Exploring the Efficacy of Peptides and Mimics against Influenza A Virus, Adenovirus, and Murine Norovirus
Abstract
1. Introduction
2. Results
2.1. Direct Inactivation
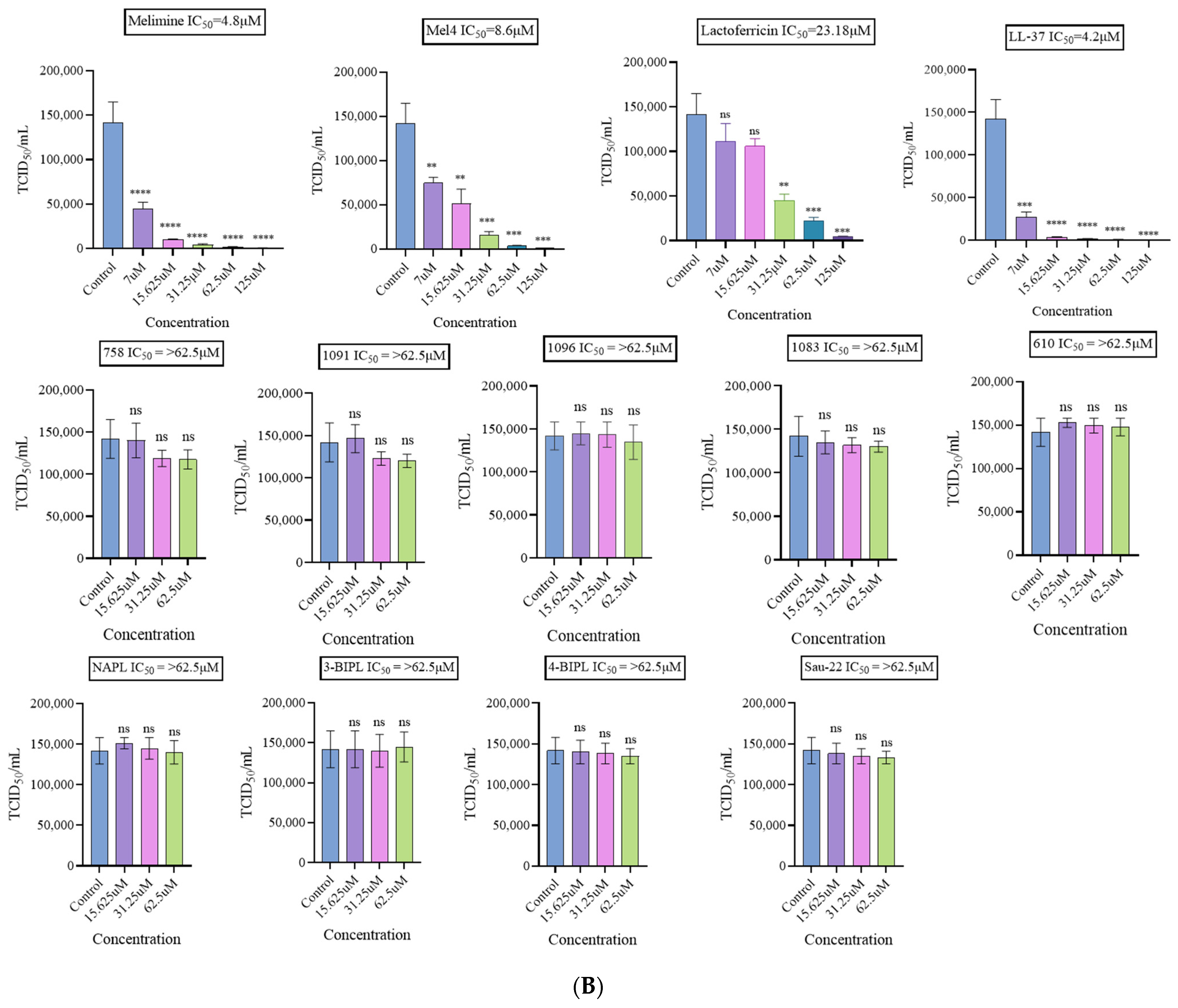
2.1.1. Antiviral Effect on H1N1
2.1.2. Antiviral Effect on H3N2
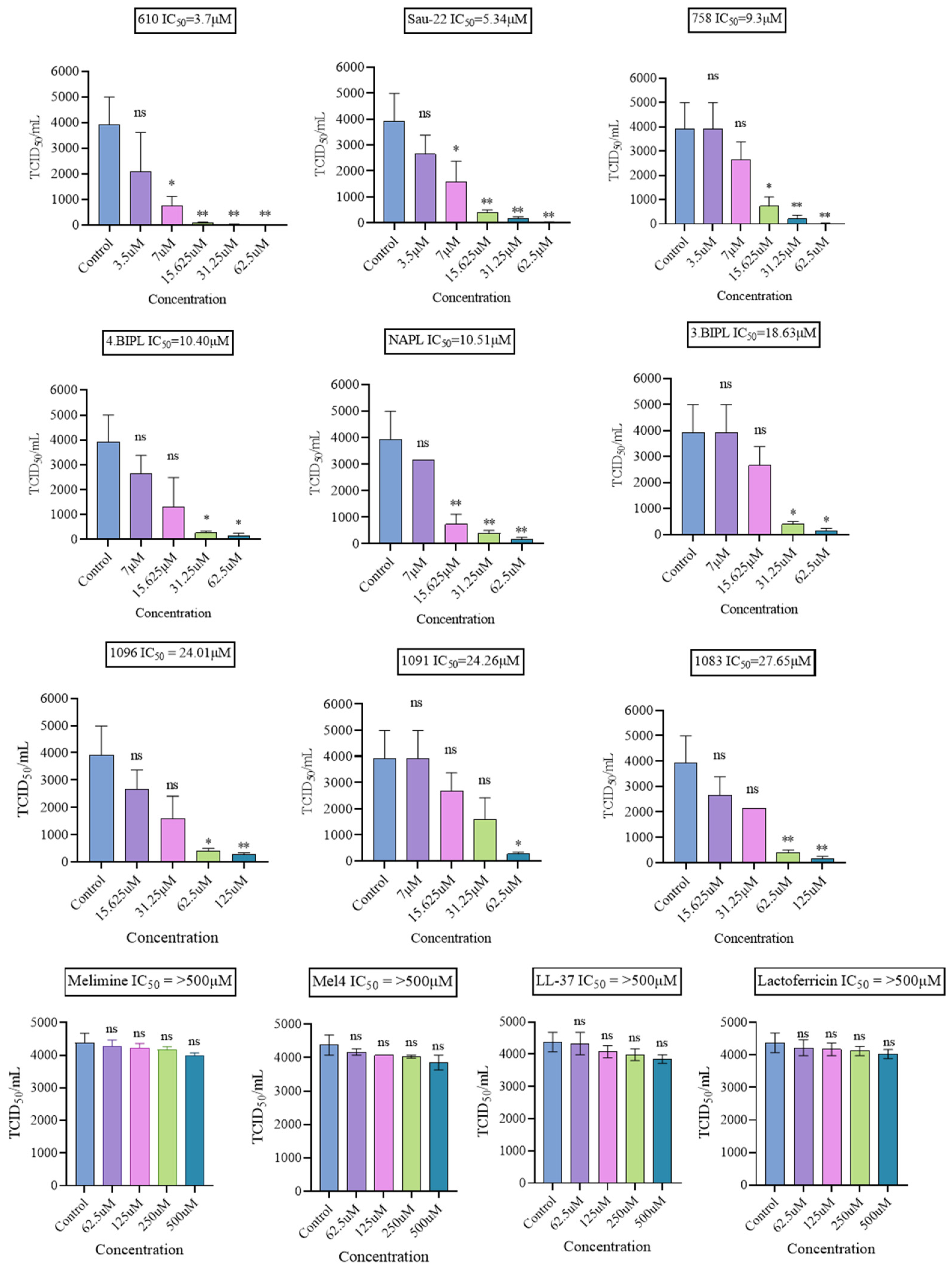
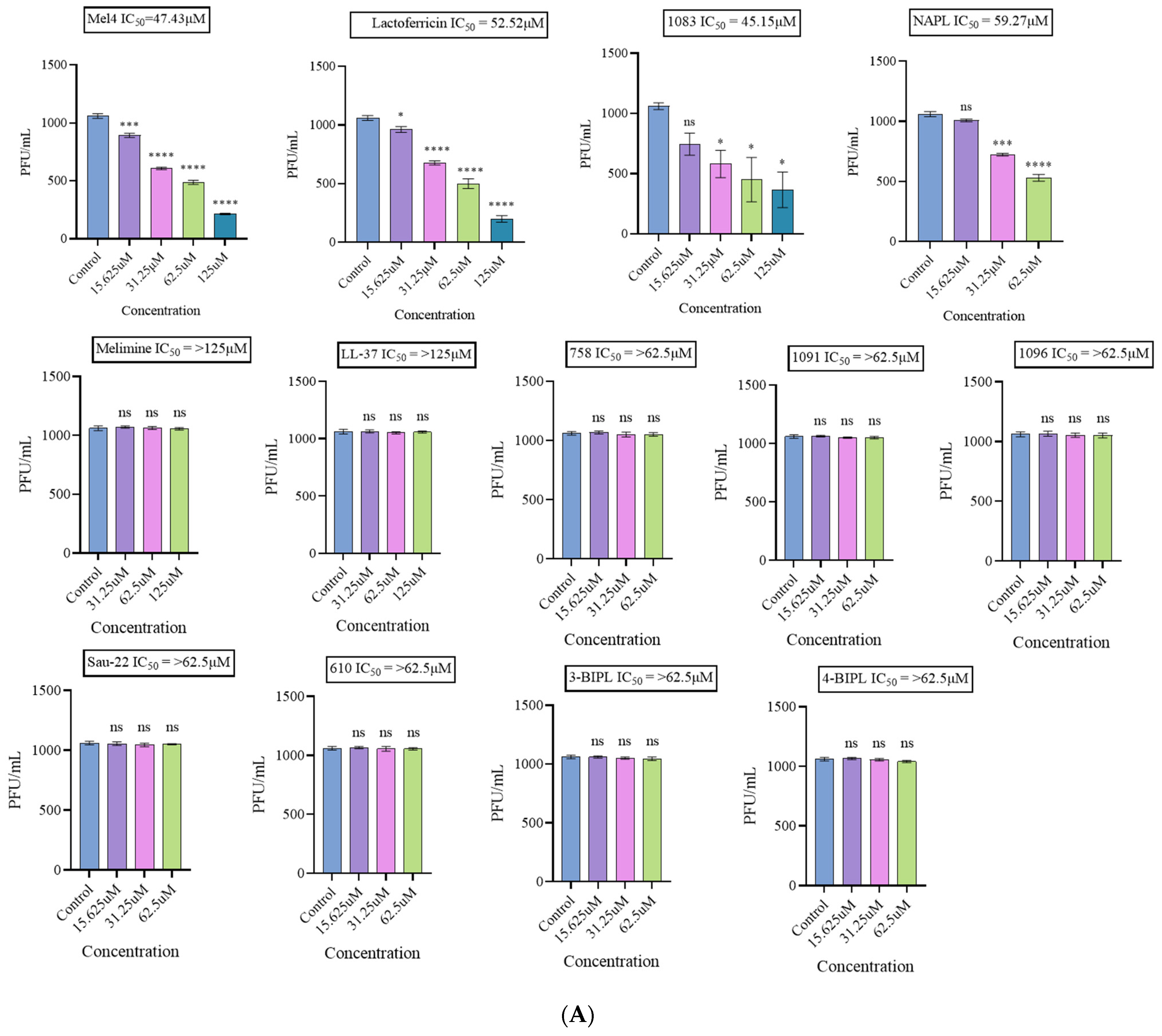
2.1.3. Antiviral Effect on the Non-Enveloped Viruses HAdV-5 and MNV-1
2.2. Indirect Inactivation
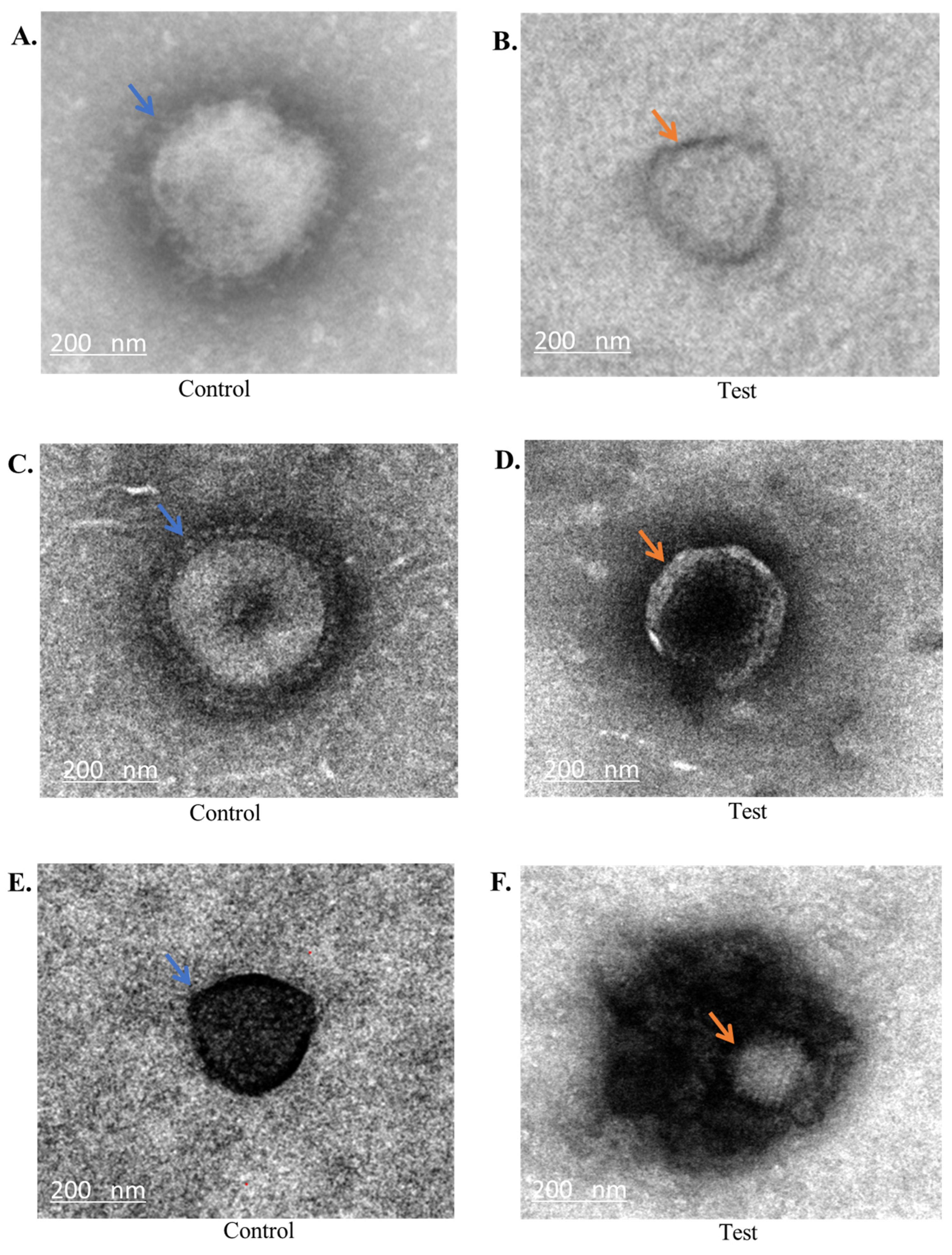
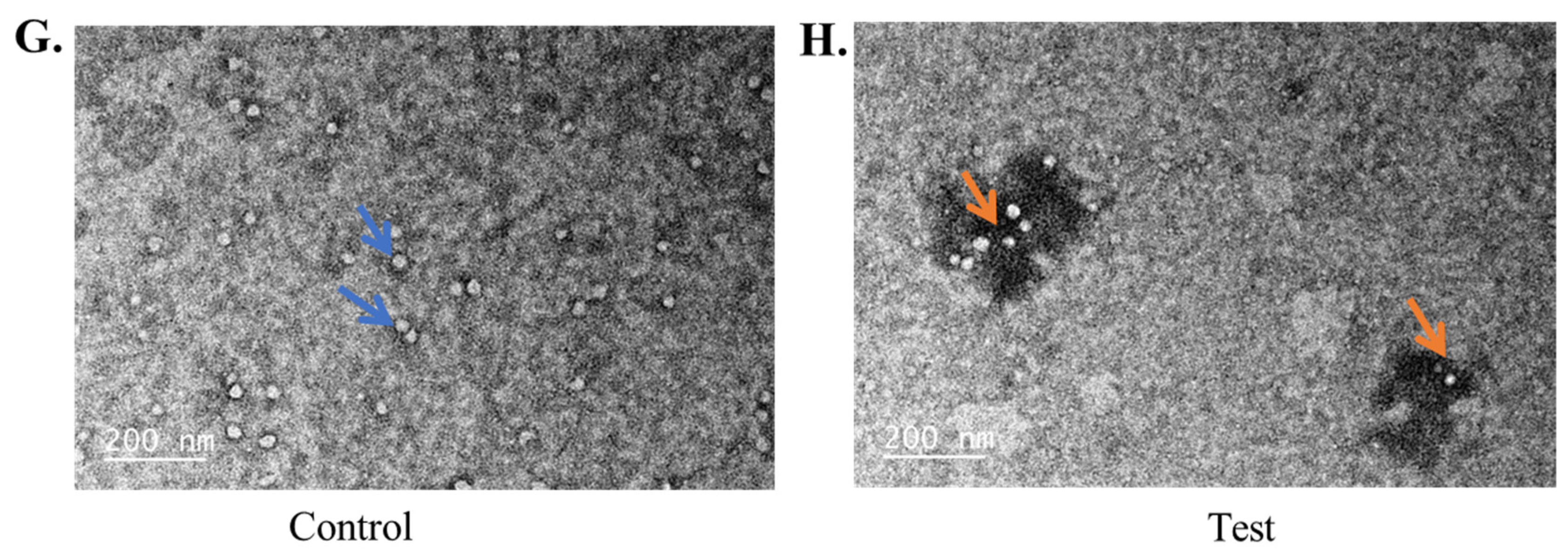
2.3. Transmission Electron Microscopy Analysis
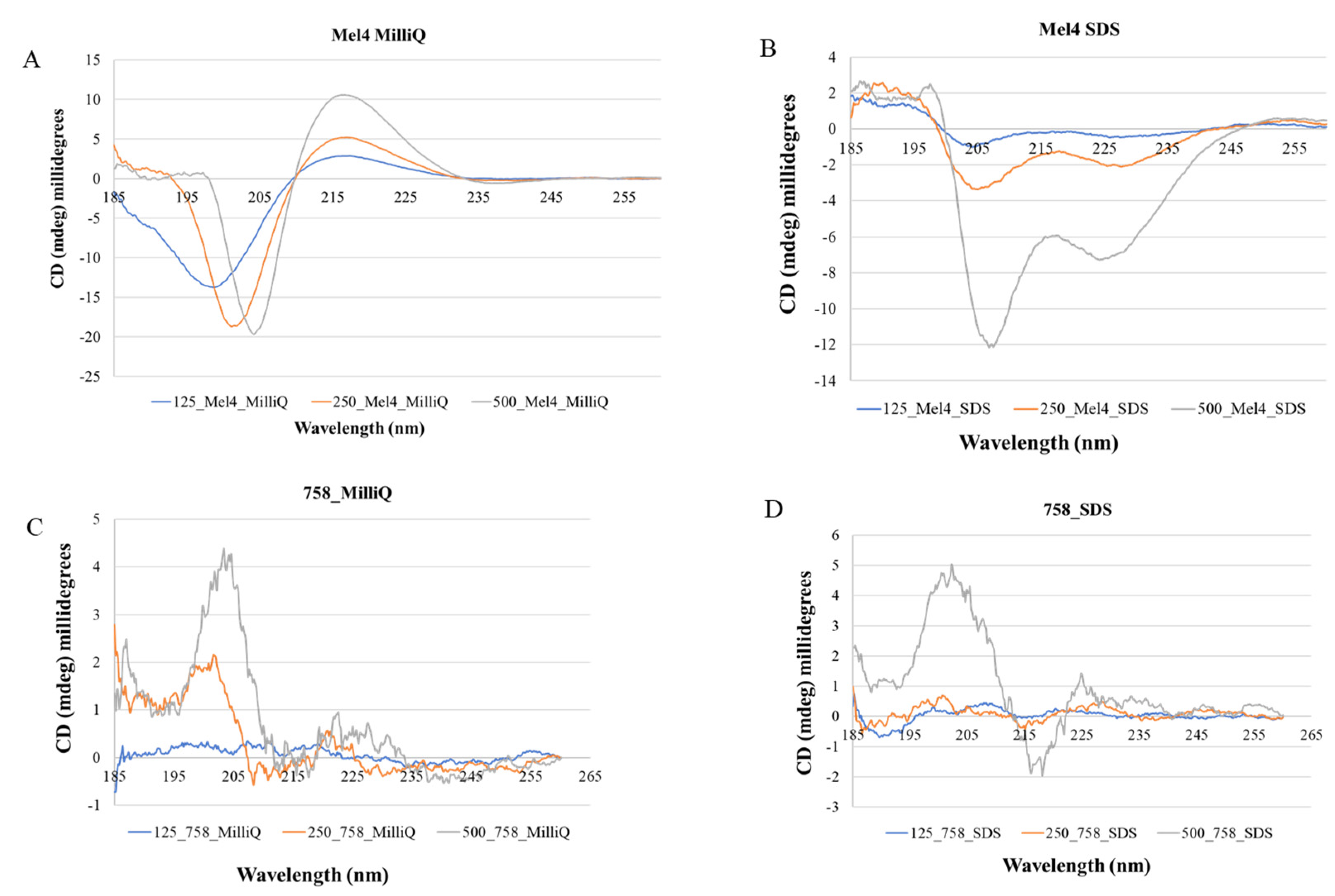
2.4. Conformational Analysis of Mel4 and 758
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Virus Infection
4.3. Peptides and Mimics
4.4. Antiviral Testing
4.5. Cytotoxicity Assay
4.6. Transmission Electron Microscopy (TEM)
4.7. Circular Dichroism
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spreeuwenberg, P.; Kroneman, M.; Paget, J. Reassessing the Global Mortality Burden of the 1918 Influenza Pandemic. Am. J. Epidemiol. 2018, 187, 2561–2567. [Google Scholar] [CrossRef]
- Wikipedia. 1957–1958 Influenza Pandemic. Available online: https://en.wikipedia.org/w/index.php?title=1957%E2%80%931958_influenza_pandemic&oldid=1198515479 (accessed on 28 March 2024).
- Honigsbaum, M. Revisiting the 1957 and 1968 influenza pandemics. Lancet 2020, 395, 1824–1826. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, B.E.; Lumi, X.; Znaor, L.; Ivastinovic, D.; Confalonieri, F.; Petrovic, M.G.; Petrovski, G. Reorganize and survive-a recommendation for healthcare services affected by COVID-19-the ophthalmology experience. Eye 2020, 34, 1177–1179. [Google Scholar] [CrossRef]
- Burkardt, H.J. Pandemic H1N1 2009 (‘swine flu’): Diagnostic and other challenges. Expert. Rev. Mol. Diagn. 2011, 11, 35–40. [Google Scholar] [CrossRef]
- Beyrer, C. A pandemic anniversary: 40 years of HIV/AIDS. Lancet 2021, 397, 2142–2143. [Google Scholar] [CrossRef]
- World Health Organization. Number of COVID-19 Deaths Reported to WHO (Cumulative Total). Available online: https://data.who.int/dashboards/covid19/deaths (accessed on 10 May 2024).
- Zhu, Z.; Fodor, E.; Keown, J.R. A structural understanding of influenza virus genome replication. Trends Microbiol. 2023, 31, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Badham, M.D.; Rossman, J.S. Filamentous Influenza viruses. Curr. Clin. Microbiol. Rep. 2016, 3, 155–161. [Google Scholar] [CrossRef]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26 (Suppl. S4), D49–D53. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, A.; Abed, Y.; Boivin, G. Influenza drug resistance. Semin. Respir. Crit. Care Med. 2011, 32, 409–422. [Google Scholar] [CrossRef]
- Greber, U.F. Adenoviruses—Infection, pathogenesis and therapy. FEBS Lett. 2020, 594, 1818–1827. [Google Scholar] [CrossRef]
- Lynch, J.P., 3rd; Fishbein, M.; Echavarria, M. Adenovirus. Semin. Respir. Crit. Care Med. 2011, 32, 494–511. [Google Scholar] [CrossRef]
- Eterpi, M.; McDonnell, G.; Thomas, V. Disinfection efficacy against parvoviruses compared with reference viruses. J. Hosp. Infect. 2009, 73, 64–70. [Google Scholar] [CrossRef]
- Kampf, G. Efficacy of ethanol in hand hygiene against adenoviruses. Am. J. Infect. Control 2016, 44, 1429. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cai, J.; Li, Y.; Ying, L.; Li, H.; Zhu, A.; Li, L.; Zhu, H.; Dong, S.; Ying, R.; et al. Outbreak of acute respiratory disease caused by human adenovirus type 7 and human coronavirus-229E in Zhejiang Province, China. J. Med. Virol. 2023, 95, e28101. [Google Scholar] [CrossRef]
- van der Zalm, M.M.; Sam-Agudu, N.A.; Verhagen, L.M. Respiratory adenovirus infections in children: A focus on Africa. Curr. Opin. Pediatr. 2024, 36, 342–348. [Google Scholar] [CrossRef]
- Afrasiabi, V.; Ghojoghi, R.; Hosseini, S.Y.; Sarvari, J.; Nekooei, F.; Joharinia, N.; Hadian, S.; Gholami, M.; Nejabat, M. The molecular epidemiology, genotyping, and clinical manifestation of prevalent adenovirus infection during the epidemic keratoconjunctivitis, South of Iran. Eur. J. Med. Res. 2023, 28, 108. [Google Scholar] [CrossRef]
- Badawi, A.E.; Kasem, M.A.; Moemen, D.; El Sayed Zaki, M. Molecular, epidemiological and clinical assessment of adenoviral keratoconjunctivitis in Egypt: Institutional study. Ocul. Immunol. Inflamm. 2023, 31, 1640–1646. [Google Scholar] [CrossRef]
- Yodmeeklin, A.; Kumthip, K.; Ukarapol, N.; Ushijima, H.; Maneekarn, N.; Khamrin, P. Diverse genotypes of human enteric and non-enteric adenoviruses circulating in children hospitalized with acute gastroenteritis in Thailand, from 2018 to 2021. Microbiol. Spectr. 2023, 11, e0117323. [Google Scholar] [CrossRef]
- Wiwanitkit, V.; Joob, B. Annual detection rate of enteric adenovirus-associated acute gastroenteritis in pediatric patients and average sunlight intensity: A medical meteorological study from an indochina country. Med. J. Dr. DY Patil Univ. 2023, 16, 914–916. [Google Scholar]
- Shieh, W.J. Human adenovirus infections in pediatric population—An update on clinico-pathologic correlation. Biomed. J. 2022, 45, 38–49. [Google Scholar] [CrossRef]
- Sarker, R.; Roknuzzaman, A.; Nazmunnahar; Islam, M.R. Risk evaluation and mitigation strategies for potential outbreaks of adenovirus infection: Evidence from the recent incidences in West Bengal, India. Clin. Pathol. 2023, 16, 2632010X231205672. [Google Scholar] [CrossRef] [PubMed]
- Tori, M.E.; Chontos-Komorowski, J.; Stacy, J.; Lamson, D.M.; George, K.S.; Lail, A.T.; Stewart-Grant, H.A.; Bell, L.J.; Kirking, H.L.; Hsu, C.H. Identification of large adenovirus infection outbreak at university by multipathogen testing, South Carolina, USA, 2022. Emerg. Infect. Dis. 2024, 30, 358. [Google Scholar] [CrossRef] [PubMed]
- Velez-Tirado, N.; Castano-Jaramillo, L.; Restrepo-Gualteros, S.; Alcala-Lozano, C.; Ruge, E.; Puente, C.; Li-Zeng, D.; Chaparro-Arce, D.; Beltran-Dimas, M.C.; Lopez, J.F.; et al. Severe adenovirus infection outbreak in Colombia: Experience from a tertiary pediatric hospital in 2022. Biomedica 2024, 44, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Qiu, S.; Zhang, L.; Wu, H.; Tian, X.; Li, X.; Xu, D.; Dai, J.; Gu, S.; Liu, Q.; et al. Analysis of severe human adenovirus infection outbreak in Guangdong Province, southern China in 2019. Virol. Sin. 2022, 37, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Tsou, T.P.; Tan, B.F.; Chang, H.Y.; Chen, W.C.; Huang, Y.P.; Lai, C.Y.; Chao, Y.N.; Wei, S.H.; Hung, M.N.; Hsu, L.C.; et al. Community outbreak of adenovirus, Taiwan, 2011. Emerg. Infect. Dis. 2012, 18, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Parks, R.J. Recent Advances in Novel Antiviral Therapies against Human Adenovirus. Microorganisms 2020, 8, 1284. [Google Scholar] [CrossRef] [PubMed]
- Romanowski, E.G.; Yates, K.A.; Gordon, Y.J. Cyclopentenylcytosine (CPE-C): In Vitro and In Vivo evaluation as an antiviral against adenoviral ocular infections. Molecules 2023, 28, 5078. [Google Scholar] [CrossRef]
- Zaczyńska, E.; Kaczmarek, K.; Zabrocki, J.; Artym, J.; Zimecki, M. Antiviral activity of a cyclic Pro-Pro-β3-HoPhe-Phe tetrapeptide against HSV-1 and HAdV-5. Molecules 2022, 27, 3552. [Google Scholar] [CrossRef]
- Chan, M.C.; Kwan, H.S.; Chan, P.K. Structure and genotypes of noroviruses. In The Norovirus; Elsevier: Amsterdam, The Netherlands, 2017; pp. 51–63. [Google Scholar]
- Randazzo, W.; D’Souza, D.H.; Sanchez, G. Norovirus: The Burden of the Unknown. Adv. Food Nutr. Res. 2018, 86, 13–53. [Google Scholar] [CrossRef]
- Marsh, Z.; Shah, M.P.; Wikswo, M.E.; Barclay, L.; Kisselburgh, H.; Kambhampati, A.; Cannon, J.L.; Parashar, U.D.; Vinje, J.; Hall, A.J. Epidemiology of foodborne norovirus outbreaks—United States, 2009–2015. Food Saf. 2018, 6, 58–66. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, B.; Yan, H.; Li, W.; Jia, L.; Tian, Y.; Chen, Y.; Wang, Q.; Pang, X. Norovirus outbreaks in Beijing, China, from 2014 to 2017. J. Infect. 2019, 79, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Udompat, P.; Srimuang, K.; Doungngern, P.; Thippamom, N.; Petcharat, S.; Rattanatumhi, K.; Khiewbanyang, S.; Taweewigyakarn, P.; Kripattanapong, S.; Ninwattana, S.; et al. An unusual diarrheal outbreak in the community in Eastern Thailand caused by Norovirus GII.3[P25]. Virol. J. 2024, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Summa, M.; Tuutti, E.; Al-Hello, H.; Huttunen, L.M.; Rimhanen-Finne, R. Norovirus GII.17 caused five outbreaks linked to frozen domestic bilberries in Finland, 2019. Food Environ. Virol. 2024, 16, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Galasiti Kankanamalage, A.C.; Chang, K.O.; Groutas, W.C. Recent advances in the discovery of norovirus therapeutics: Miniperspective. J. Med. Chem. 2015, 58, 9438–9450. [Google Scholar] [CrossRef] [PubMed]
- Van Dycke, J.; Dai, W.; Stylianidou, Z.; Li, J.; Cuvry, A.; Roux, E.; Li, B.; Rymenants, J.; Bervoets, L.; de Witte, P.; et al. A novel class of norovirus inhibitors targeting the viral protease with potent antiviral activity in vitro and in vivo. Viruses 2021, 13, 1852. [Google Scholar] [CrossRef] [PubMed]
- Amblard, F.; Zhou, S.; Liu, P.; Yoon, J.; Cox, B.; Muzzarelli, K.; Kuiper, B.D.; Kovari, L.C.; Schinazi, R.F. Synthesis and antiviral evaluation of novel peptidomimetics as norovirus protease inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 2165–2170. [Google Scholar] [CrossRef] [PubMed]
- Urmi, U.L.; Vijay, A.K.; Kuppusamy, R.; Islam, S.; Willcox, M.D.P. A review of the antiviral activity of cationic antimicrobial peptides. Peptides 2023, 166, 171024. [Google Scholar] [CrossRef] [PubMed]
- Enninful, G.N.; Kuppusamy, R.; Tiburu, E.K.; Kumar, N.; Willcox, M.D.P. Non-canonical amino acid bioincorporation into antimicrobial peptides and its challenges. J. Pept. Sci. 2024, 30, e3560. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Agerberth, B.; Lothgren, A.; Almstedt, A.; Johansson, J. Apolipoprotein A-I binds and inhibits the human antibacterial/cytotoxic peptide LL-37. J. Biol. Chem. 1998, 273, 33115–33118. [Google Scholar] [CrossRef]
- Rotem, S.; Mor, A. Antimicrobial peptide mimics for improved therapeutic properties. Biochim. Biophys. Acta 2009, 1788, 1582–1592. [Google Scholar] [CrossRef]
- Sara, M.; Yasir, M.; Kalaiselvan, P.; Hui, A.; Kuppusamy, R.; Kumar, N.; Chakraborty, S.; Yu, T.T.; Wong, E.H.H.; Molchanova, N.; et al. The activity of antimicrobial peptoids against multidrug-resistant ocular pathogens. Contact Lens Anterior Eye 2024, 47, 102124. [Google Scholar] [CrossRef]
- Yu, T.T.; Nizalapur, S.; Ho, K.K.K.; Yee, E.; Berry, T.; Cranfield, C.G.; Willcox, M.; Black, D.S.; Kumar, N. Design, synthesis and biological evaluation of N-sulfonylphenyl glyoxamide-based antimicrobial peptide mimics as novel antimicrobial agents. Chem. Sel. 2017, 2, 3452–3461. [Google Scholar] [CrossRef]
- Yu, T.T.; Kuppusamy, R.; Yasir, M.; Hassan, M.M.; Alghalayini, A.; Gadde, S.; Deplazes, E.; Cranfield, C.; Willcox, M.D.P.; Black, D.S. Design, synthesis and biological evaluation of biphenylglyoxamide-based small molecular antimicrobial peptide mimics as antibacterial agents. Int. J. Mol. Sci. 2020, 21, 6789. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.T.; Kuppusamy, R.; Yasir, M.; Hassan, M.M.; Sara, M.; Ho, J.; Willcox, M.D.P.; Black, D.S.; Kumar, N. Polyphenylglyoxamide-based amphiphilic small molecular peptidomimetics as antibacterial agents with anti-biofilm activity. Int. J. Mol. Sci. 2021, 22, 7344. [Google Scholar] [CrossRef] [PubMed]
- Nizalapur, S.; Kimyon, O.; Yee, E.; Bhadbhade, M.M.; Manefield, M.; Willcox, M.; Black, D.S.; Kumar, N. Synthesis and biological evaluation of novel acyclic and cyclic glyoxamide based derivatives as bacterial quorum sensing and biofilm inhibitors. Org. Biomol. Chem. 2017, 15, 5743–5755. [Google Scholar] [CrossRef] [PubMed]
- Nizalapur, S.; Kimyon, O.; Yee, E.; Ho, K.; Berry, T.; Manefield, M.; Cranfield, C.G.; Willcox, M.; Black, D.S.; Kumar, N. Amphipathic guanidine-embedded glyoxamide-based peptidomimetics as novel antibacterial agents and biofilm disruptors. Org. Biomol. Chem. 2017, 15, 2033–2051. [Google Scholar] [CrossRef]
- Kuppusamy, R.; Yasir, M.; Yee, E.; Willcox, M.; Black, S.S.; Kumar, N. Guanidine functionalized anthranilamides as effective antibacterials with biofilm disruption activity. Org. Biomol. Chem. 2018, 16, 5871–5888. [Google Scholar] [CrossRef]
- Bahatheg, G.; Kuppusamy, R.; Yasir, M.; Black, D.S.; Willcox, M.; Kumar, N. Short tryptamine-based peptoids as potential therapeutics for microbial keratitis: Structure-function correlation studies. Antibiotics 2022, 11, 1074. [Google Scholar] [CrossRef]
- Kuppusamy, R.; Yasir, M.; Yu, T.T.; Voli, F.; Vittorio, O.; Miller, M.J.; Lewis, P.; Black, D.S.; Willcox, M.; Kumar, N. Tuning the anthranilamide peptidomimetic design to selectively target planktonic bacteria and biofilm. Antibiotics 2023, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- Urmi, U.L.; Attard, S.; Vijay, A.K.; Willcox, M.D.P.; Kumar, N.; Islam, S.; Kuppusamy, R. Antiviral activity of anthranilamide peptidomimetics against Herpes simplex virus 1 and a coronavirus. Antibiotics 2023, 12, 1436. [Google Scholar] [CrossRef]
- Tripathi, S.; Tecle, T.; Verma, A.; Crouch, E.; White, M.; Hartshorn, K.L. The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. J. Gen. Virol. 2013, 94, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Barlow, P.G.; Svoboda, P.; Mackellar, A.; Nash, A.A.; York, I.A.; Pohl, J.; Davidson, D.J.; Donis, R.O. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS ONE 2011, 6, e25333. [Google Scholar] [CrossRef] [PubMed]
- Pietrantoni, A.; Dofrelli, E.; Tinari, A.; Ammendolia, M.G.; Puzelli, S.; Fabiani, C.; Donatelli, I.; Superti, F. Bovine lactoferrin inhibits influenza A virus induced programmed cell death in vitro. Biometals 2010, 23, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Pietrantoni, A.; Ammendolia, M.G.; Superti, F. Bovine lactoferrin: Involvement of metal saturation and carbohydrates in the inhibition of influenza virus infection. Biochem. Cell Biol. 2012, 90, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Ammendolia, M.G.; Agamennone, M.; Pietrantoni, A.; Lannutti, F.; Siciliano, R.A.; De Giulio, B.; Amici, C.; Superti, F. Bovine lactoferrin-derived peptides as novel broad-spectrum inhibitors of influenza virus. Pathog. Glob. Health 2012, 106, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Scala, M.C.; Sala, M.; Pietrantoni, A.; Spensiero, A.; Di Micco, S.; Agamennone, M.; Bertamino, A.; Novellino, E.; Bifulco, G.; Gomez-Monterrey, I.M.; et al. Lactoferrin-derived Peptides Active towards Influenza: Identification of Three Potent Tetrapeptide Inhibitors. Sci. Rep. 2017, 7, 10593. [Google Scholar] [CrossRef] [PubMed]
- Gordon, Y.J.; Huang, L.C.; Romanowski, E.G.; Yates, K.A.; Proske, R.J.; McDermott, A.M. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr. Eye Res. 2005, 30, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Seganti, L.; Di Biase, A.M.; Marchetti, M.; Pietrantoni, A.; Tinari, A.; Superti, F. Antiviral activity of lactoferrin towards naked viruses. Biometals 2004, 17, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, A.M.; Pietrantoni, A.; Tinari, A.; Siciliano, R.; Valenti, P.; Antonini, G.; Seganti, L.; Superti, F. Heparin-interacting sites of bovine lactoferrin are involved in anti-adenovirus activity. J. Med. Virol. 2003, 69, 495–502. [Google Scholar] [CrossRef]
- Galatola, E.; Agrillo, B.; Gogliettino, M.; Palmieri, G.; Maccaroni, S.; Vicenza, T.; Proroga, Y.T.R.; Mancusi, A.; Di Pasquale, S.; Suffredini, E.; et al. A reliable multifaceted solution against foodborne viral infections: The case of RiLK1 decapeptide. Molecules 2024, 29, 2305. [Google Scholar] [CrossRef]
- Oda, H.; Kolawole, A.O.; Mirabelli, C.; Wakabayashi, H.; Tanaka, M.; Yamauchi, K.; Abe, F.; Wobus, C.E. Antiviral effects of bovine lactoferrin on human norovirus. Biochem. Cell Biol. 2021, 99, 166–172. [Google Scholar] [CrossRef]
- Ishikawa, H.; Awano, N.; Fukui, T.; Sasaki, H.; Kyuwa, S. The protective effects of lactoferrin against murine norovirus infection through inhibition of both viral attachment and replication. Biochem. Biophys. Res. Commun. 2013, 434, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Dutta, D.; Hossain, K.R.; Chen, R.; Ho, K.K.K.; Kuppusamy, R.; Clarke, R.J.; Kumar, N.; Willcox, M.D.P. Mechanism of Action of Surface Immobilized Antimicrobial Peptides Against Pseudomonas aeruginosa. Front. Microbiol. 2019, 10, 3053. [Google Scholar] [CrossRef] [PubMed]
- Falcigno, L.; D’Auria, G.; Palmieri, G.; Gogliettino, M.; Agrillo, B.; Tate, R.; Dardano, P.; Nicolais, L.; Balestrieri, M. Key physicochemical determinants in the antimicrobial peptide RiLK1 promote amphipathic structures. Int. J. Mol. Sci. 2021, 22, 10011. [Google Scholar] [CrossRef]
- Paulmann, D.; Steinmann, J.; Becker, B.; Bischoff, B.; Steinmann, E.; Steinmann, J. Virucidal activity of different alcohols against murine norovirus, a surrogate of human norovirus. J. Hosp. Infect. 2011, 79, 378–379. [Google Scholar] [CrossRef]
- Iloghalu, U.; Miller, S.; Ewunkem, A.; Khatiwada, J.; Williams, L. Treatment effect of various concentration of plant extracts on murine norovirus. Adv. Microbiol. 2022, 12, 242–253. [Google Scholar] [CrossRef]
- Cozzi, L.; Vicenza, T.; Battistini, R.; Masotti, C.; Suffredini, E.; Di Pasquale, S.; Fauconnier, M.L.; Ercolini, C.; Serracca, L. Effects of essential oils and hydrolates on the infectivity of murine norovirus. Viruses 2023, 15, 682. [Google Scholar] [CrossRef] [PubMed]
- Browne, K.; Kuppusamy, R.; Walsh, W.R.; Black, D.S.; Willcox, M.D.P.; Kumar, N.; Chen, R. Antimicrobial Peptidomimetics Prevent the Development of Resistance against Gentamicin and Ciprofloxacin in Staphylococcus and Pseudomonas Bacteria. Int. J. Mol. Sci. 2023, 24, 4966. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Lee, M.F.; Anasir, M.I.; Poh, C.L. Development of novel antiviral peptides against dengue serotypes 1-4. Virology 2023, 580, 10–27. [Google Scholar] [CrossRef]
- Chianese, A.; Zannella, C.; Monti, A.; De Filippis, A.; Doti, N.; Franci, G.; Galdiero, M. The Broad-Spectrum Antiviral Potential of the Amphibian Peptide AR-23. Int. J. Mol. Sci. 2022, 23, 883. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.Y.M.; Taneja, A.K.; Hodges, R.S. Synthesis of a model protein of defined secondary and quaternary structure-effect of chain-length on the stabilization and formation of 2-stranded alpha-helical coiled-coils. J. Biol. Chem. 1984, 259, 3253–3261. [Google Scholar] [CrossRef]
- Zhou, N.E.; Kay, C.M.; Hodges, R.S. Synthetic model proteins. Positional effects of interchain hydrophobic interactions on stability of two-stranded alpha-helical coiled-coils. J. Biol. Chem. 1992, 267, 2664–2670. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Kumar Vijay, A.; Willcox, M. Antiviral effect of multipurpose contact lens disinfecting solutions against coronavirus. Contact Lens Anterior Eye 2022, 45, 101513. [Google Scholar] [CrossRef]
- Abd-Aziz, N.; Lee, M.F.; Ong, S.K.; Poh, C.L. Antiviral activity of SP81 peptide against Enterovirus A71 (EV-A71). Virology 2024, 589, 109941. [Google Scholar] [CrossRef]

| Virus/Host Cell | Influenza A Virus (H1N1)/MDCK | Influenza A Virus (H3N2)/MDCK | Human Adenovirus-5/Vero | Murine Norovirus-1/Raw 246.7 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptide/Mimics | IC50 (µM) | CC50 (µM) | SI | IC50 (µM) | CC50 (µM) | SI | IC50 (µM) | CC50 (µM) | SI | IC50 (µM) | CC50 (µM) | SI | |
| 1 | Melimine | >500 | 28.07 | - | >500 | 28.07 | - | >125 | 14.5 | - | 4.8 | 0.03 | 0 |
| 2 | Mel4 | >500 | 12.67 | - | >500 | 12.67 | - | 47.43 | 47.6 | 1 | 8.6 | 30.27 | 3.5 |
| 3 | LL-37 | >500 | 2.8 | - | >500 | 2.8 | - | >125 | 36.74 | - | 4.2 | 49.9 | 11.8 |
| 4 | Lactoferricin | >500 | 3.9 | - | >500 | 3.9 | - | 52.52 | 257.9 | 4.9 | 23.18 | 258.7 | 11.1 |
| 5 | 758 | 12.34 | 147.1 | 11.9 | 9.3 | 147.1 | 15.8 | >62.5 | 151.8 | - | >62.5 | 16.2 | - |
| 6 | 1091 | 23.09 | 127.9 | 5.5 | 24.26 | 127.9 | 5.2 | >62.5 | 105.4 | - | >62.5 | 45.60 | - |
| 7 | 1096 | 22.22 | 47.21 | 2.1 | 24.01 | 47.21 | 1.9 | >62.5 | 57.29 | - | >62.5 | 4.7 | - |
| 8 | 1083 | 28.50 | 250.5 | 8.7 | 27.65 | 250.5 | 9.05 | 45.15 | 484.7 | 10.7 | >62.5 | 75.54 | - |
| 9 | 610 | 2.35 | 33.30 | 14.1 | 3.7 | 33.30 | 9 | >62.5 | 16.7 | - | >62.5 | 13.9 | - |
| 10 | NAPL | 10.49 | 111.8 | 10.6 | 10.51 | 111.8 | 10.6 | 59.27 | 96.1 | 1.6 | >62.5 | 40.04 | - |
| 11 | 3-BIPL | 19.24 | 28.11 | 1.4 | 18.63 | 28.11 | 1.5 | >62.5 | 27.8 | - | >62.5 | 28.24 | - |
| 12 | 4-BIPL | 10.34 | 12.78 | 1.2 | 10.4 | 12.78 | 1.2 | >62.5 | 38.8 | - | >62.5 | 60.18 | - |
| 13 | Sau-22 | 6.93 | 79.43 | 11.4 | 5.34 | 79.43 | 14.8 | >62.5 | 15.18 | - | >62.5 | 9.3 | - |
LL-37—molecular mass 4493.3; charge +6 | ||
Melimine—molecular mass 3786.6; charge +15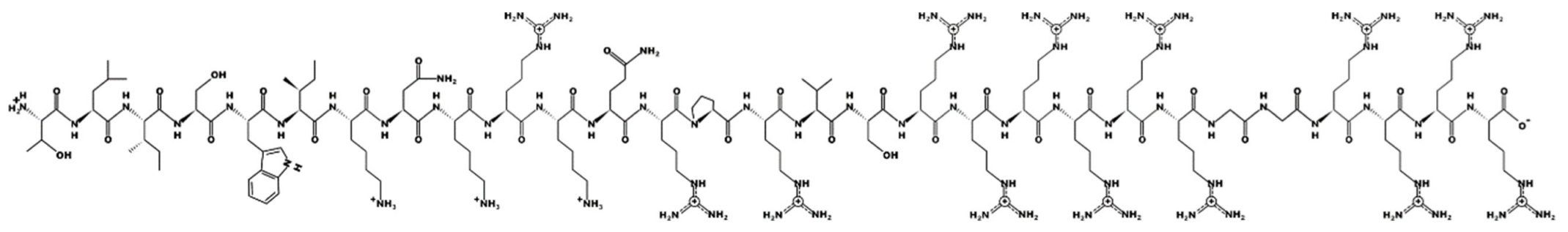 | ||
Mel4—molecular mass 2347.8; charge +14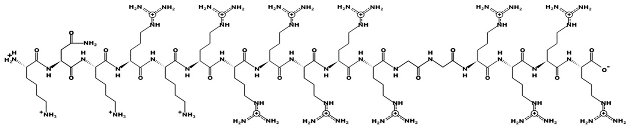 | Lactoferricin—molecular mass 1544.9; charge +4 | |
758—molecular mass 759.71; charge +2 | 1091—molecular mass 601.51; charge +1 | 1096—molecular mass 720.67; charge +2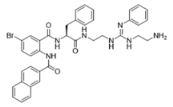 |
1083—molecular mass 726.68; charge +3 | 610—molecular mass 491.60; charge +2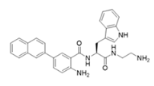 | NAPL—molecular mass 726.68; charge +2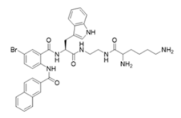 |
3-BIPL—molecular mass 752.71; charge +2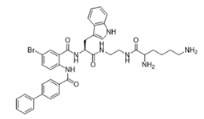 | 4-BIPL—molecular mass 752.71; charge +2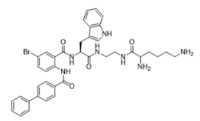 | Sau-22—molecular mass 745.68; charge +2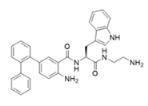 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urmi, U.L.; Vijay, A.K.; Willcox, M.D.P.; Attard, S.; Enninful, G.; Kumar, N.; Islam, S.; Kuppusamy, R. Exploring the Efficacy of Peptides and Mimics against Influenza A Virus, Adenovirus, and Murine Norovirus. Int. J. Mol. Sci. 2024, 25, 7030. https://doi.org/10.3390/ijms25137030
Urmi UL, Vijay AK, Willcox MDP, Attard S, Enninful G, Kumar N, Islam S, Kuppusamy R. Exploring the Efficacy of Peptides and Mimics against Influenza A Virus, Adenovirus, and Murine Norovirus. International Journal of Molecular Sciences. 2024; 25(13):7030. https://doi.org/10.3390/ijms25137030
Chicago/Turabian StyleUrmi, Umme Laila, Ajay Kumar Vijay, Mark D. P. Willcox, Samuel Attard, George Enninful, Naresh Kumar, Salequl Islam, and Rajesh Kuppusamy. 2024. "Exploring the Efficacy of Peptides and Mimics against Influenza A Virus, Adenovirus, and Murine Norovirus" International Journal of Molecular Sciences 25, no. 13: 7030. https://doi.org/10.3390/ijms25137030
APA StyleUrmi, U. L., Vijay, A. K., Willcox, M. D. P., Attard, S., Enninful, G., Kumar, N., Islam, S., & Kuppusamy, R. (2024). Exploring the Efficacy of Peptides and Mimics against Influenza A Virus, Adenovirus, and Murine Norovirus. International Journal of Molecular Sciences, 25(13), 7030. https://doi.org/10.3390/ijms25137030









