Investigating the Effectiveness of Ceragenins against Acinetobacter baumannii to Develop New Antimicrobial and Anti-Adhesive Strategies
Abstract
:1. Introduction
2. Results
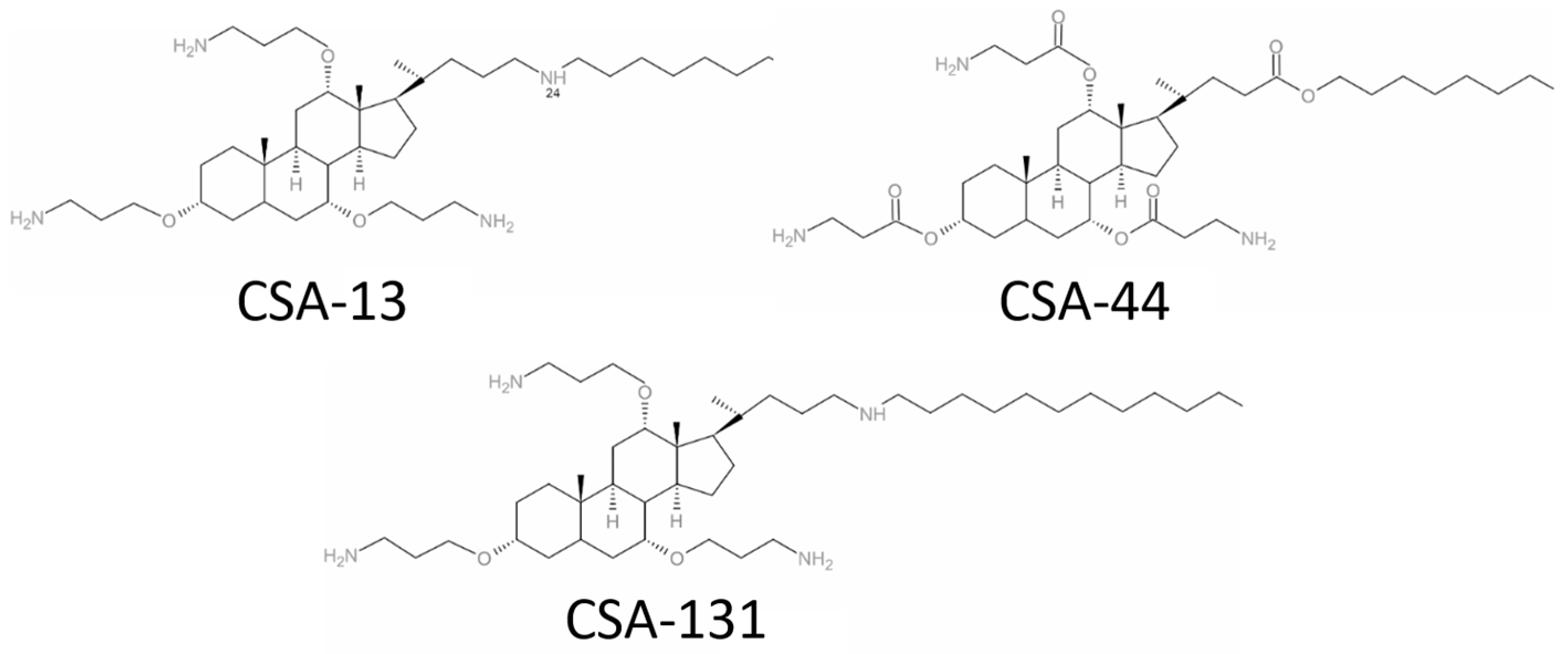
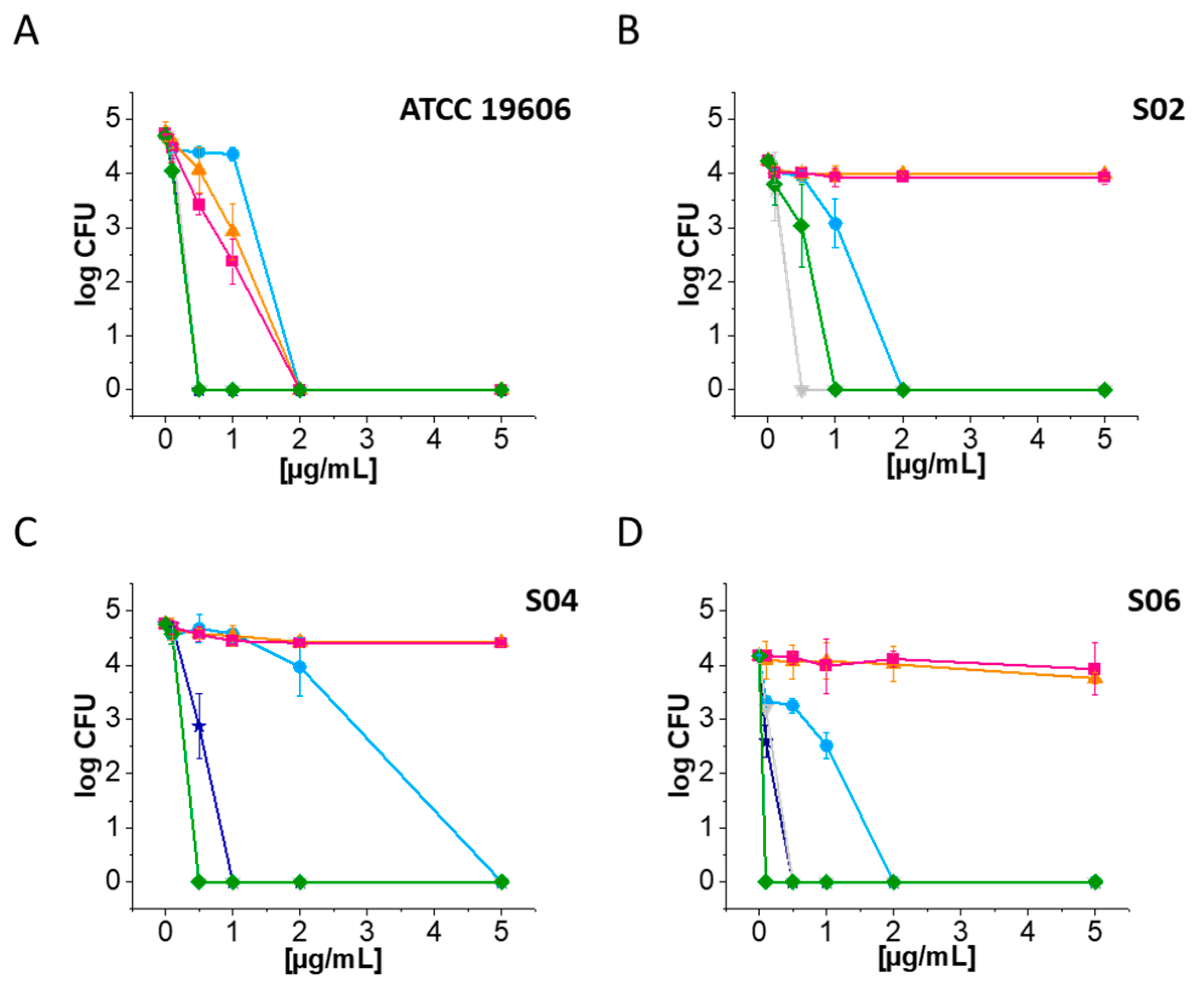
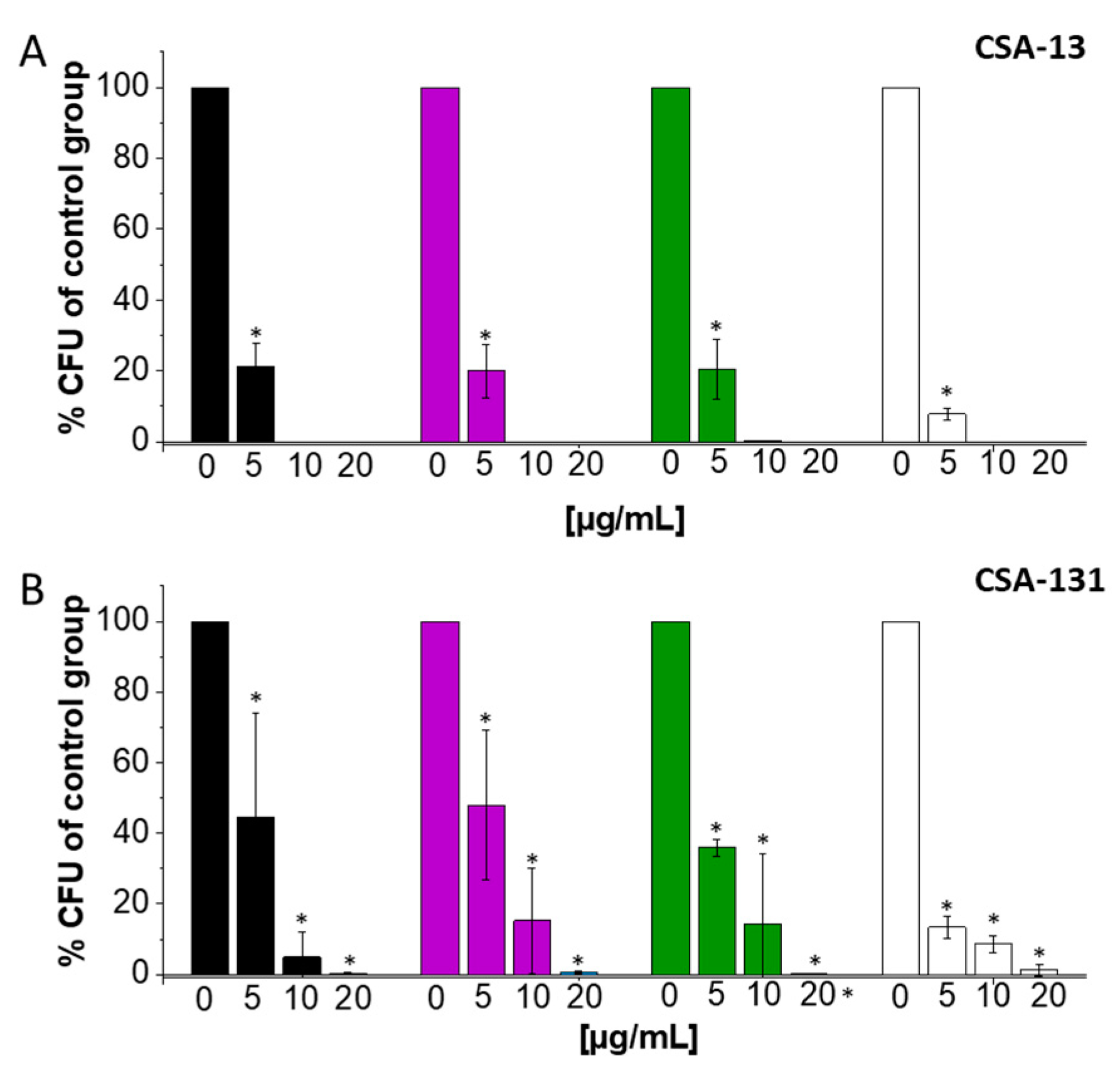
2.1. Ceragenins CSA-13 and CSA-131 Showcase Potent Antibacterial Efficacy against A. baumannii and CRAB Strains
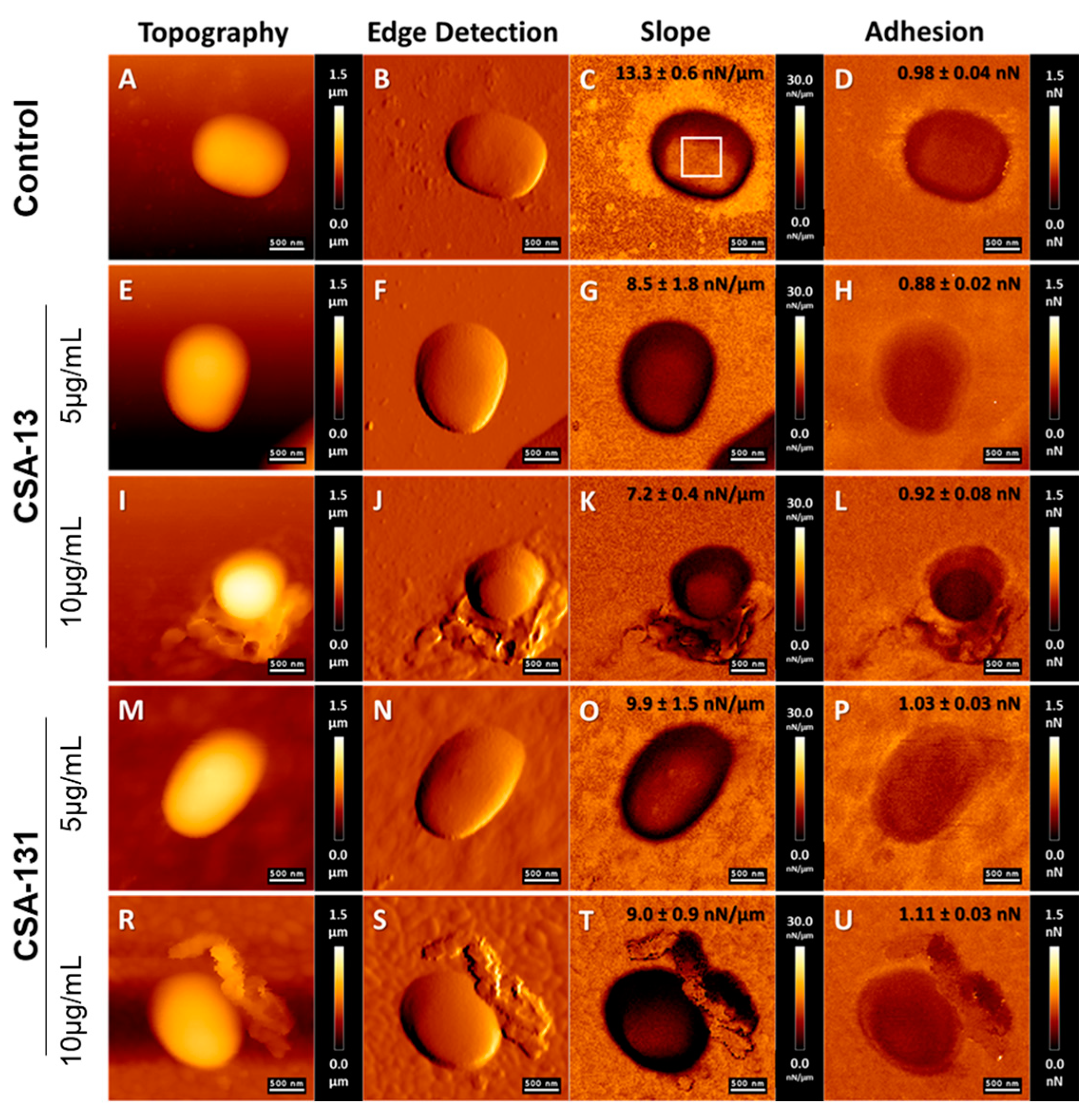
2.2. Atomic Force Microscopy Measurements of A. baumannii Cells Subjected to Deragenin Addition
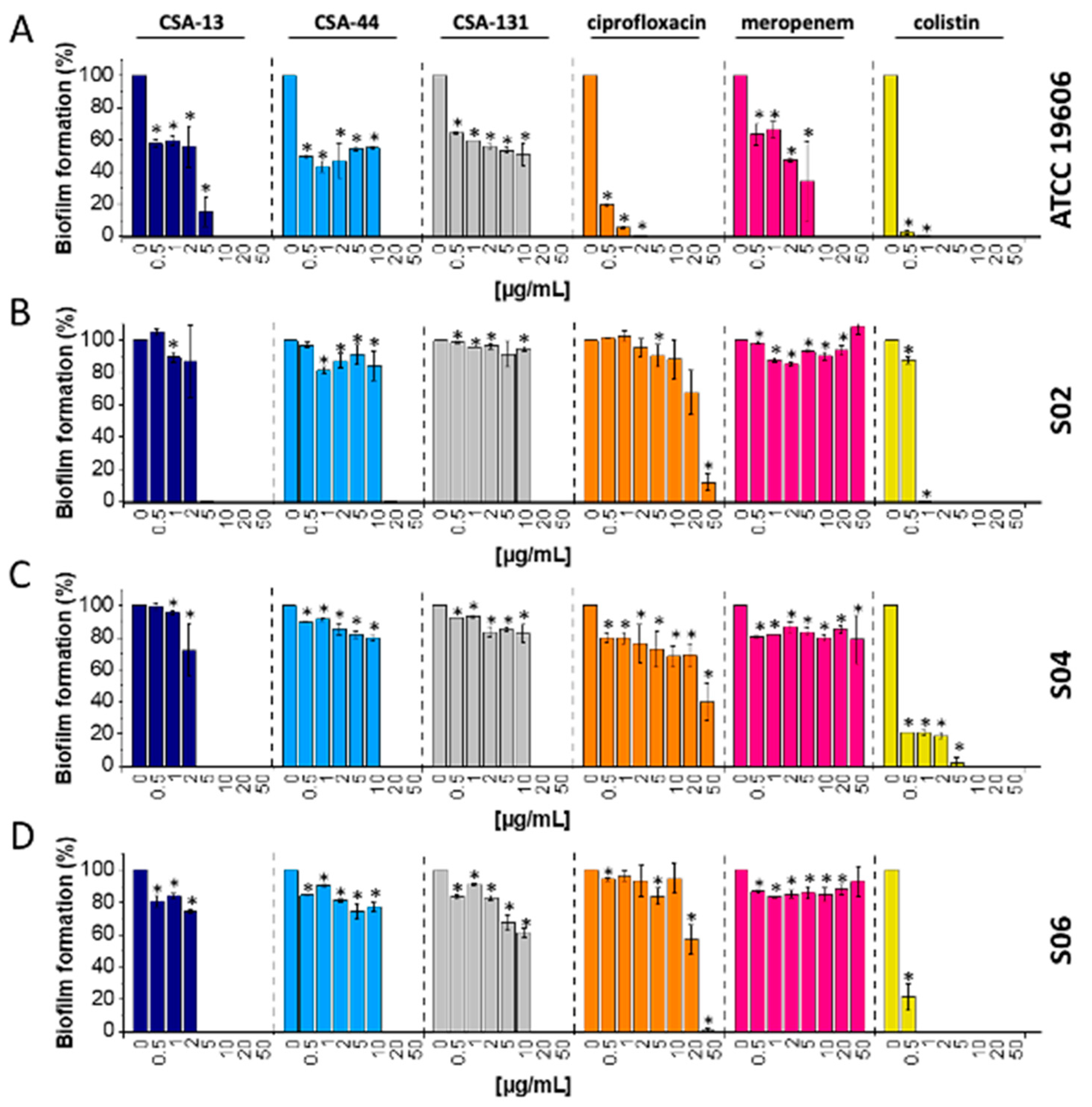
2.3. Biofilm Eradication of Carbapenem-Resistant A. baumannii after CSA-13 Addition at Low Concentration
2.4. Ceragenins Exhibited Significant Potential as Antibiofilm Agents against A. baumannii Forming Biofilm on Tracheostomy Tube Surface
2.5. Ceragenins Display Low Cytotoxicity against A549 Cells at Bactericidal Concentrations
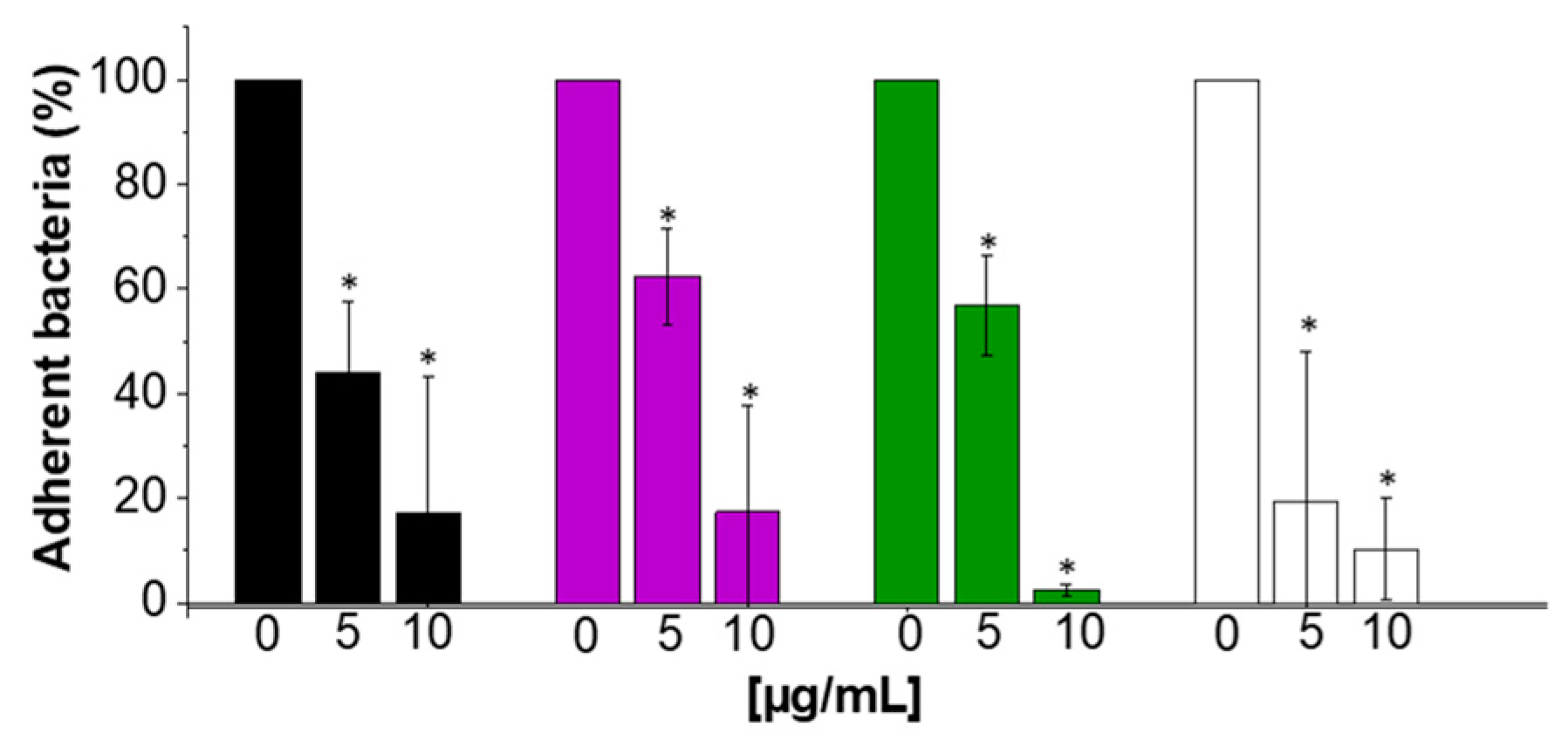
2.6. Anti-Adhesive Properties of CSA-13
3. Discussion
4. Materials and Methods
4.1. Clinical Bacterial Isolates and Antimicrobial Compounds
4.2. Mechanisms of Resistance of the Tested Clinical Strains of A. baumannii
4.3. Antimicrobial Activity Assessment
4.4. Atomic Force Microscopy Measurements of A. baumannii Cells Subjected to Ceragenin Treatment
4.5. Activity against Biofilms
4.6. Evaluation of Ceragenins’ Antibiofilm Capabilities on Tracheostomy Tubes
4.7. Cell Culture
4.8. Assessment of Ceragenin Cytotoxicity towards A549 Epithelial Cells Using MTT Assay
4.9. Adhesion Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.M.; Rovig, J.; Holden, B.S.; Taylor, M.F.; Weber, S.; Wilson, J.; Hilton, B.; Zaugg, A.L.; Ellis, S.W.; Yost, C.D.; et al. Ceragenins are active against drug-resistant Candida auris clinical isolates in planktonic and biofilm forms. J. Antimicrob. Chemother. 2018, 73, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.J.; Boswell, J.S.; Walsh, J.P.; Schellenberg, M.M.; Winter, T.W.; Li, C.; Allman, G.W.; Savage, P.B. Activities of cholic acid-derived antimicrobial agents against multidrug-resistant bacteria. J. Antimicrob. Chemother. 2001, 47, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Karasinski, M.; Wnorowska, U.; Durnas, B.; Krol, G.; Daniluk, T.; Sklodowski, K.; Gluszek, K.; Piktel, E.; Okla, S.; Bucki, R. Ceragenins and Ceragenin-Based Core-Shell Nanosystems as New Antibacterial Agents against Gram-Negative Rods Causing Nosocomial Infections. Pathogens 2023, 12, 1346. [Google Scholar] [CrossRef] [PubMed]
- Sklodowski, K.; Chmielewska, S.J.; Depciuch, J.; Deptula, P.; Piktel, E.; Daniluk, T.; Zakrzewska, M.; Czarnowski, M.; Ciesluk, M.; Durnas, B.; et al. Ceragenin-Coated Non-Spherical Gold Nanoparticles as Novel Candidacidal Agents. Pharmaceutics 2021, 13, 1940. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.; Giniyatullina, G.; Babkov, D.; Wimmer, Z. From Marine Metabolites to the Drugs of the Future: Squalamine, Trodusquemine, Their Steroid and Triterpene Analogues. Int. J. Mol. Sci. 2022, 23, 1075. [Google Scholar] [CrossRef] [PubMed]
- Paprocka, P.; Mankowska, A.; Sklodowski, K.; Krol, G.; Wollny, T.; Lesiak, A.; Gluszek, K.; Savage, P.B.; Durnas, B.; Bucki, R. Bactericidal Activity of Ceragenin in Combination with Ceftazidime, Levofloxacin, Co-Trimoxazole, and Colistin against the Opportunistic Pathogen Stenotrophomonas maltophilia. Pathogens 2022, 11, 621. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt-Guzel, C.; Inci, G.; Oyardi, O.; Savage, P.B. Synergistic Activity of Ceragenins Against Carbapenem-Resistant Acinetobacter baumannii Strains in Both Checkerboard and Dynamic Time-Kill Assays. Curr. Microbiol. 2020, 77, 1419–1428. [Google Scholar] [CrossRef]
- Hashemi, M.M.; Rovig, J.; Weber, S.; Hilton, B.; Forouzan, M.M.; Savage, P.B. Susceptibility of Colistin-Resistant, Gram-Negative Bacteria to Antimicrobial Peptides and Ceragenins. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.E.; Snarr, J.; Chaudhary, V.; Jennings, J.D.; Shaw, H.; Christiansen, B.; Wright, J.; Jia, W.; Bishop, R.E.; Savage, P.B. In vitro evaluation of the potential for resistance development to ceragenin CSA-13. J. Antimicrob. Chemother. 2012, 67, 2665–2672. [Google Scholar] [CrossRef]
- Ahuatzin-Flores, O.E.; Torres, E.; Chávez-Bravo, E. Acinetobacter baumannii, a Multidrug-Resistant Opportunistic Pathogen in New Habitats: A Systematic Review. Microorganisms 2024, 12, 644. [Google Scholar] [CrossRef]
- Bansal, K.; Saroha, T.; Patil, P.P.; Kumar, S.; Kumar, S.; Singhal, L.; Gautam, V.; Patil, P.B. Evolutionary trends of carbapenem-resistant and susceptible Acinetobacter baumannii isolates in a major tertiary care setting from North India. Infect. Genet. Evol. 2024, 117, 105542. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Huang, Y.; Pan, X.; Yao, Z.; Chen, C.; Zhong, A.; Xing, Y.; Qian, B.; Minhua, S.; Zhou, T. Nomogram for predicting 90-day mortality in patients with Acinetobacter baumannii-caused hospital-acquired and ventilator-associated pneumonia in the respiratory intensive care unit. J. Int. Med. Res. 2023, 51, 03000605231161481. [Google Scholar] [CrossRef]
- Alnimr, A. Antimicrobial Resistance in Ventilator-Associated Pneumonia: Predictive Microbiology and Evidence-Based Therapy. Infect. Dis. Ther. 2023, 12, 1527–1552. [Google Scholar] [CrossRef] [PubMed]
- Miron, M.; Blaj, M.; Ristescu, A.I.; Iosep, G.; Avădanei, A.-N.; Iosep, D.-G.; Crișan-Dabija, R.; Ciocan, A.; Perțea, M.; Manciuc, C.D.; et al. Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia: A Literature Review. Microorganisms 2024, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Shadan, A.; Pathak, A.; Ma, Y.; Pathania, R.; Singh, R.P. Deciphering the virulence factors, regulation, and immune response to Acinetobacter baumannii infection. Front. Cell. Infect. Microbiol. 2023, 13, 1053968. [Google Scholar] [CrossRef]
- Chandrasekhar, N. Hospital-Acquired Urinary Tract Infections. In Advances and Challenges in Urine Laboratory Analysis; Tomasz, J., Agnieszka, D., Eds.; IntechOpen: Rijeka, Croatia, 2023; p. Ch. 2. [Google Scholar]
- Papadopoulou, M.; Deliolanis, I.; Polemis, M.; Vatopoulos, A.; Psichogiou, M.; Giakkoupi, P. Characteristics of the Genetic Spread of Carbapenem-Resistant Acinetobacter baumannii in a Tertiary Greek Hospital. Genes 2024, 15, 458. [Google Scholar] [CrossRef]
- Choi, C.H.; Lee, E.Y.; Lee, Y.C.; Park, T.I.; Kim, H.J.; Hyun, S.H.; Kim, S.A.; Lee, S.K.; Lee, J.C. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell. Microbiol. 2005, 7, 1127–1138. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Bartal, C.; Rolston, K.V.I.; Nesher, L. Carbapenem-resistant Acinetobacter baumannii: Colonization, Infection and Current Treatment Options. Infect. Dis. Ther. 2022, 11, 683–694. [Google Scholar] [CrossRef]
- Shields, R.K.; Paterson, D.L.; Tamma, P.D. Navigating Available Treatment Options for Carbapenem-Resistant Acinetobacter baumannii-calcoaceticus Complex Infections. Clin. Infect. Dis. 2023, 76, S179–S193. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, P.; Panagopoulos, P.; Koutserimpas, C.; Samonis, G. Current Therapeutic Approaches for Multidrug-Resistant and Extensively Drug-Resistant Acinetobacter baumannii Infections. Antibiotics 2024, 13, 261. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, Q.; Chen, Z.; Li, C. Efficacy of sulbactam for the treatment of Acinetobacter baumannii complex infection: A systematic review and meta-analysis. J. Infect. Chemother. 2017, 23, 278–285. [Google Scholar] [CrossRef]
- Broncano-Lavado, A.; Santamaría-Corral, G.; Esteban, J.; García-Quintanilla, M. Advances in bacteriophage therapy against relevant multidrug-resistant pathogens. Antibiotics 2021, 10, 672. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jang, A.; Yoon, Y.K.; Kim, Y. Development of novel peptides for the antimicrobial combination therapy against carbapenem-resistant Acinetobacter baumannii infection. Pharmaceutics 2021, 13, 1800. [Google Scholar] [CrossRef] [PubMed]
- Microbiol, I.; Saikia, S.; Chetia, P. Antibiotics: From Mechanism of Action to Resistance and Beyond. Indian. J. Microbiol. 2024. [Google Scholar] [CrossRef]
- Mancuso, G.; De Gaetano, S.; Midiri, A.; Zummo, S.; Biondo, C. The Challenge of Overcoming Antibiotic Resistance in Carbapenem-Resistant Gram-Negative Bacteria: “Attack on Titan”. Microorganisms 2023, 11, 1912. [Google Scholar] [CrossRef]
- Weinberg, S.; Villedieu, A.; Bagdasarian, N.; Karah, N.; Teare, L.; Elamin, W. Control and management of multidrug resistant Acinetobacter baumannii: A review of the evidence and proposal of novel approaches. Infect. Prev. Pract. 2020, 2, 100077. [Google Scholar] [CrossRef]
- Ageitos, J.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef]
- Vila-Farrés, X.; Callarisa, A.E.; Gu, X.; Savage, P.B.; Giralt, E.; Vila, J. CSA-131, a ceragenin active against colistin-resistant Acinetobacter baumannii and Pseudomonas aeruginosa clinical isolates. Int. J. Antimicrob. Agents 2015, 46, 568–571. [Google Scholar] [CrossRef]
- Paiva, T.O.; Viljoen, A.; Dufrêne, Y.F. Seeing the unseen: High-resolution AFM imaging captures antibiotic action in bacterial membranes. Nat. Commun. 2022, 13, 6196. [Google Scholar] [CrossRef] [PubMed]
- Nasompag, S.; Siritongsuk, P.; Thammawithan, S.; Srichaiyapol, O.; Prangkio, P.; Camesano, T.A.; Sinthuvanich, C.; Patramanon, R. AFM Study of Nanoscale Membrane Perturbation Induced by Antimicrobial Lipopeptide C14 KYR. Membranes 2021, 11, 495. [Google Scholar] [CrossRef]
- Grzeszczuk, Z.; Rosillo, A.; Owens, Ó.; Bhattacharjee, S. Atomic Force Microscopy (AFM) As a Surface Mapping Tool in Microorganisms Resistant Toward Antimicrobials: A Mini-Review. Front. Pharmacol. 2020, 11, 517165. [Google Scholar] [CrossRef]
- Chmielewska, S.J.; Skłodowski, K.; Piktel, E.; Suprewicz, Ł.; Fiedoruk, K.; Daniluk, T.; Wolak, P.; Savage, P.B.; Bucki, R. NDM-1 Carbapenemase-Producing Enterobacteriaceae are Highly Susceptible to Ceragenins CSA-13, CSA-44, and CSA-131. Infect. Drug Resist. 2020, 13, 3277–3294. [Google Scholar] [CrossRef]
- Bucki, R.; Niemirowicz-Laskowska, K.; Deptuła, P.; Wilczewska, A.Z.; Misiak, P.; Durnaś, B.; Fiedoruk, K.; Piktel, E.; Mystkowska, J.; Janmey, P.A. Susceptibility of microbial cells to the modified PIP 2-binding sequence of gelsolin anchored on the surface of magnetic nanoparticles. J. Nanobiotechnol. 2019, 17, 81. [Google Scholar] [CrossRef]
- Lekka, M.; Pogoda, K.; Gostek, J.; Klymenko, O.; Prauzner-Bechcicki, S.; Wiltowska-Zuber, J.; Jaczewska, J.; Lekki, J.; Stachura, Z. Cancer cell recognition–mechanical phenotype. Micron 2012, 43, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Koh, Y.M.; Kim, J.; Lee, J.C.; Lee, Y.C.; Seol, S.Y.; Cho, D.T.; Kim, J. Capacity of multidrug-resistant clinical isolates of Acinetobacter baumannii to form biofilm and adhere to epithelial cell surfaces. Clin. Microbiol. Infect. 2008, 14, 49–54. [Google Scholar] [CrossRef]
- Wnorowska, U.; Łysik, D.; Piktel, E.; Zakrzewska, M.; Okła, S.; Lesiak, A.; Spałek, J.; Mystkowska, J.; Savage, P.B.; Janmey, P.; et al. Ceragenin-mediated disruption of Pseudomonas aeruginosa biofilms. PLoS ONE 2024, 19, e0298112. [Google Scholar] [CrossRef]
- Latorre, M.C.; Pérez-Granda, M.J.; Savage, P.B.; Alonso, B.; Martín-Rabadán, P.; Samaniego, R.; Bouza, E.; Muñoz, P.; Guembe, M. Endotracheal tubes coated with a broad-spectrum antibacterial ceragenin reduce bacterial biofilm in an in vitro bench top model. J. Antimicrob. Chemother. 2021, 76, 1168–1173. [Google Scholar] [CrossRef]
- Spałek, J.; Daniluk, T.; Godlewski, A.; Deptuła, P.; Wnorowska, U.; Ziembicka, D.; Cieśluk, M.; Fiedoruk, K.; Ciborowski, M.; Krętowski, A. Assessment of Ceragenins in prevention of damage to voice prostheses caused by Candida biofilm formation. Pathogens 2021, 10, 1371. [Google Scholar] [CrossRef]
- Choi, C.H.; Lee, J.S.; Lee, Y.C.; Park, T.I.; Lee, J.C. Acinetobacter baumannii invades epithelial cells and outer membrane protein A mediates interactions with epithelial cells. BMC Microbiol. 2008, 8, 216. [Google Scholar] [CrossRef]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Microbiol. 2018, 16, 91–102. [Google Scholar] [CrossRef]
- Weber, B.S.; Harding, C.M.; Feldman, M.F. Pathogenic Acinetobacter: From the Cell Surface to Infinity and Beyond. J. Bacteriol. 2015, 198, 880–887. [Google Scholar] [CrossRef]
- Giannouli, M.; Antunes, L.C.; Marchetti, V.; Triassi, M.; Visca, P.; Zarrilli, R. Virulence-related traits of epidemic Acinetobacter baumannii strains belonging to the international clonal lineages I-III and to the emerging genotypes ST25 and ST78. BMC Infect. Dis. 2013, 13, 282. [Google Scholar] [CrossRef]
- Bonomo, R.A.; Szabo, D. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin. Infect. Dis. 2006, 43, S49–S56. [Google Scholar] [CrossRef] [PubMed]
- Khazaal, S.S.; Al-Saryi, N.; Ibrahim, S.A. Immunomodulation by Acinetobacter baumannii of endotracheal tube biofilm in ventilator-associated pneumonia. Meta Gene 2020, 24, 100672. [Google Scholar] [CrossRef]
- Wang, M.; Lian, Y.; Wang, Y.; Zhu, L. The role and mechanism of quorum sensing on environmental antimicrobial resistance. Environ. Pollut. 2023, 322, 121238. [Google Scholar] [CrossRef]
- Roy, S.; Chowdhury, G.; Mukhopadhyay, A.K.; Dutta, S.; Basu, S. Convergence of Biofilm Formation and Antibiotic Resistance in Acinetobacter baumannii Infection. Front. Med. 2022, 9, 793615. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; He, S. Quorum Sensing Inhibition or Quenching in Acinetobacter baumannii: The Novel Therapeutic Strategies for New Drug Development. Front. Microbiol. 2021, 12, 558003. [Google Scholar] [CrossRef]
- Seleem, N.M.; Abd El Latif, H.K.; Shaldam, M.A.; El-Ganiny, A. Drugs with new lease of life as quorum sensing inhibitors: For combating MDR Acinetobacter baumannii infections. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1687–1702. [Google Scholar] [CrossRef]
- Cui, B.; Guo, Q.; Li, X.; Song, S.; Wang, M.; Wang, G.; Yan, A.; Zhou, J.; Deng, Y. A response regulator controls Acinetobacter baumannii virulence by acting as an indole receptor. PNAS Nexus 2023, 2, pgad274. [Google Scholar] [CrossRef] [PubMed]
- Gedefie, A.; Demsis, W.; Ashagrie, M.; Kassa, Y.; Tesfaye, M.; Tilahun, M.; Bisetegn, H.; Sahle, Z. Acinetobacter baumannii Biofilm Formation and Its Role in Disease Pathogenesis: A Review. Infect. Drug Resist. 2021, 14, 3711–3719. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Nadeem, A.; Mushtaq, F.; Zlatkov, N.; Shahzad, M.; Zavialov, A.V.; Wai, S.N.; Uhlin, B.E. Csu pili dependent biofilm formation and virulence of Acinetobacter baumannii. NPJ Biofilms Microbiomes 2023, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Upmanyu, K.; Haq, Q.M.R.; Singh, R. Factors mediating Acinetobacter baumannii biofilm formation: Opportunities for developing therapeutics. Curr. Res. Microb. Sci. 2022, 3, 100131. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.; Wright, J.; Feng, Y.; Geng, D.; Genberg, C.; Savage, P.B. Activities of ceragenin CSA-13 against established biofilms in an in vitro model of catheter decolonization. Anti-Infect. Agents Med. Chem. Former. Curr. Med. Chem. Anti-Infect. Agents 2009, 8, 290–294. [Google Scholar] [CrossRef]
- Williams, D.L.; Sinclair, K.D.; Jeyapalina, S.; Bloebaum, R.D. Characterization of a novel active release coating to prevent biofilm implant-related infections. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.M.; Rovig, J.; Bateman, J.; Holden, B.S.; Modelzelewski, T.; Gueorguieva, I.; von Dyck, M.; Bracken, R.; Genberg, C.; Deng, S. Preclinical testing of a broad-spectrum antimicrobial endotracheal tube coated with an innate immune synthetic mimic. J. Antimicrob. Chemother. 2018, 73, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Oyardi, Ö.; Savage, P.B.; Erturan, Z.; Bozkurt-Guzel, C. In vitro assessment of CSA-131 and CSA-131 poloxamer form for the treatment of Stenotrophomonas maltophilia infections in cystic fibrosis. J. Antimicrob. Chemother. 2021, 76, 443–450. [Google Scholar] [CrossRef]
- Wnorowska, U.; Fiedoruk, K.; Piktel, E.; Prasad, S.V.; Sulik, M.; Janion, M.; Daniluk, T.; Savage, P.B.; Bucki, R. Nanoantibiotics containing membrane-active human cathelicidin LL-37 or synthetic ceragenins attached to the surface of magnetic nanoparticles as novel and innovative therapeutic tools: Current status and potential future applications. J. Nanobiotechnol. 2020, 18, 3. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karasiński, M.; Wnorowska, U.; Daniluk, T.; Deptuła, P.; Łuckiewicz, M.; Paprocka, P.; Durnaś, B.; Skłodowski, K.; Sawczuk, B.; Savage, P.B.; et al. Investigating the Effectiveness of Ceragenins against Acinetobacter baumannii to Develop New Antimicrobial and Anti-Adhesive Strategies. Int. J. Mol. Sci. 2024, 25, 7036. https://doi.org/10.3390/ijms25137036
Karasiński M, Wnorowska U, Daniluk T, Deptuła P, Łuckiewicz M, Paprocka P, Durnaś B, Skłodowski K, Sawczuk B, Savage PB, et al. Investigating the Effectiveness of Ceragenins against Acinetobacter baumannii to Develop New Antimicrobial and Anti-Adhesive Strategies. International Journal of Molecular Sciences. 2024; 25(13):7036. https://doi.org/10.3390/ijms25137036
Chicago/Turabian StyleKarasiński, Maciej, Urszula Wnorowska, Tamara Daniluk, Piotr Deptuła, Milena Łuckiewicz, Paulina Paprocka, Bonita Durnaś, Karol Skłodowski, Beata Sawczuk, Paul B. Savage, and et al. 2024. "Investigating the Effectiveness of Ceragenins against Acinetobacter baumannii to Develop New Antimicrobial and Anti-Adhesive Strategies" International Journal of Molecular Sciences 25, no. 13: 7036. https://doi.org/10.3390/ijms25137036




