The Impact of Virgin and Aged Microstructured Plastics on Proteins: The Case of Hemoglobin Adsorption and Oxygenation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Microstructured Plastics
2.1.1. Characterization of Aged Microparticles
2.1.2. Characterization of Polypropylene Filters
2.2. Hemoglobin Adsorption on Microparticles
2.2.1. Hemoglobin Adsorption on Virgin Microparticles
2.2.2. Hemoglobin Adsorption on Aged Microparticles
2.3. Hemoglobin Oxygenation on Polypropylene Filters
3. Materials and Methods
3.1. Virgin and Aged Microstructured Plastics
3.2. Characterization of Microstructured Plastics
3.3. Hemoglobin Preparation
3.4. Adsorption Isotherms
3.5. Hemoglobin Oxygenation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wypych, G. Handbook of Polymers, 3rd ed.; ChemTec Publishing: Toronto, ON, Canada, 2022. [Google Scholar]
- Zweifel, H.; Maier, R.; Schiller, M. Plastics Additives Handbook, 6th ed.; Hanser Publications: Munich, Germany, 2009. [Google Scholar]
- Pfaendner, R. How will additives shape the future of plastics? Polym. Degrad. Stab. 2006, 91, 2249–2256. [Google Scholar] [CrossRef]
- Alegado, J.E.; Aliño, J.M.; Chow, S.C.F.; Diola, M.B.L.; Jagath, P.; Gamaralalage, D.; Gurrero, L.; Hengesbaugh, M.; Hernandez, V.; Ishimura, Y.; et al. Plastic Atlas Asia Edition, 1st ed.; Li, K., Kunze, C., Eds.; Heinrich Boell Stiftung, Break Free from Pacific Asia Pacific, Institute for Global Environmental Strategies Publishers: Hong Kong, 2021. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Ainali, N.M.; Bikiaris, D.N.; Lambropoulou, D.A. Aging effects on low- and high-density polyethylene, polypropylene and polystyrene under UV irradiation: An insight into decomposition mechanism by Py-GC/MS for microplastic analysis. J. Anal. Appl. Pyrolysis 2021, 158, 105207. [Google Scholar] [CrossRef]
- Koga, M.; Watanabe, R.; Hagihara, H.; Mizukado, J.; Tokita, M.; Shinzawa, H. Accelerated aging-induced variation of polypropylene (PP) structure studied by two-dimensional (2D) small-angle X-ray scattering (SAXS) correlation spectroscopy. J. Mol. Struct. 2020, 1207, 127764. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Tian, L.; Liu, X.; Qi, Z.; Ma, Y.; Ji, R.; Chen, W. Aging significantly affects mobility and contaminant-mobilizing ability of nanoplastics in saturated loamy sand. Environ. Sci. Technol. 2019, 53, 5805–5815. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Wagner, M. Formation of microscopic particles during the degradation of different polymers. Chemosphere 2016, 161, 510–517. [Google Scholar] [CrossRef]
- Materic, D.; Kjær, H.A.; Vallelonga, P.; Tison, J.-L.; Rockmann, T.; Holzinger, R. Nanoplastics measurements in Northern and Southern polar ice. Environ. Res. 2022, 208, 112741. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Martin, L.M.A.; Kim, S.; Zhang, Q.; Zhang, R.; Xu, W.; Luo, T. Direct observation and identification of nanoplastics in ocean water. Sci. Adv. 2024, 10, eadh1675. [Google Scholar] [CrossRef] [PubMed]
- Ivar do Sul, J.A.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef]
- Sonke, J.E.; Koenig, A.M.; Yakovenko, N.; Hagelskjær, O.; Margenat, H.; Hansson, S.V.; De Vleeschouwer, F.; Magand, O.; Le Roux, G.; Thomas, J.L. A mass budget and box model of global plastics cycling, degradation and dispersal in the land-ocean-atmosphere system. Microplastics Nanoplastics 2022, 2, 28. [Google Scholar] [CrossRef]
- Sutkar, P.R.; Gadewar, R.D.; Dhulap, V.P. Recent trends in degradation of microplastics in the environment: A state-of-the-art review. J. Hazard. Mat. Adv. 2023, 11, 100343. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Xing, B. Environmental source, fate, and toxicity of microplastics. J. Hazard. Mat. 2021, 407, 124357. [Google Scholar] [CrossRef]
- Chang, X.; Fang, Y.; Wang, Y.; Wang, F.; Shang, L.; Zhong, R. Microplastic pollution in soils, plants, and animals: A review of distributions, effects and potential mechanisms. Sci. Total Environ. 2022, 850, 157857. [Google Scholar] [CrossRef]
- Eberhard, T.; Casillas, G.; Zarus, G.M.; Barr, D.B. Systematic review of microplastics and nanoplastics in indoor and outdoor air: Identifying a framework and data needs for quantifying human inhalation exposures. J. Expo. Sci. Environ. Epidemiol. 2024, 34, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.-M.; Van den Eede, G. Review of micro- and nanoplastic contamination in the food chain. Food Addit. Contam. Part A 2019, 36, 639–673. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Chen, Y.; Yang, F.; Yao, W.; Xie, Y. Microplastics and nanoplastics: Emerging contaminants in food. J. Agric. Food Chem. 2021, 69, 10450–10468. [Google Scholar] [CrossRef]
- Mamun, A.A.; Prasetya, T.A.E.; Dewi, I.R.; Ahmad, M. Microplastics in human food chains: Food becoming a threat to health safety. Sci. Total Environ. 2023, 858, 159834. [Google Scholar] [CrossRef]
- Ziani, K.; IonitaMîndrican, C.-B.; Mititelu, M.; Neacsu, S.M.; Negrei, C.; Morosan, E.; Draganescu, D.; Preda, O.-T. Microplastics: A real global threat for environment and food safety: A state of the art review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Xu, X.; Guo, L.; Jin, R.; Lu, Y. Uptake and transport of micro/nanoplastics in terrestrial plants: Detection, mechanisms, and influencing factors. Sci. Total Environ. 2024, 907, 168155. [Google Scholar] [CrossRef]
- Sobhani, Z.; Lei, Y.; Tang, Y.; Wu, L.; Zhang, X.; Naidu, R.; Megharaj, M.; Fang, C. Microplastics generated when opening plastic packaging. Sci. Rep. 2020, 10, 4841. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Gou, X.; Xiang, J.; Huang, Q.; Liu, G. Revealing trace nanoplastics in food packages─An electrochemical approach facilitated by synergistic attraction of electrostatics and hydrophobicity. Anal. Chem. 2022, 94, 12657–12663. [Google Scholar] [CrossRef] [PubMed]
- Zangmeister, C.D.; Radney, J.G.; Benkstein, K.D.; Kalanyan, B. Common single-use consumer plastic products release trillions of sub-100 nm nanoparticles per liter into water during normal use. Environ. Sci. Technol. 2022, 56, 5448–5455. [Google Scholar] [CrossRef]
- Aslam, S.; Khurram, A.; Hussain, R.; Qadir, A.; Ahmad, S.R. Sources, distribution, and incipient threats of polymeric plastic materials. Environ. Monit. Assess. 2023, 195, 638–663. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, E.G.; Li, J.; Chen, Q.; Ma, L.; Zeng, E.Y.; Shi, H. A review of microplastics in table salt, drinking water, and air: Direct human exposure. Environ. Sci. Technol. 2020, 54, 3740–3751. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Nor, N.H.; Kooi, M.; Diepens, N.J.; Koelmans, A.A. Lifetime accumulation of microplastic in children and adults. Environ. Sci. Technol. 2021, 55, 5084–5096. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and human health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei, S.; Hanachi, P.; Walker, T.R.; Cole, M. Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ. Sci. Pollut. Res. 2018, 25, 6046–36063. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Man, Y.B.; Wong, M.H.; Owen, R.B.; Chow, K.L. Environmental health impacts of microplastics exposure on structural organization levels in the human body. Sci. Total Environ. 2022, 825, 154025. [Google Scholar] [CrossRef]
- Barcelo, D.; Pico, Y.; Alfarhan, A.H. Microplastics: Detection in human samples, cell line studies, and health impacts. Environ. Tox. Pharm. 2023, 101, 104204. [Google Scholar] [CrossRef]
- Guan, Q.; Jiang, J.; Huang, Y.; Wang, Q.; Liu, Z.; Ma, X.; Yang, X.; Li, Y.; Wang, S.; Cui, W.; et al. The landscape of micron-scale particles including microplastics in human enclosed body fluids. J. Hazard. Mat. 2023, 442, 130138. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeong, S. Approach to an answer to “How dangerous microplastics are to the human body”: A systematic review of the quantification of MPs and simultaneously exposed chemicals. J. Hazard. Mat. 2023, 460, 132404. [Google Scholar] [CrossRef] [PubMed]
- Ramsperger, A.F.R.M.; Bergamaschi, E.; Panizzolo, M.; Fenoglio, I.; Barbero, F.; Peters, R.; Undas, A.; Purker, S.; Giese, B.; Lalyer, C.R.; et al. Nano- and microplastics: A comprehensive review on their exposure routes, translocation, and fate in humans. NanoImpact 2023, 29, 100441. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mat. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Kumar, M.; Saha, M.; Kirkham, M.B.; Singh, L.; Bolan, N.S. The polymers and their additives in particulate plastics: What makes them hazardous to the fauna? Sci. Total Environ. 2022, 824, 153828. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Raps, H.; Cropper, M.; Bald, C.; Brunner, M.; Canonizado, E.M.; Charles, D.; Chiles, T.C.; Donohue, M.J.; Enck, J.; et al. The Minderoo-Monaco commission on plastics and human health. Ann. Glob. Health 2023, 89, 23. [Google Scholar] [CrossRef] [PubMed]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Enhanced desorption of persistent organic pollutants frommicroplastics under simulated physiological conditions. Environ. Pollut. 2014, 185, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-D.; Gong, B.; Chen, W.; Qian, C.; Du, M.; Yu, H.Q. In situ quantitative monitoring the organic contaminants uptake onto suspended microplastics in aquatic environments. Water Res. 2022, 215, 118235. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of nanoplastic in the environment and possible impact on human health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef] [PubMed]
- Yee, M.S.-L.; Hii, L.-W.; Looi, C.K.; Lim, W.-M.; Wong, S.-F.; Kok, Y.-Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of microplastics and nanoplastics on human health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Wu, P.; Lin, S.; Cao, G.; Wu, J.; Jin, H.; Wang, C.; Wong, M.H.; Yang, Z.; Cai, Z. Absorption, distribution, metabolism, excretion and toxicity of microplastics in the human body and health implications. J. Hazard. Mat. 2022, 437, 129361. [Google Scholar] [CrossRef]
- Rotchell, J.M.; Jenner, L.C.; Chapman, E.; Bennett, R.T.; Bolanle, I.O.; Loubani, M.; Sadofsky, L.; Palmer, T.M. Detection of microplastics in human saphenous vein tissue using μFTIR: A pilot study. PLoS ONE 2020, 18, e0280594. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Chambers, E.; Mitragotri, S. Prolonged circulation of large polymeric nanoparticles by non-covalent adsorption on erythrocytes. J. Control Release 2004, 100, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Gupta, V.; Zern, B.J.; Pan, D.; Zakrewsky, M.; Muzykantov, V.; Mitragotri, S. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano 2013, 7, 11129–11137. [Google Scholar] [CrossRef] [PubMed]
- Fleury, J.B.; Baulin, V.A. Microplastics destabilize lipid membranes by mechanical stretching. Proc. Natl. Acad. Sci. USA 2021, 118, e2104610118. [Google Scholar] [CrossRef]
- Gopinath, P.M.; Saranya, V.; Vijayakumar, S.; Meera, M.M.; Ruprekha, S.; Kunal, R.; Pranay, A.; Thomas, J.; Mukherjee, A.; Chandrasekaran, N. Assessment on interactive prospectives of nanoplastics with plasma proteins and the toxicological impacts of virgin, coronated and environmentally released-nanoplastics. Sci. Rep. 2019, 9, 8860. [Google Scholar] [CrossRef]
- Ju, P.; Zhang, Y.; Ding, J.; Zheng, Y.; Wang, S.; Jiang, F.; Sun, C. New insights into the toxic interactions of polyvinyl chloride microplastics with bovine serum albumin. Environ. Sci. Pollut. Res. 2021, 28, 5520–5531. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, D.; Chandrasekaran, N.; Waychal, Y.; Mukherjee, A. Nanoplastics alter the conformation and activity of human serum albumin. NanoImpact 2022, 27, 100412. [Google Scholar] [CrossRef]
- Saha, G.; Chandrasekaran, N. Isolation and characterization of microplastics from skin care products; interactions with albumin proteins and in-vivo toxicity studies on Artemia salina. Environ. Tox. Pharm. 2023, 99, 104112. [Google Scholar] [CrossRef]
- Dong, Z.; Hou, Y.; Han, W.; Liu, M.; Wang, J.; Qiu, Y. Protein corona-mediated transport of nanoplastics in seawater saturated porous media. Water Res. 2020, 182, 115978. [Google Scholar] [CrossRef] [PubMed]
- Kihara, S.; Köper, I.; Mata, J.P.; McGillivray, D.J. Reviewing nanoplastic toxicology: It’s an interface problem. Adv. Colloid Interface Sci. 2021, 288, 102337. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Du, Q.; Zhong, Z.; Xu, Y.; Peng, J. Protein-coated microplastics corona complex: An underestimated risk of microplastics. Sci. Total Environ. 2022, 851, 157948. [Google Scholar] [CrossRef] [PubMed]
- Schvartz, M.; Saudrais, F.; Devineau, S.; Chédin, S.; Jamme, F.; Leroy, J.; Rakotozandriny, K.; Taché, O.; Brotons, G.; Pin, S.; et al. Role of the protein corona in the colloidal behavior of microplastics. Langmuir 2023, 39, 4291–4303. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Sethson, I.; Jonsson, B.H. Protein adsorption onto silica nanoparticles: Conformational changes depend on the particles’ curvature and the protein stability. Langmuir 2004, 20, 10639–10647. [Google Scholar] [CrossRef] [PubMed]
- Sanfins, E.; Dairou, J.; Hussain, S.; Busi, F.; Chaffotte, A.F.; Rodrigues-Lima, F.; Dupret, J.-M. Carbon black nanoparticles impair acetylation of aromatic amine carcinogens through inactivation of arylamine N-acetyltransferase enzymes. ACS Nano 2011, 5, 4504–4511. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, S.; Bag, S.; Burman, M.D.; Bhowmik, S. Multispectroscopic investigations of the binding interaction between polyethylene microplastics and human Hemoglobin. J. Phys. Chem. Lett. 2023, 14, 10328–10332. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, D.; Chandrasekaran, N. Molecular interaction of functionalized nanoplastics with human hemoglobin. J. Fluoresc. 2023, 33, 2257–2272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yao, J.; Li, H.; Lan, J.; Hollert, H.; Zhao, X. Interaction of polystyrene nanoplastics and hemoglobin is determined by both particle curvature and available surface area. Sci. Total Environ. 2023, 899, 165617. [Google Scholar] [CrossRef]
- Schrurs, F.; Lison, D. Focusing the research efforts. Nat. Nanotechnol. 2012, 7, 546–548. [Google Scholar] [CrossRef]
- Guadagnini, R.; Halamoda Kenzaoui, B.; Walker, L.; Pojana, G.; Magdolenova, Z.; Bilanicova, D.; Saunders, M.; Juillerat-Jeanneret, L.; Marcomini, A.; Huk, A.; et al. Toxicity screenings of nanomaterials: Challenges due to interference with assay processes and components of classic in vitro tests. Nanotoxicology 2015, 9, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Devineau, S.; Zargarian, L.; Renault, J.P.; Pin, S. Structure and function of adsorbed hemoglobin on silica nanoparticles: Relationship between the adsorption process and the oxygen binding properties. Langmuir 2017, 33, 3241–3252. [Google Scholar] [CrossRef]
- Gardette, M.; Perthue, A.; Gardette, J.-L.; Janecska, T.; Földes, E.; Pukánszky, B.; Therias, S. Photo- and thermal-oxidation of polyethylene: Comparison of mechanisms and influence of unsaturation content. Polym. Degrad. Stab. 2013, 98, 2383–2390. [Google Scholar] [CrossRef]
- Rajakumar, K.; Sarasvathy, V.; Thamarai Chelvan, A.; Chitra, R.; Vijayakumar, C.T. Natural weathering studies of polypropylene. J. Polym. Environ. 2009, 17, 191–202. [Google Scholar] [CrossRef]
- Saudrais, F. Physico-Chemistry of Interactions between Microstructured Plastics and Proteins or Model Peptides by Experimental and In Silico Approaches. Ph.D. Thesis, Université Paris-Saclay, Saclay, France, 18 April 2023. [Google Scholar]
- Almond, J.; Sugumaar, P.; Wenzel, M.N.; Hill, G.; Wallis, C. Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy. e-Polymers 2020, 20, 369–381. [Google Scholar] [CrossRef]
- Lopez, G.P.; Castner, D.G.; Ratner, B.D. XPS O 1s binding energies for polymers containing hydroxyl, ether, ketone and ester groups. Surf. Interface Anal. 1991, 17, 267–272. [Google Scholar] [CrossRef]
- Lindner, P.; Zemb, T. Neutron, X-rays and Light: Scattering Methods Applied to Soft Condensed Matter; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Horbett, T.A.; Weathersbp, P.K.; Boffman, A.S. Hemoglobin adsorption to three polymer surfaces. Thromb. Res. 1978, 12, 319–329. [Google Scholar] [CrossRef]
- Rahmati, M.; Mozafaria, M. Protein adsorption on polymers. Mat. Today Com. 2018, 17, 527–540. [Google Scholar] [CrossRef]
- Katz, D.S.; White, S.P.; Huang, W.; Kumar, R.; Christianson, D.W. Structure determination of aquomet porcine hemoglobin at 2.8 Å resolution. J. Mol. Biol. 1994, 244, 541–553. [Google Scholar] [CrossRef]
- Malamud, D.; Drysdale, J.W. Isoelectric points of proteins: A table. Anal. Biochem. 1978, 86, 620–647. [Google Scholar] [CrossRef]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef]
- Roach, P.; Farrar, D.; Perry, C.C. Interpretation of protein adsorption: Surface induced conformational changes. J. Am. Chem. Soc. 2005, 127, 8168–8173. [Google Scholar] [CrossRef]
- Xu, M.; Hu, S.; Cui, Z.; Liu, C.; Xiao, Y.; Liu, R.; Zong, W. Characterizing the binding interactions between virgin/aged microplastics and catalase in vitro. Chemosphere 2023, 323, 138199. [Google Scholar] [CrossRef]
- Gligorijevic, N.; Lujic, T.; Mutic, T.; Vasovic, T.; de Guzman, M.K.; Acimovic, J.; Stanic-Vucinic, D.; Cirkovic Velickovic, T. Ovalbumin interaction with polystyrene and polyethylene terephthalate microplastics alters its structural properties. Int. J. Biol. Macromol. 2024, 267, 131564. [Google Scholar] [CrossRef] [PubMed]
- Azhagesan, A.; Chandrasekaran, N.; Mukherjee, A. Multispectroscopy analysis of polystyrene nanoplastic interaction with diastase α-amylase. Ecotox. Environ. Saf. 2022, 247, 114226. [Google Scholar] [CrossRef] [PubMed]
- Azhagesan, A.; Rajendran, D.; Varghese, R.P.; Doss, C.G.P.; Chandrasekarann, N. Assessment of polystyrene nano plastics effect on human salivary α-amylase structural alteration: Insights from an in vitro and in silico study. Int. J. Biol. Macromol. 2024, 57, 128650. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, Y.; Luan, Y.; Dai, W. The formation of protein Corona by nanoplastics and horseradish peroxidase. Nanomaterials 2022, 12, 4467. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Luan, Y.; Dai, W. Time evolution of protein corona formed by polystyrene nanoplastics and urease. Int. J. Biol. Macromol. 2022, 218, 72–81. [Google Scholar] [CrossRef]
- Thennadil, S.N. Relationship between the Kubelka-Munk scattering and radiative transfer coefficients. J. Opt. Soc. Am. A 2008, 25, 1480–1485. [Google Scholar] [CrossRef]
- Ellerbrock, R.H.; Gerke, H.H.; Böhm, C. In situ DRIFT characterization of organic matter composition on soil structural surfaces. Soil Sci. Soc. Am. J. 2009, 73, 531–540. [Google Scholar] [CrossRef]
- Dailianis, S.; Rouni, M.; Ainali, N.M.; Vlastos, D.; Kyzas, G.Z.; Lambropoulou, D.A.; Bikiaris, D.N. New insights into the size-independent bioactive potential of pristine and UV-B aged polyethylene microplastics. Sci. Total Environ. 2024, 918, 170616. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Qian, L.; Wang, H.; Zhan, X.; Lu, K.; Gu, C.; Gao, S. New insights into the aging behavior of microplastics accelerated by advanced oxidation processes. Environ. Sci. Technol. 2019, 53, 3579–3588. [Google Scholar] [CrossRef] [PubMed]
- Loutit, J.F.; Mollison, P.L.; Young, M. Citric acid sodium citrate glucose mixtures for blood storage. Quart. J. Exp. Physiol. Cognate Med. Sci. 1943, 32, 183–202. [Google Scholar] [CrossRef]
- Perutz, M.F. Preparation of Haemoglobin crystals. J. Cryst. Growth 1968, 2, 54–56. [Google Scholar] [CrossRef]
- Jelkmann, W.; Bauer, C. What is the best method to remove 2,3-diphosphoglycerate from hemoglobin? Anal. Biochem. 1976, 75, 382–388. [Google Scholar] [CrossRef]
- Antonini, E.; Brunori, M. Hemoglobin and Myoglobin in Their Interactions with Ligands, Frontiers of Biology; North Holland Publishing Company: Amsterdam, The Netherlands, 1971; Volume 21. [Google Scholar]
- Schvartz, M.; Saudrais, F.; Devineau, S.; Aude, J.-C.; Chédin, S.; Henry, C.; Millan-Oropeza, A.; Perrault, T.; Pieri, L.; Pin, S.; et al. A proteome scale study reveals how plastic surfaces and agitation promote protein aggregation. Sci. Rep. 2023, 13, 1227. [Google Scholar] [CrossRef]
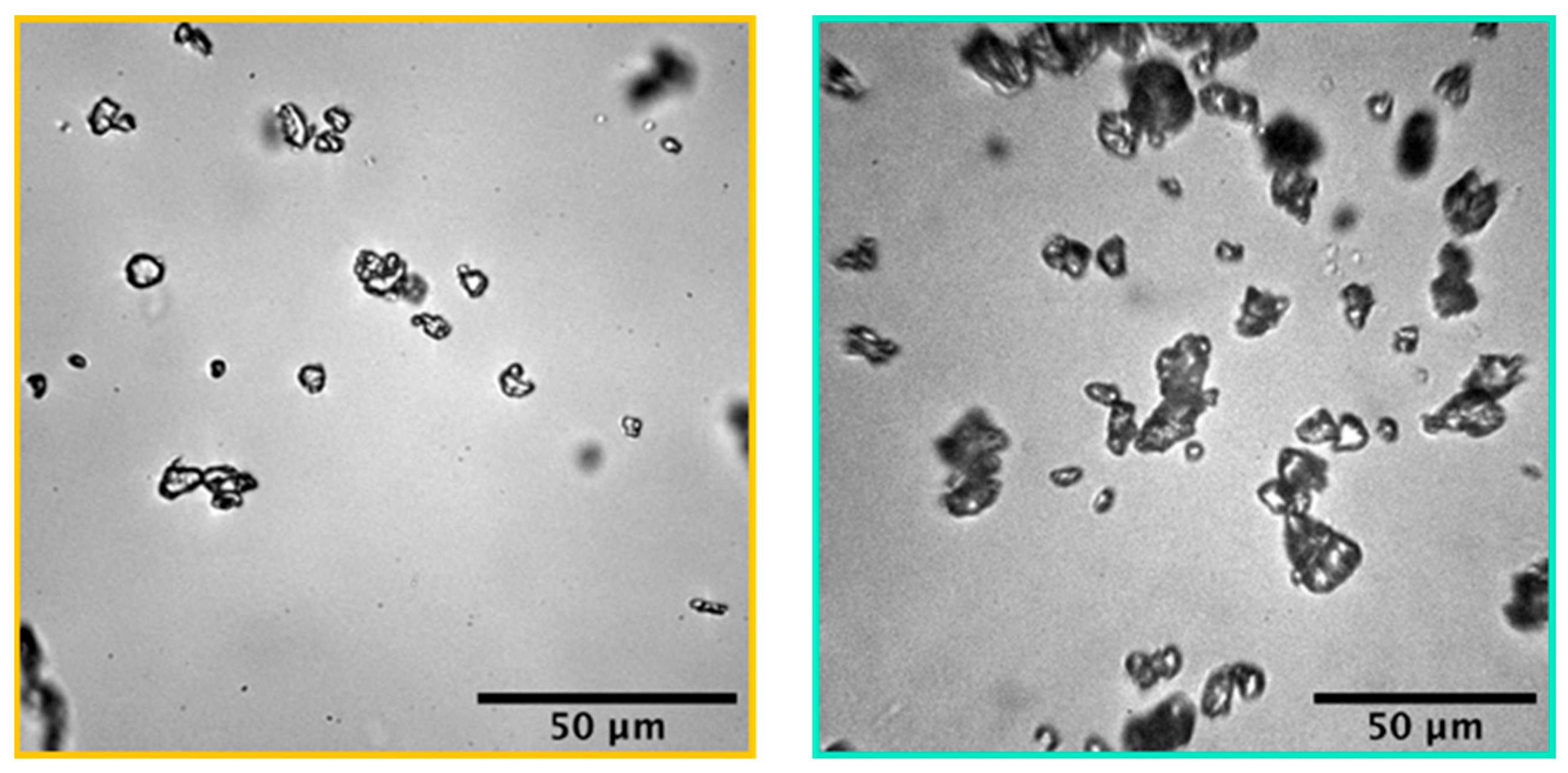
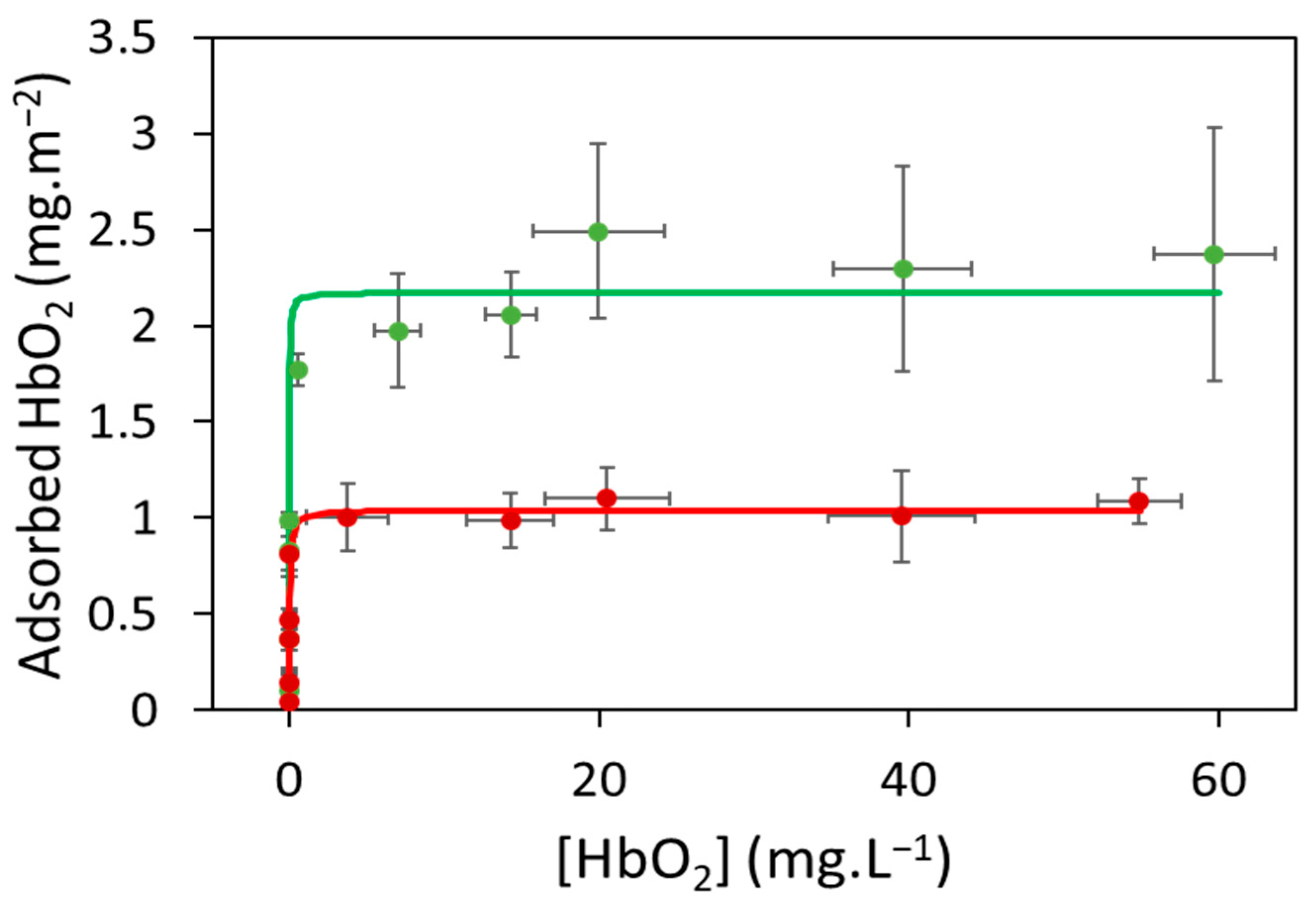


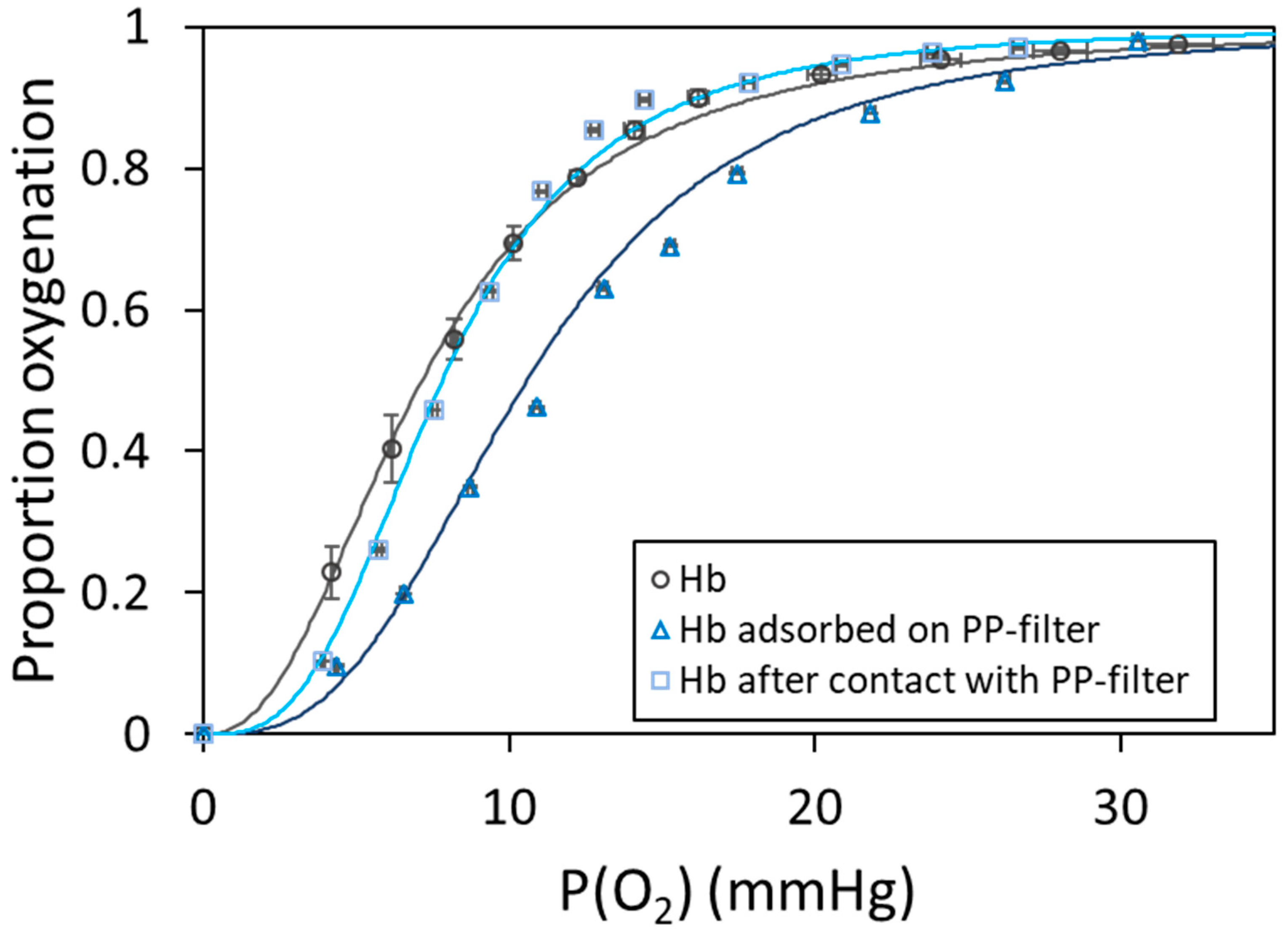
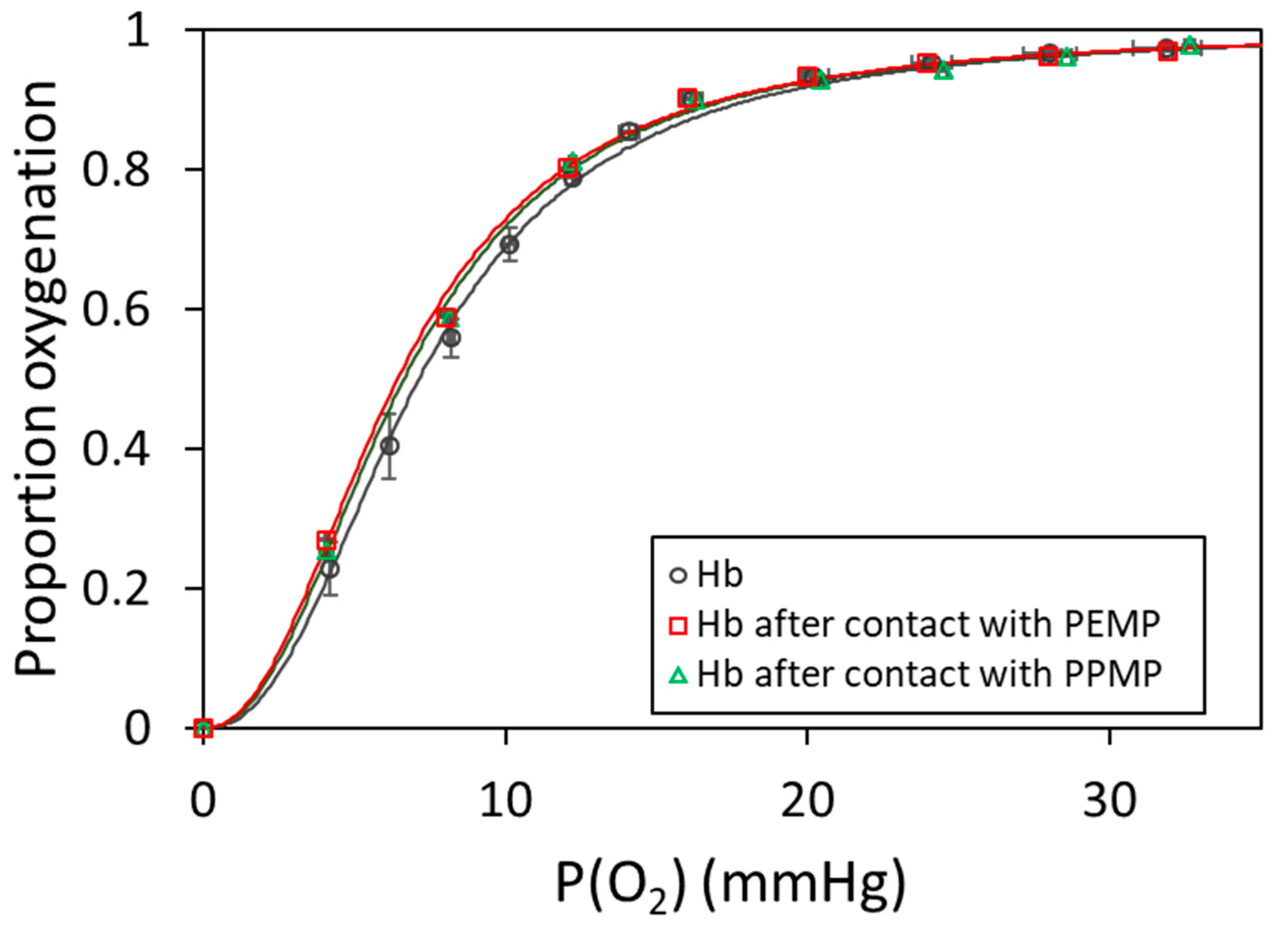
| Characterization Method | PEMP [57] | PPMP [57] | PP-Filter |
|---|---|---|---|
| Optical microscopy | d = 4.2 µm | d = 10.0 µm | |
| SAXS | S = 2.5 m2.g−1 | S = 1.1 m2.g−1 | S = 0.7 m2.g−1 |
| ATR-FTIR* | CI = 0.00–0.02 | CI = 0.02 ± 0.02 | CI = 0.09 ± 0.02 |
| XPS | 0.9% oxygen | No oxygen atoms | No oxygen atoms |
| Raman microscopy | 3 crystalline phases | 1 crystalline phase | 1 crystalline phase |
| Characterization Method | ag-PEMP | ag-PPMP |
|---|---|---|
| Optical microscopy | d = 5.8 µm | d = 9.9 µm |
| SAXS | S = 2.4 m2.g−1 | S = 1.2 m2.g−1 |
| ATR-FTIR | CI = 0.73 ± 0.04 | CI = 1.15 ± 0.04 |
| XPS | 2.6% oxygen atoms | 2.6% oxygen atoms |
| Raman microscopy | 3 crystalline phases | 1 crystalline phase |
| PEMP | ag-PEMP | PPMP | ag-PPMP | |
|---|---|---|---|---|
| m∞ (mg.m−2) | 1.0 ± 0.1 | 2.2 ± 0.2 | 2.2 ± 0.2 | 4.1 ± 0.2 |
| Kads (L.mg−1) | 26 ± 3 | 0.021 ± 0.005 | 92 ± 8 | 0.014 ± 0.002 |
| Sample | P50 (mmHg) | n |
|---|---|---|
| Hb | 7.09 ± 0.48 | 2.46 ± 0.02 |
| Hb on PP-filter | 10.57 ± 0.42 | 2.96 ± 0.10 |
| Hb after PP-filter | 7.81 ± 0.46 | 3.01 ± 0.05 |
| Hb after PEMP | 6.43 ± 0.46 | 2.24 ± 0.03 |
| Hb after PPMP | 6.64 ± 0.46 | 2.28 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saudrais, F.; Schvartz, M.; Renault, J.-P.; Vieira, J.; Devineau, S.; Leroy, J.; Taché, O.; Boulard, Y.; Pin, S. The Impact of Virgin and Aged Microstructured Plastics on Proteins: The Case of Hemoglobin Adsorption and Oxygenation. Int. J. Mol. Sci. 2024, 25, 7047. https://doi.org/10.3390/ijms25137047
Saudrais F, Schvartz M, Renault J-P, Vieira J, Devineau S, Leroy J, Taché O, Boulard Y, Pin S. The Impact of Virgin and Aged Microstructured Plastics on Proteins: The Case of Hemoglobin Adsorption and Oxygenation. International Journal of Molecular Sciences. 2024; 25(13):7047. https://doi.org/10.3390/ijms25137047
Chicago/Turabian StyleSaudrais, Florent, Marion Schvartz, Jean-Philippe Renault, Jorge Vieira, Stéphanie Devineau, Jocelyne Leroy, Olivier Taché, Yves Boulard, and Serge Pin. 2024. "The Impact of Virgin and Aged Microstructured Plastics on Proteins: The Case of Hemoglobin Adsorption and Oxygenation" International Journal of Molecular Sciences 25, no. 13: 7047. https://doi.org/10.3390/ijms25137047






