The Role of Piromelatine on Peripheral and Hippocampal Insulin Resistance in Rat Offspring Exposed to Chronic Maternal Stress
Abstract
:1. Introduction
2. Results
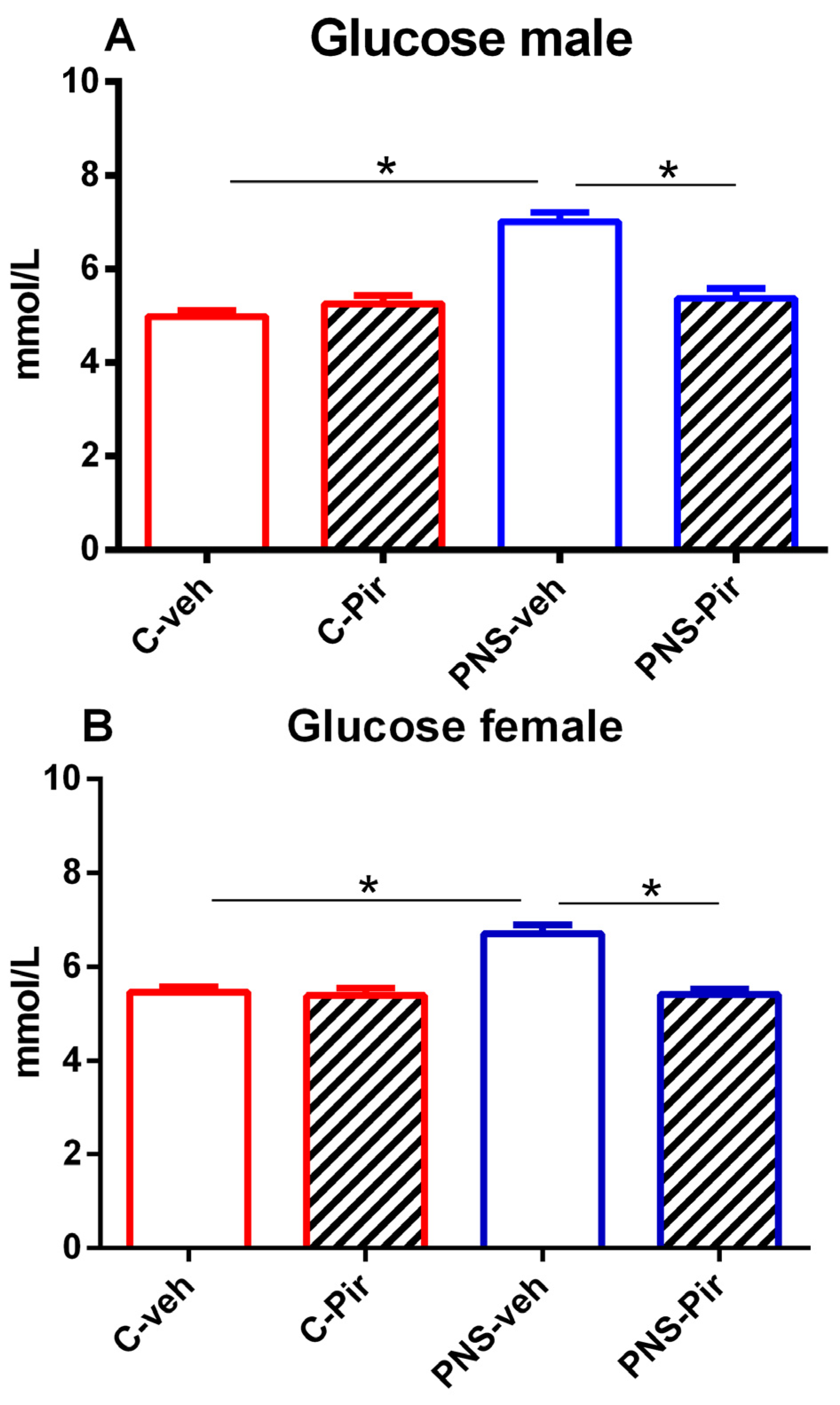
2.1. The Effects of Chronic Pir Administration on Plasma Glucose Levels in Prenatally Stressed Male and Female Offspring
2.2. The Effects of Chronic Pir Administration on Serum Insulin Levels in Prenatally Stressed Male and Female Offspring
2.3. The Effects of Chronic Pir Administration on HOMA-IR in Prenatally Stressed Male and Female Offspring (Measurement of Fasting Plasma Glucose and Serum Insulin)
2.4. The Effects of Chronic Pir Administration on GLUT4 Protein Levels in the Hippocampus of Prenatally Stressed Male and Female Offspring
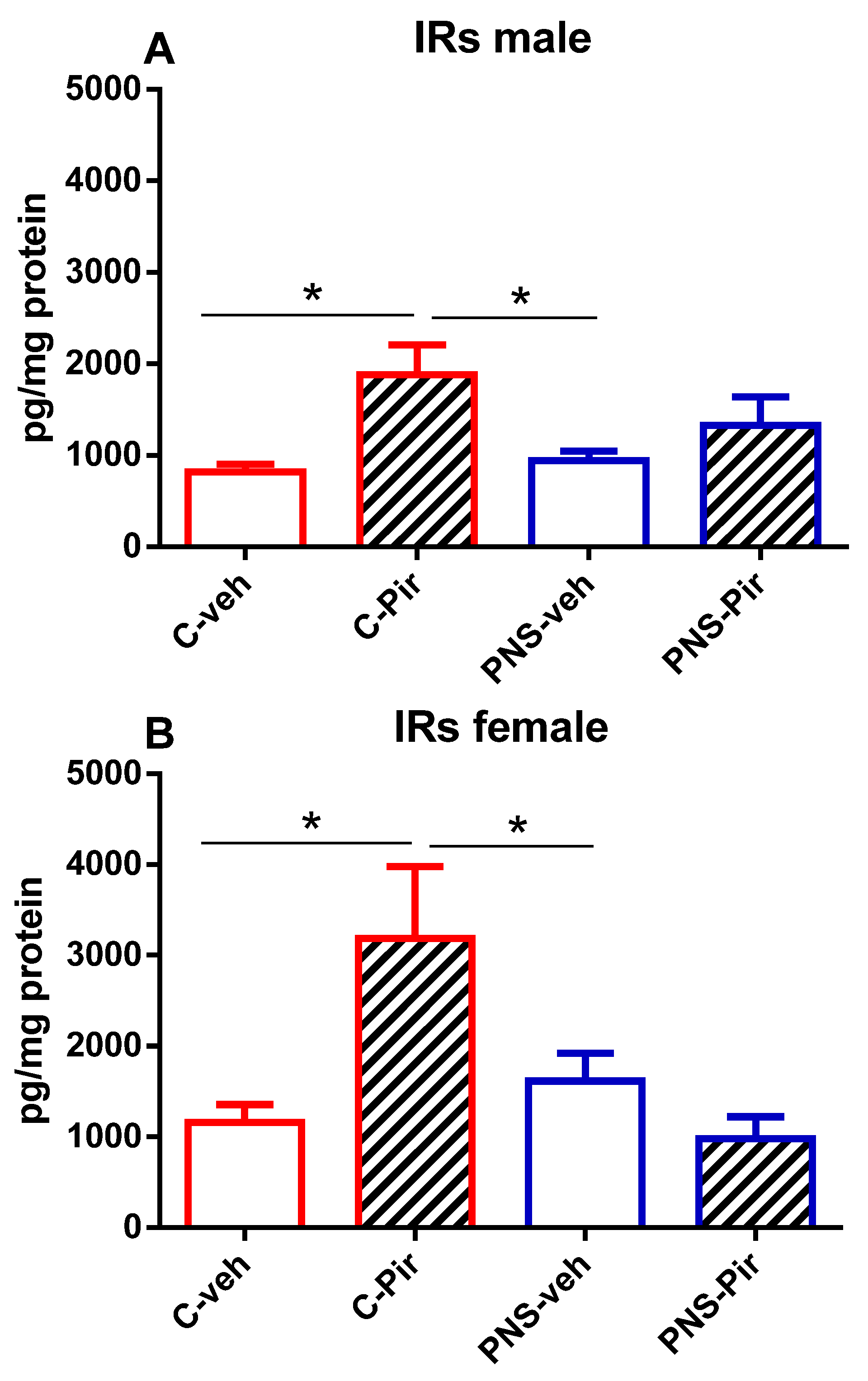
2.5. The Effects of Chronic Pir Administration on IRs Protein Levels in the Hippocampus of Prenatally Stressed Male and Female Offspring
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Procedure for PNS
4.3. Piromelatine Administration and Protocol Design
4.4. Biochemical Methods
4.4.1. Measurement of Serum Insulin and Plasma Glucose
4.4.2. HOMA-IR
4.4.3. Measurement of GLUT4 and IRs in the Hippocampus
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coussons-Read, M.E. Effects of Prenatal Stress on Pregnancy and Human Development: Mechanisms and Pathways. Obstet. Med. 2013, 6, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Soares-Cunha, C.; Coimbra, B.; Borges, S.; Domingues, A.V.; Silva, D.; Sousa, N.; Rodrigues, A.J. Mild Prenatal Stress Causes Emotional and Brain Structural Modifications in Rats of Both Sexes. Front. Behav. Neurosci. 2018, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, N.; Nenchovska, Z.; Atanasova, M.; Laudon, M.; Mitreva, R.; Tchekalarova, J. Chronic Piromelatine Treatment Alleviates Anxiety, Depressive Responses and Abnormal Hypothalamic–Pituitary–Adrenal Axis Activity in Prenatally Stressed Male and Female Rats. Cell. Mol. Neurobiol. 2021, 42, 2257–2272. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, N.; Nenchovska, Z.; Atanasova, M.; Mitreva, R.; Stoynova, T.; Kortenska, L.; Tchekalarova, J. Sex-Dependent Differences of Emotional Status in a Rat Model of Prenatal Stress. C. R. Acad. Bulg. Sci. 2022, 75, 1082–1088. [Google Scholar] [CrossRef]
- Reynolds, R.M. Corticosteroid-Mediated Programming and the Pathogenesis of Obesity and Diabetes. J. Steroid Biochem. Mol. Biol. 2010, 122, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, S.; Juárez, Y.R.; Marcone, M.P.; Vidal, M.A.; Genaro, A.M.; Burgueño, A.L. Prenatal Stress Promotes Insulin Resistance without Inflammation or Obesity in C57BL/6J Male Mice. Stress 2021, 24, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Flier, J.S. Insulin Receptors and Insulin Resistance. Annu. Rev. Med. 1983, 34, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.; Fusco, S.; Grassi, C. Brain Insulin Resistance and Hippocampal Plasticity: Mechanisms and Biomarkers of Cognitive Decline. Front. Neurosci. 2019, 13, 788. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Reagan, L.P. Hippocampal Insulin Resistance and Cognitive Dysfunction. Nat. Rev. Neurosci. 2015, 16, 660–671. [Google Scholar] [CrossRef] [PubMed]
- McNay, E.C.; Recknagel, A.K. Brain Insulin Signaling: A Key Component of Cognitive Processes and a Potential Basis for Cognitive Impairment in Type 2 Diabetes. Neurobiol. Learn. Mem. 2011, 96, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.M.; Acevedo, L.A.; Pennings, N. Insulin Resistance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Mcmahon, M.; Gerich, J.; Rizza, R. Effects of Glucocorticoids on Carbohydrate Metabolism. Diabetes Metab. Rev. 1988, 4, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Głombik, K.; Detka, J.; Góralska, J.; Kurek, A.; Solnica, B.; Budziszewska, B. Brain Metabolic Alterations in Rats Showing Depression-Like and Obesity Phenotypes. Neurotox. Res. 2020, 37, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Vallée, M.; Mayo, W.; Maccari, S.; Le Moal, M.; Simon, H. Long-Term Effects of Prenatal Stress and Handling on Metabolic Parameters: Relationship to Corticosterone Secretion Response. Brain Res. 1996, 712, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Darnaudéry, M.; Maccari, S. Epigenetic Programming of the Stress Response in Male and Female Rats by Prenatal Restraint Stress. Brain Res. Rev. 2008, 57, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Eberle, C.; Fasig, T.; Brüseke, F.; Stichling, S. Impact of Maternal Prenatal Stress by Glucocorticoids on Metabolic and Cardiovascular Outcomes in Their Offspring: A Systematic Scoping Review. PLoS ONE 2021, 16, e0245386. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, J.; Hu, X.; Huang, X.; Chen, G.-X. Current Understanding of Glucose Transporter 4 Expression and Functional Mechanisms. WJBC 2020, 11, 76–98. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Pandey, S.; Rumman, M.; Singh, B.; Mahdi, A.A. Molecular Mechanisms Underlying Hyperglycemia Associated Cognitive Decline. IBRO Neurosci. Rep. 2023, 14, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Piroli, G.G.; Grillo, C.A.; Reznikov, L.R.; Adams, S.; McEwen, B.S.; Charron, M.J.; Reagan, L.P. Corticosterone Impairs Insulin-Stimulated Translocation of GLUT4 in the Rat Hippocampus. Neuroendocrinology 2007, 85, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Schernthaner-Reiter, M.H.; Wolf, P.; Vila, G.; Luger, A. The Interaction of Insulin and Pituitary Hormone Syndromes. Front. Endocrinol. 2021, 12, 626427. [Google Scholar] [CrossRef] [PubMed]
- Beaupere, C.; Liboz, A.; Fève, B.; Blondeau, B.; Guillemain, G. Molecular Mechanisms of Glucocorticoid-Induced Insulin Resistance. IJMS 2021, 22, 623. [Google Scholar] [CrossRef] [PubMed]
- Geer, E.B.; Islam, J.; Buettner, C. Mechanisms of Glucocorticoid-Induced Insulin Resistance. Endocrinol. Metab. Clin. N. Am. 2014, 43, 75–102. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, N.; Atanasova, M.; Nenchovska, Z.; Tchekalarova, J. Sex-Dependent Effect of Chronic Piromelatine Treatment on Prenatal Stress-Induced Memory Deficits in Rats. IJMS 2023, 24, 1271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, R.; Li, Z.; Li, X.; Zhang, K.; Ge, Y.; Kong, X.; Liu, X.; Chen, G. Melatonin Improves Maternal Sleep Deprivation-induced Learning and Memory Impairment, Inflammation, and Synaptic Dysfunction in Murine Male Adult Offspring. Brain Behav. 2024, 14, e3515. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Liao, Q.; Wong, Y.K.; Chen, X.; Yang, C.; Xu, C.; Sun, J.; Wang, J. The Role of Melatonin in the Treatment of Type 2 Diabetes Mellitus and Alzheimer’s Disease. Int. J. Biol. Sci. 2022, 18, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Z. Effects of Melatonin Supplementation on Insulin Levels and Insulin Resistance: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm. Metab. Res. 2021, 53, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Laudon, M.; Nir, T.; Zisapel, N.S. 27.03 Development of Piromelatine, a Novel Multimodal Sleep Medicine. Eur. Neuropsychopharmacol. 2014, 24, S145. [Google Scholar] [CrossRef]
- Tchekalarova, J.; Kortenska, L.; Marinov, P.; Ivanova, N. Sex-Dependent Effects of Piromelatine Treatment on Sleep-Wake Cycle and Sleep Structure of Prenatally Stressed Rats. IJMS 2022, 23, 10349. [Google Scholar] [CrossRef] [PubMed]
- She, M.; Deng, X.; Guo, Z.; Laudon, M.; Hu, Z.; Liao, D.; Hu, X.; Luo, Y.; Shen, Q.; Su, Z.; et al. NEU-P11, a Novel Melatonin Agonist, Inhibits Weight Gain and Improves Insulin Sensitivity in High-Fat/High-Sucrose-Fed Rats. Pharmacol. Res. 2009, 59, 248–253. [Google Scholar] [CrossRef] [PubMed]
- She, M.; Hu, X.; Su, Z.; Zhang, C.; Yang, S.; Ding, L.; Laudon, M.; Yin, W. Piromelatine, a Novel Melatonin Receptor Agonist, Stabilizes Metabolic Profiles and Ameliorates Insulin Resistance in Chronic Sleep Restricted Rats. Eur. J. Pharmacol. 2014, 727, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, D.; Luo, X.; Jia, X.; Li, M.; Laudon, M.; Zhang, R.; Jia, Z. Melatonin Receptor Agonist Piromelatine Ameliorates Impaired Glucose Metabolism in Chronically Stressed Rats Fed a High-Fat Diet. J. Pharmacol. Exp. Ther. 2018, 364, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, J.; Li, X.; Li, Y.; Zhou, Y.; Cai, S. Neu-P11—A Novel Melatonin Receptor Agonist, Could Improve the Features of Type-2 Diabetes Mellitus in Rats. Endokrynol. Pol. 2021, 72, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, S.; Yin, W.; Hu, X. Neu-P11 Reduces Clock/Apelin Expression in Insulin-Resistant Mouse Adipocyte Model. ABBS 2013, 45, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, S.; Yin, W.; Hu, X.; Zhang, S.; Li, Z.; Li, X.; Laudon, M. Role of Neu-P11/Luzindole in the Regulation of Insulin Signaling Pathways and Insulin Resistance. ABBS 2016, 48, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Detka, J.; Kurek, A.; Basta-Kaim, A.; Kubera, M.; Lasoń, W.; Budziszewska, B. Elevated Brain Glucose and Glycogen Concentrations in an Animal Model of Depression. Neuroendocrinology 2014, 100, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Lesage, J.; Del-Favero, F.; Leonhardt, M.; Louvart, H.; Maccari, S.; Vieau, D.; Darnaudery, M. Prenatal Stress Induces Intrauterine Growth Restriction and Programmes Glucose Intolerance and Feeding Behaviour Disturbances in the Aged Rat. J. Endocrinol. 2004, 181, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Drake, A.J.; Tang, J.I.; Nyirenda, M.J. Mechanisms Underlying the Role of Glucocorticoids in the Early Life Programming of Adult Disease. Clin. Sci. 2007, 113, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Boersma, G.J.; Tamashiro, K.L. Individual Differences in the Effects of Prenatal Stress Exposure in Rodents. Neurobiol. Stress 2015, 1, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Wyrwoll, C.S.; Mark, P.J.; Mori, T.A.; Waddell, B.J. Developmental Programming of Adult Hyperinsulinemia, Increased Proinflammatory Cytokine Production, and Altered Skeletal Muscle Expression of SLC2A4 (GLUT4) and Uncoupling Protein 3. J. Endocrinol. 2008, 198, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Franko, K.L.; Forhead, A.J.; Fowden, A.L. Differential Effects of Prenatal Stress and Glucocorticoid Administration on Postnatal Growth and Glucose Metabolism in Rats. J. Endocrinol. 2010, 204, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-C.; Tiao, M.-M.; Sheen, J.-M.; Huang, L.-T.; Tain, Y.-L.; Lin, I.-C.; Lin, Y.-J.; Lai, Y.-J.; Chen, C.-C.; Chang, K.-A.; et al. Obesity Programmed by Prenatal Dexamethasone and Postnatal High-Fat Diet Leads to Distinct Alterations in Nutrition Sensory Signals and Circadian-Clock Genes in Visceral Adipose Tissue. Lipids Health Dis. 2019, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Mercau, M.E.; Calanni, J.S.; Aranda, M.L.; Caldareri, L.J.; Rosenstein, R.E.; Repetto, E.M.; Cymeryng, C.B. Melatonin Prevents Early Pituitary Dysfunction Induced by Sucrose-rich Diets. J. Pineal Res. 2019, 66, e12545. [Google Scholar] [CrossRef] [PubMed]
- Kanoski, S.E.; Davidson, T.L. Different Patterns of Memory Impairments Accompany Short- and Longer-Term Maintenance on a High-Energy Diet. J. Exp.Psychol. Anim. Behav. Process. 2010, 36, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Pearson-Leary, J.; McNay, E.C. Novel Roles for the Insulin-Regulated Glucose Transporter-4 in Hippocampally Dependent Memory. J. Neurosci. 2016, 36, 11851–11864. [Google Scholar] [CrossRef] [PubMed]
- Ter Horst, G.J. Estrogen in the Limbic System. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2010; Volume 82, pp. 319–338. ISBN 978-0-12-381515-6. [Google Scholar]
- Faustini-Fustini, M.; Rochira, V.; Carani, C. Oestrogen Deficiency in Men: Where Are We Today? Eur. J. Endocrinol. 1999, 140, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.R.; Clegg, D.J.; Prossnitz, E.R.; Barton, M. Obesity, Insulin Resistance and Diabetes: Sex Differences and Role of Oestrogen Receptors: Obesity, Insulin Resistance and Diabetes: Role of Oestrogen. Acta Physiol. 2011, 203, 259–269. [Google Scholar] [CrossRef] [PubMed]


| Group | Male | Female |
|---|---|---|
| C-veh | 2.05 ± 0.13 | 2.43 ± 0.03 |
| C-Pir | 2.30 ± 0.10 | 2.32 ± 0.07 |
| PNS-veh | 4.58 ± 0.32 * | 4.35 ± 0.25 * |
| PNS-Pir | 1.97 ± 0.14 o | 2.47 ± 0.05 o |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, N.; Atanasova, M.; Terzieva, D.; Georgieva, K.; Tchekalarova, J. The Role of Piromelatine on Peripheral and Hippocampal Insulin Resistance in Rat Offspring Exposed to Chronic Maternal Stress. Int. J. Mol. Sci. 2024, 25, 7022. https://doi.org/10.3390/ijms25137022
Ivanova N, Atanasova M, Terzieva D, Georgieva K, Tchekalarova J. The Role of Piromelatine on Peripheral and Hippocampal Insulin Resistance in Rat Offspring Exposed to Chronic Maternal Stress. International Journal of Molecular Sciences. 2024; 25(13):7022. https://doi.org/10.3390/ijms25137022
Chicago/Turabian StyleIvanova, Natasha, Milena Atanasova, Dora Terzieva, Katerina Georgieva, and Jana Tchekalarova. 2024. "The Role of Piromelatine on Peripheral and Hippocampal Insulin Resistance in Rat Offspring Exposed to Chronic Maternal Stress" International Journal of Molecular Sciences 25, no. 13: 7022. https://doi.org/10.3390/ijms25137022
APA StyleIvanova, N., Atanasova, M., Terzieva, D., Georgieva, K., & Tchekalarova, J. (2024). The Role of Piromelatine on Peripheral and Hippocampal Insulin Resistance in Rat Offspring Exposed to Chronic Maternal Stress. International Journal of Molecular Sciences, 25(13), 7022. https://doi.org/10.3390/ijms25137022







