scholar] [CrossRef]
- Lerner, N.; Chen, I.; Schreiber-Avissar, S.; Beit-Yannai, E. Extracellular Vesicles Mediate Anti-Oxidative Response-In Vitro Study in the Ocular Drainage System. Int. J. Mol. Sci. 2020, 21, 6105. [Google Scholar] [CrossRef] [PubMed]
- Aires, I.D.; Ribeiro-Rodrigues, T.; Boia, R.; Catarino, S.; Girao, H.; Ambrosio, A.F.; Santiago, A.R. Exosomes derived from microglia exposed to elevated pressure amplify the neuroinflammatory response in retinal cells. Glia 2020, 68, 2705–2724. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Zhang, X.; Li, Q.; Wang, H.; Zhang, Z.; Han, Z.; Ke, M.; Chen, X. Identification of proteins in the aqueous humor associated with cataract development using iTRAQ methodology. Mol. Med. Rep. 2017, 15, 3111–3120. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jung, J.H.; Park, T.K.; Moon, C.E.; Han, K.; Lee, J.; Lee, H.K.; Ji, Y.W.; Kim, C.Y. Proteome alterations in the aqueous humor reflect structural and functional phenotypes in patients with advanced normal-tension glaucoma. Sci. Rep. 2022, 12, 1221. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Wang, L.; Feng, Q.; Zhang, D.; Chen, K.; Yiming, G.H.; Wang, Q.; Hong, Y.; Whelchel, A.; Zhang, X.; et al. Low expression of GSTP1 in the aqueous humour of patients with primary open-angle glaucoma. J. Cell. Mol. Med. 2021, 25, 3063–3079. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.M.; Williams, P.A.; Braine, C.E.; Yang, H.S.; Thomas, J.M.; Foxworth, N.E.; John, S.W.M.; Howell, G.R. Complement peptide C3a receptor 1 promotes optic nerve degeneration in DBA/2J mice. J. Neuroinflamm. 2020, 17, 336. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, S.; Doerner, J.D.; Mueller-Buehl, A.M.; Koch, D.; Fuchshofer, R.; Dick, H.B.; Joachim, S.C. Cytokine and Complement Response in the Glaucomatous betaB1-CTGF Mouse Model. Front. Cell. Neurosci. 2021, 15, 718087. [Google Scholar] [CrossRef] [PubMed]
- Dietlein, T.S.; Jacobi, P.C.; Paulsson, M.; Smyth, N.; Krieglstein, G.K. Laminin heterogeneity around Schlemm’s canal in normal humans and glaucoma patients. Ophthalmic Res. 1998, 30, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Carrara, N.; Weaver, M.; Piedade, W.P.; Vocking, O.; Famulski, J.K. Temporal characterization of optic fissure basement membrane composition suggests nidogen may be an initial target of remodeling. Dev. Biol. 2019, 452, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, Y.; Shao, M.; Tang, B.; Cao, W.; Sun, X. Association between coagulation function and patients with primary angle closure glaucoma: A 5-year retrospective case-control study. BMJ Open 2017, 7, e016719. [Google Scholar] [CrossRef]
- Szweras, M.; Liu, D.; Partridge, E.A.; Pawling, J.; Sukhu, B.; Clokie, C.; Jahnen-Dechent, W.; Tenenbaum, H.C.; Swallow, C.J.; Grynpas, M.D.; et al. alpha 2-HS glycoprotein/fetuin, a transforming growth factor-beta/bone morphogenetic protein antagonist, regulates postnatal bone growth and remodeling. J. Biol. Chem. 2002, 277, 19991–19997. [Google Scholar] [CrossRef] [PubMed]
- Montecchi-Palmer, M.; Bermudez, J.Y.; Webber, H.C.; Patel, G.C.; Clark, A.F.; Mao, W. TGFbeta2 Induces the Formation of Cross-Linked Actin Networks (CLANs) in Human Trabecular Meshwork Cells through the Smad and Non-Smad Dependent Pathways. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, K.; Torne, O.; Sun, D.; Moon, A.K.B.; Kiland, J.A.; Trane, R.M.; McLellan, G.J. Aqueous Humor TGF-beta2 and Its Association with Intraocular Pressure in a Naturally Occurring Large Animal Model of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2023, 64, 18. [Google Scholar] [CrossRef] [PubMed]
- Funding, M.; Vorum, H.; Nexo, E.; Ehlers, N. Alpha-1--antitrypsin in aqueous humour from patients with corneal allograft rejection. Acta Ophthalmol. Scand. 2005, 83, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Giachelli, C.M.; Steitz, S. Osteopontin: A versatile regulator of inflammation and biomineralization. Matrix Biol. 2000, 19, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Toma, K.; Kinde, B.; Li, L.; Patel, A.K.; Wu, K.Y.; Lum, M.R.; Tan, C.; Hooper, J.E.; Kriegstein, A.R.; et al. Osteopontin drives retinal ganglion cell resiliency in glaucomatous optic neuropathy. Cell Rep. 2023, 42, 113038. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jakobs, T.C. Secreted phosphoprotein 1 slows neurodegeneration and rescues visual function in mouse models of aging and glaucoma. Cell Rep. 2022, 41, 111880. [Google Scholar] [CrossRef]
- Chowdhury, U.R.; Jea, S.Y.; Oh, D.J.; Rhee, D.J.; Fautsch, M.P. Expression profile of the matricellular protein osteopontin in primary open-angle glaucoma and the normal human eye. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6443–6451. [Google Scholar] [CrossRef]
- Godowski, P.J.; Mark, M.R.; Chen, J.; Sadick, M.D.; Raab, H.; Hammonds, R.G. Reevaluation of the roles of protein S and Gas6 as ligands for the receptor tyrosine kinase Rse/Tyro 3. Cell 1995, 82, 355–358. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jung, S.H.; Jung, D.H.; Choi, S.J.; Lee, Y.R.; Kim, J.S. Gas6 stimulates angiogenesis of human retinal endothelial cells and of zebrafish embryos via ERK1/2 signaling. PLoS ONE 2014, 9, e83901. [Google Scholar] [CrossRef]
- Cruz-Herranz, A.; Oertel, F.C.; Kim, K.; Canto, E.; Timmons, G.; Sin, J.H.; Devereux, M.; Baker, N.; Michel, B.; Schubert, R.D.; et al. Distinctive waves of innate immune response in the retina in experimental autoimmune encephalomyelitis. JCI Insight 2021, 6, e149228. [Google Scholar] [CrossRef] [PubMed]
- Sainaghi, P.P.; Collimedaglia, L.; Alciato, F.; Molinari, R.; Sola, D.; Ranza, E.; Naldi, P.; Monaco, F.; Leone, M.; Pirisi, M.; et al. Growth arrest specific gene 6 protein concentration in cerebrospinal fluid correlates with relapse severity in multiple sclerosis. Mediat. Inflamm. 2013, 2013, 406483. [Google Scholar] [CrossRef] [PubMed]
- Sainaghi, P.P.; Bellan, M.; Lombino, F.; Alciato, F.; Carecchio, M.; Galimberti, D.; Fenoglio, C.; Scarpini, E.; Cantello, R.; Pirisi, M.; et al. Growth Arrest Specific 6 Concentration is Increased in the Cerebrospinal Fluid of Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2017, 55, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Sacca, S.C.; Paluan, F.; Gandolfi, S.; Manni, G.; Cutolo, C.A.; Izzotti, A. Common aspects between glaucoma and brain neurodegeneration. Mutat. Res. Rev. Mutat. Res. 2020, 786, 108323. [Google Scholar] [CrossRef] [PubMed]
- Lledo, V.E.; Alkozi, H.A.; Sanchez-Naves, J.; Fernandez-Torres, M.A.; Guzman-Aranguez, A. Modulation of aqueous humor melatonin levels by yellow-filter and its protective effect on lens. J. Photochem. Photobiol. B 2021, 221, 112248. [Google Scholar] [CrossRef] [PubMed]
- Montero-Calle, A.; Aranguren-Abeigon, I.; Garranzo-Asensio, M.; Poves, C.; Fernández-Aceñero, M.J.; Martínez-Useros, J.; Sanz, R.; Dziaková, J.; Rodriguez-Cobos, J.; Solís-Fernández, G.; et al. Multiplexed biosensing diagnostic platforms detecting autoantibodies to tumor-associated antigens from exosomes released by CRC cells and tissue samples showed high diagnostic ability for colorectal cancer. Engineering 2021, 7, 1393–1412. [Google Scholar] [CrossRef]
- Garranzo-Asensio, M.; Rodriguez-Cobos, J.; San Millan, C.; Poves, C.; Fernandez-Acenero, M.J.; Pastor-Morate, D.; Vinal, D.; Montero-Calle, A.; Solis-Fernandez, G.; Ceron, M.A.; et al. In-depth proteomics characterization of ∆Np73 effectors identifies key proteins with diagnostic potential implicated in lymphangiogenesis, vasculogenesis and metastasis in colorectal cancer. Mol. Oncol. 2022, 16, 2672–2692. [Google Scholar] [CrossRef] [PubMed]
- Montero-Calle, A.; Lopez-Janeiro, A.; Mendes, M.L.; Perez-Hernandez, D.; Echevarria, I.; Ruz-Caracuel, I.; Heredia-Soto, V.; Mendiola, M.; Hardisson, D.; Argueso, P.; et al. In-depth quantitative proteomics analysis revealed C1GALT1 depletion in ECC-1 cells mimics an aggressive endometrial cancer phenotype observed in cancer patients with low C1GALT1 expression. Cell. Oncol. 2023, 46, 697–715. [Google Scholar] [CrossRef]
- Montero-Calle, A.; Coronel, R.; Garranzo-Asensio, M.; Solis-Fernandez, G.; Rabano, A.; de Los Rios, V.; Fernandez-Acenero, M.J.; Mendes, M.L.; Martinez-Useros, J.; Megias, D.; et al. Proteomics analysis of prefrontal cortex of Alzheimer’s disease patients revealed dysregulated proteins in the disease and novel proteins associated with amyloid-beta pathology. Cell. Mol. Life Sci. 2023, 80, 141. [Google Scholar] [CrossRef]
- Solis-Fernandez, G.; Montero-Calle, A.; Martinez-Useros, J.; Lopez-Janeiro, A.; de Los Rios, V.; Sanz, R.; Dziakova, J.; Milagrosa, E.; Fernandez-Acenero, M.J.; Pelaez-Garcia, A.; et al. Spatial Proteomic Analysis of Isogenic Metastatic Colorectal Cancer Cells Reveals Key Dysregulated Proteins Associated with Lymph Node, Liver, and Lung Metastasis. Cells 2022, 11, 447. [Google Scholar] [CrossRef]
- Montero-Calle, A.; Garranzo-Asensio, M.; Rejas-Gonzalez, R.; Feliu, J.; Mendiola, M.; Pelaez-Garcia, A.; Barderas, R. Benefits of FAIMS to Improve the Proteome Coverage of Deteriorated and/or Cross-Linked TMT 10-Plex FFPE Tissue and Plasma-Derived Exosomes Samples. Proteomes 2023, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Garranzo-Asensio, M.; Solis-Fernandez, G.; Montero-Calle, A.; Garcia-Martinez, J.M.; Fiuza, M.C.; Pallares, P.; Palacios-Garcia, N.; Garcia-Jimenez, C.; Guzman-Aranguez, A.; Barderas, R. Seroreactivity against Tyrosine Phosphatase PTPRN Links Type 2 Diabetes and Colorectal Cancer and Identifies a Potential Diagnostic and Therapeutic Target. Diabetes 2022, 71, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Garranzo-Asensio, M.; Guzman-Aranguez, A.; Poves, C.; Fernandez-Acenero, M.J.; Montero-Calle, A.; Ceron, M.A.; Fernandez-Diez, S.; Rodriguez, N.; Gomez de Cedron, M.; Ramirez de Molina, A.; et al. The specific seroreactivity to ∆Np73 isoforms shows higher diagnostic ability in colorectal cancer patients than the canonical p73 protein. Sci. Rep. 2019, 9, 13547. [Google Scholar] [CrossRef] [PubMed]
- Garranzo-Asensio, M.; San Segundo-Acosta, P.; Poves, C.; Fernandez-Acenero, M.J.; Martinez-Useros, J.; Montero-Calle, A.; Solis-Fernandez, G.; Sanchez-Martinez, M.; Rodriguez, N.; Ceron, M.A.; et al. Identification of tumor-associated antigens with diagnostic ability of colorectal cancer by in-depth immunomic and seroproteomic analysis. J. Proteom. 2020, 214, 103635. [Google Scholar] [CrossRef] [PubMed]
| Samples | Number (N) | Age Average ± SD (Years) | Min. Age (Years) | Max. Age (Years) | Gender (N) | ||
|---|---|---|---|---|---|---|---|
| M | F | ||||||
| TMT | ICL | 19 | 42.1 (±11.42) | 22 | 59 | 8 | 11 |
| Cataracts | 40 | 67.27 (±10.03) | 40 | 82 | 15 | 25 | |
| Glaucoma | 13 | 67.54 (±15.72) | 26 | 89 | 4 | 9 | |
| WB | ICL | 4 | 47.5 (±14.43) | 26 | 57 | 1 | 3 |
| Cataracts | 5 | 68.6 (±7.73) | 60 | 79 | 3 | 2 | |
| Glaucoma | 5 | 66.4 (±12.66) | 51 | 80 | 1 | 4 | |
| Pool ICL | 4 | 45.25 (±12.34) | 31 | 57 | 1 | 3 | |
| Pool cataracts | 5 | 73.6 (±14.10) | 55 | 89 | 2 | 3 | |
| Pool glaucoma | 4 | 62.25 (±11.00) | 55 | 74 | 1 | 3 | |
| ELISA | ICL | 39 | 43.38 (±12.05) | 22 | 59 | 10 | 29 |
| Cataracts | 96 | 69.32 (±10.67) | 41 | 91 | 35 | 57 | |
| Glaucoma | 31 | 70.19 (±20) | 26 | 89 | 10 | 21 | |
| Protein Name | Analysis | Dysregulation | Log2 Ratio * | p-Value | Exocarta |
|---|---|---|---|---|---|
| APOA1 | ICL–Cataracts | Upregulated | 1.21 | 0.024 | Colorectal cancer cells, liver cancer cells, melanoma cells, etc. |
| DBF4B | ICL–Cataracts | Upregulated | 2.00 | 0.031 | Mesenchymal stem cells |
| SPP1 | ICL–Cataracts | Upregulated | 2.15 | 0.002 | Ovarian cancer cells |
| Cataracts–Glaucoma | Downregulated | −1.42 | 0.007 | ||
| GAS6 | ICL–Cataracts | Upregulated | 1.17 | 0.049 | Colorectal cancer cells, mesenchymal stem cells, nasopharyngeal carcinoma cells |
| Cataracts–Glaucoma | Downregulated | −1.29 | 0.008 | ||
| APLP2 | ICL–Cataracts | Upregulated | 2.85 | 0.025 | Colorectal cancer cells, ovarian cancer cells, saliva |
| FGB | ICL–Glaucoma | Downregulated | −1.97 | 0.021 | Hepatocellular carcinoma cells, malignant pleural effusions, mesenchymal stem cells, plasma, etc. |
| SERPINA1 | Cataracts–Glaucoma | Downregulated | −1.11 | 0.03 | Colorectal cancer cells, hepatocellular carcinoma cells, liver cancer cells, plasma, platelets, urine, etc. |
| SPTA1 | Cataracts–Glaucoma | Downregulated | −1.98 | 0.021 | - |
| AHSG | Cataracts–Glaucoma | Downregulated | −1.96 | 0.026 | B-cells, colorectal cancer cells, hepatocellular carcinoma cells, melanoma, mesenchymal stem cells, platelets, prostate cancer cells, urine, etc. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rejas-González, R.; Montero-Calle, A.; Valverde, A.; Salvador, N.P.; Carballés, M.J.C.; Ausín-González, E.; Sánchez-Naves, J.; Campuzano, S.; Barderas, R.; Guzman-Aranguez, A. Proteomics Analyses of Small Extracellular Vesicles of Aqueous Humor: Identification and Validation of GAS6 and SPP1 as Glaucoma Markers. Int. J. Mol. Sci. 2024, 25, 6995. https://doi.org/10.3390/ijms25136995
Rejas-González R, Montero-Calle A, Valverde A, Salvador NP, Carballés MJC, Ausín-González E, Sánchez-Naves J, Campuzano S, Barderas R, Guzman-Aranguez A. Proteomics Analyses of Small Extracellular Vesicles of Aqueous Humor: Identification and Validation of GAS6 and SPP1 as Glaucoma Markers. International Journal of Molecular Sciences. 2024; 25(13):6995. https://doi.org/10.3390/ijms25136995
Chicago/Turabian StyleRejas-González, Raquel, Ana Montero-Calle, Alejandro Valverde, Natalia Pastora Salvador, María José Crespo Carballés, Emma Ausín-González, Juan Sánchez-Naves, Susana Campuzano, Rodrigo Barderas, and Ana Guzman-Aranguez. 2024. "Proteomics Analyses of Small Extracellular Vesicles of Aqueous Humor: Identification and Validation of GAS6 and SPP1 as Glaucoma Markers" International Journal of Molecular Sciences 25, no. 13: 6995. https://doi.org/10.3390/ijms25136995






