Designed De Novo α-Sheet Peptides Destabilize Bacterial Biofilms and Increase the Susceptibility of E. coli and S. aureus to Antibiotics
Abstract
:1. Introduction
2. Results
2.1. E. coli UTI89 and S. aureus MN8 Show Low Biofilm Antibiotic Susceptibility
2.2. α-Sheet Peptides Inhibit Amyloid Formation and Reduce Biofilm Density
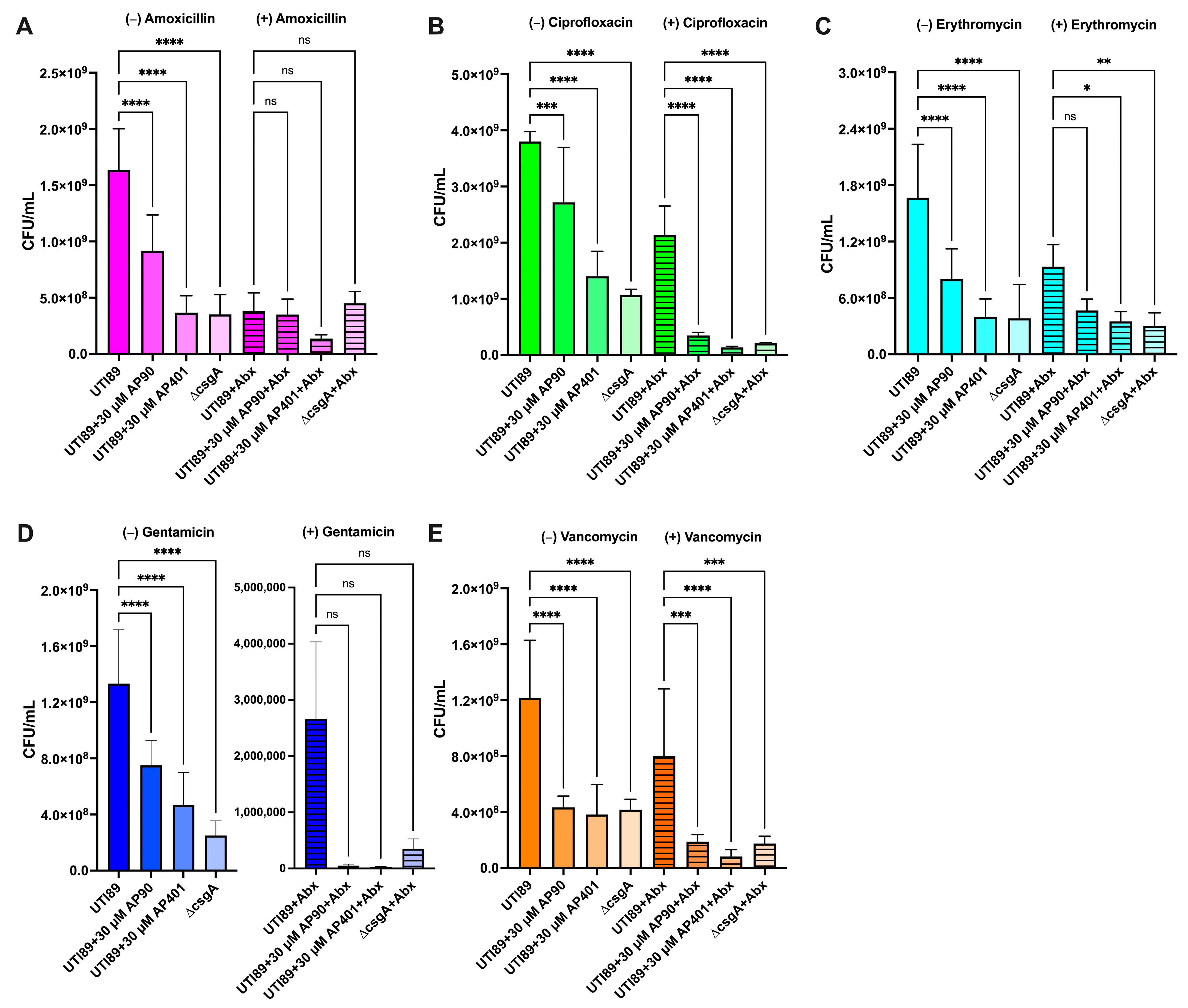
2.3. Curli Fibril Inhibition by AP90 and AP401 Render E. coli More Susceptible to Antibiotics
2.3.1. UTI89 Susceptibility: Gentamicin
2.3.2. UTI89 Susceptibility: Ciprofloxacin
2.3.3. UTI89 Susceptibility: Vancomycin
2.3.4. UTI89 Susceptibility: Amoxicillin
2.3.5. UTI89 Susceptibility: Erythromycin
2.4. AP90 and AP401 Increase S. aureus Biofilm Susceptibility to Antibiotics
2.4.1. MN8 Susceptibility: Vancomycin
2.4.2. MN8 Susceptibility: Erythromycin
2.4.3. MN8 Susceptibility: Ciprofloxacin
2.4.4. MN8 Susceptibility: Amoxicillin
2.4.5. MN8 Susceptibility: Gentamicin
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis
4.2. E. coli Biofilm Growth
4.3. S. aureus Biofilm Growth
4.4. Thioflavin T (ThT) Assay, Biofilm and Total Density Measurements
4.5. Growth Curves
4.6. Antibiotic Susceptibility
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.J.; Vendruscolo, M.; Dobson, C.M. The Amyloid State and Its Association with Protein Misfolding Diseases. Nat. Rev. Mol. Cell Biol. 2014, 15, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Prosswimmer, T.; Daggett, V. The Role of α-Sheet Structure in Amyloidogenesis: Characterization and Implications. Open Biol. 2022, 12, 220261. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.F.; Hellewell, A.L.; Gosal, W.S.; Homans, S.W.; Hewitt, E.W.; Radford, S.E. Fibril Fragmentation Enhances Amyloid Cytotoxicity. J. Biol. Chem. 2009, 284, 34272–34282. [Google Scholar] [CrossRef] [PubMed]
- Tomic, J.L.; Pensalfini, A.; Head, E.; Glabe, C.G. Soluble Fibrillar Oligomer Levels Are Elevated in Alzheimer’s Disease Brain and Correlate with Cognitive Dysfunction. Neurobiol. Dis. 2009, 35, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Bucciantini, M.; Giannoni, E.; Chiti, F.; Baroni, F.; Formigli, L.; Zurdo, J.; Taddei, N.; Ramponi, G.; Dobson, C.M.; Stefani, M. Inherent Toxicity of Aggregates Implies a Common Mechanism for Protein Misfolding Diseases. Nature 2002, 416, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Hopping, G.; Kellock, J.; Barnwal, R.P.; Law, P.; Bryers, J.; Varani, G.; Caughey, B.; Daggett, V. Designed α-Sheet Peptides Inhibit Amyloid Formation by Targeting Toxic Oligomers. eLife 2014, 3, e01681. [Google Scholar] [CrossRef] [PubMed]
- Shea, D.; Hsu, C.-C.; Bi, T.M.; Paranjapye, N.; Childers, M.C.; Cochran, J.; Tomberlin, C.P.; Wang, L.; Paris, D.; Zonderman, J.; et al. α-Sheet Secondary Structure in Amyloid β-Peptide Drives Aggregation and Toxicity in Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2019, 116, 8895–8900. [Google Scholar] [CrossRef]
- Bleem, A.; Prosswimmer, T.; Chen, R.; Hady, T.F.; Li, J.; Bryers, J.D.; Daggett, V. Designed α-Sheet Peptides Disrupt Uropathogenic E. coli Biofilms Rendering Bacteria Susceptible to Antibiotics and Immune Cells. Sci. Rep. 2023, 13, 9272. [Google Scholar] [CrossRef]
- Evans, M.L.; Chapman, M.R. Curli Biogenesis: Order out of Disorder. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 1551–1558. [Google Scholar] [CrossRef]
- Bleem, A.; Francisco, R.; Bryers, J.D.; Daggett, V. Designed α-Sheet Peptides Suppress Amyloid Formation in Staphylococcus Aureus Biofilms. NPJ Biofilms Microbiomes 2017, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Andreasen, M. Cross-Talk between Individual Phenol- Soluble Modulins in Staphylococcus Aureus Biofilm Enables Rapid and Efficient Amyloid Formation. eLife 2020, 9, e59776. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.; Syed, A.K.; Stephenson, R.E.; Rickard, A.H.; Boles, B.R. Functional Amyloids Composed of Phenol Soluble Modulins Stabilize Staphylococcus Aureus Biofilms. PLoS Pathog. 2012, 8, e1002744. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, S.; Xu, H.; Walsh, D.M.; Selkoe, D.J. Large Soluble Oligomers of Amyloid β-Protein from Alzheimer Brain Are Far Less Neuroactive Than the Smaller Oligomers to Which They Dissociate. J. Neurosci. 2017, 37, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Gurlo, T.; Kayed, R.; Butler, A.E.; Haataja, L.; Glabe, C.G.; Butler, P.C. Toxic Human Islet Amyloid Polypeptide (h-IAPP) Oligomers Are Intracellular, and Vaccination to Induce Anti-Toxic Oligomer Antibodies Does Not Prevent h-IAPP–Induced β-Cell Apoptosis in h-IAPP Transgenic Mice. Diabetes 2007, 56, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Haataja, L.; Gurlo, T.; Huang, C.J.; Butler, P.C. Islet Amyloid in Type 2 Diabetes, and the Toxic Oligomer Hypothesis. Endocr. Rev. 2008, 29, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Kayed, R.; Head, E.; Thompson, J.L.; McIntire, T.M.; Milton, S.C.; Cotman, C.W.; Glabe, C.G. Common Structure of Soluble Amyloid Oligomers Implies Common Mechanism of Pathogenesis. Science 2003, 300, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Paranjapye, N.; Daggett, V. De Novo Designed α-Sheet Peptides Inhibit Functional Amyloid Formation of Streptococcus Mutans Biofilms. J. Mol. Biol. 2018, 430, 3764–3773. [Google Scholar] [CrossRef]
- Kellock, J.; Hopping, G.; Caughey, B.; Daggett, V. Peptides Composed of Alternating L- and D-Amino Acids Inhibit Amyloidogenesis in Three Distinct Amyloid Systems Independent of Sequence. J. Mol. Biol. 2016, 428, 2317–2328. [Google Scholar] [CrossRef] [PubMed]
- Bi, T.M.; Daggett, V. The Role of α-Sheet in Amyloid Oligomer Aggregation and Toxicity. Yale J. Biol. Med. 2018, 91, 247–255. [Google Scholar] [PubMed]
- Maris, N.L.; Shea, D.; Bleem, A.; Bryers, J.D.; Daggett, V. Chemical and Physical Variability in Structural Isomers of an l/d α-Sheet Peptide Designed to Inhibit Amyloidogenesis. Biochemistry 2018, 57, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Daggett, V. Alpha-Sheet: The Toxic Conformer in Amyloid Diseases? Acc. Chem. Res. 2006, 39, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus Aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent Bacterial Infections and Persister Cells. Nat. Rev. Microbiol. 2017, 15, 453–464. [Google Scholar] [CrossRef]
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial Infections: Epidemiology, Prevention, Control and Surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-Resistant Pathogens Associated with Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control. Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.M.; Fridkin, S.K.; Aponte-Torres, Z.; Avery, L.; Coffin, N.; Dudeck, M.A.; Edwards, J.R.; Jernigan, J.A.; Konnor, R.; Soe, M.M.; et al. Vital Signs: Preventing Antibiotic-Resistant Infections in Hospitals—United States, 2014. Am. J. Transplant. 2016, 16, 2224–2230. [Google Scholar] [CrossRef]
- CDC. Antibiotic Resistance Threats in the United States, 2019; CDC: Atlanta, GA, USA, 2019.
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, VMBF-0016-2015. [Google Scholar] [CrossRef]
- Lewis, K. Multidrug Tolerance of Biofilms and Persister Cells. Bact. Biofilms 2008, 322, 107–131. [Google Scholar] [CrossRef]
- Banin, E.; Brady, K.M.; Greenberg, E.P. Chelator-Induced Dispersal and Killing of Pseudomonas Aeruginosa Cells in a Biofilm. Appl. Environ. Microbiol. 2006, 72, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial Exo-Polysaccharides in Biofilms: Role in Antimicrobial Resistance and Treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Kot, B. Antibiotic Resistance among Uropathogenic Escherichia coli. Pol. J. Microbiol. 2019, 68, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Al-Talib, H.; Yean, C.; Al-Jashamy, K.; Hasan, H. Methicillin-Resistant Staphylococcus Aureus Nosocomial Infection Trends in Hospital Universiti Sains Malaysia during 2002–2007. Ann. Saudi Med. 2010, 30, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Su, T.; Wu, H.; Liu, S.; Wang, D.; Zhao, T.; Jin, Z.; Du, W.; Zhu, M.-J.; Chua, S.L.; et al. PslG, a Self-Produced Glycosyl Hydrolase, Triggers Biofilm Disassembly by Disrupting Exopolysaccharide Matrix. Cell Res. 2015, 25, 1352–1367. [Google Scholar] [CrossRef] [PubMed]
- Devlin, H.; Fulaz, S.; Hiebner, D.W.; O’Gara, J.P.; Casey, E. Enzyme-Functionalized Mesoporous Silica Nanoparticles to Target Staphylococcus Aureus and Disperse Biofilms. Int. J. Nanomed. 2021, 16, 1929–1942. [Google Scholar] [CrossRef] [PubMed]
- Okshevsky, M.; Regina, V.R.; Meyer, R.L. Extracellular DNA as a Target for Biofilm Control. Curr. Opin. Biotechnol. 2015, 33, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, Y.; Kim, D.; Li, Y.; Hwang, G.; Naha, P.C.; Cormode, D.P.; Koo, H. Nanocatalysts Promote Streptococcus Mutans Biofilm Matrix Degradation and Enhance Bacterial Killing to Suppress Dental Caries in Vivo. Biomaterials. 2016, 101, 272–284. [Google Scholar] [CrossRef]
- Wang, Y.; Kadiyala, U.; Qu, Z.; Elvati, P.; Altheim, C.; Kotov, N.A.; Violi, A.; VanEpps, J.S. Anti-Biofilm Activity of Graphene Quantum Dots via Self-Assembly with Bacterial Amyloid Proteins. ACS Nano 2019, 13, 4278–4289. [Google Scholar] [CrossRef]
- Powell, L.C.; Pritchard, M.F.; Ferguson, E.L.; Powell, K.A.; Patel, S.U.; Rye, P.D.; Sakellakou, S.-M.; Buurma, N.J.; Brilliant, C.D.; Copping, J.M.; et al. Targeted Disruption of the Extracellular Polymeric Network of Pseudomonas Aeruginosa Biofilms by Alginate Oligosaccharides. NPJ Biofilms Microbiomes 2018, 4, 13. [Google Scholar] [CrossRef]
- Cegelski, L.; Pinkner, J.S.; Hammer, N.D.; Cusumano, C.K.; Hung, C.S.; Chorell, E.; Åberg, V.; Walker, J.N.; Seed, P.C.; Almqvist, F.; et al. Small-Molecule Inhibitors Target Escherichia coli Amyloid Biogenesis and Biofilm Formation. Nat. Chem. Biol. 2009, 5, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Shea, D.; Colasurdo, E.; Smith, A.; Paschall, C.; Jayadev, S.; Keene, C.D.; Galasko, D.; Ko, A.; Li, G.; Peskind, E.; et al. SOBA: Development and Testing of a Soluble Oligomer Binding Assay for Detection of Amyloidogenic Toxic Oligomers. Proc. Natl. Acad. Sci. USA 2022, 119, e2213157119. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, M.A.; Schilling, J.D.; Hultgren, S.J. Establishment of a Persistent Escherichia coli Reservoir during the Acute Phase of a Bladder Infection. Infect. Immun. 2001, 69, 4572–4579. [Google Scholar] [CrossRef] [PubMed]
- García, V.; Grønnemose, R.B.; Torres-Puig, S.; Kudirkiene, E.; Piantelli, M.; Ahmed, S.; Andersen, T.E.; Møller-Jensen, J.; Olsen, J.E.; Herrero-Fresno, A. Genome-Wide Analysis of Fitness-Factors in Uropathogenic Escherichia coli during Growth in Laboratory Media and during Urinary Tract Infections. Microb. Genom. 2021, 7, 000719. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, B.J.; Khanna, N.R.; Vijhani, P. Amoxicillin. In StatPearls [Internet]; StatPearls Publishin: Treasure Island, FL, USA, 2023. [Google Scholar]
- Sharma, D.; Patel, R.P.; Zaidi, S.T.R.; Sarker, M.M.R.; Lean, Q.Y.; Ming, L.C. Interplay of the Quality of Ciprofloxacin and Antibiotic Resistance in Developing Countries. Front. Pharmacol. 2017, 8, 266703. [Google Scholar] [CrossRef] [PubMed]
- Washington, J.A.; Wilson, W.R. Erythromycin: A Microbial and Clinical Perspective after 30 Years of Clinical Use (First of Two Parts). Mayo Clin. Proc. 1985, 60, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Chaves, B.J.; Tadi, P. Gentamicin. In StatPearls [Internet]; StatPearls Publishin: Treasure Island, FL, USA, 2012. [Google Scholar]
- Patel, S.; Preuss, C.V.; Bernice, F. Vancomycin. In StatPearls [Internet]; StatPearls Publishin: Treasure Island, FL, USA, 2023. [Google Scholar]
- Rafaque, Z.; Abid, N.; Liaqat, N.; Afridi, P.; Siddique, S.; Masood, S.; Kanwal, S.; Dasti, J.I. In-Vitro Investigation of Antibiotics Efficacy against Uropathogenic Escherichia coli Biofilms and Antibiotic Induced Biofilm Formation at Subminimum Inhibitory Concentration of Ciprofloxacin. Infect. Drug Resist. 2020, 13, 2801–2810. [Google Scholar] [CrossRef] [PubMed]
- Thieme, L.; Hartung, A.; Tramm, K.; Klinger-Strobel, M.; Jandt, K.D.; Makarewicz, O.; Pletz, M.W. MBEC versus MBIC: The Lack of Differentiation between Biofilm Reducing and Inhibitory Effects as a Current Problem in Biofilm Methodology. Biol. Proced. Online 2019, 21, 18. [Google Scholar] [CrossRef]
- Okae, Y.; Nishitani, K.; Sakamoto, A.; Kawai, T.; Tomizawa, T.; Saito, M.; Kuroda, Y.; Matsuda, S. Estimation of Minimum Biofilm Eradication Concentration (MBEC) on In Vivo Biofilm on Orthopedic Implants in a Rodent Femoral Infection Model. Front. Cell. Infect. Microbiol. 2022, 12, 896978. [Google Scholar] [CrossRef]
- Mandell, J.B.; Orr, S.; Koch, J.; Nourie, B.; Ma, D.; Bonar, D.D.; Shah, N.; Urish, K.L. Large Variations in Clinical Antibiotic Activity against Staphylococcus Aureus Biofilms of Periprosthetic Joint Infection Isolates. J. Orthop. Res. 2019, 37, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Pettit, R.K.; Weber, C.A.; Pettit, G.R. Application of a High Throughput Alamar Blue Biofilm Susceptibility Assay to Staphylococcus Aureus Biofilms. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, P.; McLaren, A.; Tavaziva, G.; Overstreet, D. Biofilm Antimicrobial Susceptibility Increases with Antimicrobial Exposure Time. Clin. Orthop. Relat. Res. 2016, 474, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.D.; Shah, S.; Tammela, P. Defining Conditions for Biofilm Inhibition and Eradication Assays for Gram-Positive Clinical Reference Strains. BMC Microbiol. 2018, 18, 173. [Google Scholar] [CrossRef] [PubMed]
- Adamus-Białek, W.; Wawszczak, M.; Arabski, M.; Majchrzak, M.; Gulba, M.; Jarych, D.; Parniewski, P.; Głuszek, S. Ciprofloxacin, Amoxicillin, and Aminoglycosides Stimulate Genetic and Phenotypic Changes in Uropathogenic Escherichia coli Strains. Virulence 2019, 10, 260–276. [Google Scholar] [CrossRef]
- Verderosa, A.D.; Harris, J.; Dhouib, R.; Totsika, M.; Fairfull-Smith, K.E. Eradicating Uropathogenic Escherichia coli Biofilms with a Ciprofloxacin–Dinitroxide Conjugate. Medchemcomm 2019, 10, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Goneau, L.W.; Yeoh, N.S.; MacDonald, K.W.; Cadieux, P.A.; Burton, J.P.; Razvi, H.; Reid, G. Selective Target Inactivation Rather than Global Metabolic Dormancy Causes Antibiotic Tolerance in Uropathogens. Antimicrob. Agents Chemother. 2014, 58, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Biancalana, M.; Koide, S. Molecular Mechanism of Thioflavin-T Binding to Amyloid Fibrils. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2010, 1804, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold. Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Alreshidi, M.M.; Dunstan, R.H.; Macdonald, M.M.; Gottfries, J.; Roberts, T.K. The Uptake and Release of Amino Acids by Staphylococcus Aureus at Mid-Exponential and Stationary Phases and Their Corresponding Responses to Changes in Temperature, PH and Osmolality. Front. Microbiol. 2020, 10, 3059. [Google Scholar] [CrossRef]
- Perov, S.; Lidor, O.; Salinas, N.; Golan, N.; Tayeb-Fligelman, E.; Deshmukh, M.; Willbold, D.; Landau, M. Structural Insights into Curli CsgA Cross-β Fibril Architecture Inspire Repurposing of Anti-Amyloid Compounds as Anti-Biofilm Agents. PLoS Pathog. 2019, 15, e1007978. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, J.; Pan, T.; Wu, R.; Tao, Y.; Lin, H. The Broad-Spectrum Antibiofilm Activity of Amyloid-Forming Hexapeptides. Microb. Biotechnol. 2021, 14, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.O.; Mika, F.; Richter, A.M.; Hengge, R. The Green Tea Polyphenol EGCG Inhibits E. coli Biofilm Formation by Impairing Amyloid Curli Fibre Assembly and Downregulating the Biofilm Regulator CsgD via the σ(E)-Dependent SRNA RybB. Mol. Microbiol. 2016, 101, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Pruteanu, M.; Hernández Lobato, J.I.; Stach, T.; Hengge, R. Common Plant Flavonoids Prevent the Assembly of Amyloid Curli Fibres and Can Interfere with Bacterial Biofilm Formation. Environ. Microbiol. 2020, 22, 5280–5299. [Google Scholar] [CrossRef] [PubMed]
- Bikels-Goshen, T.; Landau, E.; Saguy, S.; Shapira, R. Staphylococcal Strains Adapted to Epigallocathechin Gallate (EGCG) Show Reduced Susceptibility to Vancomycin, Oxacillin and Ampicillin, Increased Heat Tolerance, and Altered Cell Morphology. Int. J. Food. Microbiol. 2010, 138, 26–31. [Google Scholar] [CrossRef] [PubMed]
- O’May, C.; Ciobanu, A.; Lam, H.; Tufenkji, N. Tannin Derived Materials Can Block Swarming Motility and Enhance Biofilm Formation in Pseudomonas Aeruginosa. Biofouling 2012, 28, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Stenvang, M.; Dueholm, M.S.; Vad, B.S.; Seviour, T.; Zeng, G.; Geifman-Shochat, S.; Søndergaard, M.T.; Christiansen, G.; Meyer, R.L.; Kjelleberg, S.; et al. Epigallocatechin Gallate Remodels Overexpressed Functional Amyloids in Pseudomonas Aeruginosa and Increases Biofilm Susceptibility to Antibiotic Treatment. J. Biol. Chem. 2016, 291, 26540–26553. [Google Scholar] [CrossRef]
- van der Kamp, M.W.; Schaeffer, R.D.; Jonsson, A.L.; Scouras, A.D.; Simms, A.M.; Toofanny, R.D.; Benson, N.C.; Anderson, P.C.; Merkley, E.D.; Rysavy, S.; et al. Dynameomics: A Comprehensive Database of Protein Dynamics. Structure 2010, 18, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Beck, D.A.C.; Alonso, D.O.V.; Inoyama, D.; Daggett, V. The Intrinsic Conformational Propensities of the 20 Naturally Occurring Amino Acids and Reflection of These Propensities in Proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 12259–12264. [Google Scholar] [CrossRef]
- Lim, J.Y.; May, J.M.; Cegelski, L. Dimethyl Sulfoxide and Ethanol Elicit Increased Amyloid Biogenesis and Amyloid-Integrated Biofilm Formation in Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 3369–3378. [Google Scholar] [CrossRef]
- Blomster-Hautamaa, D.A.; Schlievert, P.M. Preparation of Toxic Shock Syndrome Toxin-1; Academic Press: Cambridge, MA, USA, 1988. [Google Scholar]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to Optimize the Drop Plate Method for Enumerating Bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef] [PubMed]



| Antibiotic Concentration (µg/mL) | |||||
|---|---|---|---|---|---|
| 100 | 300 | 500 * | 1000 * | ||
| E. coli UTI89 | Amoxicillin | 39 | 78 | 88 | 56 |
| Ciprofloxacin | 44 | 70 | 79 | 60 | |
| Erythromycin | 47 | 40 | 42 | 48 | |
| Gentamicin | 99 | 99.9 | 99.99 | 99.99 | |
| Vancomycin | 38 | 43 | 47 | 53 | |
| S. aureus MN8 | Amoxicillin | 48 | 31 | 26 | 18 |
| Ciprofloxacin | 40 | 54 | 72 | 81 | |
| Erythromycin | 11 | 45 | 37 | 51 | |
| Gentamicin | 45 | 53 | 59 | 63 | |
| Vancomycin | 59 | 88 | 86 | 87 | |
| Increased Susceptibility? | |||
|---|---|---|---|
| AP90 + abx | AP401 + abx | ||
| E. coli UTI89 | Amoxicillin | ✓ | ✓✓✓ |
| Ciprofloxacin | ✓✓✓ | ✓✓✓ | |
| Erythromycin | ✓ | ✓ | |
| Gentamicin | ✓✓✓✓✓ | ✓✓✓✓✓ | |
| Vancomycin | ✓✓ | ✓✓✓ | |
| S. aureus MN8 | Amoxicillin | ✓ | ✓ |
| Ciprofloxacin | ✓ | ✓ | |
| Erythromycin | ✓✓ | ✓✓ | |
| Gentamicin | ✓ | ✓ | |
| Vancomycin | ✓✓✓✓ | ✓✓✓ | |
| Peptide Sequences | |||
|---|---|---|---|
| Name a | Sequence b | Description | Source |
| AP90 | Ac-RGEmNlSwMNEYSGWtMnLkMGR-NH2 | α-sheet monomer | Hopping et al., 2014 [8] |
| AP401 | Ac-rGeMnLsWmneysGwTmNlKmGr-NH2 | α-sheet monomer | Bleem et al., 2017 [12] |
| E. coli Strains | |||
| UTI89 | UPEC strain; cystitis isolate | Mulvey et al., 2001 [45] | |
| UTI89 ΔcsgA | UPEC strain; cystitis isolate with chromosomal deletion of csgA gene | Cegelski et al., 2009 [43] | |
| S. aureus Strain | |||
| MN8 | Clinically relevant strain; toxic shock isolate, urogenital tract | Schwartz et al., 2012 [14] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prosswimmer, T.; Nick, S.E.; Bryers, J.D.; Daggett, V. Designed De Novo α-Sheet Peptides Destabilize Bacterial Biofilms and Increase the Susceptibility of E. coli and S. aureus to Antibiotics. Int. J. Mol. Sci. 2024, 25, 7024. https://doi.org/10.3390/ijms25137024
Prosswimmer T, Nick SE, Bryers JD, Daggett V. Designed De Novo α-Sheet Peptides Destabilize Bacterial Biofilms and Increase the Susceptibility of E. coli and S. aureus to Antibiotics. International Journal of Molecular Sciences. 2024; 25(13):7024. https://doi.org/10.3390/ijms25137024
Chicago/Turabian StyleProsswimmer, Tatum, Sarah E. Nick, James D. Bryers, and Valerie Daggett. 2024. "Designed De Novo α-Sheet Peptides Destabilize Bacterial Biofilms and Increase the Susceptibility of E. coli and S. aureus to Antibiotics" International Journal of Molecular Sciences 25, no. 13: 7024. https://doi.org/10.3390/ijms25137024






