Modeling Host–Pathogen Interactions in C. elegans: Lessons Learned from Pseudomonas aeruginosa Infection
Abstract
:1. Introduction
2. Pseudomonas aeruginosa Virulence Factors and Infection
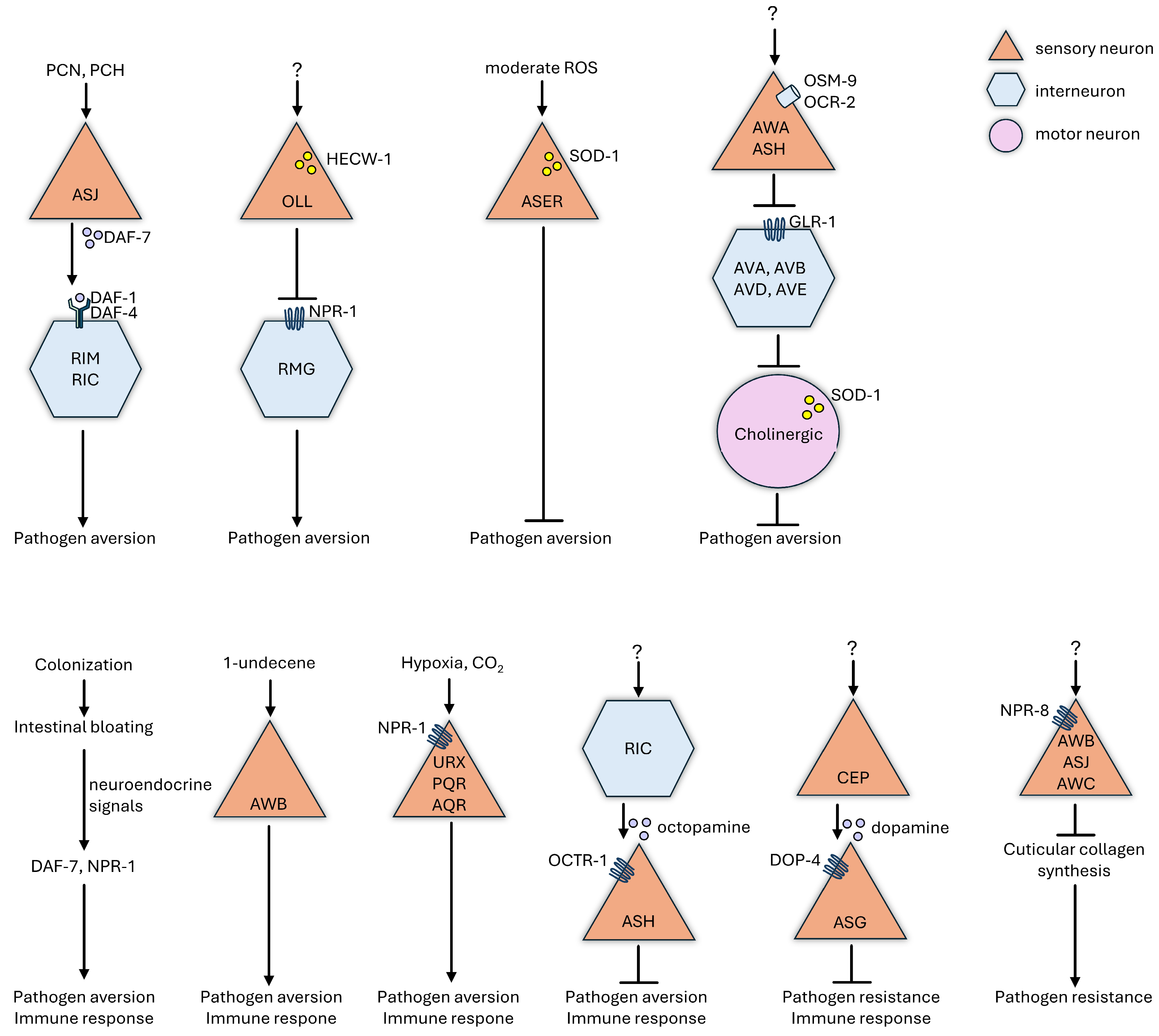
3. Routes of Pathogen Recognition
4. Cellular Stress and Immune Responses

| Key Regulators (Pathways) | Activatory Signals | Specific to P. aeruginosa | Relevance in Mammals | Ref. |
|---|---|---|---|---|
| TIR-1/SARM1 PMK-1/p38 MAPK (p38 MAPK pathway) | ROS, pyocyanin-induced LRO stress | No | Yes | [15,89,90] |
| DAF-16/FOXO (ILS and oxidative stress response) | ROS, proteostasis disturbance, suppressed DAF-2 signaling | No | Yes | [15,67,91] |
| SKN-1/Nrf-2 (Oxidative and electrophilic stress response) | ROS, PMK-1 | No | Yes | [15,92,93,94,95] |
| HSF-1 (Heat shock response) | Proteostasis disturbance, ROS | No | Yes | [96] |
| FSHR-1 | n.d. | No | No | [97,98] |
| ZIP-2 (Immune response, ESRE) | ExoA-induced suppression of mRNA translation, pyoverdine-induced mitochondrial dysfunction | Yes | No | [99,100,101,102] |
| ATFS-1/ATF4 (UPRMT) | Disturbance of mitochondrial proteostasis | n.d. | Yes | [103,104] |
| HIF-1, AMPK | Mitochondrial dysfunction due to pyoverdine-induced iron depletion | No | Yes | [56,77,105,106] |
| NHR-86/HNF4 | Phenazin-1-caroboxamide | n.d. | No | [61,107] |
4.1. The p38 MAPK Pathway
4.2. TIR-1/SARM Activation and Immune Surveillance of Lysosome-Related Organelles
4.3. The DAF-16/FOXO, SKN-1/Nrf2, and HSF-1 Pathways
4.4. The FSHR-1 Pathway
5. Surveillance Immunity of ExoA-Mediated Translation Inhibition
6. Surveillance Immunity of Mitochondrial Function
6.1. Stress Response Element (ESRE)
6.2. Mitochondrial Unfolded Protein Response (UPRMT)
6.3. Siderophore-Specific Mitophagy
6.4. Mitochondrial Reactive Oxygen Species (mtROS)
7. Alternative Pattern Recognition by the Pathogen Metabolite Checkpoint
8. Neuroendocrine Regulation of Physiological and Behavioral Defenses
9. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brockhurst, M.A.; Koskella, B. Experimental coevolution of species interactions. Trends Ecol. Evol. 2013, 28, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Gandon, S.; Buckling, A.; Decaestecker, E.; Day, T. Host-parasite coevolution and patterns of adaptation across time and space. J. Evol. Biol. 2008, 21, 1861–1866. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.; Fields, P.D. Host-parasite co-evolution and its genomic signature. Nat. Rev. Genet. 2020, 21, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Baruah, A.; Tomioka, M.; Iino, Y.; Kalita, M.C.; Khan, M. Caenorhabditis elegans: A model to understand host–microbe interactions. Cell. Mol. Life Sci. 2019, 77, 1229–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Holdorf, A.D.; Walhout, A.J. C. elegans and its bacterial diet as a model for systems-level understanding of host–microbiota interactions. Curr. Opin. Biotechnol. 2017, 46, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Flavell, S.W. Host-microbe interactions and the behavior of Caenorhabditis elegans. J. Neurogenetics 2020, 34, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Millet, A.C.; Ewbank, J.J. Immunity in Caenorhabditis elegans. Curr. Opin. Immunol. 2003, 16, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Gravato-Nobre, M.J.; Hodgkin, J. Caenorhabditis elegans as a model for innate immunity to pathogens. Cell. Microbiol. 2005, 7, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Vance, R.E.; Isberg, R.R.; Portnoy, D.A. Patterns of Pathogenesis: Discrimination of Pathogenic and Nonpathogenic Microbes by the Innate Immune System. Cell Host Microbe 2009, 6, 10–21. [Google Scholar] [CrossRef]
- Cohen, L.B.; Troemel, E.R. Microbial pathogenesis and host defense in the nematode C. elegans. Curr. Opin. Microbiol. 2015, 23, 94–101. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J. Detection of Pathogens and Regulation of Immunity by the Caenorhabditis elegans Nervous System. mBio 2021, 12, e02301-20. [Google Scholar] [CrossRef] [PubMed]
- Ermolaeva, M.A.; Schumacher, B. Insights from the worm: The C. elegans model for innate immunity. Semin. Immunol. 2014, 26, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Aballay, A.; Ausubel, F.M. Caenorhabditis elegans as a host for the study of host–pathogen interactions. Curr. Opin. Microbiol. 2002, 5, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Tan, Y.; Tu, H.; Tan, W. Neuronal basis and diverse mechanisms of pathogen avoidance in Caenorhabditis elegans. Front. Immunol. 2024, 15, 1353747. [Google Scholar] [CrossRef] [PubMed]
- Martineau, C.N.; Kirienko, N.V.; Pujol, N. Innate immunity in C. elegans. Curr. Top. Dev. Biol. 2021, 144, 309–351. [Google Scholar] [CrossRef] [PubMed]
- Kirienko, N.V.; Cezairliyan, B.O.; Ausubel, F.M.; Powell, J.R. Pseudomonas aeruginosa PA14 pathogenesis in Caenorhabditis elegans. Methods Mol. Biol. 2014, 1149, 653–669. [Google Scholar] [CrossRef]
- A Driscoll, J.; Brody, S.L.; Kollef, M.H. The Epidemiology, Pathogenesis and Treatment of Pseudomonas aeruginosa Infections. Drugs 2007, 67, 351–368. [Google Scholar] [CrossRef]
- Garcia-Clemente, M.; de la Rosa, D.; Máiz, L.; Girón, R.; Blanco, M.; Olveira, C.; Canton, R.; Martinez-García, M.A. Impact of Pseudomonas aeruginosa Infection on Patients with Chronic Inflammatory Airway Diseases. J. Clin. Med. 2020, 9, 3800. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.; Sahu, R.; Owen, D.R.; Dennis, V.A. Pseudomonas aeruginosa reference strains PAO1 and PA14: A genomic, phenotypic, and therapeutic review. Front. Microbiol. 2022, 13, 1023523. [Google Scholar] [CrossRef]
- Mikkelsen, H.; McMullan, R.; Filloux, A. The Pseudomonas aeruginosa Reference Strain PA14 Displays Increased Virulence Due to a Mutation in ladS. PLoS ONE 2011, 6, e29113. [Google Scholar] [CrossRef]
- Mahajan-Miklos, S.; Tan, M.-W.; Rahme, L.G.; Ausubel, F.M. Molecular Mechanisms of Bacterial Virulence Elucidated Using a Pseudomonas aeruginosa– Caenorhabditis elegans Pathogenesis Model. Cell 1999, 96, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.-W.; Mahajan-Miklos, S.; Ausubel, F.M. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 715–720. [Google Scholar] [CrossRef]
- Liao, C.; Huang, X.; Wang, Q.; Yao, D.; Lu, W. Virulence Factors of Pseudomonas Aeruginosa and Antivirulence Strategies to Combat Its Drug Resistance. Front. Cell. Infect. Microbiol. 2022, 12, 926758. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef] [PubMed]
- Kilmury, S.L.N.; Burrows, L.L. The Pseudomonas aeruginosa PilSR Two-Component System Regulates Both Twitching and Swimming Motilities. mBio 2018, 9, e01310-18. [Google Scholar] [CrossRef] [PubMed]
- Pena, J.; Fu, Z.; Schwarzer, C.; Machen, T.E. Pseudomonas aeruginosa Inhibition of Flagellin-Activated NF-κB and Interleukin-8 by Human Airway Epithelial Cells. Infect. Immun. 2009, 77, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Feinbaum, R.L.; Urbach, J.M.; Liberati, N.T.; Djonovic, S.; Adonizio, A.; Carvunis, A.-R.; Ausubel, F.M. Genome-Wide Identification of Pseudomonas aeruginosa Virulence-Related Genes Using a Caenorhabditis elegans Infection Model. PLoS Pathog. 2012, 8, e1002813. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Kays, M.; Prince, A. Role of Pseudomonas aeruginosa pili in acute pulmonary infection. Infect. Immun. 1995, 63, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Vigneshkumar, B.; Pandian, S.K.; Balamurugan, K. Regulation of Caenorhabditis elegans and Pseudomonas aeruginosa machinery during interactions. Arch. Microbiol. 2011, 194, 229–242. [Google Scholar] [CrossRef]
- Wieland, C.W.; Siegmund, B.; Senaldi, G.; Vasil, M.L.; Dinarello, C.A.; Fantuzzi, G. Pulmonary Inflammation Induced by Pseudomonas aeruginosa Lipopolysaccharide, Phospholipase C, and Exotoxin A: Role of Interferon Regulatory Factor 1. Infect. Immun. 2002, 70, 1352–1358. [Google Scholar] [CrossRef]
- Mateu-Borrás, M.; Zamorano, L.; González-Alsina, A.; Sánchez-Diener, I.; Doménech-Sánchez, A.; Oliver, A.; Albertí, S. Molecular Analysis of the Contribution of Alkaline Protease A and Elastase B to the Virulence of Pseudomonas aeruginosa Bloodstream Infections. Front. Cell. Infect. Microbiol. 2022, 11, 816356. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Liu, C.; Liu, Y.; Feng, R.; Cao, R.; Guan, Z.; Xuan, B.; Gao, Y.; Wang, Q.; Yang, N.; et al. Antibodies Against Pseudomonas aeruginosa Alkaline Protease Directly Enhance Disruption of Neutrophil Extracellular Traps Mediated by This Enzyme. Front. Immunol. 2021, 12, 654649. [Google Scholar] [CrossRef]
- Duong, F.; Bonnet, E.; Géli, V.; Lazdunski, A.; Murgier, M.; Filloux, A. The AprX protein of Pseudomonas aeruginosa: A new substrate for the Apr type I secretion system. Gene 2001, 262, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Otero-Asman, J.R.; García-García, A.I.; Civantos, C.; Quesada, J.M.; Llamas, M.A. Pseudomonas aeruginosa possesses three distinct systems for sensing and using the host molecule haem. Environ. Microbiol. 2019, 21, 4629–4647. [Google Scholar] [CrossRef] [PubMed]
- McEwan, D.L.; Kirienko, N.V.; Ausubel, F.M. Host Translational Inhibition by Pseudomonas aeruginosa Exotoxin A Triggers an Immune Response in Caenorhabditis elegans. Cell Host Microbe 2012, 11, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Pollack, M.; E Anderson, S. Toxicity of Pseudomonas aeruginosa exotoxin A for human macrophages. Infect. Immun. 1978, 19, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Tielen, P.; Kuhn, H.; Rosenau, F.; Jaeger, K.-E.; Flemming, H.-C.; Wingender, J. Interaction between extracellular lipase LipA and the polysaccharide alginate of Pseudomonas aeruginosa. BMC Microbiol. 2013, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Rosenau, F.; Isenhardt, S.; Gdynia, A.; Tielker, D.; Schmidt, E.; Tielen, P.; Schobert, M.; Jahn, D.; Wilhelm, S.; Jaeger, K.-E. Lipase LipC affects motility, biofilm formation and rhamnolipid production in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2010, 309, 25–34. [Google Scholar] [CrossRef]
- Terada, L.S.; Johansen, K.A.; Nowbar, S.; Vasil, A.I.; Vasil, M.L. Pseudomonas aeruginosa Hemolytic Phospholipase C Suppresses Neutrophil Respiratory Burst Activity. Infect. Immun. 1999, 67, 2371–2376. [Google Scholar] [CrossRef]
- Malloy, J.L.; Veldhuizen, R.A.W.; Thibodeaux, B.A.; O’Callaghan, R.J.; Wright, J.R. Pseudomonas aeruginosa protease IV degrades surfactant proteins and inhibits surfactant host defense and biophysical functions. Am. J. Physiol. Cell. Mol. Physiol. 2005, 288, L409–L418. [Google Scholar] [CrossRef]
- Cowell, B.A.; Twining, S.S.; Hobden, J.A.; Kwong, M.S.F.; Fleiszig, S.M.J. Mutation of lasA and lasB reduces Pseudomonas aeruginosa invasion of epithelial cells. Microbiology 2003, 149, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Shaver, C.M.; Hauser, A.R. Relative Contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to Virulence in the Lung. Infect. Immun. 2004, 72, 6969–6977. [Google Scholar] [CrossRef] [PubMed]
- Zelikman, S.; Dudkevich, R.; Korenfeld-Tzemach, H.; Shmidov, E.; Levi-Ferber, M.; Shoshani, S.; Ben-Aroya, S.; Henis-Korenblit, S.; Banin, E. PemB, a type III secretion effector in Pseudomonas aeruginosa, affects Caenorhabditis elegans life span. Heliyon 2024, 10, e29751. [Google Scholar] [CrossRef] [PubMed]
- Kloth, C.; Schirmer, B.; Munder, A.; Stelzer, T.; Rothschuh, J.; Seifert, R. The Role of Pseudomonas aeruginosa ExoY in an Acute Mouse Lung Infection Model. Toxins 2018, 10, 185. [Google Scholar] [CrossRef]
- Burstein, D.; Satanower, S.; Simovitch, M.; Belnik, Y.; Zehavi, M.; Yerushalmi, G.; Ben-Aroya, S.; Pupko, T.; Banin, E. Novel Type III Effectors in Pseudomonas aeruginosa. mBio 2015, 6, e00161-15–e00161. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Gdynia, A.; Tielen, P.; Rosenau, F.; Jaeger, K.-E. The Autotransporter Esterase EstA of Pseudomonas aeruginosa Is Required for Rhamnolipid Production, Cell Motility, and Biofilm Formation. J. Bacteriol. 2007, 189, 6695–6703. [Google Scholar] [CrossRef] [PubMed]
- Leo, J.C.; Grin, I.; Linke, D. Type V secretion: Mechanism(s) of autotransport through the bacterial outer membrane. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Kida, Y.; Higashimoto, Y.; Inoue, H.; Shimizu, T.; Kuwano, K. A novel secreted protease from Pseudomonas aeruginosa activates NF-κB through protease-activated receptors. Cell. Microbiol. 2008, 10, 1491–1504. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.B.; Hood, R.D.; Bui, N.K.; LeRoux, M.; Vollmer, W.; Mougous, J.D. Type VI secretion delivers bacteriolytic effectors to target cells. Nature 2011, 475, 343–347. [Google Scholar] [CrossRef]
- Hood, R.D.; Singh, P.; Hsu, F.; Güvener, T.; Carl, M.A.; Trinidad, R.R.; Silverman, J.M.; Ohlson, B.B.; Hicks, K.G.; Plemel, R.L.; et al. A Type VI Secretion System of Pseudomonas aeruginosa Targets a Toxin to Bacteria. Cell Host Microbe 2010, 7, 25–37. [Google Scholar] [CrossRef]
- Yorgey, P.; Rahme, L.G.; Tan, M.; Ausubel, F.M. The roles of mucD and alginate in the virulence of Pseudomonas aeruginosa in plants, nematodes and mice. Mol. Microbiol. 2001, 41, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.S.; Kharazmi, A.; Espersen, F.; Høiby, N. Pseudomonas aeruginosa alginate in cystic fibrosis sputum and the inflammatory response. Infect. Immun. 1990, 58, 3363–3368. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, E.v.T.; Charron-Mazenod, L.; Reading, D.J.; Reckseidler-Zenteno, S.L.; Lewenza, S. Exopolysaccharide-Repressing Small Molecules with Antibiofilm and Antivirulence Activity against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2017, 61, e01997-16. [Google Scholar] [CrossRef]
- Fleming, D.; Niese, B.; Redman, W.; Vanderpool, E.; Gordon, V.; Rumbaugh, K.P. Contribution of Pseudomonas aeruginosa Exopolysaccharides Pel and Psl to Wound Infections. Front. Cell. Infect. Microbiol. 2022, 12, 835754. [Google Scholar] [CrossRef] [PubMed]
- Minandri, F.; Imperi, F.; Frangipani, E.; Bonchi, C.; Visaggio, D.; Facchini, M.; Pasquali, P.; Bragonzi, A.; Visca, P. Role of Iron Uptake Systems in Pseudomonas aeruginosa Virulence and Airway Infection. Infect. Immun. 2016, 84, 2324–2335. [Google Scholar] [CrossRef] [PubMed]
- Kirienko, N.V.; Kirienko, D.R.; Larkins-Ford, J.; Wählby, C.; Ruvkun, G.; Ausubel, F.M. Pseudomonas aeruginosa Disrupts Caenorhabditis elegans Iron Homeostasis, Causing a Hypoxic Response and Death. Cell Host Microbe 2013, 13, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Meisel, J.D.; Panda, O.; Mahanti, P.; Schroeder, F.C.; Kim, D.H. Chemosensation of Bacterial Secondary Metabolites Modulates Neuroendocrine Signaling and Behavior of C. elegans. Cell 2014, 159, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Morello, E.; Pérez-Berezo, T.; Boisseau, C.; Baranek, T.; Guillon, A.; Bréa, D.; Lanotte, P.; Carpena, X.; Pietrancosta, N.; Hervé, V.; et al. Pseudomonas aeruginosa Lipoxygenase LoxA Contributes to Lung Infection by Altering the Host Immune Lipid Signaling. Front. Microbiol. 2019, 10, 1826. [Google Scholar] [CrossRef]
- Cezairliyan, B.; Vinayavekhin, N.; Grenfell-Lee, D.; Yuen, G.J.; Saghatelian, A.; Ausubel, F.M. Identification of Pseudomonas aeruginosa Phenazines that Kill Caenorhabditis elegans. PLoS Pathog. 2013, 9, e1003101. [Google Scholar] [CrossRef]
- Denning, G.M.; Iyer, S.S.; Reszka, K.J.; O’Malley, Y.; Rasmussen, G.T.; Britigan, B.E. Phenazine-1-carboxylic acid, a secondary metabolite of Pseudomonas aeruginosa, alters expression of immunomodulatory proteins by human airway epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2003, 285, L584–L592. [Google Scholar] [CrossRef]
- Peterson, N.D.; Tse, S.Y.; Huang, Q.J.; Wani, K.A.; Schiffer, C.A.; Pukkila-Worley, R. Non-canonical pattern recognition of a pathogen-derived metabolite by a nuclear hormone receptor identifies virulent bacteria in C. elegans. Immunity 2023, 56, 768–782.e9. [Google Scholar] [CrossRef] [PubMed]
- Stewart-Tull, D.E.S.; Armstrong, A.V. The Effect Of 1-Hydroxyphenazine and Pyocyanin from Pseudomonas Aeruginosa On Mammalian Cell Respiration. J. Med. Microbiol. 1972, 5, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.W.; Ran, H.; Kong, F.; Hassett, D.J.; Mavrodi, D. Pseudomonas aeruginosa Pyocyanin Is Critical for Lung Infection in Mice. Infect. Immun. 2004, 72, 4275–4278. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.-W.; Rahme, L.G.; Sternberg, J.A.; Tompkins, R.G.; Ausubel, F.M. Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc. Natl. Acad. Sci. USA 1999, 96, 2408–2413. [Google Scholar] [CrossRef] [PubMed]
- Hennemann, L.C.; LaFayette, S.L.; Malet, J.K.; Bortolotti, P.; Yang, T.; McKay, G.A.; Houle, D.; Radzioch, D.; Rousseau, S.; Nguyen, D. LasR-deficient Pseudomonas aeruginosa variants increase airway epithelial mICAM-1 expression and enhance neutrophilic lung inflammation. PLoS Pathog. 2021, 17, e1009375. [Google Scholar] [CrossRef]
- Mukherjee, S.; Moustafa, D.; Smith, C.D.; Goldberg, J.B.; Bassler, B.L. The RhlR quorum-sensing receptor controls Pseudomonas aeruginosa pathogenesis and biofilm development independently of its canonical homoserine lactone autoinducer. PLoS Pathog. 2017, 13, e1006504. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.A.; Kawli, T.; Tan, M.-W. Pseudomonas aeruginosa Suppresses Host Immunity by Activating the DAF-2 Insulin-Like Signaling Pathway in Caenorhabditis elegans. PLoS Pathog. 2008, 4, e1000175. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.; Balachandran, P. Role of Pseudomonas aeruginosa type III effectors in disease. Curr. Opin. Microbiol. 2009, 12, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Aiello, D.; Williams, J.D.; Majgier-Baranowska, H.; Patel, I.; Peet, N.P.; Huang, J.; Lory, S.; Bowlin, T.L.; Moir, D.T. Discovery and Characterization of Inhibitors of Pseudomonas aeruginosa Type III Secretion. Antimicrob. Agents Chemother. 2010, 54, 1988–1999. [Google Scholar] [CrossRef]
- Alouf, J.E.; Ladant, D.; Popoff, M.R. The Comprehensive Sourcebook of Bacterial Protein Toxins; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Morlon-Guyot, J.; Méré, J.; Bonhoure, A.; Beaumelle, B. Processing of Pseudomonas aeruginosa Exotoxin A Is Dispensable for Cell Intoxication. Infect. Immun. 2009, 77, 3090–3099. [Google Scholar] [CrossRef]
- Schultz, M.J.; Speelman, P.; Zaat, S.A.; Hack, C.E.; Deventer, S.J.; Poll, T. The effect of Pseudomonas exotoxin A on cytokine production in whole blood exposed to Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol. 2000, 29, 227–232. [Google Scholar] [CrossRef]
- Lavoie, E.G.; Wangdi, T.; Kazmierczak, B.I. Innate immune responses to Pseudomonas aeruginosa infection. Microbes Infect. 2011, 13, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- E Wedekind, J.; Trame, C.B.; Dorywalska, M.; Koehl, P.; Raschke, T.M.; McKee, M.; FitzGerald, D.; Collier, R.; McKay, D.B. Refined crystallographic structure of Pseudomonas aeruginosa exotoxin A and its implications for the molecular mechanism of toxicity 1 1Edited by D. Rees. J. Mol. Biol. 2001, 314, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Hummell, N.A.; Revtovich, A.V.; Kirienko, N.V. Novel Immune Modulators Enhance Caenorhabditis elegans Resistance to Multiple Pathogens. mSphere 2021, 6, e00950-20. [Google Scholar] [CrossRef] [PubMed]
- Schalk, I.J.; Guillon, L. Pyoverdine biosynthesis and secretion in Pseudomonas aeruginosa: Implications for metal homeostasis. Environ. Microbiol. 2012, 15, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Kirienko, D.R.; Webster, P.; Fisher, A.L.; Kirienko, N.V. Pyoverdine, a siderophore from Pseudomonas aeruginosa, translocates into C. elegans, removes iron, and activates a distinct host response. Virulence 2018, 9, 804–817. [Google Scholar] [CrossRef]
- Khasheii, B.; Mahmoodi, P.; Mohammadzadeh, A. Siderophores: Importance in bacterial pathogenesis and applications in medicine and industry. Microbiol. Res. 2021, 250, 126790. [Google Scholar] [CrossRef]
- Ghssein, G.; Ezzeddine, Z. A Review of Pseudomonas aeruginosa Metallophores: Pyoverdine, Pyochelin and Pseudopaline. Biology 2022, 11, 1711. [Google Scholar] [CrossRef]
- Niu, Q.; Huang, X.; Zhang, L.; Xu, J.; Yang, D.; Wei, K.; Niu, X.; An, Z.; Bennett, J.W.; Zou, C.; et al. A Trojan horse mechanism of bacterial pathogenesis against nematodes. Proc. Natl. Acad. Sci. USA 2010, 107, 16631–16636. [Google Scholar] [CrossRef]
- Meisel, J.D.; Kim, D.H. Behavioral avoidance of pathogenic bacteria by Caenorhabditis elegans. Trends Immunol. 2014, 35, 465–470. [Google Scholar] [CrossRef]
- Irazoqui, J.E.; Urbach, J.M.; Ausubel, F.M. Evolution of host innate defence: Insights from Caenorhabditis elegans and primitive invertebrates. Nat. Rev. Immunol. 2010, 10, 47–58. [Google Scholar] [CrossRef]
- Pukkila-Worley, R. Surveillance Immunity: An Emerging Paradigm of Innate Defense Activation in Caenorhabditis elegans. PLoS Pathog. 2016, 12, e1005795. [Google Scholar] [CrossRef]
- Rua, R.; Pujol, N. Pathogen metabolite checkpoint: NHR on guard. Immunity 2023, 56, 744–746. [Google Scholar] [CrossRef]
- Stuart, L.M.; Paquette, N.; Boyer, L. Effector-triggered versus pattern-triggered immunity: How animals sense pathogens. Nat. Rev. Immunol. 2013, 13, 199–206. [Google Scholar] [CrossRef]
- Tran, T.D.; Luallen, R.J. An organismal understanding of C. elegans innate immune responses, from pathogen recognition to multigenerational resistance. Semin. Cell Dev. Biol. 2024, 154, 77–84. [Google Scholar] [CrossRef]
- Singh, J.; Aballay, A. Neural control of behavioral and molecular defenses in C. elegans. Curr. Opin. Neurobiol. 2019, 62, 34–40. [Google Scholar] [CrossRef]
- Wong, D.; Bazopoulou, D.; Pujol, N.; Tavernarakis, N.; Ewbank, J.J. Genome-wide investigation reveals pathogen-specific and shared signatures in the response of Caenorhabditis elegans to infection. Genome Biol. 2007, 8, R194. [Google Scholar] [CrossRef]
- Chai, W.; Zhang, J.; Duan, Y.; Pan, D.; Liu, W.; Li, Y.; Yan, X.; Chen, B. Pseudomonas pyocyanin stimulates IL-8 expression through MAPK and NF-κB pathways in differentiated U937 cells. BMC Microbiol. 2014, 14, 26. [Google Scholar] [CrossRef]
- Tse-Kang, S.Y.; Wani, K.A.; Peterson, N.D.; Page, A.; Pukkila-Worley, R. Activation of intestinal immunity by pathogen effector-triggered aggregation of lysosomal TIR-1/SARM1. bioRxiv 2024. [Google Scholar] [CrossRef]
- Seiler, F.; Hellberg, J.; Lepper, P.M.; Kamyschnikow, A.; Herr, C.; Bischoff, M.; Langer, F.; Schäfers, H.-J.; Lammert, F.; Menger, M.D.; et al. FOXO Transcription Factors Regulate Innate Immune Mechanisms in Respiratory Epithelial Cells. J. Immunol. 2013, 190, 1603–1613. [Google Scholar] [CrossRef]
- Papp, D.; Csermely, P.; Sőti, C. A Role for SKN-1/Nrf in Pathogen Resistance and Immunosenescence in Caenorhabditis elegans. PLoS Pathog. 2012, 8, e1002673. [Google Scholar] [CrossRef]
- van der Hoeven, R.; McCallum, K.C.; Cruz, M.R.; Garsin, D.A. Ce-Duox1/BLI-3 Generated Reactive Oxygen Species Trigger Protective SKN-1 Activity via p38 MAPK Signaling during Infection in C. elegans. PLoS Pathog. 2011, 7, e1002453. [Google Scholar] [CrossRef]
- Reddy, N.M.; Suryanarayana, V.; Kalvakolanu, D.V.; Yamamoto, M.; Kensler, T.W.; Hassoun, P.M.; Kleeberger, S.R.; Reddy, S.P. Innate Immunity against Bacterial Infection following Hyperoxia Exposure Is Impaired in NRF2-Deficient Mice. J. Immunol. 2009, 183, 4601–4608. [Google Scholar] [CrossRef]
- Xu, Y.; Duan, C.; Kuang, Z.; Hao, Y.; Jeffries, J.L.; Lau, G.W. Pseudomonas aeruginosa Pyocyanin Activates NRF2-ARE-Mediated Transcriptional Response via the ROS-EGFR-PI3K-AKT/MEK-ERK MAP Kinase Signaling in Pulmonary Epithelial Cells. PLoS ONE 2013, 8, e72528. [Google Scholar] [CrossRef]
- Singh, V.; Aballay, A. Heat-shock transcription factor (HSF)-1 pathway required for Caenorhabditis elegans immunity. Proc. Natl. Acad. Sci. USA 2006, 103, 13092–13097. [Google Scholar] [CrossRef]
- Powell, J.R.; Kim, D.H.; Ausubel, F.M. The G protein-coupled receptor FSHR-1 is required for the Caenorhabditis elegans innate immune response. Proc. Natl. Acad. Sci. USA 2009, 106, 2782–2787. [Google Scholar] [CrossRef]
- Bhartiya, D.; Patel, H. An overview of FSH-FSHR biology and explaining the existing conundrums. J. Ovarian Res. 2021, 14, 144. [Google Scholar] [CrossRef]
- Dunbar, T.L.; Yan, Z.; Balla, K.M.; Smelkinson, M.G.; Troemel, E.R. C. elegans Detects Pathogen-Induced Translational Inhibition to Activate Immune Signaling. Cell Host Microbe 2012, 11, 375–386. [Google Scholar] [CrossRef]
- Tjahjono, E.; Kirienko, N.V. A conserved mitochondrial surveillance pathway is required for defense against Pseudomonas aeruginosa. PLoS Genet. 2017, 13, e1006876. [Google Scholar] [CrossRef]
- Estes, K.A.; Dunbar, T.L.; Powell, J.R.; Ausubel, F.M.; Troemel, E.R. bZIP transcription factor zip-2 mediates an early response to Pseudomonas aeruginosa infection in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2010, 107, 2153–2158. [Google Scholar] [CrossRef]
- Cavaillon, J.-M. Exotoxins and endotoxins: Inducers of inflammatory cytokines. Toxicon 2018, 149, 45–53. [Google Scholar] [CrossRef]
- Pellegrino, M.W.; Nargund, A.M.; Kirienko, N.V.; Gillis, R.; Fiorese, C.J.; Haynes, C.M. Mitochondrial UPR-regulated innate immunity provides resistance to pathogen infection. Nature 2014, 516, 414–417. [Google Scholar] [CrossRef]
- van’t Wout, E.F.A.; van Schadewijk, A.; van Boxtel, R.; Dalton, L.E.; Clarke, H.J.; Tommassen, J.; Marciniak, S.J.; Hiemstra, P.S. Virulence Factors of Pseudomonas aeruginosa Induce Both the Unfolded Protein and Integrated Stress Responses in Airway Epithelial Cells. PLoS Pathog. 2015, 11, e1004946. [Google Scholar] [CrossRef]
- Hwang, A.B.; Ryu, E.-A.; Artan, M.; Chang, H.-W.; Kabir, M.H.; Nam, H.-J.; Lee, D.; Yang, J.-S.; Kim, S.; Mair, W.B.; et al. Feedback regulation via AMPK and HIF-1 mediates ROS-dependent longevity in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2014, 111, E4458–E4467. [Google Scholar] [CrossRef]
- Palazon, A.; Goldrath, A.W.; Nizet, V.; Johnson, R.S. HIF Transcription Factors, Inflammation, and Immunity. Immunity 2014, 41, 518–528. [Google Scholar] [CrossRef]
- Peterson, N.D.; Cheesman, H.K.; Liu, P.; Anderson, S.M.; Foster, K.J.; Chhaya, R.; Perrat, P.; Thekkiniath, J.; Yang, Q.; Haynes, C.M.; et al. The nuclear hormone receptor NHR-86 controls anti-pathogen responses in C. elegans. PLoS Genet. 2019, 15, e1007935. [Google Scholar] [CrossRef]
- Park, W.S.; Lee, J.; Na, G.; Park, S.; Seo, S.-K.; Choi, J.S.; Jung, W.-K.; Choi, I.-W. Benzyl Isothiocyanate Attenuates Inflammasome Activation in Pseudomonas aeruginosa LPS-Stimulated THP-1 Cells and Exerts Regulation through the MAPKs/NF-κB Pathway. Int. J. Mol. Sci. 2022, 23, 1228. [Google Scholar] [CrossRef]
- Kato, K.; Hanss, A.D.; Zemskova, M.A.; Morgan, N.E.; Kim, M.; Knox, K.S.; Lin, Y.; Lillehoj, E.P.; Kim, K.C. Pseudomonas aeruginosa increases MUC1 expression in macrophages through the TLR4-p38 pathway. Biochem. Biophys. Res. Commun. 2017, 492, 231–235. [Google Scholar] [CrossRef]
- Troemel, E.R.; Chu, S.W.; Reinke, V.; Lee, S.S.; Ausubel, F.M.; Kim, D.H. p38 MAPK Regulates Expression of Immune Response Genes and Contributes to Longevity in C. elegans. PLoS Genet. 2006, 2, e183. [Google Scholar] [CrossRef]
- Fletcher, M.; Tillman, E.J.; Butty, V.L.; Levine, S.S.; Kim, D.H. Global transcriptional regulation of innate immunity by ATF-7 in C. elegans. PLoS Genet. 2019, 15, e1007830. [Google Scholar] [CrossRef]
- Kim, D.H.; Feinbaum, R.; Alloing, G.; Emerson, F.E.; Garsin, D.A.; Inoue, H.; Tanaka-Hino, M.; Hisamoto, N.; Matsumoto, K.; Tan, M.-W.; et al. A Conserved p38 MAP Kinase Pathway in Caenorhabditis elegans Innate Immunity. Science 2002, 297, 623–626. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, F.; Li, S.; Jiang, N.; Yu, C.; Zhu, X.; Qin, Y.; Hui, J.; Meng, L.; Song, C.; et al. Metformin promotes innate immunity through a conserved PMK-1/p38 MAPK pathway. Virulence 2019, 11, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Chikka, M.R.; Anbalagan, C.; Dvorak, K.; Dombeck, K.; Prahlad, V. The Mitochondria-Regulated Immune Pathway Activated in the C. elegans Intestine Is Neuroprotective. Cell Rep. 2016, 16, 2399–2414. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.E.; Kooistra, T.; Kim, D.H. An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature 2010, 463, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Hermann, G.J.; Schroeder, L.K.; Hieb, C.A.; Kershner, A.M.; Rabbitts, B.M.; Fonarev, P.; Grant, B.D.; Priess, J.R. Genetic Analysis of Lysosomal Trafficking in Caenorhabditis elegans. Mol. Biol. Cell 2005, 16, 3273–3288. [Google Scholar] [CrossRef] [PubMed]
- Hajdú, G.; Somogyvári, M.; Csermely, P.; Sőti, C. Lysosome-related organelles promote stress and immune responses in C. elegans. Commun. Biol. 2023, 6, 936. [Google Scholar] [CrossRef] [PubMed]
- Peterson, N.D.; Icso, J.D.; Salisbury, J.E.; Rodríguez, T.; Thompson, P.R.; Pukkila-Worley, R. Pathogen infection and cholesterol deficiency activate the C. elegans p38 immune pathway through a TIR-1/SARM1 phase transition. eLife 2022, 11, e74206. [Google Scholar] [CrossRef] [PubMed]
- Hajdú, G.; Gecse, E.; Taisz, I.; Móra, I.; Sőti, C. Toxic stress-specific cytoprotective responses regulate learned behavioral decisions in C. elegans. BMC Biol. 2021, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Huizing, M.; Helip-Wooley, A.; Westbroek, W.; Gunay-Aygun, M.; Gahl, W.A. Disorders of Lysosome-Related Organelle Biogenesis: Clinical and Molecular Genetics. Annu. Rev. Genom. Hum. Genet. 2008, 9, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Garsin, D.A.; Villanueva, J.M.; Begun, J.; Kim, D.H.; Sifri, C.D.; Calderwood, S.B.; Ruvkun, G.; Ausubel, F.M. Long-lived C. elegans daf-2 Mutants are resistant to bacterial pathogens. Science 2003, 300, 1921. [Google Scholar] [CrossRef]
- Inoue, H.; Hisamoto, N.; An, J.H.; Oliveira, R.P.; Nishida, E.; Blackwell, T.K.; Matsumoto, K. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 2005, 19, 2278–2283. [Google Scholar] [CrossRef] [PubMed]
- Thimmulappa, R.K.; Lee, H.; Rangasamy, T.; Reddy, S.P.; Yamamoto, M.; Kensler, T.W.; Biswal, S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Investig. 2006, 116, 984–995. [Google Scholar] [CrossRef]
- Evans, E.A.; Chen, W.C.; Tan, M. The DAF-2 insulin-like signaling pathway independently regulates aging and immunity in C. elegans. Aging Cell 2008, 7, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Aballay, A. Regulation of DAF-16-mediated Innate Immunity in Caenorhabditis elegans. J. Biol. Chem. 2009, 284, 35580–35587. [Google Scholar] [CrossRef]
- Zhi, L.; Yu, Y.; Li, X.; Wang, D. Molecular Control of Innate Immune Response to Pseudomonas aeruginosa Infection by Intestinal let-7 in Caenorhabditis elegans. PLoS Pathog. 2017, 13, e1006152. [Google Scholar] [CrossRef]
- Hoffman, C.; Aballay, A. Role of neurons in the control of immune defense. Curr. Opin. Immunol. 2019, 60, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Barna, J.; Csermely, P.; Vellai, T. Roles of heat shock factor 1 beyond the heat shock response. Cell. Mol. Life Sci. 2018, 75, 2897–2916. [Google Scholar] [CrossRef]
- Sural, S.; Lu, T.-C.; Jung, S.A.; Hsu, A.-L. HSB-1 Inhibition and HSF-1 Overexpression Trigger Overlapping Transcriptional Changes to Promote Longevity in Caenorhabditis elegans. G3 Genes|Genomes|Genetics 2019, 9, 1679–1692. [Google Scholar] [CrossRef]
- Garigan, D.; Hsu, A.-L.; Fraser, A.G.; Kamath, R.S.; Ahringer, J.; Kenyon, C. Genetic Analysis of Tissue Aging in Caenorhabditis elegans: A Role for Heat-Shock Factor and Bacterial Proliferation. Genetics 2002, 161, 1101–1112. [Google Scholar] [CrossRef]
- Mohri-Shiomi, A.; Garsin, D.A. Insulin Signaling and the Heat Shock Response Modulate Protein Homeostasis in the Caenorhabditis elegans Intestine during Infection. J. Biol. Chem. 2008, 283, 194–201. [Google Scholar] [CrossRef]
- Ooi, F.K.; Prahlad, V. Olfactory experience primes the heat shock transcription factor HSF-1 to enhance the expression of molecular chaperones in C. elegans. Sci. Signal. 2017, 10, eaan4893. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Kountourakis, P.; Kottorou, A.E.; Antonacopoulou, A.G.; Rolfo, C.; Peeters, M.; Kalofonos, H.P. Follicle-Stimulating Hormone Receptor (FSHR): A Promising Tool in Oncology? Mol. Diagn. Ther. 2016, 20, 523–530. [Google Scholar] [CrossRef]
- Miller, E.V.; Grandi, L.N.; Giannini, J.A.; Robinson, J.D.; Powell, J.R. The Conserved G-Protein Coupled Receptor FSHR-1 Regulates Protective Host Responses to Infection and Oxidative Stress. PLoS ONE 2015, 10, e0137403. [Google Scholar] [CrossRef]
- Kim, S.; Sieburth, D. FSHR-1/GPCR Regulates the Mitochondrial Unfolded Protein Response in Caenorhabditis elegans. Genetics 2020, 214, 409–418. [Google Scholar] [CrossRef]
- Wibisono, P.; Sun, J. Neuro-immune communication in C. elegans defense against pathogen infection. Curr. Res. Immunol. 2021, 2, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhao, N.; Yang, Z.; Li, Y.; Bai, H.; Zou, W.; Zhang, K.-Q.; Huang, X. A trade-off switch of two immunological memories in Caenorhabditis elegans reinfected by bacterial pathogens. J. Biol. Chem. 2020, 295, 17323–17336. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Aballay, A. Intestinal infection regulates behavior and learning via neuroendocrine signaling. eLife 2019, 8, e50033. [Google Scholar] [CrossRef] [PubMed]
- Irazoqui, J.E.; Troemel, E.R.; Feinbaum, R.L.; Luhachack, L.G.; Cezairliyan, B.O.; Ausubel, F.M. Distinct Pathogenesis and Host Responses during Infection of C. elegans by P. aeruginosa and S. aureus. PLoS Pathog. 2010, 6, e1000982. [Google Scholar] [CrossRef]
- A Wani, K.; Goswamy, D.; E Irazoqui, J. Nervous system control of intestinal host defense in C. elegans. Curr. Opin. Neurobiol. 2019, 62, 1–9. [Google Scholar] [CrossRef]
- Horspool, A.M.; Chang, H.C. Superoxide dismutase SOD-1 modulates C. elegans pathogen avoidance behavior. Sci. Rep. 2017, 7, srep45128. [Google Scholar] [CrossRef]
- Lee, K.; Mylonakis, E. An Intestine-Derived Neuropeptide Controls Avoidance Behavior in Caenorhabditis elegans. Cell Rep. 2017, 20, 2501–2512. [Google Scholar] [CrossRef]
- Vasquez-Rifo, A.; Ricci, E.P.; Ambros, V. Pseudomonas aeruginosa cleaves the decoding center of Caenorhabditis elegans ribosomes. PLoS Biol. 2020, 18, e3000969. [Google Scholar] [CrossRef] [PubMed]
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Establishment of Pseudomonas aeruginosa infection: Lessons from a versatile opportunist. Microbes Infect. 2000, 2, 1051–1060. [Google Scholar] [CrossRef]
- Prakash, D.; Ms, A.; Radhika, B.; Venkatesan, R.; Chalasani, S.H.; Singh, V. 1-Undecene from Pseudomonas aeruginosa is an olfactory signal for flight-or-fight response in Caenorhabditis elegans. EMBO J. 2021, 40, e106938. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Samuel, B.S.; Breen, P.C.; Ruvkun, G. Caenorhabditis elegans pathways that surveil and defend mitochondria. Nature 2014, 508, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Kim, E.J.E.; Lee, S.-J.V. Mitochondria-mediated defense mechanisms against pathogens in Caenorhabditis elegans. BMB Rep. 2018, 51, 274–279. [Google Scholar] [CrossRef]
- Haynes, C.M.; Hekimi, S. Mitochondrial dysfunction, aging, and the mitochondrial unfolded protein response in Caenorhabditis elegans. Genetics 2022, 222, iyac160. [Google Scholar] [CrossRef]
- Campos, J.C.; Wu, Z.; Rudich, P.D.; Soo, S.K.; Mistry, M.; Ferreira, J.C.; Blackwell, T.K.; Van Raamsdonk, J.M. Mild mitochondrial impairment enhances innate immunity and longevity through ATFS-1 and p38 signaling. EMBO Rep. 2021, 22, e52964. [Google Scholar] [CrossRef]
- Melber, A.; Haynes, C.M. UPRmt regulation and output: A stress response mediated by mitochondrial-nuclear communication. Cell Res. 2018, 28, 281–295. [Google Scholar] [CrossRef]
- Mao, K.; Ji, F.; Breen, P.; Sewell, A.; Han, M.; Sadreyev, R.; Ruvkun, G. Mitochondrial Dysfunction in C. elegans Activates Mitochondrial Relocalization and Nuclear Hormone Receptor-Dependent Detoxification Genes. Cell Metab. 2019, 29, 1182–1191.e4. [Google Scholar] [CrossRef]
- Jeong, D.; Lee, D.; Hwang, S.; Lee, Y.; Lee, J.; Seo, M.; Hwang, W.; Seo, K.; Hwang, A.B.; Artan, M.; et al. Mitochondrial chaperone HSP-60 regulates anti-bacterial immunity via p38 MAP kinase signaling. EMBO J. 2017, 36, 1046–1065. [Google Scholar] [CrossRef] [PubMed]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018, 20, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Kirienko, N.V.; Ausubel, F.M.; Ruvkun, G. Mitophagy confers resistance to siderophore-mediated killing by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2015, 112, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS Signaling in Organismal Homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Lukácsi, S.; Farkas, Z.; Saskői, É.; Bajtay, Z.; Takács-Vellai, K. Conserved and Distinct Elements of Phagocytosis in Human and C. elegans. Int. J. Mol. Sci. 2021, 22, 8934. [Google Scholar] [CrossRef] [PubMed]
- Ewald, C.Y. Redox Signaling of NADPH Oxidases Regulates Oxidative Stress Responses, Immunity and Aging. Antioxidants 2018, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Rigoulet, M.; Yoboue, E.D.; Devin, A. Mitochondrial ROS Generation and Its Regulation: Mechanisms Involved in H2O2Signaling. Antioxidants Redox Signal. 2011, 14, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef]
- McCallum, K.C.; Garsin, D.A. The Role of Reactive Oxygen Species in Modulating the Caenorhabditis elegans Immune Response. PLoS Pathog. 2016, 12, e1005923. [Google Scholar] [CrossRef]
- Shao, Z.; Zhang, Y.; Ye, Q.; Saldanha, J.N.; Powell-Coffman, J.A. C. elegans SWAN-1 Binds to EGL-9 and Regulates HIF-1-Mediated Resistance to the Bacterial Pathogen Pseudomonas aeruginosa PAO1. PLoS Pathog. 2010, 6, e1001075. [Google Scholar] [CrossRef]
- Deng, P.; Naresh, N.U.; Du, Y.; Lamech, L.T.; Yu, J.; Zhu, L.J.; Pukkila-Worley, R.; Haynes, C.M. Mitochondrial UPR repression during Pseudomonas aeruginosa infection requires the bZIP protein ZIP-3. Proc. Natl. Acad. Sci. USA 2019, 116, 6146–6151. [Google Scholar] [CrossRef] [PubMed]
- Naim, N.; Amrit, F.R.G.; Ratnappan, R.; DelBuono, N.; Loose, J.A.; Ghazi, A. Cell nonautonomous roles of NHR-49 in promoting longevity and innate immunity. Aging Cell 2021, 20, e13413. [Google Scholar] [CrossRef] [PubMed]
- Rajan, M.; Anderson, C.P.; Rindler, P.M.; Romney, S.J.; dos Santos, M.C.F.; Gertz, J.; A Leibold, E. NHR-14 loss of function couples intestinal iron uptake with innate immunity in C. elegans through PQM-1 signaling. eLife 2019, 8, e44674. [Google Scholar] [CrossRef] [PubMed]
- Sellegounder, D.; Liu, Y.; Wibisono, P.; Chen, C.H.; Leap, D.; Sun, J. Neuronal GPCR NPR-8 regulates C. elegans defense against pathogen infection. Sci. Adv. 2019, 5, eaaw4717. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.C.; Hunter, R.C.; Bhatla, N.; Newman, D.K.; Kim, D.H. Caenorhabditis elegans NPR-1–mediated behaviors are suppressed in the presence of mucoid bacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 12887–12892. [Google Scholar] [CrossRef]
- Brandt, J.P.; Ringstad, N. Toll-like Receptor Signaling Promotes Development and Function of Sensory Neurons Required for a C. elegans Pathogen-Avoidance Behavior. Curr. Biol. 2015, 25, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Hallem, E.A.; Sternberg, P.W. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2008, 105, 8038–8043. [Google Scholar] [CrossRef]
- Prakash, D.; Siddiqui, R.; Chalasani, S.H.; Singh, V. Pyrrole produced by Pseudomonas aeruginosa influences olfactory food choice of Caenorhabditis elegans. bioRxiv 2022. [Google Scholar] [CrossRef]
- Ha, H.-I.; Hendricks, M.; Shen, Y.; Gabel, C.V.; Fang-Yen, C.; Qin, Y.; Colón-Ramos, D.; Shen, K.; Samuel, A.D.; Zhang, Y. Functional Organization of a Neural Network for Aversive Olfactory Learning in Caenorhabditis elegans. Neuron 2010, 68, 1173–1186. [Google Scholar] [CrossRef]
- Reddy, K.C.; Andersen, E.C.; Kruglyak, L.; Kim, D.H. A Polymorphism in npr-1 Is a Behavioral Determinant of Pathogen Susceptibility in C. elegans. Science 2009, 323, 382–384. [Google Scholar] [CrossRef]
- Sellegounder, D.; Yuan, C.-H.; Wibisono, P.; Liu, Y.; Sun, J. Octopaminergic Signaling Mediates Neural Regulation of Innate Immunity in Caenorhabditis elegans. mBio 2018, 9, e01645-18. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Aballay, A. Neural Inhibition of Dopaminergic Signaling Enhances Immunity in a Cell-Non-autonomous Manner. Curr. Biol. 2016, 26, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-Y.; Chang, H.C. Glutamate signaling mediates C. elegans behavioral plasticity to pathogens. iScience 2022, 25, 103919. [Google Scholar] [CrossRef]
- Shore, D.E.; Ruvkun, G. A cytoprotective perspective on longevity regulation. Trends Cell Biol. 2013, 23, 409–420. [Google Scholar] [CrossRef]
- Chang, H.C.; Paek, J.; Kim, D.H. Natural polymorphisms in C. elegans HECW-1 E3 ligase affect pathogen avoidance behaviour. Nature 2011, 480, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Horspool, A.M.; Chang, H.C. Neuron-specific regulation of superoxide dismutase amid pathogen-induced gut dysbiosis. Redox Biol. 2018, 17, 377–385. [Google Scholar] [CrossRef]
- Melo, J.A.; Ruvkun, G. Inactivation of Conserved C. elegans Genes Engages Pathogen- and Xenobiotic-Associated Defenses. Cell 2012, 149, 452–466. [Google Scholar] [CrossRef]
- Singh, J.; Aballay, A. Microbial Colonization Activates an Immune Fight-and-Flight Response via Neuroendocrine Signaling. Dev. Cell 2019, 49, 89–99.e4. [Google Scholar] [CrossRef]
- Hong, C.; Lalsiamthara, J.; Ren, J.; Sang, Y.; Aballay, A. Microbial colonization induces histone acetylation critical for inherited gut-germline-neural signaling. PLoS Biol. 2021, 19, e3001169. [Google Scholar] [CrossRef]
- Liao, C.-P.; Chiang, Y.-C.; Tam, W.H.; Chen, Y.-J.; Chou, S.-H.; Pan, C.-L. Neurophysiological basis of stress-induced aversive memory in the nematode Caenorhabditis elegans. Curr. Biol. 2022, 32, 5309–5322.e6. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]

| Virulence Factors | C. elegans | Mammals | Ref. |
|---|---|---|---|
| Bacterial surface structure | |||
| Flagella (surface appendages) | + | + | [25,26] |
| Type IV pili (surface appendages) | + | + | [27,28] |
| Lipopolysaccharide (OMC) | + | + | [29,30] |
| Secreted factors | |||
| Alkaline protease A (protease, T1SS) | − | + | [31,32] |
| Alkaline protease X (protease, T1SS) | n.d. | n.d. | [33] |
| HasAp (heme acquisition protein, T1SS) | n.d. | n.d. | [34] |
| ExoA (toxin, T2SS) | + | + | [35,36] |
| Lipase A (toxin, T2SS) | n.d. | n.d. | [37] |
| Lipase C (toxin, T2SS) | n.d. | n.d. | [38] |
| Phospholipase C (toxin, T2SS) | n.d. | + | [39] |
| Protease IV (protease, T2SS) | n.d. | + | [40] |
| Elastase A (protease, T2SS) | n.d. | + | [41] |
| Elastase B (protease, T2SS) | − | + | [31,41] |
| ExoS (toxin, T3SS) | n.d. | + | [42] |
| ExoT (toxin, T3SS) | + | + | [42,43] |
| ExoU (toxin, T3SS) | + | + | [42,43] |
| ExoY (toxin, T3SS) | + | + | [43,44] |
| PemA (effector, T3SS) | − | − | [43,45] |
| PemB (effector, T3SS) | + | − | [43,45] |
| EstA (esterase, T5SS) | n.d. | n.d. | [46] |
| TpsA (exoprotein, T5SS) | n.d. | n.d. | [47] |
| TpsB (β-barrel transport protein, T5SS) | n.d. | n.d. | [47] |
| LepA (protease, T5SS) | n.d. | + | [48] |
| Tse1 (bacteriolytic effector, T6SS) | n.d. | n.d. | [49] |
| Tse2 (bacteriolytic effector, T6SS) | n.d. | − | [50] |
| Tse3 (bacteriolytic effector, T6SS) | n.d. | n.d. | [49] |
| Alginate (exopolysaccharide) | − | + | [51,52] |
| Pel (exopolysaccharide) | + | − | [53,54] |
| Psl (exopolysaccharide) | + | − | [53,54] |
| Pyoverdine (siderophore) | + | + | [55,56] |
| Pyochelin (siderophore) | + | + | [55,57] |
| Lipoxygenase (toxin) | n.d. | + | [58] |
| Phenazine-1-carboxylic acid (toxin) | + | + | [59,60] |
| Phenazine-1-carboxamide (toxin) | + | n.d | [61] |
| 1-hydroxyphenazine | + | + | [59,62] |
| Pyocyanin | + | + | [21,63] |
| Bacterial cell–cell interaction | |||
| LasR (quorum sensing) | + | + | [64,65] |
| RhlR (quorum sensing) | + | + | [66,67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajdú, G.; Szathmári, C.; Sőti, C. Modeling Host–Pathogen Interactions in C. elegans: Lessons Learned from Pseudomonas aeruginosa Infection. Int. J. Mol. Sci. 2024, 25, 7034. https://doi.org/10.3390/ijms25137034
Hajdú G, Szathmári C, Sőti C. Modeling Host–Pathogen Interactions in C. elegans: Lessons Learned from Pseudomonas aeruginosa Infection. International Journal of Molecular Sciences. 2024; 25(13):7034. https://doi.org/10.3390/ijms25137034
Chicago/Turabian StyleHajdú, Gábor, Csenge Szathmári, and Csaba Sőti. 2024. "Modeling Host–Pathogen Interactions in C. elegans: Lessons Learned from Pseudomonas aeruginosa Infection" International Journal of Molecular Sciences 25, no. 13: 7034. https://doi.org/10.3390/ijms25137034
APA StyleHajdú, G., Szathmári, C., & Sőti, C. (2024). Modeling Host–Pathogen Interactions in C. elegans: Lessons Learned from Pseudomonas aeruginosa Infection. International Journal of Molecular Sciences, 25(13), 7034. https://doi.org/10.3390/ijms25137034






